Abstract
Objective
Cytogenetic analysis of spontaneous abortions is frequently complicated by culture failure and maternal cell contamination. The objective of the study is to demonstrate that multiplex FISH can increase the yield and accuracy of karyotypes from spontaneous abortion specimens.
Method
A multiplex interphase FISH probe set was used to analyze two sample sets: First, uncultured tissues from 153 abortions samples with a normal 46,XX karyotype; and Second, a series of 171 samples that either failed to grow or were contaminated. Maternal cell contamination (MCC) studies were performed on 70 cultures where both karyotype and FISH indicated a normal female karyotype.
Results
FISH showed 31% (53/171) of the specimens karyotyped as 46,XX were either male or abnormal. 23% (40/118) of these specimens were found to have an abnormal chromosome complement. In specimens with culture failure, FISH showed an abnormal complement in 44.4% (68/153). MCC studies showed 41.49% (29/70) cultures of maternal origin, 45.7% (32/70) fetal, 11.4% (8/70) a maternal/fetal mixture and 1 diploid mole.
Conclusion
Results demonstrate the utility of a simple FISH panel in increasing the detection rate of abnormal karyotypes. They also reveal the high frequency of overgrowth of maternal cells in cultured specimens from villi after embryonic loss.
Introduction
Cytogenetic analysis of a spontaneously aborted conceptus provides valuable information for patients. Knowledge of a chromosome abnormality in the abortus specimen precludes further testing regarding the cause of the abortion and provides a better assessment of recurrence risk for the couple. Standard cytogenetic analysis of spontaneous abortions requires culture and karyotype of chorionic villi or fetal tissues. Success rates for culture and karyotype of the products of conceptions vary among laboratories, ranging from 60 to 90% (Benkhalifa et al., 2005, Bruno et al., 2006, Donaghue et al., 2010, Fritz et al., 2001, Lomax et al., 2000). Although successful culture and karyotype can be achieved in the majority of specimens, there are still two major problems associated with culture of fetal tissues: (a) failure of growth and/or microbial contamination in culture and (b) maternal cell contamination of the culture. The latter is often revealed by a preponderance of apparent females among the specimens with normal karyotypes and by the common phenomenon of observing a 46,XX cell line along with an abnormal karyotype.
Aneuploidy is the major cause of reproductive loss, accounting for up to 50% of early spontaneous abortions (Warburton, 2000), depending upon the maternal age distribution of the population. In a previous study (Jobanputra et al., 2002) we validated a multiplex interphase FISH probe set for chromosomes 13, 15, 16, 18, 21, 22, X and Y as a screen for common aneuploidies in uncultured tissues from spontaneous abortions. This probe set detected polyploidy as well as all common monosomies and trisomies and was able to detect ∼80% of all chromosome abnormalities in our series.
We have now used this approach in a research study involving karyotyped spontaneous abortions and have also introduced it into our clinical cytogenetics laboratory, with the goal of increasing the yield and accuracy of karyotypes from spontaneous abortion specimens. Here we report the results of this approach.
Materials and methods
Study Specimens
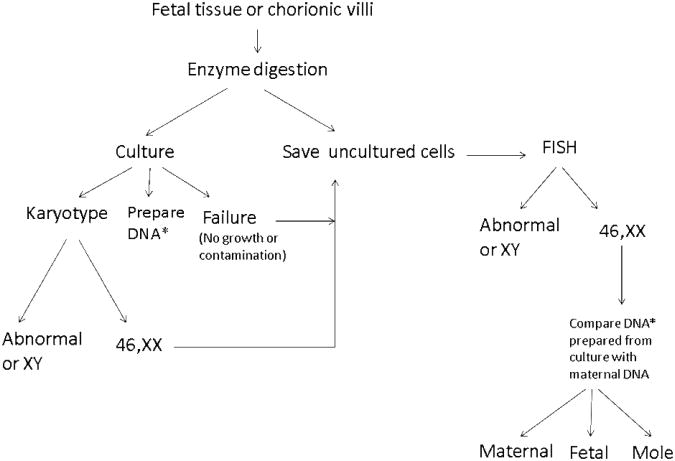
Fetal tissue specimens were obtained from (1) a research study of consecutive spontaneous abortions (Warburton et al., 2009) derived from a hospital-based sample identified here as TVH (n = 157) and (2) specimens submitted to our clinical cytogenetic laboratory identified as NYP (n = 167). The maternal age distributions of the two study populations were comparable. The mean maternal age for the TVH sample was 34.8 years (range: 18-48 years) and the mean maternal age of the NYP sample was 34.1 years (range: 19-45 years). In the TVH study sample, 44.2% of women were older than 35 years and 14% were older than 40 years. In the NYP population 42.2% of women were older than 35 years and 17.5% were older than 40 years. The sample processing strategy for culture, karyotype, FISH and MCC studies is shown in Figure 1.
