Abstract
Semaphorin-3A (Sema3a), a guidance protein secreted by podocytes, is essential for normal kidney patterning and glomerular filtration barrier development. Here, we report that podocyte-specific Sema3a gain-of-function in adult mice leads to proteinuric glomerular disease involving the three layers of the glomerular filtration barrier. Reversibility of the glomerular phenotype upon removal of the transgene induction provided proof-of-principle of the cause-and-effect relationship between podocyte Sema3a excess and glomerular disease. Mechanistically, excess Sema3a induces dysregulation of nephrin, matrix metalloproteinase 9, and αvβ3 integrin in vivo. Sema3a cell-autonomously disrupts podocyte shape. We identified a novel direct interaction between the Sema3a signaling receptor plexinA1 and nephrin, linking extracellular Sema3a signals to the slit-diaphragm signaling complex. We conclude that Sema3a functions as an extracellular negative regulator of the structure and function of the glomerular filtration barrier in the adult kidney. Our findings demonstrate a crosstalk between Sema3a and nephrin signaling pathways that is functionally relevant both in vivo and in vitro.
The glomerular filter is a size-selective barrier composed of three layers: fenestrated endothelium, glomerular basement membrane (GBM), and podocyte foot processes.1 Disruption of any of these components of the glomerular filtration barrier causes loss of permselectivity, proteinuria, and glomerular disease.1 Podocyte foot processes are linked by slit diaphragms, which are modified adherens junctions composed of extracellular domains of nephrin molecules associated to a multiprotein complex.2,3 Gene mutations in slit-diaphragm proteins and their actin-associated proteins cause familial nephrotic syndrome.4–7 The GBM is a complex of type IV collagen (α3, α4, and α5) and laminin 521 (α5β2γ1) chains, perlecan, syndecan, entactin, and agrin. Imbalance of collagen and laminin chain expression results in abnormalities of GBM ultrastructure and proteinuria.8,9 Loss of glomerular endothelial fenestration due to inhibition of vascular endothelial growth factor (VEGF-A) signaling or to excess soluble Flt-1 causes proteinuria and preeclampsia.10,11
Semaphorin-3A (Sema3a) is a secreted guidance protein involved in axon pathfinding and in cardiovascular, lung, and kidney patterning.12,13 In the normal kidney, Sema3a is expressed in podocytes and collecting ducts.7 Loss-of-function studies during kidney development showed that Sema3a inhibits endothelial cell migration into glomeruli and limits ureteric bud branching.14,15 Sema3a gain-of-function during development resulted in glomerular hypoplasia, delayed podocyte differentiation, and absent slit diaphragms.15 Exposure of cultured podocytes to recombinant Sema3a induced down-regulation of podocin and decreased the interactions among nephrin, podocin, and CD2AP.16 Systemic administration of Sema3a to adult mice induced transient, reversible foot-process effacement and proteinuria similar to that induced by protamine sulfate.17,18 We observed increased podocyte Sema3a protein and mRNA expression in mice with diabetic nephropathy.13,19 Taken together, our previous studies suggested that excess Sema3a might disrupt the glomerular filtration barrier in the mature kidney, particularly in the setting of diabetes.
The goal of the present study was to define whether excess podocyte Sema3a per se causes glomerular disease in adult mice, and to examine the mechanism involved. Here, we report that induction of podocyte-specific Sema3a overexpression in adult mice causes a proteinuric glomerular disease involving the three layers of the glomerular filtration barrier. Mechanistically, we show that excess Sema3a induces dysregulation of nephrin, MMP-9, and αvβ3 integrin in vivo, and we identify a novel interaction between the Sema3a signaling receptor plexinA1 and nephrin that links Sema3a signals to the slit-diaphragm signaling complex. Collectively, these findings establish that Sema3a functions as an extracellular negative regulator of the integrity and function of the glomerular filtration barrier.
Materials and Methods
Animal protocols and procedures were approved by the institutional animal care and use committees at Albert Einstein College of Medicine and Yale University School of Medicine. Mice were housed in a pathogen-free environment. Mice were anesthetized with ketamine–xylazine (0.1 mg/kg body weight i.p.), and kidneys were removed before euthanasia. Blood and urine were obtained by cardiac and bladder puncture, respectively.
Sema3a-Overexpressing Mice
Generation of doxycycline-regulated podocin-rtTA:tet-O-Sema3a mice (hereafter referred to as Sema3a+) was performed as described previously.15 Six-week-old male Sema3a+ mice were fed doxycycline chow (625 mg/kg chow; Harlan-Teklad, Madison, WI) for 1 month (n = 16). Controls were age-matched uninduced Sema3a+ mice (n = 18) fed standard chow and single-transgenic (podocin-rtTA or tet-O-Sema3a) mice fed doxycycline chow for 1 month (n = 4). For reversibility experiments, Sema3a+ mice were induced with doxycycline for 1 month and then were fed standard chow for 2 weeks (n = 10).
Noninvasive Blood Pressure Monitoring
Systolic and diastolic blood pressure were noninvasively measured on nonanesthetized mice (n = 4 per experimental group) previously acclimated to the procedure, by determining the tail blood volume with a volume pressure recording sensor and an occlusion tail-cuff (CODA system; Kent Scientific, Torrington, CT). Mice were acclimated before blood pressure readings were obtained (at least 15 readings per mouse).
Albuminuria and Creatinine Clearance
Equal volumes of urine were resolved by SDS-PAGE and were stained with Coomassie Blue or immunoblotted with anti–bovine serum albumin antibody (Upstate 07-248; 1:1000; EMD Millipore, Billerica, MA). Creatinine was measured in plasma and in 24-hour urine samples by high-performance liquid chromatography.19
Histology and Immunohistochemistry
Kidneys were fixed in 10% formalin and were paraffin embedded or processed for cryosectioning. PAS staining was performed and examined by light microscopy. Fluorescent immunostaining was performed for total laminin (Sigma-Aldrich, St. Louis, MO) and collagen IV (SouthernBiotech, Birmingham, AL) in formalin-fixed deparaffinized sections, and for nephrin (Fitzgerald Industries International, Acton, MA), podocin (Sigma-Aldrich), Sema3a (R&D Systems, Minneapolis, MN), αvβ3 integrin (EMD Millipore), and Wow-1 fragment antigen-binding region (Fab)20 in acetone-fixed cryosections, as described previously.11,15,19,21 Appropriate Cy2 and Cy3 fluorescent-tagged donkey secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were used to visualize immunoreactive signals, and confocal images were acquired (FluoView 300; Olympus, Tokyo, Japan). Quantitation of immunofluorescence signal was performed using ImageJ software version 1.47 (NIH, Bethesda, MD), as described by Yu et al22 with minor modifications. In brief, the integrated density of immunofluorescence-positive signals was measured and normalized for the glomerular area in 5 to 10 glomeruli per mouse (n = 4 to 6 mice per experimental group).
TEM
Kidney cortex was fixed and processed for transmission electron microscopy (TEM), and samples were examined on a JEOL 1200EX microscope as described previously.15 High-resolution digitized images (2000 dpi) were used to measure foot process width using NIH ImageJ software, as described previously.21 Approximately 100 to 150 foot processes per kidney were measured, adjacent to ≥50 μm GBM per kidney (n = 3 or 4 mice per experimental group).
qPCR
Total RNA was isolated from whole-kidney tissue using TRIzol reagent (Life Technologies-Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. One microgram of isolated RNA from each animal was used to generate cDNA, using a QuantiTect kit (Qiagen, Valencia, CA). PCR reactions used pooled cDNA (n = 4 or 5 mice per experimental group), and amplification was performed using an Applied Biosystems SYBR Green master mix (Life Technologies) with a Mastercycler ep realplex2 system (Eppendorf, Hauppauge, NY) . PCR primers were designed with Primer Express software version 2.0 (Life Technologies), as described previously,15 with the following additional primers (forward and reverse): Lmx1b 5′-CATCCTTTGAGGTCTCCTCCAA-3′ and 5′-GCCAGCTTCTTCATCTTTGCTC-3′; collagen IV α2 5′-CCCATCTGACATCACACTTGTTG-3′ and 5′-TGAGATTACGCCGGGTATCC-3′; α2 laminin 5′-GACCTCTCTGGCCGCATTC-3′ and 5′-CCTCAGAGTTACTGATGCTGATCTG-3′; and β2 laminin 5′-GGGTTCCAATGGTCACCATC-3′ and 5′-TGAAATGAAACTCAGCTTCCAGG-3′. Reactions were performed in duplicate, and each experiment was repeated three times. Gene expression relative to the housekeeping gene GAPDH or ubiquitin was determined with the 2−ΔΔCt method, as described previously.15
Western Blot Analysis, Immunoprecipitation, GST, and Overlay Assays
Kidneys were lysed in modified radioimmunoprecipitation assay buffer, and pooled samples of whole-kidney lysates were generated using equal amounts of protein from each mouse (n = 4 to 5 mice per experimental group).15 Proteins were resolved on 8% to 15% SDS-PAGE gels, and immunoblotting was performed using standard technique with the following primary antibodies: WT1 (sc-192; Santa Cruz Biotechnology, Santa Cruz, CA), nephrin (20R-NP002; Fitzgerald Industries International), podocin (P0372; Sigma-Aldrich), CD2AP (sc-9137; Santa Cruz Biotechnology), neuropilin-123; Sema3a (sc-28867; Santa Cruz Biotechnology), β1 and αvβ3 integrins (44-870G and MAB 1976Z; EMD Millipore), hMMP-9 (Chemicon AB-19016; EMD-Millipore), tubulin (T6074; Sigma-Aldrich), and actin (A2066; Sigma-Aldrich).
