Abstract
Despite important progress in adjuvant and neoadjuvant therapies, metastatic disease often develops in breast cancer patients and remains the leading cause of their deaths. For patients with established metastatic disease, therapy is palliative, with few breaks and with mounting adverse effects. Many have hypothesized that a personalized or precision approach (the terms are used interchangeably) to cancer therapy, in which treatment is based on the individual characteristics of each patient, will provide better outcomes. Here, we discuss the molecular basis of breast cancer metastasis and the challenges in personalization of treatment. The instability of metastatic tumors remains a leading obstacle to personalization, because information from a patient’s primary tumor may not accurately reflect the metastasis, and one metastasis may vary from another. Furthermore, the variable presence of tumor subpopulations, such as stem cells and dormant cells, may increase the complexity of the targeted treatments needed. Although molecular signatures and circulating biomarkers have been identified in breast cancer, there is lack of validated predictive molecular markers to optimize treatment choices for either prevention or treatment of metastatic disease. Finally, to maximize the information that can be obtained, increased attention to clinical trial design in the metastasis preventive setting is needed.
CME Accreditation Statement: This activity (“ASIP 2013 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2013 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Although personalizing the treatment of breast and other cancers is a promising goal, individualizing treatments will require a wealth of new molecular data and therapeutic options. Much of the recent progress has been in personalizing treatment of early breast cancer. These efforts have moved beyond already established therapies for patients with estrogen receptor–positive (ER+) and/or progesterone receptor–positive (PR+) and HER2-overexpressing (HER2+) disease, primarily using multigene assays that are prognostic for risk of recurrence and predictive for response to cytotoxic chemotherapy.
Personalized medicine for metastatic disease presents greater hurdles, however. The complexity, heterogeneity, and genomic instability of metastatic breast cancer cells make their evaluation and therapy a challenging process (Table 1). Thus, although this review sheds light on potentially important aspects of personalized medicine in metastatic breast cancer, the potential remains to be realized.
Table 1.
Hallmarks of Metastasis and Their Implications for Personalized Medicine
| Hallmark | Implications for Personalized Medicine |
|---|---|
| Heterogeneity between primary tumors and metastases, and among metastases | Therapy based on primary tumor characteristics may not be effective |
| Redundancy of mechanistic pathways | Need for combination therapies |
| Variable dormancy | Clinical trials must address delayed relapses |
| Contributions of cancer-initiating cells | Incorporation of therapies that target stem cells |
Metastasis has been described mechanistically as the migration of tumor cells from the primary tumor, followed by intravasation, survival, extravasation of the circulatory system, and progressive colonization of a distant site.1–3 This mechanistic description does not capture other equally valid characteristics, however. In a second definition, that of parallel progression, the defining feature is tumor cell genomic instability, promoting selection for characteristics that enable invasion and distant organ colonization.4 From this perspective, it is not the steps in metastasis that are critical, but rather the instability that fuels the process. In yet another definition, metastasis is described in terms of seed and soil.5 Tumor cells (seeds) spread widely through the body, but grow only in supportive locations (congenial soil). Thus the various microenvironments (soils) of metastases contribute to the observed heterogeneity. Layered over the fundamentals of the metastatic process is acquired or innate resistance to therapies. Because only 5% of breast cancer patients have stage IV disease at initial diagnosis (http://www.seer.cancer.gov/statfacts/html/breast.html; accessed February 1, 2013), in the majority of metastatic patients the metastatic disease develops after hormone therapy, chemotherapy, or biologics have been used in adjuvant treatment.
Another hallmark of metastasis is the redundancy of pathways that mediate the process or its component steps. Genes promoting breast cancer metastasis abound, including ERBB2 (alias HER2, NEU), CTNNB1, KRAS, PI3KCA (alias PI3K), EGFR, MYC, TWIST1, SNAI1 (alias SNAIL), SNAI2, MET, and ID1.6 Some genes are involved in tumor cell survival and colonization in the metastatic site in a generalized manner, including PTGS2, EREG, MMP1, LOX, ANGPTL4, and CCL5. Other genes, such as PTHLH (alias PTHRP), IL11, CSF2RB, IL6, and TNF (previously TNFA) function in a more organ-specific manner.6 Several of these pathways in genetically engineered mouse models exhibit oncogene addiction, with the ablation of the expression of a single gene causing tumor regression.7 Does this portend better responses to pathway inhibitors? Most pathways are only partial contributors to the metastatic process, meaning that their inhibition would have at-best partial effects and could be overcome by other contributory pathways. Additional pathways suppress metastasis, either by inhibiting tumorigenesis8 or by specifically suppressing the metastatic process, the latter defined as metastasis suppressor genes.9,10 Successful personalized medicine approaches will have to deal with the instability, complexity, and multifactorial nature of the metastatic process.
Research into the metastatic process is relevant to personalizing both the adjuvant and metastatic clinical settings. In the adjuvant setting, micrometastases are thought to be present, and systemic therapy is administered to prevent their outgrowth. In the metastatic setting, treatment aims to shrink lesions that have completed the metastatic process and to prevent the outgrowth of further metastases. Both preclinical and clinical data suggest that a given drug may not be equally effective in both settings.
Factors Important to Personalizing Therapy for Preventing and Treating Metastatic Disease
Semipersonalized Medicine
There appear to be degrees of personalized medicine. Semipersonalized medicine is based on the identification of large groups of patients with certain tumor characteristics that can direct a given patient to corresponding specific types of therapy. True personalized medicine would be based on an individual patient’s tumor, directing to a tailored therapy maximized for effectiveness for that one patient in particular. Semipersonalized medicine has already generated effective therapies for groups of patients and thus can provide a basis for personalized approaches. Here, we address three examples: HER2-directed therapies, anti-estrogenic therapies, and bisphosphonate and antibody therapies.
HER2-Directed Therapies
The tyrosine kinase receptor proto-oncogene c-ErbB-2 (hereafter referred to by the familiar alias HER2) is overexpressed or amplified in approximately 25% of breast cancers, and is a significant prognostic marker of shorter relapse-free and overall survival.11 HER2 is a transmembrane tyrosine kinase receptor and a member of the EGFR family, which also includes HER1 (EGFR), HER3, and HER4. Additionally, HER2 can interact reversibly with ligand-activated family members to form active heterodimers, leading to phosphorylation of intracellular tyrosine residues. This activation recruits cytoplasmatic signal transducers such as STAT, p85-PI3K, PLC-γ, and Src. Two of the main downstream pathways activated by HER2 are the MAPK and PI3K–AKT pathways promoting cell survival, cell proliferation, and migration.12
HER2+ breast cancer patients derive significant benefit from HER2-targeted therapy, such as the humanized monoclonal antibody trastuzumab combined with chemotherapy in the adjuvant and metastatic settings.13 Although the benefit of trastuzumab-based therapy is undeniable, approximately 50% of HER2-overexpressing breast cancers do not respond to trastuzumab,13 suggesting the need for greater precision. Lapatinib, a small-molecule inhibitor of HER2 and EGFR, has shown efficacy in the metastatic setting after relapse on trastuzumab-based therapy.14 Several newer therapies aimed at the HER superfamily (EGFR and HER2 to HER4) have been approved or are in late development; these include pertuzumab15 and trastuzumab emtansine (T-DM1).16 These vary in potency and specific target within the superfamily.
In animal models, overexpression of HER2 promotes metastasis to lymph node, lung, bone, and brain.17–19 Studies of HER2 promotion of metastasis have identified several pathways, including a bidirectional interaction with the TGF-β/Smad pathway,20 an increase in expression and stability of the homing chemokine receptor CXCR4,21 an activation of Src with consequent phosphorylation of FAKtyr861 and activation of p120/Rac1/Cdc42,22 and an increase in angiogenesis through up-regulation of VEGF23 and angiopoietin-2.24 It will be of interest to determine whether inhibitors of these pathways, in combination with HER2 therapy, provide a better degree of metastasis prevention or shrinkage of established lesions.