Figure 1.
Protocol for processing specimens from spontaneous abortions in the laboratory: Fetal tissue (usually chorionic villi) are carefully dissected and enzyme digested to obtain a single cell suspension. This cell suspension is used to set up cultures for karyotyping and the uncultured cells are saved at 4°C for FISH if required. DNA is also prepared from the cultured cells when MCC studies are required. FISH is performed on the uncultured cells if the karyotype is 46,XX or no karyotype results are obtained because of growth failure or contamination. *MCC studies are performed using DNA from culture when both karyotype and FISH show a normal female chromosome complement.
Cytogenetic and FISH analysis
All villous specimens were examined under a dissecting microscope, freed as far as possible of decidual tissues, and washed clean of blood. Fifteen to 25 mg of tissue were digested with trypsin and collagenase and cultured using standard procedures. There was no attempt to separate trophoblast from mesenchymal tissue. The digested tissue suspension was divided into two parts, one used for tissue culture and an aliquot that was fixed and saved for possible FISH. The protocol and FISH probes are described more fully in our validation study (Jobanputra et al., 2002, Warburton et al., 2009). G-banded karyotype analysis was usually performed on coverslips harvested in situ, although occasionally trypsinization was necessary before harvest. When the karyotype was abnormal or normal male, 10 cells were scored. When the karyotype was normal female, 20 cells were scored to search for a second cell line.
Interphase FISH with the probe panel for chromosomes 13, 15, 16, 18, 21, 22, X and Y was performed on 171 uncultured cell suspensions from spontaneous abortions samples showing a pure 46,XX karyotype and in 153 samples that failed to grow or were contaminated in culture. For each probe mix, 20 interphase cells were scored, and those cases in which no abnormalities were seen with the FISH panel were scored as normal male or female.
Maternal Cell Contamination (MCC) studies
To determine whether normal female fetal specimens identified by both karyotype and FISH analysis were misclassified because of overgrowth of maternal cell in the culture, we carried out MCC studies. Maternal bloods were available only for TVH samples. A total of 70 samples with both maternal and fetal DNA were available for these studies. The fetal DNA could not be made from the same dish that was used for culture and karyotype; rather, it was made from another dish set up at the same time from the same suspension. Thus differences between the samples should reflect only stochastic sampling differences.
DNA was extracted following trypsin treatment and transfer of the released cells to a separate tube. A previously described set of 18 polymorphic PCR markers (Brown et al., 2006) was used in conjunction with capillary electrophoresis to analyze maternal-fetal DNA pairs for evidence of maternal cell contamination. All fetal samples were scored as “pure fetal”, “pure maternal contamination” or “mixed”. In order to be scored as “pure fetal”, an abortion sample had to have at least two heterozygous markers, each with one maternal and one clearly non-maternal allele. To be scored as “pure maternal contamination” all alleles for all markers had to be consistent with being maternally derived. To be scored as “mixed”, both maternal alleles as well as a third non-maternally derived allele had to be present for at least two loci. By testing artificial mixtures of maternal and fetal DNA, we determined that the limit of detection was approximately 25% maternal cell contamination (data not shown).
Results
The two sample populations showed very similar results. Thus, we combined the data in most analyses.
From the 171 samples in which cultured cells showed a 46,XX karyotype (Table 1), FISH showed a normal female in 118 (69%), a normal male in 13 (7.6%) and an abnormal complement in 40 (23.4%). There were 2 cases of monosomy 15 and 3 cases of monosomy 21 among the abnormals. Otherwise the abnormalities were distributed as expected among spontaneous abortions.
Table 1. Interphase FISH on uncultured cells for samples that were 46,XX by karyotype.