Whole kidney and immortalized podocytes were lysed in immunoprecipitation buffer, precleared, and incubated with purified 1 μg FLAG-tagged nephrin overnight at 4°C, and immunoprecipitated with anti-FLAG antibody (F7425; Sigma-Aldrich), as described previously.21 Immunoprecipitates were analyzed by Western blotting using anti-nephrin (Fitzgerald Industries International), anti-plexinA1 (Cell Signaling Technology, Danvers, MA), and anti-FLAG (Sigma-Aldrich) antibodies. HEK cells were transiently transfected with plexinA1–Myc and nephrin–FLAG using Lipofectamine 2000 reagent (Life Technologies), according to the manufacturer’s protocol; cells were lysed 24 hours later in immunoprecipitation buffer. Lysates were precleared and immunoprecipitated with nephrin24 and plexinA1 antibodies, as described previously.11,25 Immunoprecipitates were analyzed by Western blotting using anti-nephrin (Fitzgerald Industries International) and anti-plexinA1. Lysates from HEK cells transfected with plexinA1–Myc and nephrin–FLAG were used as positive controls.
Glutathione S-transferase (GST) binding assays were performed as described previously,25 with minor modifications. In brief, GST–nephrin cytoplasmic domain (GST-CD-nephrin) and GST–control fusion proteins expressed in Escherichia coli BL21 (Stratagene, La Jolla, CA) were purified using batch purification on glutathione–Sepharose 4B beads (GE Healthcare, Little Chalfont, UK). PlexinA1–FLAG was purified from HEK cells transiently transfected with pFlag–CMV–plexinA1 construct26 using anti-FLAG M2 affinity gel and was eluted by competition with 3× FLAG peptide (F4799; Sigma-Aldrich). One microgram of purified plexinA1–FLAG was incubated overnight with 0.25 μg GST fusion proteins in PBS and 0.05% Triton-X, washed, eluted by boiling in Laemmli buffer, resolved, immunoblotted with anti-FLAG (Sigma-Aldrich) and anti-GST (sc-33613; Santa Cruz Biotechnology) antibodies, and detected by enhanced chemiluminescence.
Blot overlay assays were performed as described previously.25 Two to ten micrograms of GST-tagged fusion proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes, probed with 0.2 μg/mL purified plexinA1–FLAG, immunoblotted with anti-FLAG antibody, and detected by enhanced chemiluminescence.
Generation of the Sema3a+ Podocyte Cell Line
The Sema3a+ mice (podocin-rtTA:tet-O-Sema3a15) were bred with H-2Kb-tsA58 mice27 (Immortomouse; Jackson Laboratory, Bar Harbor, ME) to generate a conditionally immortalized podocyte cell line overexpressing Sema3a in a doxycycline-regulated manner. Glomeruli were isolated from triple-transgenic mice using magnetic beads under sterile conditions,22,28,29 plated on collagen I–coated dishes, and cultured in RPMI 1640 medium (Life Technologies) under permissive conditions (33°C), as described previously.25 Podocyte primary cultures were propagated and subjected to dilution cloning. Clones were selected on the basis of podocyte-specific protein expression, morphology, and doxycycline-regulated Sema3a expression and then were propagated and induced to differentiate at 37°C for at least 7 days before experiments.
Differentiated Sema3a+ podocytes plated on collagen I–coated glass slide chambers were kept in standard medium or were exposed to 50 to 500 ng/mL recombinant mouse Sema3a16 for 1 to 12 hours, fixed in 4% paraformaldehyde, and stained with rhodamine phalloidin. Images were acquired using a Zeiss Axiovert microscope (Carl Zeiss Microscopy, Jena, Germany) equipped with an ApoTome imaging system. Podocyte area (μm2) was measured using Zeiss AxioVision software version 4.8 freehand area selection on images acquired at ×600 magnification (n = 43 ± 8 cells per experimental condition in four independent experiments). Data are expressed as means ± SEM.
Statistical Analysis
Student’s unpaired t-test or analysis of variance followed by Bonferroni correction was used to compare groups, as appropriate. P < 0.05 was deemed statistically significant.
Results
Podocyte Sema3a Gain-of-Function in Adult Mice Causes Glomerular Disease
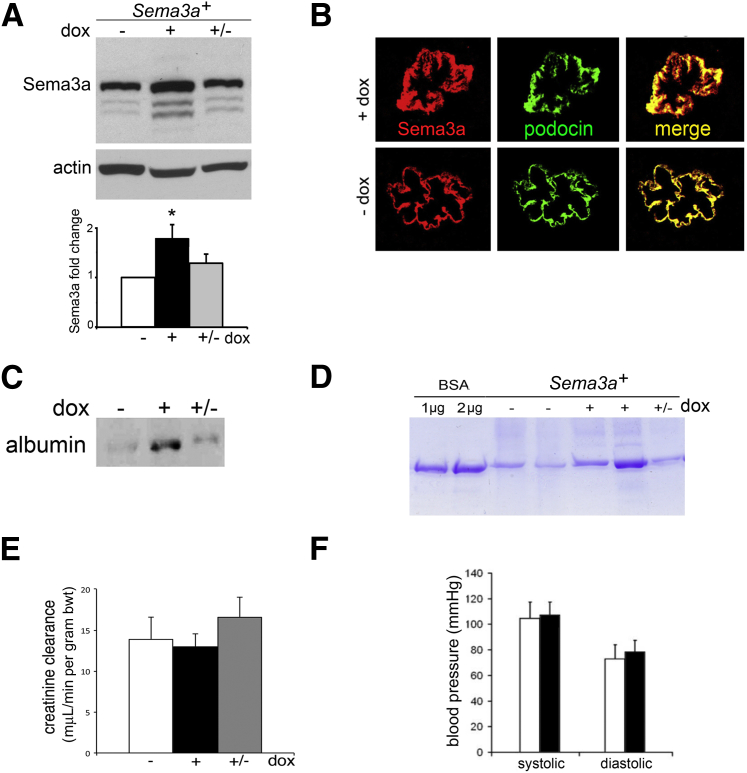
To determine whether increased podocyte Sema3a has a pathogenic role, we used Sema3a+ podocyte-specific, inducible Sema3a gain-of-function adult mice.15 In these mice, the Sema3a transgene is expressed in podocytes in a doxycycline-regulated manner15 (Figure 1, A and B). Uninduced adult Sema3a+ mice are fertile and have normal life spans, normal renal histology, and normal glomerular ultrastructure, as assessed by TEM (Figure 2, B, E, and G). Sema3a+ mice were induced with doxycycline for 1 month. Uninduced Sema3a+ and single-transgenic mice receiving doxycycline served as controls.
Figure 1.
Podocyte Sema3a gain-of-function induces proteinuria. A: Western blotting indicates that Sema3a+ mice overexpress kidney Sema3a approximately twofold after doxycycline-induction for 1 month. Smaller molecular weight bands represent cleaved Sema3a. Quantitation is based on at least three independent experiments. B: Dual immunofluorescence reveals Sema3a (red) and podocin (green) expression in developing mouse glomeruli; colocalization (yellow) indicates Sema3a expression in podocytes. C and D: Western blotting (C) and Coomassie Blue gel (D) indicate that excess Sema3a induces reversible proteinuria, based on albumin detected in urine samples corrected for creatinine. E and F: Excess Sema3a does not alter creatinine clearance (E) or blood pressure (F) within 1 month. Data are expressed as means ± SEM. White bars, control (−dox); black bars, Sema3a excess (+dox); gray bars, reversibility (+/−dox). ∗P < 0.05. bwt, body weight. Original magnification, ×400 (E).