Anti-Estrogenic Therapies
At diagnosis, 75% of breast tumors are ER+ and can potentially respond to tamoxifen, aromatase inhibitors, or other hormonal therapies.25,26 ER+ tumors tend to metastasize to the bone,27 and often metastasize late.28 A recent long-term follow-up study after 5 years of tamoxifen therapy showed that metastatic relapses continue over the next 10 years and have not leveled off at that time point, suggesting a continuous break from dormancy.29
Estrogen effects are mediated by two specific nuclear receptors, estrogen receptor α (ER-α) and estrogen receptor β (ER-β). ER-α is expressed in breast and is associated with increased proliferation and metastasis. On binding to the ligand, ER regulates the transcription of target genes. ER can also form multiprotein complexes with membrane-related factors such as Src, G-proteins, RTK, and PELP1.30 Downstream of ER lies the activation of Src, Ras, and MAPK signaling to promote cell proliferation, PI3K–Akt to induce survival and invasion, and the Rho family GTPases Rac and Cdc42 to promote cell migration, invasion of the extracellular matrix, and metastasis.31 Crosstalk between ER signaling and growth factor pathways is correlated with both cancer progression and resistance to hormonal therapy.32 A link between ER signaling and the epithelial–mesenchymal transition has been described.33 Finally, estrogen affects the cytokine milieu in the cancer microenvironment.34
The wide range of endocrine therapy options provides an opportunity to select the optimal sequence and combination of therapeutic agents after recurrence or relapse. Current strategies focus on combinations with growth factor and PI3 kinase pathway targeting agents, such as gefitinib and everolimus.35,36 Combinations with other pathway inhibitors may hold promise. The potential of gene signatures for individualizing therapy in the ER+ setting is under testing in the TAILORx trial. The purpose of this randomized phase III trial is to identify the best individual therapy for node-negative, ER+ breast cancer patients, classifying them by using the 21-gene signature Oncotype DX.37 If validated, this signature could provide patients with an individualized estimate of therapeutic benefit.
Bisphosphonates and RANKL Antibody
Breast cancer patients with bone metastases have distinct therapeutic options, including bisphosphonates and (more recently) denosumab, typically in combination with endocrine or HER2-directed therapies. The bone metastatic process has been described as a vicious cycle.38 Alterations in the bone microenvironment are the initiators of this cycle; major changes include hypoxia, acidic pH, and increased levels of extracellular calcium and growth factors.39 The osteolytic vicious cycle is characterized as bone lysis with concurrent infiltration of metastatic tumor cells. TGF-β and parathyroid hormone-related protein (PTHrP) both play an important role in osteolysis. PTHrP, produced by tumor cells, activates osteoblasts and osteoclasts via the RANK ligand (RANKL) pathway, resulting in bone resorption. Bone resorption releases growth factors, ionized calcium, and ionized phosphate. Release of ionized calcium results in an elevation of circulating PTHrP, increasing the propensity for osteolysis by osteoclasts and thus promoting the vicious cycle. Release of growth factors from the bone matrix, such as TGF-β, in turn activates tumor cells, contributing to the cycle.39 Denosumab is a human monoclonal antibody against RANKL. In a phase III trial, denosumab reduced the risk of developing multiple skeletal-related events (time to first and subsequent events) by 23%, compared with the bisphosphonate zoledronic acid.40
It must be noted that one consequence of semipersonalized medicine is that some patients will be overtreated, because not all members of the large groups on which semipersonalized treatment is based will benefit alike.
Factors That We Ignore at Our Peril
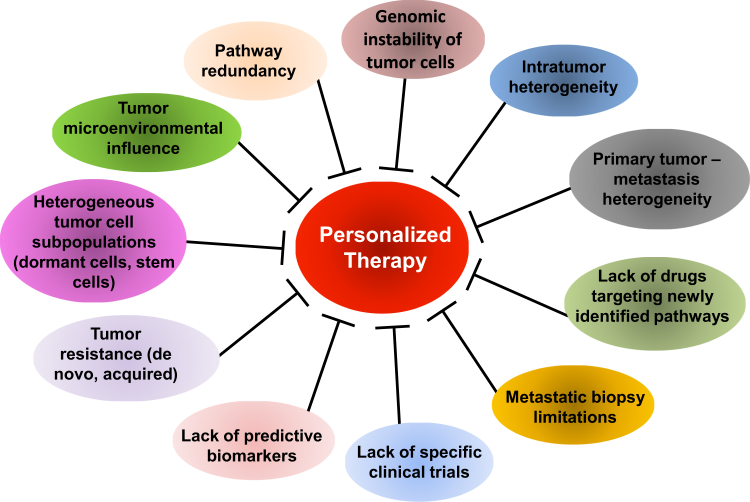
Metastatic disease is largely incurable because of the very factors that define it (Figure 1). As precision treatment approaches develop in the adjuvant and metastatic settings, consideration must be given in trial design to these factors: heterogeneity, genomic instability, sites of metastasis, tumor subpopulations, and microenvironmental influences.
Figure 1.
The complex of challenges in personalizing therapy in breast cancer metastasis. Metastasis is an intricate disease state, and many aspects of its biology and origin remain poorly understood. Thus, the complexity of the disease (with its genetic instability, tumor heterogeneity, and dormancy phenotype) limits accurate diagnosis and tailoring of treatments for patients. The lack of validated predictive biomarkers able to determine treatment choice and scarcity of metastasis-specific clinical trials constitutes a further limitation for implementation of personalized medicine in breast cancer metastasis.
Heterogeneity
Recent research has demonstrated heterogeneity in primary tumors and corresponding breast cancer metastases at the morphological, molecular, and genomic levels, and such heterogeneity may be a significant determinant of anticancer therapy response.41 Studies evaluating the traditional markers HER2, ER, and PR indicate 5% to 22%, 13% to 33%, and 31% to 32% discordance, respectively, between primary tumor and distant metastases.42–44 A retrospective study of 255 patients with matched primary breast cancer and liver metastatic samples available for evaluation reported discordance of 14.5%, 48.6%, and 13.9% for ER, PR, and HER2, respectively.45 Interestingly, the authors reported the influence of receptor status discordance on the therapeutic regimen: based on the metastatic biopsy, treatment was changed for 12.1% of the patients. Larger studies will be needed to fully assess the frequency and potential benefits of altering therapy because of discordance of tumor characteristics in metastatic sites.
For other therapeutic targets, discordance between primary tumors and metastases reigns. Akcakanat et al46 determined whether primary tumors differed from their metastases in their expression of p-Akt and p-4E-BP1, components of the therapeutically relevant PI3K pathway. They observed poor concordance between immunohistochemical levels of p-Akt and p-4E-BP1 expression in primary tumors and metastases. Wu et al47 observed extensive heterogeneity between primary breast carcinomas and their paired metastases, as well as among multiple metastatic breast carcinomas from the same patient. They observed down-regulation of ER and PR and overexpression of COX-2, MET, EGFR, and mesothelin in metastatic versus primary lesions, and they concluded that therapeutic targets identified in the primary breast carcinoma, or even in some metastatic breast carcinomas, might not reflect targets present in all metastatic sites.47 DNA methylation of five marker genes [CCND2, RARB, TWIST1, SCGB3A1 (alias HIN1), and RASSF1] has been reported to be discordant between primary tumor and metastasis, and between metastases in a warm autopsy study.48
Mutational profiling of breast cancer metastases and either matched or unmatched primary tumors has recently identified a number of genetic alterations that, although not unique to metastases, occur more frequently in secondary lesions. For example, mutations in the tumor-suppressor gene TP53, which occur in roughly a quarter of primary breast carcinomas, were found at higher frequency (87%) in a series of 23 brain metastases of breast cancer, with a striking preponderance of complex TP53 mutations, such as frameshift, splice, and nonsense mutations, as well as in-frame insertions and deletions.49 Increased amplification of MYC in systemic metastases compared with primary breast tumors has been described.50 In brain metastases, the tumor-suppressor PTEN was more frequently mutated or lost because of allelic imbalance, compared with primary breast tumors.51
Recently, advanced next-generation sequencing techniques have been used to interrogate whole cancer genomes at the single-nucleotide level and have distinguished between mutations in breast cancer metastases that are present in rare cell populations of the primary tumor and to those arising de novo during metastatic progression. In the brain-metastatic progression of a basal-like breast cancer, most mutations were shared between the primary tumor and the metastasis, although a significant enrichment of missense mutations in NRK (a JNK activating protein kinase), PTPRJ (a protein tyrosine phosphatase), and WWRTR1 (a modulator of mesenchymal stem cell differentiation) was observed in the metastatic lesion.52 In contrast, the majority of mutations present in a metastatic lobular breast cancer specimen were not present in the primary tumor, suggesting substantial genetic evolution during the metastatic process.53
These data highlight two concerning findings. First, we cannot accurately predict the molecular profile of metastatic disease by profiling the primary tumor. Second, one metastasis may be distinct from another within the same individual. Effective personalized medicine will have to account not only for a person’s individual primary tumor data, but also for variances within and between metastases. Moreover, we still lack drugs targeting many of the newly identified pathways that are altered in metastatic lesions. The creation of new therapies based on detailed molecular profiling remains challenging.