| N | FISH Result | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XX | XY | +13 | +15 | −15 | +16 | +18 | +21 | −21 | +22 | 45,X | Triploid | Tetraploid | Sex trisomy | Double Anomaly | Differs from Karyotype (%) | Abnormal (%) | ||
| NYP | 62 | 39 | 6 | 1 | 2 | 2 | 1 | 0 | 3 | 0 | 3 | 2 | 2 | 0 | 0 | 1A | 23 (37.0%) | 17 (27.4%) |
| TVH | 109 | 79 | 7 | 2 | 3 | 0 | 3 | 1 | 2 | 2 | 2 | 4 | 0 | 2B | 0 | 2C | 30 (27.5%) | 23 (21.1%) |
| Total for all data sets | 171 | 118 | 13 | 3 | 5 | 2 | 4 | 1 | 5 | 2 | 5 | 6 | 2 | 2 | 0 | 3 | 53 (31.0%) | 40 (23.4%) |
This case had an XYY sex chromosome complement and monosomy 21
One case was mosaic with both XY and XXYY cells
XX,+15,+15 and XXYY,+15
Among 153 specimens that failed to grow or were microbially contaminated (Table 2), FISH showed a normal female in 46 (30%), a normal male in 39 (25.4%) and an abnormal complement in 68 (44.4%). Even allowing for the 20% of abnormalities that FISH would have missed, there is no indication that the specimens failing to grow had a higher incidence of abnormalities than those that grew normally.
Table 2. Interphase FISH on uncultured cells for samples that did not yield a karyotype result.
| N | FISH Result | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XX | XY | +13 | +15 | −15 | +16 | +18 | +21 | −21 | +22 | 45,X | Triploid | Tetraploid | Sex trisomy | Double anomaly | Abnormal (%) | ||
| NYP | 105 | 31 | 30 | 0 | 3 | 0 | 7 | 3 | 8 | 2 | 1 | 12B | 4 | 0 | 2 | 1A | 44 (41.9%) |
| TVH | 48 | 15 | 9 | 0 | 4 | 0 | 2 | 2 | 0 | 2 | 3 | 2 | 6 | 1 | 1 | 1C | 24 (50%) |
| Total | 153 | 46 | 39 | 0 | 7 | 1 | 9 | 5 | 8 | 4 | 4 | 14 | 10 | 1 | 3 | 2 | 68 (44.4%) |
This case had both monosomy 15 and trisomy 21
Two of these cases were mosaic with both X and XX cells present
XX,+18,+21
The skewed sex ratio (109 apparently normal females: 62 normal males) among the TVH cultured samples prior to analysis by FISH (Warburton et al. 2009) implies that maternal cell contamination is a common occurrence, despite our careful efforts to prevent it. We set out to determine how frequently 46,XX cultures actually represented overgrowth of maternal cells, by using PCR based polymorphic markers to look for marker segregation in a series of samples where both maternal and putative fetal DNA was available.
We analyzed 70 samples where both the karyotype on the cultured material and the FISH studies on uncultured material showed 46,XX. Thirty-two (45.7%) abortus tissue samples had only one maternal allele for at least two informative loci and were therefore confirmed as pure fetal. Another 29 (41.4%) samples included only maternal alleles and eight (11.4%) were mixed. One sample was a diploid mole with only non-maternal alleles. Overall, about half of all cultured samples were compromised by maternal cell contamination.
Discussion
The incidence of chromosome abnormalities in spontaneous abortions, and their distribution by type, are often quoted statistics. However, there are many factors that may influence the perceived and the actual proportion of abortuses that have chromosome abnormalities: (1) selection bias (e.g., over-representation of recurrent abortions or fetuses with ultrasound abnormalities), (2) the developmental age distribution, (3) the maternal age distribution of the pregnant population being sampled, (4) the quality of tissue samples that are received, which may depend upon whether the sample is selected by a pathologist, obstetrician or a cytogenetics laboratory technician with access to the complete specimen, and (5) the expertise of the cytogenetic laboratory staff in selecting fetal material for culture, culture methods and culture success rate. As an example, the percentage trisomy among spontaneous abortion specimens in research studies performed in the New York area increased from 26% for studies done in the 1970's-1980's, to 44% for more recent studies from 1990 to the present. A small part of this increase is due to improved culture success rates and selection of samples for earlier developmental age. However, most of the change can be attributed to the older age at pregnancy in the later sample; the mean age at miscarriage was 6.6 years older in the latter time period (Warburton, 2007).
Chromosome studies of the abortus provide important information for the recurrence risk of chromosome abnormality and miscarriage in subsequent pregnancies of couples experiencing spontaneous abortions. Following a pregnancy loss with trisomy, there is an increased recurrence risk for other trisomies in subsequent pregnancies (Warburton et al., 2004). However, the recurrence risk for spontaneous abortion is lower if the fetus has a chromosome abnormality (Carp et al., 2001, Stephenson et al., 2002, Warburton et al., 1987) and the finding of a chromosome abnormality in the fetus eliminates the need for other testing to find the cause of the miscarriage. Given the clinical significance of the chromosome findings, it is important for the cytogenetics laboratory to provide accurate and cost effective results.