Figure 2.
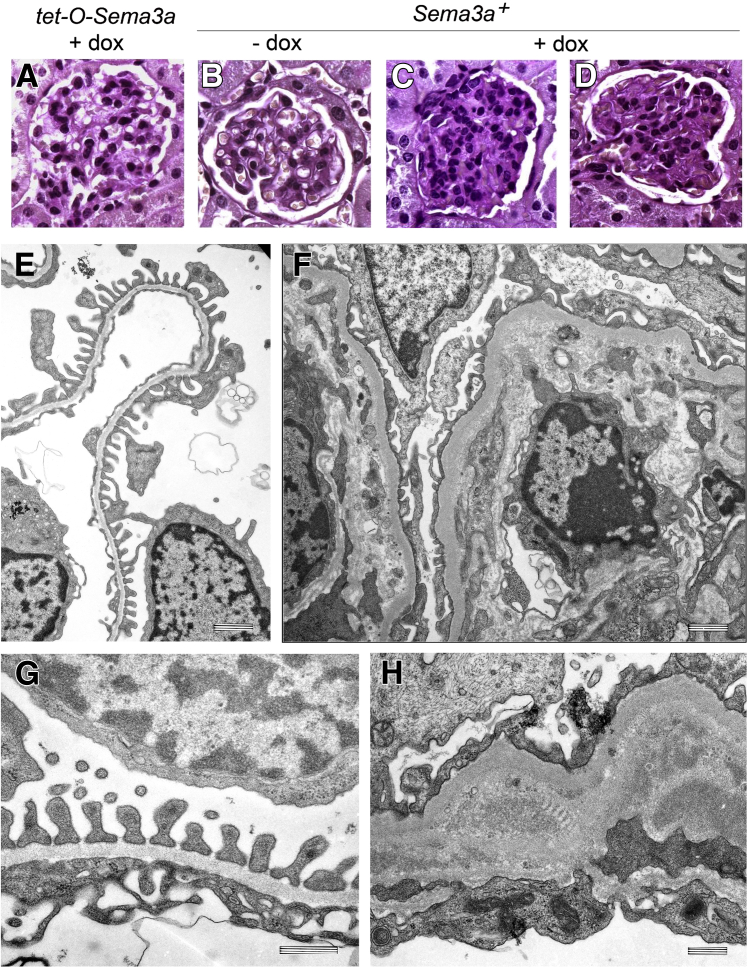
Podocyte Sema3a gain-of-function induces mesangial expansion foot process effacement and endothelial injury. A and B: PAS staining reveals normal glomeruli in kidneys of single-transgenic mice with doxycycline (+dox) and in uninduced Sema3a+ mice (−dox). C and D: Two representative Sema3a-overexpressing glomeruli (+dox) exhibit mesangial expansion. E–H: TEM reveals normal glomerular filtration barrier ultrastructure in uninduced Sema3a+ kidneys (E and G). Sema3a overexpression induces foot process effacement, endothelial cell swelling, and marked GBM expansion (F and H). Original magnification, ×400 (A–D). Scale bars: 1 μm (E and F) and 500 nm (G and H).
Podocyte Sema3a-overexpressing mice developed proteinuria within 4 weeks (Figure 1, C and D); their creatinine clearance and blood pressure were normal (Figure 1, E and F). Light microscopy revealed mesangial matrix expansion and mild hypercellularity (Figure 2, C and D), and TEM revealed marked glomerular abnormalities in Sema3a-overexpressing kidneys (Figure 2, F and H). Expansion of the mesangial matrix was confirmed by TEM (Supplemental Figure S1). Glomerular endothelial cells were swollen and exhibited interdigitation (Figure 2, F and H), indicating that podocyte Sema3a overexpression induces endothelial cell injury. The GBM was thickened; the lamina rara interna was widened by lamination, electron-dense material, and mesangial matrix interposition (Figure 2, F and H). Significant podocyte foot process effacement was observed adjacent to the GBM abnormalities (Figure 2, F and H). Morphometric analysis of TEM images indicated increased foot process width in Sema3a-overexpressing mice, compared with uninduced mice of identical genotype or single-transgenic tet-O-Sema3a mice (737 ± 77 nm versus 370 ± 13 nm or 333 ± 50 nm, respectively) (P < 0.05). Thus, Sema3a-overexpressing mice developed a distinct glomerular disease characterized by mesangial matrix and GBM expansion, focal foot process effacement, endothelial injury, and proteinuria.
Sema3a-Induced Glomerular Disease Is Partially Reversible
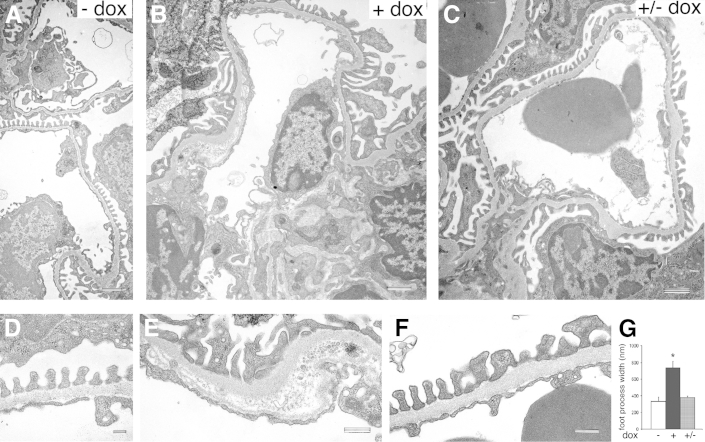
To determine whether the effects of podocyte Sema3a gain-of-function are reversible, Sema3a+ mice were induced for 1 month with doxycycline and examined 2 weeks after doxycycline removal (+/−dox). Sema3a-induced albuminuria resolved 2 weeks after doxycycline removal in approximately half of the mice (6 of 11 mice) (Figure 1, C and D). Notably, TEM revealed that the Sema3a-induced foot process effacement and the GBM and endothelial damage (Figure 3, B and E) also resolved upon removal of transgene induction (Figure 3, C and F), although some mesangial matrix expansion and focal GBM changes remained (Supplemental Figure S1). TEM morphometric analysis confirmed that foot process width returned to baseline (from 737 ± 77 to 380 ± 15 nm; P < 0.05) (Figure 3G). Although Sema3a-induced abnormalities were only partially reversible, these findings provide a proof-of-principle of the cause-and-effect relationship between podocyte Sema3a excess and glomerular disease.
Figure 3.
Sema3a-induced glomerular ultrastructural abnormalities are reversible. A and D: TEM reveals normal glomerular filtration barrier ultrastructure in uninduced Sema3a+ kidneys. B and E: Sema3a podocyte overexpression induces foot process effacement, mesangial matrix expansion, and GBM lamination. C and F: At 2 weeks after doxycycline removal (+/−dox), the Sema3a-induced foot process effacement, GBM, and endothelial cell abnormalities resolve. G: Morphometry confirms reversibility of foot process effacement. Data are expressed as means ± SEM. ∗P < 0.05. Scale bars: 1 μm (A–C); 200 nm (D); 500 nm (E and F).
Podocyte Sema3a Gain-of-Function Disrupts GBM Composition
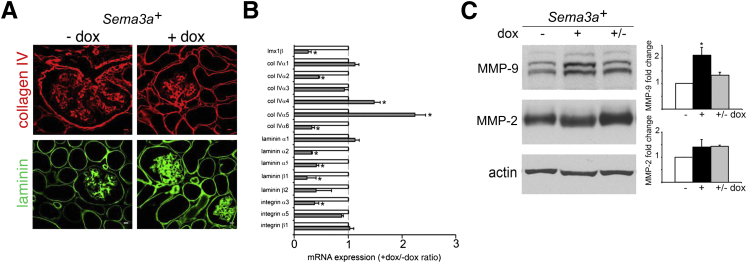
To further define the GBM phenotype induced by excess Sema3a, we examined mRNA and protein expression of GBM components in induced Sema3a+ and uninduced (control) kidneys. Expression of total collagen IV and laminin, as assessed by immunohistochemistry, was similar in induced Sema3a+ and control kidneys (Figure 4A). Collagen IV and laminin chain immunostaining revealed no change in localization of individual chains (data not shown); however, α2 laminin expression was decreased in Sema3a-overexpressing kidneys, compared with controls (data not shown). qPCR demonstrated a significant increase in α4 and α5 collagen IV mRNA, whereas laminin α2, α5, β1, and β2 mRNAs were decreased to less than half their control levels (Figure 4B), suggesting that the total laminin observed with IHC may include abnormal laminin chains. Expression level of the LIM homeobox transcription factor 1-beta, a major regulator of the GBM encoded by Lmx1b, decreased significantly in Sema3a-overexpressing kidneys, as determined by qPCR (Figure 4B). Next, we examined the glomerular metalloproteinases MMP-2 and MMP-9 by immunoblotting. MMP-9 was up-regulated in Sema3a-overexpressing kidneys and returned to baseline on removal of the transgene induction, whereas expression of MMP-2 did not change (Figure 4C). Taken together, these findings suggest that the Sema3a-induced GBM phenotype in adult kidney results from both increased MMP-9 expression and subtle changes in collagen and laminin chain composition.