Genomic Instability
The instability of metastatic breast cancer cells likely drives heterogeneity. Comparative genomic hybridization has been used as a tool to identify large scale genomic instability and identified two classes of breast cancer genomic structure: monogenomic, with one major clonal subpopulation with high chromosomal stability, and polygenomic, with multiple clonal subpopulations. Polygenomic clones may have shared or segregated anatomical distributions. Disparate clonal evolution may explain molecular discordance at the time of disease relapse.54 Comparison of the primary tumor and concurrent lymph node infiltrates using comparative genomic hybridization revealed extensive clonal genomic heterogeneity, indicating that the evolution of tumors can begin early.55 In addition, adjuvant therapy may contribute to biological discordance by clonal selection pressure, exemplified by the evolution of ER− subpopulations after endocrine therapy for predominantly ER+ disease.
Failure in the DNA break repair system, mitotic chromosome transmission, or the spindle mitotic checkpoint can cause the chromosomal lesions that are hallmarks of genomic instability. Consequently, high mutation rates and chromosomal rearrangements (deletions, duplications, and amplifications) may drive tumor progression by disruption of tumor-suppressor genes, formation of fusion proteins, constitutive activation of enzymes, or amplification of oncogenes.56 Whole-genome sequencing analyses of breast cancers revealed unexpectedly high levels of somatic mutations,57 which may confer a selective advantage on the tumor cells and thus promote their clonal expansion.
Sites of Metastasis
The extent to which metastases are site specific remains poorly understood. Certainly the vicious cycle has been established as being important to bone metastasis.39 However, other pathways also contribute to bone metastasis, and PTHrP, involved in the vicious cycle for bone, has been implicated in metastasis to other sites.58 Specific therapies are available for patients with bone metastases, and these need to be factored in for a personalized regimen. Other pathways have been reported to mediate lung59 and liver metastasis.60
Our research group and others have investigated brain-permeable compounds that prevent the formation of brain metastases or could potentially prevent the development of additional brain metastases in patients with a limited number of lesions. Lapatinib is the only traditional breast cancer drug, of 18 drugs tested, that has brain metastasis preventive activity in the HER2+ setting.61,62 In the model used, however, HER3 activation was lacking in the tumor cells; other models with activated HER3 signaling may show more resistance. Personalization of these trends would require the identification of those patients with HER2+ tumors at highest risk for development of brain metastases, as well as an understanding of the complex signaling pathways involved. A prospective study showed that 37.3% of all metastatic breast cancer patients with HER2+ tumors developed brain metastases over 7.1 to 13.3 months of follow-up,63 but further personalization of this trend is needed. Several attempts have been reported in preliminary form, but none have been prospectively confirmed.64 Other drugs that can partially prevent the formation of brain metastases in preclinical models but are not part of the breast cancer armamentarium include pazopanib,65 a Plk1 inhibitor,66 vorinostat,67 and TPI-287.68 Guidance is needed to identify which patients would benefit from these nontraditional therapies.
Tumor Subpopulations
In addition to molecular heterogeneity, subpopulations of tumor cells with distinct functional capabilities may exist, and these would need to be factored in for any successful personalized treatment.
Tumor stem cells or cancer-initiating cells may be one subpopulation. Stem cells are defined as cells that have the ability to perpetuate themselves through self-renewal and to generate mature cells of a particular tissue through differentiation.69 A small subpopulation of tumor cells characterized by CD44+CD24−/low was enriched in stem cell-like properties.70 In addition to the CD44+CD24−/low subpopulation, aldehyde dehydrogenase 1 [encoded by ALDH1A1 (previously ALDH1)] was reported to identify stem cell-like properties on breast cancer cells. Both aldehyde dehydrogenase and CD44/CD24 expression have been linked to aggressive metastatic behavior.71 Metastasis is an inefficient process, and very few of the cells released from a primary tumor can reinitiate tumor growth at distant sites.72 The formation of metastases has been thought to result from the dissemination of cancer cells possessing stem cell-like properties and their proliferation at distant sites. Stem cell-like properties have been proposed to confer not only metastatic potential but also chemotherapy resistance.73 Recently, a plasticity model was proposed by Polyak, Weinberg, and colleagues.74 They demonstrated in mammalian breast cells that undifferentiated CD44−/lowCD24+ epithelial cells can revert to a stem-like state (CD44+CD24−/low), driven by the epithelial–mesenchymal transition. To our knowledge, it remains uncertain whether tumor stem cells also evolve in the metastatic process, which would determine whether the stem cell subpopulation from the primary tumor resection is adequate.
Another tumor subpopulation that we ignore at our peril is dormant tumor cells. For some breast cancer patients, metastasis occurs soon after a primary tumor develops, whereas for other patients metastases emerge years or even decades after initial treatment. Tumor cells that remain latent for a prolonged period of time are termed dormant. Dormancy itself can be heterogeneous, reflecting a balance of proliferation and apoptosis, cell cycle quiescence, and/or antiangiogenic mechanisms.75,76 An experimental study demonstrated doxorubicin resistance in dormant breast cancer cells,77 which suggests that these cells can survive initial chemotherapy and awaken later.
To address the chemoresistance of dormant tumor cells, novel therapeutic strategies based on mechanistic pathways mediating dormancy are needed. Several clues have emerged from the preclinical literature. When HEp3 cells were xenografted into the immunodeficient chick embryo, dormancy was accompanied by a balance between reduced activation of ERK proliferative MAP kinase and elevated activation of p38 stress MAP kinase; this balance was regulated by the urokinase receptor and by interactions with fibronectin.78 A similar inverse balance between ERK and p38 was observed in other models.79 The data suggest that p38-activating drugs or ERK inhibitors hold benefit. The lysophosphatidic acid receptor 1 (LPA-1) inhibitor Debio 0719 significantly decreased metastatic progression in two triple-negative breast cancer model systems using the murine mammary carcinoma cell line 4T1 and human breast cancer cell line MDA-MB-231. The inhibitor prevented these cells from proliferating in distant organs such as liver and lung, as evidenced by reduced Ki-67 staining; cancer cells in distant organs in the inhibitor-treated mice showed a reduction in ERK activation and an increase in p38 activation.79 In a three-dimensional culture system, inhibition of integrin β1 or MLCK prevented transition from a quiescent to a proliferative state,80 which has been reported also in other models.81 These studies suggest that integrin β1 inhibitors may control dormancy.
Regardless of personalized medicine approaches, clinical trial designs to validate dormancy-maintenance regimens will need new designs and endpoints. We currently have no validated mechanism to determine whether a patient harbors dormant tumor cells, nor for evaluating the potential of any such cells for awakening or their molecular characteristics. Disseminated tumor cells in the bone marrow have been proposed as a potential reservoir for dormant tumor cells to nest, and their presence is prognostic.82 However, we do not know what proportion of tumor cells reside in bone marrow versus in the secondary organs, and the invasiveness of bone marrow biopsies precludes their routine use.