Although array-based methods and the multiplexed ligation dependent probe amplification (MLPA) assay have significant advantages over conventional karyotyping, they are not efficient for a routine and robust test to detect chromosome abnormalities in products of conception. Major limitations of the CGH, microarray analysis and MLPA assays include inability to detect polyploidy, which is common in miscarriage (∼7 to 10%), mosaic aneuploidies and balanced rearrangements. The additional costs of doing FISH and MCC are about $200 for those cases that require it (46,XX and growth failure). This cost is similar to that of a low resolution microarray analysis. While microarray analysis may become the method of choice soon, its use in abortus specimens awaits results from studies addressing the issues of finding small copy number variations of unknown clinical significance. Our FISH procedure has the advantage that it is easy for cytogenetic laboratories to use and it increases the number of abnormalities detected by 10%.
A multiplex FISH panel of 8 chromosome specific probes (13, 15, 16, 18, 21, 22, X and Y) is simple to use and provides significant additional information for cases with culture failure and 46,XX cases that might represent maternal cell contamination of the culture. This probe set can detect not only the most common trisomies (15, 16, 21 and 22), but also other frequent chromosomal anomalies in spontaneous abortions: monosomy X, triploidy and tetraploidy. Additionally it is also an efficient method of detecting mosaicism on the interphase nuclei and can be used on paraffin-embedded fetal tissues. The latter is particularly useful in providing results when the products of conceptions are fixed at the time of pathological examination and the need for karyotype analysis is only recognized later. In this study we scored 20 interphase nuclei, which detects 14% mosaicism with 95% confidence (Hook, 1977). Increasing the number of cells scored to 50 would allow detection of lower level (6%) mosaicism.
In this study, FISH on uncultured cells demonstrated an incorrect karyotype in 31% (53/171) of the specimens with 46,XX karyotype, of which 40 (75%) were abnormal. The MCC studies also detected a very high rate of maternal cells in cultures that were 46,XX both by karyotype and our FISH panel. Even after a careful and meticulous dissection of fetal tissues by our experienced laboratory staff, almost 40% (29/70) of the 46,XX specimens appear to have been maternal karyotypes. This contamination rate is comparable to that noted by other studies (Bell et al., 1999, Jarrett et al., 2001, Menten et al., 2009) that used digested villous tissues.
In the TVH sample, we initially ascertained by karyotyping alone 62 normal males and 109 normal females (sex ratio M/F = 0.56). Eliminating the 30 female cases shown by FISH not to be normal female and the 29 cases presumed maternal based on MCC studies, leaves 50 normal females. An additional seven specimens were re-classified as normal males based on FISH, yielding 69 males and 50 females. Among samples in which tissue culture failed, we identified by FISH 9 normal males and 15 normal females. Adding these samples to the 69 normal males and 50 normal females above, the overall sex ratio to 78 males:65 females (sex ratio=1.2), closer to the expected sex ratio of 1.0.
Chromosome abnormalities were found in approximately 44% of the culture failures. It has been speculated that specimens that fail to grow would include the rarely seen anomalies that do not sustain culture growth. In our study the frequency of chromosome anomalies detected is not higher than that from karyotyped specimens. However, our FISH probes did not test for the rarer trisomies. We observed an unusually high frequency of monosomy 21 and 15 (10 or 2.7% of all specimens) in both the culture failures and the 46,XX specimens.
Our study shows the utility of FISH and MCC studies for increasing the informativeness of cytogenetic studies on spontaneous abortion specimens. Our data from the TVH sample show that culture and karyotype revealed chromosome abnormalities in 54% of specimens, while using FISH showed that at least 65.5% of specimens are abnormal. Traditional methods of culture and karyotype would provide correct results in 84.4% of cases whereas the combined approach will provide correct results in 95.2% of cases. Our integrated approach increases the yield and accuracy of karyotypes from spontaneous abortion specimens with minimal additional expense and effort.
Acknowledgments
This work was supported by a grant from the National Institutes on Child Health and Development (R01 HD 42725) to DW. VJ received support from a grant (KL2 RR024157) from the National Center for Research Resources. We thank Ann Kinney for help in summarizing the TVH data.