Figure 4.
Sema3a gain-of-function disrupts GBM composition. A: IHC reveals similar distribution of collagen IV and laminin. B: qPCR indicates Sema3a-induced dysregulation of individual collagen IV and laminin chain mRNA, normalized for control mRNA expression levels. C: Western blotting indicates Sema3a-induced reversible MMP-9 up-regulation; MMP-2 remains unchanged. Quantitation of fold change is based on at least four independent experiments. Data are expressed as means ± SEM. ∗P < 0.05. col, collagen. Scale bar = 400 μm (A).
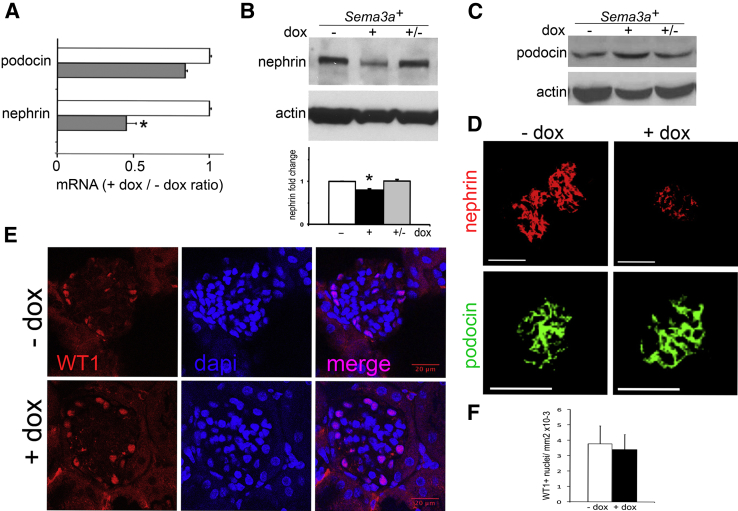
Sema3a Gain-of-Function Decreases Nephrin Expression
To define the molecular basis of podocyte effacement in Sema3a+ adult mice, we examined the expression of slit-diaphragm proteins. Nephrin expression decreased to approximately 50% of control levels, as demonstrated by qPCR, immunoblotting, and immunohistochemistry, whereas podocin expression level was not altered by Sema3a excess (Figure 5, A–C), suggesting that the decreased nephrin expression was not a result of podocyte loss. Similar podocyte numbers in control and Sema3a-overexpressing glomeruli was confirmed by counts of WT1+ nuclei (Figure 5, D and E). On removal of doxycycline induction, nephrin expression returned to control levels (Figure 5B), demonstrating a cause-and-effect relationship between Sema3a excess and nephrin down-regulation. The concomitant reversibility of podocyte foot process effacement and proteinuria independently confirmed the lack of podocyte loss and the Sema3a-induced pathogenic mechanism (ie, nephrin loss disrupts slit-diaphragm integrity, leading to increased glomerular filtration barrier permeability).
Figure 5.
Sema3a gain-of-function down-regulates nephrin expression. A: Quantitative PCR indicates decreased nephrin mRNA and stable podocin mRNA in Sema3a-overexpressing mice. B and C: Western blotting for nephrin (B) and podocin (C) in whole-kidney lysate indicates that excess Sema3a induces reversible nephrin down-regulation but does not affect podocin expression. Quantitation is based on at least four independent experiments. D: Immunofluorescence reveals down-regulation of nephrin and stable expression of podocin localized to glomerular podocytes. E: Immunofluorescence reveals no change in podocyte number (WT1+ nuclei) in Sema3a-overexpressing mice versus controls. DAPI labels nuclei; merged images confirm nuclear WT1 staining. F: Quantitation of WT1+ cells per standard area. Data are expressed as means ± SEM. n = 3 mice per experimental group (approximately 20 glomeruli quantified per mouse). Scale bars: 100 μm (D); 20 μm (E).
The Sema3a Signaling Receptor PlexinA1 Interacts with Nephrin
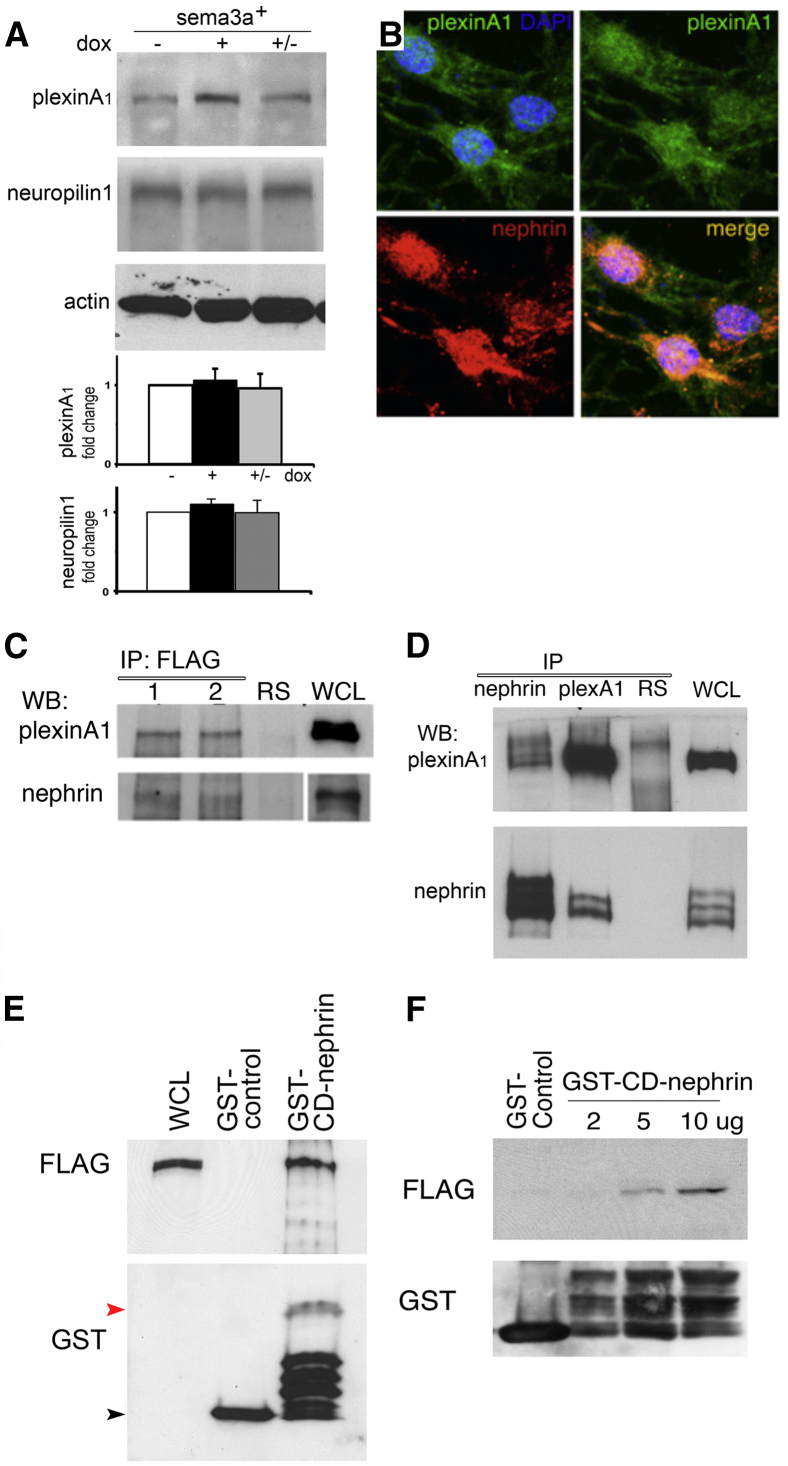
We examined the molecular mechanisms involved in the crosstalk between Sema3a and nephrin signaling. Neuropilin-1 and plexinA1, Sema3a binding, and signaling receptors12,30,31 were detected in kidney lysates and cultured podocytes by immunoblotting and immunofluorescence (Figure 6, A and B, and Supplemental Figure S2). Purified nephrin–FLAG was found to associate with endogenous plexinA1 in vivo and in cultured mouse podocytes, as determined by co-immunoprecipitation (Figure 6C). Furthermore, plexinA1 co-immunoprecipitated with nephrin in HEK cells transiently transfected with the corresponding constructs (Figure 6D). Overlay and GST binding assays were performed to further evaluate plexinA1–nephrin interaction. These experiments showed that purified plexinA1–FLAG physically associates with the cytoplasmic domain of nephrin (GST-CD-nephrin) (Figure 6, E and F). Taken together, these findings demonstrate a direct plexinA1–nephrin interaction.