Microenvironmental Influences
A major contributor to tumor metastasis is the tumor microenvironment, which includes fibroblasts, vasculature, immune and inflammatory cells, and extracellular matrix. Microenvironments (also known as niches) participate in reciprocal interactions between tumor cells and their surroundings.83 Microenvironments are not static. They are modified by tumor cells and infiltrated by immune and other circulating cells, a state often referred to as reactive; in turn, the reactive microenvironment can fuel tumor progression. To date, a complete portrait of the metastatic microenvironment by organ site, through time, is lacking even in experimental models. Layered onto this lack of basic information is limited understanding of the role that the microenvironment plays in chemotherapeutic resistance.83
Simplistically, aspects of the microenvironment have been targeted for interruption (eg, angiogenesis). Again simplistically, this complex process has been reduced to the angiogenesis promoter VEGF (targeted by bevacizumab) or a handful of angiogenesis receptors such as VEGFRs and PDGFRs (targeted by sorafenib, pazopanib, and other kinase inhibitors). Although the U.S. Food and Drug Administration (FDA) has approved bevacizumab for certain metastatic cancer indications, conditional approval in metastatic breast cancer was withdrawn over efficacy and toxicity concerns (http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab, last accessed February 1, 2013). In terms of efficacy, there is much that we do not understand. For example, the reliance of metastasis on angiogenesis (as opposed to co-option of the existing vasculature or other mechanisms) is debated,84 an effect of angiogenesis on hypoxia-induced tumor progression has been reported,85 and other angiogenic factors and receptors are known to exist.84 Such detailed knowledge of microenvironmental evolution will be critical to identifying newer and more effective metastasis preventive and therapeutic strategies and to their eventual personalization.
Oligometastatic or Limited Metastatic Disease?
The clinical state of oligometastatic disease, as described in 1995 by Hellman and Weichselbaum,86 refers to a subgroup of cancer patients with a limited metastatic burden. In general, only one organ is involved, and with only a single detectable metastatic lesion. Does the oligometastatic state benefit from distinct treatments, and would its personalization be different? Given their relatively limited nature, oligometastases could be removed by local therapy, such as surgical excision or stereotactic radiotherapy. In some cases, local therapy is followed by a systemic treatment similar to adjuvant therapy, to reduce possible subclinical systemic spreading of the metastatic disease. For a subgroup of oligometastatic breast cancer patients, the multidisciplinary approach may be curative. The selection of a subgroup of oligometastatic patients that may benefit from aggressive local treatment, currently based on a long disease-free interval, young age, good performance status, negative HER2 status, and a low number of involved sites,87 can be refined to more personalized levels.
Approaches to Personalized Medicine for Metastatic Breast Cancer
Can Prognostic and Predictive Signatures Guide Individualized Therapy?
In addition to standard histopathological tools, a number of multigene expression signatures have been identified for estimating the natural history of the disease. Although there is generally little overlap among the genes in these signatures, most prognostic signatures are related to tumor proliferation phenotypes. The two most commonly considered platforms, MammaPrint (Agendia, Amsterdam, Netherlands; Irvine, CA) and Oncotype DX (Genomic Health, Redwood City, CA), for example, have only one gene in common.88,89 Immunohistochemical detection of Ki-67 is a relatively inexpensive, well-established proliferation marker in breast cancer with demonstrated predictive power.90 The predictive ability of these platforms may be useful for personalization of therapy to prevent metastasis.
Another relevant question is whether signatures based on characteristics other than proliferation (eg, dormancy, stemness, or metastatic colonization potential) may hold greater potential for personalizing the prevention of metastasis. A starting point would be to determine what tumor cohorts with what follow-up data are needed, to be collected as a centralized resource for validation of potential signatures.
Metastatic Biopsies
As noted above, a substantial number of patients have discordant findings between matched biopsies of primary tumor and metastatic sites. Furthermore, one metastasis may differ from another within the same individual, raising the question of how many biopsies are needed. Biopsy of metastatic tissue could potentially improve outcome by identifying what new genetic or molecular pathways are activated, leading to altered and ideally more efficacious therapy. In 2011, the guidelines of the National Comprehensive Cancer Network (http://www.nccn.com/files/cancer-guidelines/breast/index.html#/1, last accessed February 1, 2013) indicated the importance of biopsy to confirm recurrence, if possible, and its necessity if either HER2 or hormone receptor testing was negative or never performed. The extent to which these guidelines are practiced is unknown. Issues include reimbursement, the pain and morbidity for the patient, and technical difficulties (especially for the biopsy of bone metastases).
Botteri et al91 reported that biopsies of liver metastasis were useful for confirmation or exclusion of advanced disease and for reassessment of the biology of the metastatic disease, and thus can contribute to defining a more effective treatment strategy, either by proposing new treatment options or avoiding ineffective therapies. Indeed, they observed a positive effect of liver biopsy on survival in patients with early metastases. Clearly, additional larger studies will be needed to validate the technique.
Can CTCs Guide Personalized Therapy?
A minimally invasive tumor assessment would be preferable to an invasive biopsy. Serial reconfirmation of disease biocharacteristics before commencing new therapy and at relapse could potentially personalize and optimize therapeutic decisions. Detection and biocharacterization of circulating tumor cells (CTCs) in the peripheral blood of patients with advanced breast cancer could optimally serve as a real-time tumor biopsy.92 The cardinal feature not only of breast cancer metastases but of all cancer metastases is the circulation of cancer cells from a primary tumor to distant organs such as lung, liver, bone, or brain.
CTCs are rare events, occurring at rates as low as one cell per 105 to 107 peripheral blood mononucleated cells. Their detection is complicated by significant leukocyte contamination.92 Recent advanced methodologies in the detection and characterization of CTCs include microchips, filtration, microscopic approaches, highly sensitive quantitative RT-PCR, the FDA-approved CellSearch system (Janssen Diagnostics, Raritan, NJ) system, or a combination of molecular and imaging methods.93 Detection based on EpCAM or cytokeratin expression on CTCs is potentially complicated by the epithelial–mesenchymal transition, where expression of these proteins is lost.94
Prospective studies have demonstrated that detection of CTCs in metastatic breast cancer can successfully predict progression-free survival and overall survival.95 The German SUCCESS trial is the largest study (performed with the CellSearch system) to evaluate the prognostic relevance of CTCs in breast cancer patients in the adjuvant setting.96 In 2007, CTCs were cited for the first time in the American Society of Clinical Oncology recommendations on tumor markers.97
Although CTCs are promising, several issues in the literature need resolution before CTCs can be proposed as a part of a personalized medicine regimen. The proportion of CTCs that are metastatically competent (as opposed to shed tumor cells destined to die) is unclear. According to the experimental literature, the vast majority of tumor cells shed into the circulation never form a metastatic lesion.72 Can therapeutic decisions be made on the characteristics of all CTCs obtained, or do we need to know which ones are metastatically competent? In addition, it is known that the molecular characteristics of CTCs do not always match those of the primary tumor and that in the same blood sample, heterogeneous CTC subpopulations with different hormone receptor and other phenotypes coexist.98,99 Given our increasing ability to profile CTCs not only for standard histopathological markers but also for gene expression, mutations, and epigenetic alterations, the prognostic and predictive ability of these subpopulations of tumor cells must be confirmed. This may be best accomplished by the collection of longitudinal banks of CTCs with associated patient follow-up data to semipersonalized therapy, for use in multiple molecular platforms. Finally, we note that CTCs are not obtained in all patients, even some with metastatic disease; necessarily, CTCs can provide information only when they are available.
miRNAs
Other components of blood, such as standard cancer markers and microRNAs (miRNAs) may also have predictive ability. miRNAs, which are small noncoding RNA molecules consisting of approximately 22 nucleotides, have been identified in the serum of cancer patients.100 miRNAs are thought to regulate the expression of multiple genes, based on their binding sites, and are involved in different cellular processes, including apoptosis, differentiation, metabolism, and cancer.101 It will be of interest to determine whether a specific set of miRNAs control metastasis and/or therapy resistance pathways.
New Trial Designs Will Be Needed
To what extent is our failure to improve the lives of metastatic breast cancer patients, or those at high risk of developing metastases, a failure not of drug development, but of clinical trial design?