Footnotes
Conflict of interest statement: None declared
References
- Bell KA, Van Deerlin PG, Haddad BR, Feinberg RF. Cytogenetic diagnosis of “normal 46,XX” karyotypes in spontaneous abortions frequently may be misleading. Fertil Steril. 1999;71:334–41. doi: 10.1016/s0015-0282(98)00445-2. [DOI] [PubMed] [Google Scholar]
- Benkhalifa M, Kasakyan S, Clement P, Baldi M, Tachdjian G, Demirol A, Gurgan T, Fiorentino F, Mohammed M, Qumsiyeh MB. Array comparative genomic hybridization profiling of first-trimester spontaneous abortions that fail to grow in vitro. Prenat Diagn. 2005;25:894–900. doi: 10.1002/pd.1230. [DOI] [PubMed] [Google Scholar]
- Brown L, Abigania M, Warburton D, Brown S. Validation of QF-PCR for prenatal aneuploidy screening in the United States. Prenat Diagn. 2006;26:1068–74. doi: 10.1002/pd.1558. [DOI] [PubMed] [Google Scholar]
- Bruno DL, Burgess T, Ren H, Nouri S, Pertile MD, Francis DI, Norris F, Kenney BK, Schouten J, Andy Choo KH, Slater HR. High-throughput analysis of chromosome abnormality in spontaneous miscarriage using an MLPA subtelomere assay with an ancillary FISH test for polyploidy. Am J Med Genet A. 2006;140:2786–93. doi: 10.1002/ajmg.a.31552. [DOI] [PubMed] [Google Scholar]
- Carp H, Toder V, Aviram A, Daniely M, Mashiach S, BARKAI G. Karyotype of the abortus in recurrent miscarriage. Fertil Steril. 2001;75:678–82. doi: 10.1016/s0015-0282(00)01801-x. [DOI] [PubMed] [Google Scholar]
- Donaghue C, Mann K, Docherty Z, Mazzaschi R, Fear C, Ogilvie C. Combined QF-PCR and MLPA molecular analysis of miscarriage products: an efficient and robust alternative to karyotype analysis. Prenat Diagn. 2010;30:133–7. doi: 10.1002/pd.2424. [DOI] [PubMed] [Google Scholar]
- Fritz B, Aslan M, Kalscheuer V, Ramsing M, Saar K, Fuchs B, Rehder H. Low incidence of UPD in spontaneous abortions beyond the 5th gestational week. Eur J Hum Genet. 2001;9:910–6. doi: 10.1038/sj.ejhg.5200741. [DOI] [PubMed] [Google Scholar]
- Hook EB. Exclusion of chromosomal mosaicism: tables of 90%, 95% and 99% confidence limits and comments on use. Am J Hum Genet. 1977;29:94–7. [PMC free article] [PubMed] [Google Scholar]
- Jarrett KL, Michaelis RC, Phelan MC, Vincent VA, Best RG. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46,XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198–203. doi: 10.1067/mob.2001.114692. [DOI] [PubMed] [Google Scholar]
- Jobanputra V, Sobrino A, Kinney A, Kline J, Warburton D. Multiplex interphase FISH as a screen for common aneuploidies in spontaneous abortions. Hum Reprod. 2002;17:1166–70. doi: 10.1093/humrep/17.5.1166. [DOI] [PubMed] [Google Scholar]
- Lomax B, Tang S, Separovic E, Phillips D, Hillard E, Thomson T, Kalousek DK. Comparative genomic hybridization in combination with flow cytometry improves results of cytogenetic analysis of spontaneous abortions. Am J Hum Genet. 2000;66:1516–21. doi: 10.1086/302878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menten B, Swerts K, Delle Chiaie B, Janssens S, Buysse K, Philippe J, Speleman F. Array comparative genomic hybridization and flow cytometry analysis of spontaneous abortions and mors in utero samples. BMC Med Genet. 2009;10:89. doi: 10.1186/1471-2350-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Hum Reprod. 2002;17:446–51. doi: 10.1093/humrep/17.2.446. [DOI] [PubMed] [Google Scholar]
- Warburton D, Kline J, Stein Z, Hutzler M, Chin A, Hassold T. Does the karyotype of a spontaneous abortion predict the karyotype of a subsequent abortion? Evidence from 273 women with two karyotyped spontaneous abortions. Am J Hum Genet. 1987;41:465–83. [PMC free article] [PubMed] [Google Scholar]
- Warburton D. Cytogenetics of reproductive wastage: from conception to birth. Marcel Dekker; New York: 2000. [Google Scholar]
- Warburton D, Dallaire L, Thangavelu M, Ross L, Levin B, Kline J. Trisomy recurrence: a reconsideration based on North American data. Am J Hum Genet. 2004;75:376–85. doi: 10.1086/423331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D. 2006 William Allan Award Address. Having it all. Am J Hum Genet. 2007;81:648. doi: 10.1086/521405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, Kline J, Kinney A, Yu CY, Levin B, Brown S. Skewed X chromosome inactivation and trisomic spontaneous abortion: no association. Am J Hum Genet. 2009;85:179–93. doi: 10.1016/j.ajhg.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]