Figure 6.
Sema3a signaling receptor plexinA1 interacts with nephrin. A: Representative Western blots and quantitation indicate no significant changes in plexinA1 or neuropilin 1 expression in whole kidneys after doxycycline induction. B: Immunofluorescence reveals colocalization of plexinA1 and nephrin in cultured podocytes. C: Co-immunoprecipitation reveals association of endogenous plexinA1 and FLAG-nephrin. Lane 1, cultured podocytes; lane 2, whole kidney. D: Reciprocal nephrin and plexinA1 coimmunoprecipitation reveals nephrin–plexinA1 interaction in transfected HEK cells. E and F: GST binding assay (E) indicates direct interaction of purified FLAG-plexinA1 with nephrin cytoplasmic domain (GST-CD-nephrin, approximately 60 kDa; red arrowhead); the GST-control is approximately 25 kDa (black arrowhead). Overlay assay (F) indicates that plexinA1–nephrin interaction is direct. Purified FLAG-plexinA1 binds increasing amounts of GST-CD-nephrin blotted on cellulose membrane, as detected by FLAG immunoblotting; the GST Western blot confirms equal loading. Data are representative of at least three independent experiments. IP, immunoprecipitation; RS, rabbit serum; WB, Western blot; WCL, whole-cell lysate. Original magnification, ×400 (B).
Sema3a Decreases Glomerular αvβ3 Integrin Activity and Modulates Podocyte Shape
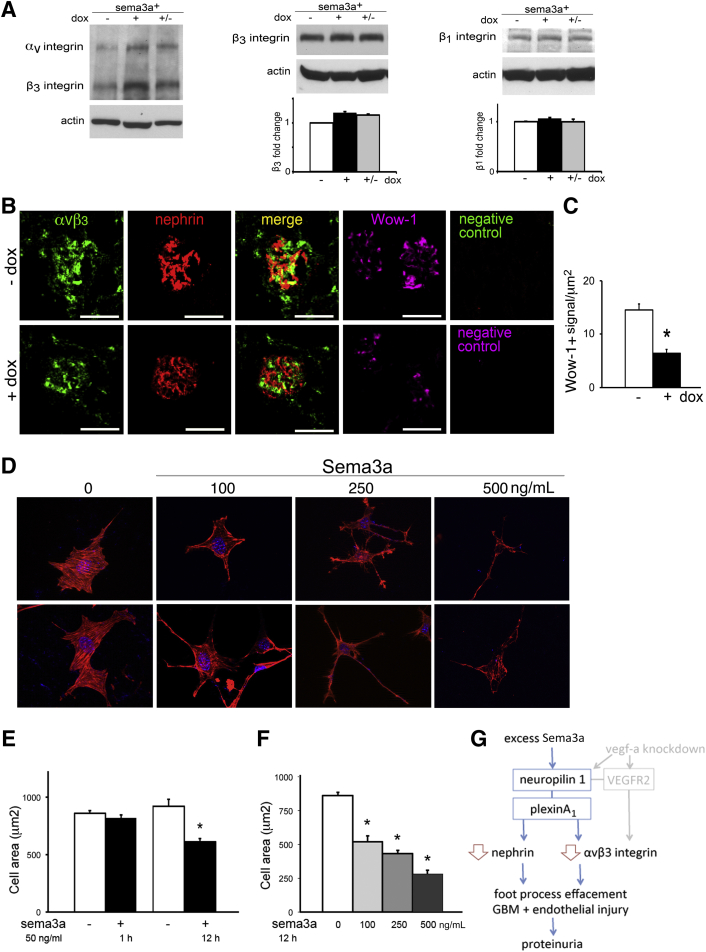
Sema3a signaling inhibits integrin activity and thereby modulates cell adhesion and motility.12,32 We therefore compared αvβ3 and β1 integrin expression and activity in Sema3a-overexpressing and control kidneys. Although quantitation of αvβ3, β1, or β3 integrin expression in whole-kidney lysates was similar (Figure 7A), Sema3a gain-of-function decreased glomerular αvβ3 integrin expression and activity, as assessed by immunolabeling with αvβ3 integrin and Wow-1 antibodies, which recognize total and activated αvβ3 integrin, respectively (Figure 7B). Quantitation of Wow-1 signals by morphometric analysis confirmed the observation that Sema3a gain-of-function results in an approximately 50% decrease in glomerular active αvβ3 integrin (Figure 7C), suggesting that low αvβ3 integrin activity contributes to the observed glomerular phenotype.
Figure 7.
Sema3a decreases αvβ3 integrin glomerular activity and induces podocyte contraction and shape change. A: Western blotting and quantitation indicate that αvβ3, β3, and β1 integrin whole-kidney expression levels were not changed by Sema3a overexpression. B: Immunofluorescence reveals decrease of both glomerular total αvβ3 integrin and Wow-1 (active αvβ3 integrin) in Sema3a-overexpressing kidneys. The αvβ3 integrin and nephrin partially colocalize; negative controls demonstrate no signal in the absence of the primary antibodies (total αvβ3 integrin, nephrin, and active αvβ3 integrin). C: Quantitation of active αvβ3-positive signals (Wow-1), normalized for glomerular area (integrated density/μm2) indicates an approximately 50% decrease in αvβ3 integrin activity. D: Sema3a induced cell contraction and shape change in a dose-dependent manner; two representative images from each condition are shown (rhodamine phalloidin staining). Note decreasing F-actin staining at higher Sema3a concentration. E: Differentiated Sema3a podocytes exposed to vehicle or 50 ng/mL recombinant mouse Sema3a for 1 hour or 12 hours exhibited significant change in cell size. F: Quantitation after 12 hours of Sema3a exposure indicates dose-dependent decrease in cell area. G: Model for Sema3a-induced glomerular disease mechanism. Data are expressed as means ± SEM, representative of four independent experiments (approximately 50 cells per experiment). ∗P < 0.05. Scale bar = 100 μm (B). Original magnification, ×600 (D).
To determine whether Sema3a influences podocyte shape cell-autonomously, we exposed cultured mouse podocytes to recombinant Sema3a16 and examined cell shape, size, and actin cytoskeleton by rhodamine phalloidin staining (Figure 7, D–F). We observed that podocyte shape changes develop within 1 to 12 hours of Sema3a exposure. Morphometric analysis of time- and dose-response experiments demonstrated that Sema3a induces significant podocyte contraction and F-actin collapse, resulting in decreased size and dramatic shape changes (Figure 7, D–F).
Discussion
While our previous studies showed that excess Sema3a disrupts slit-diaphragm development, the present findings demonstrate that a tight regulation of podocyte Sema3a is important for the maintenance of glomerular filtration barrier structure and function in the adult kidney. In the present study, we report that podocyte Sema3a gain-of-function in adult mice causes glomerular disease, and Sema3a overexpression induces reversible dysregulation of nephrin, MMP-9, and αvβ3 integrin, resulting in foot process effacement, a striking GBM phenotype, mesangial expansion, and endothelial cell injury.