Even before personalized medicine is developed, new trial designs will be needed to address the goal of metastasis prevention, both in the adjuvant setting and for prevention of additional metastases in the limited metastatic setting (secondary prevention). The preclinical literature abounds with evidence that compounds currently in clinical trials prevent the formation of metastases (they are given early and continuously, and fewer metastases develop at the endpoint).102,103 We have advocated for phase II randomized primary and secondary metastasis prevention trials.104 In brief, for semipersonalized groups, patients could receive standard of care and be randomized to a metastasis preventive agent or placebo. The endpoint of interest would be time to the development of a first metastasis, or time to the development of a new metastasis. This type of trial design could validate drugs with efficacy to hold single tumor cells or micrometastases in check, but that cannot shrink an established lesion in standard phase II metastatic setting trials. It is noteworthy that the primary prevention scheme dovetails with recent FDA guidance on neoadjuvant clinical trial designs.105 Thus, patients who do not achieve a complete pathological response could be optimal candidates for primary metastasis prevention trials.
New trials will also be needed to address the induction and breakdown of metastatic dormancy as this phenotype is drugged.106 Long-term therapy may induce drug insensitivity (resistance) as well as prolonged toxicity.
Trials with other designs that may be particularly germane to the personalization of treatment are underway. In the SAFIR-01 trial, coordinated by Dr. Fabrice André, high-throughput technologies (microarray gene expression profiling and comparative genomic hybridization array, OncoMap platform, and next-generation sequencing) are used to identify metastatic breast cancer patients whose tumor metastases present specific molecular alterations to add a targeted regimen to the standard treatment. More than 400 mutations are analyzed. Moreover, these technologies determine whether such genomic alterations are single (expected efficacy of a single agent) or multiple (rationale for a combination). This approach attempts to improve personalized medicine and to lower risk, compared with a single-biomarker trial. However, the high cost of the high-throughput technologies and the small size that a personalized approach can reach must be considered as limitations.107
The role of adaptive trial designs is being developed in the I-SPY trial series, although in the neoadjuvant setting. Coordinated by Drs. Laura Esserman and Donald Berry, the I-SPY 2 trial uses biomarkers (HR status, HER2 status, and the MammaPrint 70-gene signature status) to stratify patients based on their predicted potential response to treatment, and evaluates phase II drugs in combination with standard chemotherapy.108 An adaptive trial can use more than one type of adaptation, such as stopping a treatment early, changing or dropping arms or doses, and changing the proportion of patients randomized to each arm. This approach allows a rapid identification of effective new agents and drug combinations, as well as of the breast cancer subtypes that will benefit from the new therapy. It is hoped that this trial design will also reduce adverse effects and spare patients from enrolling in trials from which they will not benefit. How adaptive designs fit into the adjuvant and metastatic settings remains incompletely resolved.
Redundancy
The metastatic process is inherently redundant, in that multiple pathways can accomplish the same task. For example, tumor cell motility may be mediated by integrin, Rac1, Rho, MMP, FAK, multiple growth factors, and other signaling pathways.1 Although each of these pathways is mechanistically validated, an inhibitor to one pathway likely selects for the rare tumor cell that can use an alternative pathway for the same function. The same genomic instability that fuels this adaptive response to the mechanistic requirements of metastasis likely also promotes an adaptive response for the development of resistance to chemotherapies and hormonal therapies. Personalized medicine may actually be most effective when administered as a cocktail of therapies to combat both the complex molecular wiring and the predicted resistance mechanisms, similar to the cocktails effective for the treatment of AIDS.
Patient Advocate Perspective
The life expectancy for most individuals with metastatic breast cancer is still less than 5 years. Although patients receive semipersonalized treatment, the life-saving efficacy observed in the adjuvant setting is absent from the metastatic setting. Treatment of metastatic breast cancer is largely a hit-or-miss proposition characterized by increasing resistance with successive lines of therapy. Although some guidelines are available, they are based by necessity on consensus of what has been shown to be best for groups of patients in clinical trials.
Apart from the predictive value of HER2 and ER status in governing treatment choices, treatment of metastatic disease is almost wholly an empirical process, especially in the later stages of the disease, involving a series of trial-and-error attempts to control the cancer. The lack of specific predictive biomarkers for treatment response means that weeks to months of toxic and expensive treatments are undergone before scans indicate whether a treatment is working.
We still understand little about why one treatment works and another does not. A much more personalized approach would include other factors than the ER and HER2 expression of a tumor. Because treatment efficacy and both de novo and acquired resistance may be affected by other than genomic factors, perhaps we need to use the term personalized medicine more inclusively.
Many relevant and urgent questions need answering. What drives the dormant cancer cells to grow again or send outgrowths from quiescent, stable lesions? Is it an individual’s metabolism? Is it the microenvironment and stroma surrounding the cells? Why do some patients get only bone metastases? Or only one lesion in one organ? It seems as if sequencing the genome of one individual lesion may not answer such questions.
If a more truly personalized approach comes within financial and logistic reach, the hope is that it could greatly boost not only the quantity but also the quality of lives. The dream of living a relatively normal life span could become a reality. The challenges, however, seem too steep, and for many reasons. How will researchers, each with tiny pieces of a giant puzzle, come together to make sense of the increasing amounts of data? Who is going to develop drugs that may have quite a limited market? Who will the payers be? Who will benefit from the personalized genomic approach? Finally, how will personalized medicine, and treatment of metastatic breast cancer in particular, reach those living in low-resource countries?
Footnotes
Supported by the Intramural Program of the National Cancer Institute.
This article is dedicated to the memory of Maria Wetzel, who died of breast cancer in May 2013.
Current address of J.N., Department of Biological Sciences, National University of Singapore, Singapore.
This article is part of a review series on the molecular pathogenesis of breast cancer.
References
- 1.Steeg P.S. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 2.Comen E., Norton L., Massagué J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8:369–377. doi: 10.1038/nrclinonc.2011.64. [DOI] [PubMed] [Google Scholar]
- 3.Welch D.R. Do we need to redefine a cancer metastasis and staging definitions? Breast Dis. 2006;26:3–12. doi: 10.3233/bd-2007-26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein C.A. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 5.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 6.Nguyen D.X., Bos P.D., Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 7.Vernon A.E., Bakewell S.J., Chodosh L.A. Deciphering the molecular basis of breast cancer metastasis with mouse models. Rev Endocr Metab Disord. 2007;8:199–213. doi: 10.1007/s11154-007-9041-5. [DOI] [PubMed] [Google Scholar]