An important finding of the present study is that the glomerular disease caused by excess podocyte Sema3a in adult mice is partially reversible. The evidence is that Sema3a-induced foot process effacement and endothelial injury are reversible 2 weeks after removal of the transgene induction. Moreover, we demonstrated that down-regulation of nephrin induced by Sema3a gain-of-function is also reversible, and the latter results in recovery of glomerular permselectivity. Proteinuria resolved in more than half of the mice; persistent proteinuria in the remainder was likely due to the observed persistent excess extracellular matrix and focal GBM abnormalities. Taken together, these findings provide proof-of-principle of the cause-and-effect relationship between podocyte Sema3a excess and glomerular disease. Consistent with these data, we had previously found that mice overexpressing Sema3a during glomerular development lacked slit diaphragms at birth and that their nephrin and WT-1 expression was decreased.15 We had also found that cultured podocytes exposed to recombinant Sema3a had decreased association of nephrin–podocin–CD2AP and down-regulated podocin, indicating that Sema3a dysregulates slit-diaphragm proteins16 (albeit not precisely in the same manner as it does in vivo, likely because of cell culture conditions).
Another key finding of the present study is that in podocytes the Sema3a signaling receptor plexinA1 interacts with nephrin in vivo and in vitro, as shown by co-immunoprecipitation of native plexinA1 and purified nephrin and confirmed using transfected cells and purified proteins. Overlay and GST binding assays demonstrated that plexinA1–nephrin interaction is direct. Taken together, these findings indicate that plexinA1 is a novel component of the nephrin signaling complex. PlexinA1–nephrin interaction links Sema3a extracellular signals to the nephrin signaling pathway and provides a mechanism for Sema3a-induced foot process effacement. The plexinA1 extracellular domain is known to be autoinhibitory, and the inhibition is released on Sema3a binding to neuropilin-1.24,33 Downstream plexinA1 signaling is complex and involves microtubule and actin cytoskeleton regulation via integrins, CRMPs, Rho-GTPases, and protein-methionine sulfoxide oxidase MICAL1.12,33–35 Further studies would be required for detailed characterization of the downstream intracellular signaling pathways mediating the Sema3a effects on podocytes described here. Nonetheless, consistent with previous reports documenting Sema3a-induced growth-cone collapse in neurons,12 as well as F-actin collapse and inhibition of motility and migration in endothelial cells,15,36 our present findings indicate that Sema3a causes podocyte F-actin collapse and podocyte shape change, in a time- and concentration-dependent manner. Sema3a-induced cell shape changes may be mediated in part by αvβ3 integrin inactivation, and may also involve microtubules and myosin.37,38
Sema3a gain-of-function induces a laminated GBM phenotype similar to that of Alport or Pierson syndrome and that of integrin knockout and Vegf-a knockdown mice, likely through its effects on podocytes and endothelial cells, which together secrete the GBM components.39 The mechanisms that regulate GBM assembly are not fully understood, and changes in GBM composition are involved in the pathogenesis of a variety of human proteinuric kidney diseases. These include classic GBM diseases such as Alport syndrome, diabetic nephropathy, and lupus nephritis.9,40 Interestingly, our research group and others have reported Sema3a dysregulation in diabetic nephropathy and in lupus nephritis, respectively.19,41
A proper balance of collagen chain synthesis appears to be required for generation of the triple helix of collagen IV and laminin chains; indeed, permeability defects in the Alport mouse model are exacerbated by inappropriate laminin chain synthesis in response to α3 collagen IV deficit.8 Our present findings suggest that excess Sema3a dysregulates MMP-9, as well as various extracellular matrix components (α2, α5, and β1 laminins) and integrin receptors (α3 and αvβ3) in the glomerulus. Laminin α5, a major component of the adult glomerular basement membrane laminin 521 (α5β2γ1), is required for mesangial cell adhesion and glomerular development and is down-regulated in diabetic nephropathy.42,43 Mutations of β2 laminin cause Pierson syndrome, a rare form of nephrotic syndrome.44,45 Abnormal glomerular deposition of α2 laminin has been reported in Alport syndrome.46 Down-regulation of the Lmx1b protein suggests that Sema3a might regulate the molecular composition of the GBM, although similar to podocyte-specific Lmx1b-knockout mice and to patients with nail–patella syndrome, we did not identify the decreased expression of podocin, CD2AP, or α3 collagen IV that has been described in Lmx1b-null mice.47–50
Integrin α3 facilitates podocyte attachment to the GBM and is required during development to organize glomerular capillary loops and GBM assembly.51,52 Deletion of podocyte β1 integrin induces podocyte effacement and laminated GBM, demonstrating a role in podocyte attachment; glomerular endothelium remains normal.53 Deletion of the tetraspanin CD151 (which regulates α3 integrin–mediated adhesion) induces GBM abnormalities similar to those observed in Alport mice and similar to our present findings, suggesting that defects in podocyte adhesion can induce abnormalities in GBM structure.54 Sema3a inhibits endothelial cell adhesion to the extracellular matrix and cell motility via inhibition of integrin activity in vitro.32,35 In the kidney, αvβ3 integrin is expressed by the glomerular endothelium and podocytes, the target cells for Sema3a paracrine13,36 and autocrine7,15,16 effects. Podocyte Sema3a gain-of-function down-regulates glomerular αvβ3 integrin expression and activity in vivo. However, Sema3a-induced changes in podocyte shape, cytoskeleton, and αvβ3 integrin did not result in podocyte loss in vivo.
Remarkably, podocyte Vegfa knockdown induces a glomerular phenotype similar to that of Sema3a gain-of-function, mediated at least in part by down-regulation of glomerular αvβ3 integrin expression and activity.11 Vegf-a and Sema3a compete to bind neuropilin-1, which functions as a coreceptor for Vegf-a and a binding receptor for Sema3a.12,23,36 Of note, Vegf-a and neuropilin-1 expression are not altered in Sema3a gain-of-function mice, and Sema3a expression is not dysregulated in mice with podocyte Vegfa knockdown (data not shown). Taken together, these findings suggest that down-regulation of αvβ3 integrin expression and activity plays a key role in disrupting the integrity and function of the glomerular filtration barrier. Notably, this occurs downstream of both Sema3a excess (the present study) and Vegfa knockdown,11 suggesting that these two proteins secreted by podocytes mediate opposite signals (in the same or independent pathways) that merge to down-regulate integrin activity in vivo (Figure 7G), as described in cultured cells,32,36 resulting in glomerular phenotypes similar to integrin deletion models.
We have previously shown that Sema3a expression in the kidney is developmentally regulated and that Sema3a loss or gain-of-function disrupts normal glomerular filtration barrier development.7,15 The transcriptional and translational regulation of Sema3a in health and disease are poorly understood. Similarly, the regulation of Sema3a secretion (if any) is unknown; most studies of semaphorins have focused on the downstream signaling, developmental, and pathological effects.12,13,56,58 Our research group previously reported up-regulation of Sema3a protein expression in type 1 diabetic mice,19 and we have also observed increased Sema3a in renal biopsies from patients with advanced diabetic nephropathy (D. Veron and A. Tufro, unpublished data), raising the possibility that excess Sema3a may contribute to the pathogenesis of proteinuric kidney disease, including diabetic nephropathy. Quantitation of Sema3a mRNA expression in glomeruli from db/db mice reported by our group and others showed discrepant results (up-regulation and down-regulation, respectively),13,55 whereas neuropilin-1 mRNA decreased in diabetic mice and humans,55 providing further indication that the complexity of glomerular Sema3a biology warrants further study.
Accumulating evidence suggests that Sema3a negatively regulates immune responses via Sema3a, neuropilin-1 and plexinA4 expressed in T cells, regulatory B cells, and activated monocytes.41,56 Sema3a dysregulation was recently reported in human systemic lupus erythematosus; serum Sema3a was mildly decreased, whereas Sema3a was increased in renal tubules.56,57 Further studies are needed to elucidate whether dysregulation of Sema3a signaling in T cells or B cells is pathogenic in systemic lupus erythematosus and whether it is mechanistically involved in lupus nephritis. However, given the role of Sema3a as negative regulator of immune response and angiogenesis, as well as its osteoprotective effect, it has been suggested that Sema3a could be used therapeutically in immune diseases, cancer, and osteoporosis.56,58,59 Off-target effects of Sema3a should therefore be carefully examined.
Taken together, our present findings demonstrate that excess podocyte Sema3a causes glomerular disease in adult mice. Thus, tight Sema3a regulation is critical for the maintenance of the glomerular filtration barrier structure and function. Our findings suggest that, while Sema3a-induced down-regulation of nephrin leads to podocyte effacement, up-regulation of MMP-9 and decreased αvβ3 integrin activity in the glomerulus result in disruption of the integrity of the GBM and endothelial injury. Direct Sema3a effects on podocyte and endothelial cell actin cytoskeleton are likely to contribute to podocyte effacement and endotheliosis in vivo. PlexinA1–nephrin interaction links Sema3a signals to the nephrin signaling pathway, providing a mechanism for Sema3a-induced foot process effacement. Insight into precisely how plexinA1–nephrin interaction modulates nephrin turnover and podocyte cytoskeleton will require additional studies, including identification of the responsible actin-binding protein. Elucidation of the Sema3a signaling pathway downstream from plexinA1 in podocytes may reveal novel molecular and cellular mechanisms involved in the pathogenesis of proteinuric renal disease and diabetic nephropathy, and may identify novel therapeutic targets as well.
Acknowledgments
We thank Alex Kolodkin (John Hopkins University) for providing NP1 antibody, Lawrence Holzman (University of Pennsylvania Perelman School of Medicine) for providing GST–nephrin constructs and nephrin antibody, Valerie Castellani (Centre de Génétique et de Physiologie Moléculaire et Cellulaire, Lyon, France) and Stephen Strittmatter (Yale School of Medicine) for providing plexinA1 constructs, and Jeffrey Miner (Washington University in St. Louis) for laminin chain immunostaining and for critical review of the manuscript.
Footnotes
Supported by NIH grants R01-DK64187 (A.T.), R01-DK59333 (A.T.), and T32-DK007110 (K.R.) and a grant from the Emerald Foundation (A.T.).
K.J.R. and P.K.A. contributed equally to this work.
Current address of J.J.J., Department of Pathology, Mount Sinai School of Medicine, New York, NY; of D.B.T., Department of Pathology, University of Miami, Miami, FL.
Supplemental Data
Podocyte Sema3a overexpression in mice induces mesangial expansion, as seen in TEM micrographs. A: Uninduced Sema3a+ mice (without doxycycline; −dox) have normal glomerular ultrastructure. B: Induced Sema3a+ mice (+dox) exhibit extensive mesangial matrix expansion. C: Mesangial expansion is not reversible after doxycycline removal for 2 weeks (±dox). Scale bars = 2 μm (A and C); 1 μm (B).
Differentiated Sema3a+ podocytes express the podocyte-specific proteins nephrin, podocin, and WT1, as well as Sema3a and its binding and signaling receptors neuropilin 1 and plexinA1, as detected by Western blot analysis.
References
- 1.Haraldsson B., Nyström J., Deen W.M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 2.Reiser J., Kriz W., Kretzler M., Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- 3.Tryggvason K., Patrakka J., Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 4.Kestilä M., Lenkkeri U., Männikkö M., Lamerdin J., McCready P., Putaala H., Ruotsalainen V., Morita T., Nissinen M., Herva R., Kashtan C.E., Peltonen L., Holmberg C., Olsen A., Tryggvason K. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 5.Zenker M., Machuca E., Antignac C. Genetics of nephrotic syndrome: new insights into molecules acting at the glomerular filtration barrier. J Mol Med (Berl) 2009;87:849–857. doi: 10.1007/s00109-009-0505-9. [DOI] [PubMed] [Google Scholar]
- 6.Boute N., Gribouval O., Roselli S., Benessy F., Lee H., Fuchshuber A., Dahan K., Gubler M.C., Niaudet P., Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [Erratum appeared in Nat Genet 2000, 25:125] [DOI] [PubMed] [Google Scholar]
- 7.Villegas G., Tufro A. Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Mech Dev. 2002;119(Suppl 1):S149–S153. doi: 10.1016/s0925-4773(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 8.Abrahamson D.R., Isom K., Roach E., Stroganova L., Zelenchuk A., Miner J.H., St John P.L. Laminin compensation in collagen alpha3(IV) knockout (Alport) glomeruli contributes to permeability defects. J Am Soc Nephrol. 2007;18:2465–2472. doi: 10.1681/ASN.2007030328. [DOI] [PubMed] [Google Scholar]
- 9.Gubler M.C. Inherited diseases of the glomerular basement membrane. Nat Clin Pract Nephrol. 2008;4:24–37. doi: 10.1038/ncpneph0671. [DOI] [PubMed] [Google Scholar]
- 10.Eremina V., Jefferson J.A., Kowalewska J., Hochster H., Haas M., Weisstuch J., Richardson C., Kopp J.B., Kabir M.G., Backx P.H., Gerber H.P., Ferrara N., Barisoni L., Alpers C.E., Quaggin S.E. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veron D., Villegas G., Aggarwal P.K., Bertuccio C., Jimenez J., Velazquez H., Reidy K., Abrahamson D.R., Moeckel G., Kashgarian M., Tufro A. Acute podocyte vascular endothelial growth factor (VEGF-A) knockdown disrupts alpha(V)beta(3) integrin signaling in the glomerulus. PLoS One. 2012;7:e40589. doi: 10.1371/journal.pone.0040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran T.S., Kolodkin A.L., Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 13.Reidy K., Tufro A. Semaphorins in kidney development and disease: modulators of ureteric budbranching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr Nephrol. 2011;26:1407–1412. doi: 10.1007/s00467-011-1769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tufro A., Teichman J., Woda C., Villegas G. Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech Dev. 2008;125:558–568. doi: 10.1016/j.mod.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reidy K.J., Villegas G., Teichman J., Veron D., Shen W., Jimenez J., Thomas D., Tufro A. Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development. 2009;136:3979–3989. doi: 10.1242/dev.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan F., Villegas G., Teichman J., Mundel P., Tufro A. Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int. 2006;69:1564–1569. doi: 10.1038/sj.ki.5000313. [DOI] [PubMed] [Google Scholar]
- 17.Tapia R., Guan F., Gershin I., Teichman J., Villegas G., Tufro A. Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int. 2008;73:733–740. doi: 10.1038/sj.ki.5002726. [DOI] [PubMed] [Google Scholar]
- 18.Seiler M.W., Venkatachalam M.A., Cotran R.S. Glomerular epithelium: structural alterations induced by polycations. Science. 1975;189:390–393. doi: 10.1126/science.1145209. [DOI] [PubMed] [Google Scholar]
- 19.Veron D., Bertuccio C.A., Marlier A., Reidy K., Garcia A.M., Jimenez J., Velazquez H., Kashgarian M., Moeckel G.W., Tufro A. Podocyte vascular endothelial growth factor (Vegf164) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia. 2011;54:1227–1241. doi: 10.1007/s00125-010-2034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pampori N., Hato T., Stupack D.G., Aidoudi S., Cheresh D.A., Nemerow G.R., Shattil S.J. Mechanisms and consequences of affinity modulation of integrin alpha(V)beta(3) detected with a novel patch-engineered monovalent ligand. J Biol Chem. 1999;274:21609–21616. doi: 10.1074/jbc.274.31.21609. [DOI] [PubMed] [Google Scholar]
- 21.Veron D., Reidy K.J., Bertuccio C., Teichman J., Villegas G., Jimenez J., Shen W., Kopp J.B., Thomas D.B., Tufro A. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 2010;77:989–999. doi: 10.1038/ki.2010.64. [DOI] [PubMed] [Google Scholar]
- 22.Yu L., Su Y., Paueksakon P., Cheng H., Chen X., Wang H., Harris R.C., Zent R., Pozzi A. Integrin α1/Akita double-knockout mice on a Balb/c background develop advanced features of human diabetic nephropathy. Kidney Int. 2012;81:1086–1097. doi: 10.1038/ki.2011.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolodkin A.L., Levengood D.V., Rowe E.G., Tai Y.T., Giger R.J., Ginty D.D. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 24.Verma R., Wharram B., Kovari I., Kunkel R., Nihalani D., Wary K.K., Wiggins R.C., Killen P., Holzman L.B. Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J Biol Chem. 2003;278:20716–20723. doi: 10.1074/jbc.M301689200. [Erratum appeared in J Biol Chem 2005, 280:26640] [DOI] [PubMed] [Google Scholar]
- 25.Bertuccio C., Veron D., Aggarwal P.K., Holzman L., Tufro A. Vascular endothelial growth factor receptor 2 direct interaction with nephrin links VEGF-A signals to actin in kidney podocytes. J Biol Chem. 2011;286:39933–39944. doi: 10.1074/jbc.M111.241620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawabi H., Briançon-Marjollet A., Clark C., Sanyas I., Takamatsu H., Okuno T., Kumanogoh A., Bozon M., Takeshima K., Yoshida Y., Moret F., Abouzid K., Castellani V. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev. 2010;224:396–410. doi: 10.1101/gad.542510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shigehara T., Zaragoza C., Kitiyakara C., Takahashi H., Lu H., Moeller M., Holzman L.B., Kopp J.B. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol. 2003;14:1998–2003. doi: 10.1681/ASN.V1481998. [DOI] [PubMed] [Google Scholar]