- 8.Sun W., Yang J. Functional mechanisms for human tumor suppressors. J Cancer. 2010;1:136–140. doi: 10.7150/jca.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steeg P.S. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 10.Smith S.C., Theodorescu D. Learning therapeutic lessons from metastasis suppressor proteins. Nat Rev Cancer. 2009;9:253–264. doi: 10.1038/nrc2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 12.Hynes N.E., Lane H.A. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [Erratum appeared in Nat Rev Cancer 2005, 5:580] [DOI] [PubMed] [Google Scholar]
- 13.Vogel C.L., Cobleigh M.A., Tripathy D., Gutheil J.C., Harris L.N., Fehrenbacher L., Slamon D.J., Murphy M., Novotny W.F., Burchmore M., Shak S., Stewart S.J., Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell K.L., Burstein H.J., Storniolo A.M., Rugo H.S., Sledge G., Aktan G., Ellis C., Florance A., Vukelja S., Bischoff J., Baselga J., O’Shaughnessy J. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J., Cortés J., Kim S.B., Im S.A., Hegg R., Im Y.H., Roman L., Pedrini J.L., Pienkowski T., Knott A., Clark E., Benyunes M.C., Ross G., Swain S.M., CLEOPATRA Study Group Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J., Pegram M., Oh D.Y., Diéras V., Guardino E., Fang L., Lu M.W., Olsen S., Blackwell K., EMILIA Study Group Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [Erratum appeared in N Engl J Med 2013, 368:2442] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmieri D., Bronder J.L., Herring J.M., Yoneda T., Weil R.J., Stark A.M., Kurek R., Vega-Valle E., Feigenbaum L., Halverson D., Vortmeyer A.O., Steinberg S.M., Aldape K., Steeg P.S. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 18.Moody S.E., Sarkisian C.J., Hahn K.T., Gunther E.J., Pickup S., Dugan K.D., Innocent N., Cardiff R.D., Schnall M.D., Chodosh L.A. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–461. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 19.Khalili P., Arakelian A., Chen G., Singh G., Rabbani S.A. Effect of Herceptin on the development and progression of skeletal metastases in a xenograft model of human breast cancer. Oncogene. 2005;24:6657–6666. doi: 10.1038/sj.onc.1208790. [Erratum appeared in Oncogene 2006, 25:492] [DOI] [PubMed] [Google Scholar]
- 20.Chow A., Arteaga C.L., Wang S.E. When tumor suppressor TGFbeta meets the HER2 (ERBB2) oncogene. J Mammary Gland Biol Neoplasia. 2011;16:81–88. doi: 10.1007/s10911-011-9206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y.M., Pan Y., Wei Y., Cheng X., Zhou B.P., Tan M., Zhou X., Xia W., Hortobagyi G.N., Yu D., Hung M.C. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Vadlamudi R.K., Sahin A.A., Adam L., Wang R.A., Kumar R. Heregulin and HER2 signaling selectively activates c-Src phosphorylation at tyrosine 215. FEBS Lett. 2003;543:76–80. doi: 10.1016/s0014-5793(03)00404-6. [DOI] [PubMed] [Google Scholar]
- 23.Yen L., You X.L., Al Moustafa A.E., Batist G., Hynes N.E., Mader S., Meloche S., Alaoui-Jamali M.A. Heregulin selectively upregulates vascular endothelial growth factor secretion in cancer cells and stimulates angiogenesis. Oncogene. 2000;19:3460–3469. doi: 10.1038/sj.onc.1203685. [DOI] [PubMed] [Google Scholar]
- 24.Niu G., Carter W.B. Human epidermal growth factor receptor 2 regulates angiopoietin-2 expression in breast cancer via AKT and mitogen-activated protein kinase pathways. Cancer Res. 2007;67:1487–1493. doi: 10.1158/0008-5472.CAN-06-3155. [DOI] [PubMed] [Google Scholar]
- 25.Early Breast Cancer Trialists’ Collaborative Group Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 26.Mehta R.S., Barlow W.E., Albain K.S., Vandenberg T.A., Dakhil S.R., Tirumali N.R., Lew D.L., Hayes D.F., Gralow J.R., Livingston R.B., Hortobagyi G.N. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–444. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James J.J., Evans A.J., Pinder S.E., Gutteridge E., Cheung K.L., Chan S., Robertson J.F. Bone metastases from breast carcinoma: histopathological–radiological correlations and prognostic features. Br J Cancer. 2003;89:660–665. doi: 10.1038/sj.bjc.6601198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim R.S., Avivar-Valderas A., Estrada Y., Bragado P., Sosa M.S., Aguirre-Ghiso J.A., Segall J.E. Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PLoS One. 2012;7:e35569. doi: 10.1371/journal.pone.0035569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hackshaw A., Roughton M., Forsyth S., Monson K., Reczko K., Sainsbury R., Baum M. Long-term benefits of 5 years of tamoxifen: 10-year follow-up of a large randomized trial in women at least 50 years of age with early breast cancer. J Clin Oncol. 2011;29:1657–1663. doi: 10.1200/JCO.2010.32.2933. [DOI] [PubMed] [Google Scholar]
- 30.Chakravarty D., Nair S.S., Santhamma B., Nair B.C., Wang L., Bandyopadhyay A., Agyin J.K., Brann D., Sun L.Z., Yeh I.T., Lee F.Y., Tekmal R.R., Kumar R., Vadlamudi R.K. Extranuclear functions of ER impact invasive migration and metastasis by breast cancer cells. Cancer Res. 2010;70:4092–4101. doi: 10.1158/0008-5472.CAN-09-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuqua S.A., Cui Y. Targeting the estrogen receptor in clinical breast cancer. Breast Dis. 2002;15:3–11. doi: 10.3233/bd-2002-15102. [DOI] [PubMed] [Google Scholar]
- 32.Sabnis G.J., Jelovac D., Long B., Brodie A. The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res. 2005;65:3903–3910. doi: 10.1158/0008-5472.CAN-04-4092. [DOI] [PubMed] [Google Scholar]
- 33.Oesterreich S., Deng W., Jiang S., Cui X., Ivanova M., Schiff R., Kang K., Hadsell D.L., Behrens J., Lee A.V. Estrogen-mediated down-regulation of E-cadherin in breast cancer cells. Cancer Res. 2003;63:5203–5208. [PubMed] [Google Scholar]
- 34.Rhodes L.V., Short S.P., Neel N.F., Salvo V.A., Zhu Y., Elliott S., Wei Y., Yu D., Sun M., Muir S.E., Fonseca J.P., Bratton M.R., Segar C., Tilghman S.L., Sobolik-Delmaire T., Horton L.W., Zaja-Milatovic S., Collins-Burow B.M., Wadsworth S., Beckman B.S., Wood C.E., Fuqua S.A., Nephew K.P., Dent P., Worthylake R.A., Curiel T.J., Hung M.C., Richmond A., Burow M.E. Cytokine receptor CXCR4 mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human breast cancer. Cancer Res. 2011;71:603–613. doi: 10.1158/0008-5472.CAN-10-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Leo A., Malorni L. Polyendocrine treatment in estrogen receptor-positive breast cancer: a “FACT” yet to be proven. J Clin Oncol. 2012;30:1897–1900. doi: 10.1200/JCO.2012.41.7394. [DOI] [PubMed] [Google Scholar]
- 36.Loi S., Michiels S., Baselga J., Bartlett J.M., Singhal S.K., Sabine V.S., Sims A.H., Sahmoud T., Dixon J.M., Piccart M.J., Sotiriou C. PIK3CA genotype and a PIK3CA mutation-related gene signature and response to everolimus and letrozole in estrogen receptor positive breast cancer. PLoS One. 2013;8:e53292. doi: 10.1371/journal.pone.0053292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sparano J.A. TAILORx: trial assigning individualized options for treatment (Rx) Clin Breast Cancer. 2006;7:347–350. doi: 10.3816/CBC.2006.n.051. [DOI] [PubMed] [Google Scholar]
- 38.Kang Y., Siegel P.M., Shu W., Drobnjak M., Kakonen S.M., Cordón-Cardo C., Guise T.A., Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 39.Kingsley L.A., Fournier P.G., Chirgwin J.M., Guise T.A. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 40.Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M., Fan M., Jiang Q., Dansey R., Jun S., Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 41.Jin K., Teng L., Shen Y., He K., Xu Z., Li G. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol. 2010;12:473–480. doi: 10.1007/s12094-010-0540-6. [DOI] [PubMed] [Google Scholar]