- 28.Jat P.S., Noble M.D., Ataliotis P., Tanaka Y., Yannoutsos N., Larsen L., Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mundel P., Reiser J., Zúñiga Mejía Borja A., Pavenstädt H., Davidson G.R., Kriz W., Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 30.Tamagnone L., Artigiani S., Chen H., He Z., Ming G.I., Song H., Chedotal A., Winberg M.L., Goodman C.S., Poo M., Tessier-Lavigne M., Comoglio P.M. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [Erratum appeared in Cell 2001, 104:following 320] [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T., Fournier A., Nakamura F., Wang L.H., Murakami Y., Kalb R.G., Fujisawa H., Strittmatter S.M. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 32.Serini G., Valdembri D., Zanivan S., Morterra G., Burkhardt C., Caccavari F., Zammataro L., Primo L., Tamagnone L., Logan M., Tessier-Lavigne M., Taniguchi M., Püschel A.W., Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [Erratum appeared in Nature 2003, 424:974] [DOI] [PubMed] [Google Scholar]
- 33.Takahashi T., Strittmatter S.M. PlexinA1 autoinhibition by the plexin sema domain. Neuron. 2001;29:429–439. doi: 10.1016/s0896-6273(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 34.Hung R.J., Terman J.R. Extracellular inhibitors, repellents, and semaphorin/plexin/MICAL-mediated actin filament disassembly. Cytoskeleton (Hoboken) 2011;68:415–433. doi: 10.1002/cm.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valdembri D., Sandri C., Santambrogio M., Serini G. Regulation of integrins by conformation and traffic: it takes two to tango. Mol Biosyst. 2011;7:2539–2546. doi: 10.1039/c1mb05066d. [DOI] [PubMed] [Google Scholar]
- 36.Miao H.Q., Soker S., Feiner L., Alonso J.L., Raper J.A., Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi N., Mundel P. A role of microtubules during the formation of cell processes in neuronal and non-neuronal cells. Cell Tissue Res. 1998;291:163–174. doi: 10.1007/s004410050988. [DOI] [PubMed] [Google Scholar]
- 38.Takamatsu H., Takegahara N., Nakagawa Y., Tomura M., Taniguchi M., Friedel R.H., Rayburn H., Tessier-Lavigne M., Yoshida Y., Okuno T., Mizui M., Kang S., Nojima S., Tsujimura T., Nakatsuji Y., Katayama I., Toyofuku T., Kikutani H., Kumanogoh A. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol. 2010;11:594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.St John P.L., Abrahamson D.R. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60:1037–1046. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- 40.Herbach N., Schairer I., Blutke A., Kautz S., Siebert A., Göke B., Wolf E., Wanke R. Diabetic kidney lesions of GIPRdn transgenic mice: podocyte hypertrophy and thickening of the GBM precede glomerular hypertrophy and glomerulosclerosis. Am J Physiol Renal Physiol. 2009;296:F819–F829. doi: 10.1152/ajprenal.90665.2008. [DOI] [PubMed] [Google Scholar]
- 41.Vadasz Z., Haj T., Halasz K., Rosner I., Slobodin G., Attias D., Kessel A., Kessler O., Neufeld G., Toubi E. Semaphorin 3A is a marker for disease activity and a potential immunoregulator in systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R146. doi: 10.1186/ar3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberg S., Adair-Kirk T.L., Senior R.M., Miner J.H. Maintenance of glomerular filtration barrier integrity requires laminin alpha5. J Am Soc Nephrol. 2010;21:579–586. doi: 10.1681/ASN.2009091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ha T.S., Barnes J.L., Stewart J.L., Ko C.W., Miner J.H., Abrahamson D.R., Sanes J.R., Kasinath B.S. Regulation of renal laminin in mice with type II diabetes. J Am Soc Nephrol. 1999;10:1931–1939. doi: 10.1681/ASN.V1091931. [DOI] [PubMed] [Google Scholar]
- 44.Miner J.H., Li C. Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol. 2000;217:278–289. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- 45.Kikkawa Y., Virtanen I., Miner J.H. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol. 2003;161:187–196. doi: 10.1083/jcb.200211121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kashtan C.E., Kim Y., Lees G.E., Thorner P.S., Virtanen I., Miner J.H. Abnormal glomerular basement membrane laminins in murine, canine, and human Alport syndrome: aberrant laminin alpha2 deposition is species independent. J Am Soc Nephrol. 2001;12:252–260. doi: 10.1681/ASN.V122252. [DOI] [PubMed] [Google Scholar]
- 47.Morello R., Zhou G., Dreyer S.D., Harvey S.J., Ninomiya Y., Thorner P.S., Miner J.H., Cole W., Winterpacht A., Zabel B., Oberg K.C., Lee B. Regulation of glomerular basement membrane collagen expression by LMX1B contributes to renal disease in nail patella syndrome. Nat Genet. 2001;27:205–208. doi: 10.1038/84853. [DOI] [PubMed] [Google Scholar]
- 48.Chen H., Lun Y., Ovchinnikov D., Kokubo H., Oberg K.C., Pepicelli C.V., Gan L., Lee B., Johnson R.L. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51–55. doi: 10.1038/ng0598-51. [DOI] [PubMed] [Google Scholar]
- 49.Miner J.H., Morello R., Andrews K.L., Li C., Antignac C., Shaw A.S., Lee B. Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J Clin Invest. 2002;109:1065–1072. doi: 10.1172/JCI13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rohr C., Prestel J., Heidet L., Hosser H., Kriz W., Johnson R.L., Antignac C., Witzgall R. The LIM-homeodomain transcription factor Lmx1b plays a crucial role in podocytes. J Clin Invest. 2002;109:1073–1082. doi: 10.1172/JCI13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kreidberg J.A., Donovan M.J., Goldstein S.L., Rennke H., Shepherd K., Jones R.C., Jaenisch R. alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 52.Borza C.M., Borza D.B., Pedchenko V., Saleem M.A., Mathieson P.W., Sado Y., Hudson H.M., Pozzi A., Saus J., Abrahamson D.R., Zent R., Hudson B.G. Human podocytes adhere to the KRGDS motif of the alpha3alpha4alpha5 collagen IV network. J Am Soc Nephrol. 2008;19:677–684. doi: 10.1681/ASN.2007070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanasaki K., Kanda Y., Palmsten K., Tanjore H., Lee S.B., Lebleu V.S., Gattone V.H., Jr., Kalluri R. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313:584–593. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baleato R.M., Guthrie P.L., Gubler M.C., Ashman L.K., Roselli S. Deletion of CD151 results in a strain-dependent glomerular disease due to severe alterations of the glomerular basement membrane. Am J Pathol. 2008;173:927–937. doi: 10.2353/ajpath.2008.071149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bondeva T., Rüster C., Franke S., Hammerschmid E., Klagsbrun M., Cohen C.D., Wolf G. Advanced glycation end-products suppress neuropilin-1 expression in podocytes. Kidney Int. 2009;75:605–616. doi: 10.1038/ki.2008.603. [DOI] [PubMed] [Google Scholar]
- 56.Takamatsu H., Kumanogoh A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends Immunol. 2012;33:127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Vadasz Z., Ben-Izhak O., Bejar J., Sabo E., Kessel A., Storch S., Toubi E. The involvement of immune semaphorins and neuropilin-1 in lupus nephritis. Lupus. 2011;20:1466–1473. doi: 10.1177/0961203311417034. [DOI] [PubMed] [Google Scholar]
- 58.Maione F., Capano S., Regano D., Zentilin L., Giacca M., Casanovas O., Bussolino F., Serini G., Giraudo E. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J Clin Invest. 2012;122:1832–1848. doi: 10.1172/JCI58976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi M., Nakashima T., Taniguchi M., Kodama T., Kumanogoh A., Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Podocyte Sema3a overexpression in mice induces mesangial expansion, as seen in TEM micrographs. A: Uninduced Sema3a+ mice (without doxycycline; −dox) have normal glomerular ultrastructure. B: Induced Sema3a+ mice (+dox) exhibit extensive mesangial matrix expansion. C: Mesangial expansion is not reversible after doxycycline removal for 2 weeks (±dox). Scale bars = 2 μm (A and C); 1 μm (B).
Differentiated Sema3a+ podocytes express the podocyte-specific proteins nephrin, podocin, and WT1, as well as Sema3a and its binding and signaling receptors neuropilin 1 and plexinA1, as detected by Western blot analysis.