- 42.Niikura N., Liu J., Hayashi N., Mittendorf E.A., Gong Y., Palla S.L., Tokuda Y., Gonzalez-Angulo A.M., Hortobagyi G.N., Ueno N.T. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30:593–599. doi: 10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindström L.S., Karlsson E., Wilking U.M., Johansson U., Hartman J., Lidbrink E.K., Hatschek T., Skoog L., Bergh J. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 44.Amir E., Clemons M., Purdie C.A., Miller N., Quinlan P., Geddie W., Coleman R.E., Freedman O.C., Jordan L.B., Thompson A.M. Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev. 2012;38:708–714. doi: 10.1016/j.ctrv.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Curigliano G., Bagnardi V., Viale G., Fumagalli L., Rotmensz N., Aurilio G., Locatelli M., Pruneri G., Giudici S., Bellomi M., Della Vigna P., Monfardini L., Orsi F., Nole F., Munzone E., Goldhirsch A. Should liver metastases of breast cancer be biopsied to improve treatment choice? Ann Oncol. 2011;22:2227–2233. doi: 10.1093/annonc/mdq751. [DOI] [PubMed] [Google Scholar]
- 46.Akcakanat A., Sahin A., Shaye A.N., Velasco M.A., Meric-Bernstam F. Comparison of Akt/mTOR signaling in primary breast tumors and matched distant metastases. Cancer. 2008;112:2352–2358. doi: 10.1002/cncr.23456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J.M., Fackler M.J., Halushka M.K., Molavi D.W., Taylor M.E., Teo W.W., Griffin C., Fetting J., Davidson N.E., De Marzo A.M., Hicks J.L., Chitale D., Ladanyi M., Sukumar S., Argani P. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14:1938–1946. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehrotra J., Vali M., McVeigh M., Kominsky S.L., Fackler M.J., Lahti-Domenici J., Polyak K., Sacchi N., Garrett-Mayer E., Argani P., Sukumar S. Very high frequency of hypermethylated genes in breast cancer metastasis to the bone, brain, and lung. Clin Cancer Res. 2004;10:3104–3109. doi: 10.1158/1078-0432.ccr-03-0118. [DOI] [PubMed] [Google Scholar]
- 49.Lo Nigro C., Vivenza D., Monteverde M., Lattanzio L., Gojis O., Garrone O., Comino A., Merlano M., Quinlan P.R., Syed N., Purdie C.A., Thompson A., Palmieri C., Crook T. High frequency of complex TP53 mutations in CNS metastases from breast cancer. Br J Cancer. 2012;106:397–404. doi: 10.1038/bjc.2011.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singhi A.D., Cimino-Mathews A., Jenkins R.B., Lan F., Fink S.R., Nassar H., Vang R., Fetting J.H., Hicks J., Sukumar S., De Marzo A.M., Argani P. MYC gene amplification is often acquired in lethal distant breast cancer metastases of unamplified primary tumors. Mod Pathol. 2012;25:378–387. doi: 10.1038/modpathol.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wikman H., Lamszus K., Detels N., Uslar L., Wrage M., Benner C., Hohensee I., Ylstra B., Eylmann K., Zapatka M., Sauter G., Kemming D., Glatzel M., Müller V., Westphal M., Pantel K. Relevance of PTEN loss in brain metastasis formation in breast cancer patients. Breast Cancer Res. 2012;14:R49. doi: 10.1186/bcr3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding L., Ellis M.J., Li S., Larson D.E., Chen K., Wallis J.W. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah S.P., Morin R.D., Khattra J., Prentice L., Pugh T., Burleigh A., Delaney A., Gelmon K., Guliany R., Senz J., Steidl C., Holt R.A., Jones S., Sun M., Leung G., Moore R., Severson T., Taylor G.A., Teschendorff A.E., Tse K., Turashvili G., Varhol R., Warren R.L., Watson P., Zhao Y., Caldas C., Huntsman D., Hirst M., Marra M.A., Aparicio S. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–813. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 54.Nik-Zainal S., Van Loo P., Wedge D.C., Alexandrov L.B., Greenman C.D., Lau K.W., Breast Cancer Working Group of the International Cancer Genome Consortium The life history of 21 breast cancers. Cell. 2012;149:994–1007. doi: 10.1016/j.cell.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navin N., Krasnitz A., Rodgers L., Cook K., Meth J., Kendall J., Riggs M., Eberling Y., Troge J., Grubor V., Levy D., Lundin P., Maner S., Zetterberg A., Hicks J., Wigler M. Inferring tumor progression from genomic heterogeneity. Genome Res. 2010;20:68–80. doi: 10.1101/gr.099622.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephens P.J., McBride D.J., Lin M.L., Varela I., Pleasance E.D., Simpson J.T., Stebbings L.A., Leroy C., Edkins S., Mudie L.J., Greenman C.D., Jia M., Latimer C., Teague J.W., Lau K.W., Burton J., Quail M.A., Swerdlow H., Churcher C., Natrajan R., Sieuwerts A.M., Martens J.W., Silver D.P., Langerød A., Russnes H.E., Foekens J.A., Reis-Filho J.S., van 't Veer L., Richardson A.L., Børresen-Dale A.L., Campbell P.J., Futreal P.A., Stratton M.R. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature. 2009;462:1005–1010. doi: 10.1038/nature08645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banerji S., Cibulskis K., Rangel-Escareno C., Brown K.K., Carter S.L., Frederick A.M. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J., Karaplis A.C., Huang D.C., Siegel P.M., Camirand A., Yang X.F., Muller W.J., Kremer R. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. J Clin Invest. 2011;121:4655–4669. doi: 10.1172/JCI46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minn A.J., Gupta G.P., Siegel P.M., Bos P.D., Shu W., Giri D.D., Viale A., Olshen A.B., Gerald W.L., Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vlastos G., Smith D.L., Singletary S.E., Mirza N.Q., Tuttle T.M., Popat R.J., Curley S.A., Ellis L.M., Roh M.S., Vauthey J.N. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol. 2004;11:869–874. doi: 10.1245/ASO.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Gril B., Palmieri D., Bronder J.L., Herring J.M., Vega-Valle E., Feigenbaum L., Liewehr D.J., Steinberg S.M., Merino M.J., Rubin S.D., Steeg P.S. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bachelot T., Romieu G., Campone M., Diéras V., Cropet C., Dalenc F., Jimenez M., Le Rhun E., Pierga J.Y., Gonçalves A., Leheurteur M., Domont J., Gutierrez M., Curé H., Ferrero J.M., Labbe-Devilliers C. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 63.Brufsky A.M., Mayer M., Rugo H.S., Kaufman P.A., Tan-Chiu E., Tripathy D., Tudor I.C., Wang L.I., Brammer M.G., Shing M., Yood M.U., Yardley D.A. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 64.Frati A., Chereau E., Coutant C., Bezu C., Antoine M., Chopier J., Daraï E., Uzan S., Gligorov J., Rouzier R. Comparison of two nomograms to predict pathologic complete responses to neoadjuvant chemotherapy for breast cancer: evidence that HER2-positive tumors need specific predictors. Breast Cancer Res Treat. 2012;132:601–607. doi: 10.1007/s10549-011-1897-0. [DOI] [PubMed] [Google Scholar]
- 65.Gril B., Palmieri D., Qian Y., Smart D., Ileva L., Liewehr D.J., Steinberg S.M., Steeg P.S. Pazopanib reveals a role for tumor cell B-Raf in the prevention of HER2+ breast cancer brain metastasis. Clin Cancer Res. 2011;17:142–153. doi: 10.1158/1078-0432.CCR-10-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qian Y., Hua E., Bisht K., Woditschka S., Skordos K.W., Liewehr D.J., Steinberg S.M., Brogi E., Akram M.M., Killian J.K., Edelman D.C., Pineda M., Scurci S., Degenhardt Y.Y., Laquerre S., Lampkin T.A., Meltzer P.S., Camphausen K., Steeg P.S., Palmieri D. Inhibition of Polo-like kinase 1 prevents the growth of metastatic breast cancer cells in the brain. Clin Exp Metastasis. 2011;28:899–908. doi: 10.1007/s10585-011-9421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palmieri D., Lockman P.R., Thomas F.C., Hua E., Herring J., Hargrave E., Johnson M., Flores N., Qian Y., Vega-Valle E., Taskar K.S., Rudraraju V., Mittapalli R.K., Gaasch J.A., Bohn K.A., Thorsheim H.R., Liewehr D.J., Davis S., Reilly J.F., Walker R., Bronder J.L., Feigenbaum L., Steinberg S.M., Camphausen K., Meltzer P.S., Richon V.M., Smith Q.R., Steeg P.S. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res. 2009;15:6148–6157. doi: 10.1158/1078-0432.CCR-09-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fitzgerald D.P., Emerson D.L., Qian Y., Anwar T., Liewehr D.J., Steinberg S.M., Silberman S., Palmieri D., Steeg P.S. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol Cancer Ther. 2012;11:1959–1967. doi: 10.1158/1535-7163.MCT-12-0061. [Erratum appeared in Mol Cancer Ther 2013, 12:241] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 70.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [Erratum appeared in Proc Natl Acad Sci USA 2003, 100:6890] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Croker A.K., Goodale D., Chu J., Postenka C., Hedley B.D., Hess D.A., Allan A.L. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med. 2009;13:2236–2252. doi: 10.1111/j.1582-4934.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chambers A.F., Naumov G.N., Vantyghem S.A., Tuck A.B. Molecular biology of breast cancer metastasis. Clinical implications of experimental studies on metastatic inefficiency. Breast Cancer Res. 2000;2:400–407. doi: 10.1186/bcr86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X., Lewis M.T., Huang J., Gutierrez C., Osborne C.K., Wu M.F., Hilsenbeck S.G., Pavlick A., Zhang X., Chamness G.C., Wong H., Rosen J., Chang J.C. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 74.Chaffer C.L., Brueckmann I., Scheel C., Kaestli A.J., Wiggins P.A., Rodrigues L.O., Brooks M., Reinhardt F., Su Y., Polyak K., Arendt L.M., Kuperwasser C., Bierie B., Weinberg R.A. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naumov G.N., Bender E., Zurakowski D., Kang S.Y., Sampson D., Flynn E., Watnick R.S., Straume O., Akslen L.A., Folkman J., Almog N. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006;98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- 76.Chambers A.F., Groom A.C., MacDonald I.C. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 77.Naumov G.N., Townson J.L., MacDonald I.C., Wilson S.M., Bramwell V.H., Groom A.C., Chambers A.F. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat. 2003;82:199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- 78.Ranganathan A.C., Adam A.P., Zhang L., Aguirre-Ghiso J.A. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol Ther. 2006;5:729–735. doi: 10.4161/cbt.5.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marshall J.C., Collins J.W., Nakayama J., Horak C.E., Liewehr D.J., Steinberg S.M., Albaugh M., Vidal-Vanaclocha F., Palmieri D., Barbier M., Murone M., Steeg P.S. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J Natl Cancer Inst. 2012;104:1306–1319. doi: 10.1093/jnci/djs319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barkan D., Kleinman H., Simmons J.L., Asmussen H., Kamaraju A.K., Hoenorhoff M.J., Liu Z.Y., Costes S.V., Cho E.H., Lockett S., Khanna C., Chambers A.F., Green J.E. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White D.E., Kurpios N.A., Zuo D., Hassell J.A., Blaess S., Mueller U., Muller W.J. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 82.Slade M.J., Coombes R.C. The clinical significance of disseminated tumor cells in breast cancer. Nat Clin Pract Oncol. 2007;4:30–41. doi: 10.1038/ncponc0685. [DOI] [PubMed] [Google Scholar]
- 83.Bissell M.J., Hines W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ebos J.M., Kerbel R.S. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [Erratum appeared in Nat Rev Clin Oncol 2011, 8:221 and in Nat Rev Clin Oncol 2011, 8:316] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 87.Nieto Y., Nawaz S., Jones R.B., Shpall E.J., Cagnoni P.J., McSweeney P.A., Barón A., Razook C., Matthes S., Bearman S.I. Prognostic model for relapse after high-dose chemotherapy with autologous stem-cell transplantation for stage IV oligometastatic breast cancer. J Clin Oncol. 2002;20:707–718. doi: 10.1200/JCO.2002.20.3.707. [DOI] [PubMed] [Google Scholar]
- 88.Knauer M., Mook S., Rutgers E.J., Bender R.A., Hauptmann M., van de Vijver M.J., Koornstra R.H., Bueno-de-Mesquita J.M., Linn S.C., van ’t Veer L.J. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat. 2010;120:655–661. doi: 10.1007/s10549-010-0814-2. [DOI] [PubMed] [Google Scholar]
- 89.Paik S., Shak S., Tang G., Kim C., Baker J., Cronin M., Baehner F.L., Walker M.G., Watson D., Park T., Hiller W., Fisher E.R., Wickerham D.L., Bryant J., Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 90.Niikura N., Iwamoto T., Masuda S., Kumaki N., Xiaoyan T., Shirane M., Mori K., Tsuda B., Okamura T., Saito Y., Suzuki Y., Tokuda Y. Immunohistochemical Ki67 labeling index has similar proliferation predictive power to various gene signatures in breast cancer. Cancer Sci. 2012;103:1508–1512. doi: 10.1111/j.1349-7006.2012.02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Botteri E., Disalvatore D., Curigliano G., Brollo J., Bagnardi V., Viale G., Orsi F., Goldhirsch A., Rotmensz N. Biopsy of liver metastasis for women with breast cancer: impact on survival. Breast. 2012;21:284–288. doi: 10.1016/j.breast.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 92.Smerage J.B., Hayes D.F. The measurement and therapeutic implications of circulating tumour cells in breast cancer. Br J Cancer. 2006;94:8–12. doi: 10.1038/sj.bjc.6602871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alix-Panabières C., Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110–118. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 94.Kallergi G., Papadaki M.A., Politaki E., Mavroudis D., Georgoulias V., Agelaki S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13:R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wallwiener M., Hartkopf A.D., Baccelli I., Riethdorf S., Schott S., Pantel K., Marme F., Sohn C., Trumpp A., Rack B., Aktas B., Solomayer E.F., Müller V., Janni W., Schneeweiss A., Fehm T.N. The prognostic impact of circulating tumor cells in subtypes of metastatic breast cancer. Breast Cancer Res Treat. 2013;137:503–510. doi: 10.1007/s10549-012-2382-0. [DOI] [PubMed] [Google Scholar]
- 96.Riethdorf S., Fritsche H., Müller V., Rau T., Schindlbeck C., Rack B., Janni W., Coith C., Beck K., Janicke F., Jackson S., Gornet T., Cristofanilli M., Pantel K. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 97.Harris L., Fritsche H., Mennel R., Norton L., Ravdin P., Taube S., Somerfield M.R., Hayes D.F., Bast R.C., Jr., American Society of Clinical Oncology American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 98.Powell A.A., Talasaz A.H., Zhang H., Coram M.A., Reddy A., Deng G., Telli M.L., Advani R.H., Carlson R.W., Mollick J.A., Sheth S., Kurian A.W., Ford J.M., Stockdale F.E., Quake S.R., Pease R.F., Mindrinos M.N., Bhanot G., Dairkee S.H., Davis R.W., Jeffrey S.S. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One. 2012;7:e33788. doi: 10.1371/journal.pone.0033788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pestrin M., Bessi S., Galardi F., Truglia M., Biggeri A., Biagioni C., Cappadona S., Biganzoli L., Giannini A., Di Leo A. Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat. 2009;118:523–530. doi: 10.1007/s10549-009-0461-7. [DOI] [PubMed] [Google Scholar]
- 100.Madhavan D., Zucknick M., Wallwiener M., Cuk K., Modugno C., Scharpff M., Schott S., Heil J., Turchinovich A., Yang R., Benner A., Riethdorf S., Trumpp A., Sohn C., Pantel K., Schneeweiss A., Burwinkel B. Circulating miRNAs as surrogate markers for circulating tumor cells and prognostic markers in metastatic breast cancer. Clin Cancer Res. 2012;18:5972–5982. doi: 10.1158/1078-0432.CCR-12-1407. [DOI] [PubMed] [Google Scholar]
- 101.Kloosterman W.P., Plasterk R.H. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Kim K.J., Li B., Winer J., Armanini M., Gillett N., Phillips H.S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 103.Yang Y.A., Dukhanina O., Tang B., Mamura M., Letterio J.J., MacGregor J., Patel S.C., Khozin S., Liu Z.Y., Green J., Anver M.R., Merlino G., Wakefield L.M. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–1615. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steeg P.S. Perspective: the right trials. Nature. 2012;485:S58–S59. doi: 10.1038/485S58a. [DOI] [PubMed] [Google Scholar]
- 105.Sherman R.B., Woodcock J., Norden J., Grandinetti C., Temple R.J. New FDA regulation to improve safety reporting in clinical trials. N Engl J Med. 2011;365:3–5. doi: 10.1056/NEJMp1103464. [DOI] [PubMed] [Google Scholar]
- 106.Goss P.E., Chambers A.F. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer. 2010;10:871–877. doi: 10.1038/nrc2933. [DOI] [PubMed] [Google Scholar]
- 107.Andre F., Delaloge S., Soria J.C. Biology-driven phase II trials: what is the optimal model for molecular selection? J Clin Oncol. 2011;29:1236–1238. doi: 10.1200/JCO.2010.31.6877. [DOI] [PubMed] [Google Scholar]
- 108.Barker A.D., Sigman C.C., Kelloff G.J., Hylton N.M., Berry D.A., Esserman L.J. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86:97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]