Abstract
Glioblastoma is a highly vascularized brain tumor, and antiangiogenic therapy improves its progression-free survival. However, current antiangiogenic therapy induces serious adverse effects including neuronal cytotoxicity and tumor invasiveness and resistance to therapy. Although it has been suggested that the physical microenvironment has a key role in tumor angiogenesis and progression, the mechanism by which physical properties of extracellular matrix control tumor angiogenesis and glioblastoma progression is not completely understood. Herein we show that physical compaction (the process in which cells gather and pack together and cause associated changes in cell shape and size) of human glioblastoma cell lines U87MG, U251, and LN229 induces expression of collagen types IV and VI and the collagen crosslinking enzyme lysyl oxidase and up-regulates in vitro expression of the angiogenic factor vascular endothelial growth factor. The lysyl oxidase inhibitor β-aminopropionitrile disrupts collagen structure in the tumor and inhibits tumor angiogenesis and glioblastoma multiforme growth in a mouse orthotopic brain tumor model. Similarly, d-penicillamine, which inhibits lysyl oxidase enzymatic activity by depleting intracerebral copper, also exhibits antiangiogenic effects on brain tumor growth in mice. These findings suggest that tumor microenvironment controlled by collagen structure is important in tumor angiogenesis and brain tumor progression.
Glioblastoma multiforme (GBM), a highly malignant glioma that exhibits various glial lineages such as a mixed oligodendroglial–astrocytic phenotype,1 is the most common primary brain tumor. Every year in the United States, GBM is diagnosed in about 8000 persons. Despite a great deal of effort to develop effective therapies targeting GBM, current approaches including surgery, radiotherapy, and chemotherapy have not been successful, with median survival of only 1 to 2 years.2 Angiogenesis, the formation and development of new blood vessels, is an important step in tumor growth and metastasis.3,4 Since GBM is a highly vascularized tumor and the presence of neovascularization is a key diagnostic criteria for GBM, antiangiogenic therapy is thought to represent a promising strategy for treatment of GBM.4 Indeed, the antivascular endothelial growth factor (VEGF) agent bevacizumab improves progression-free survival of GBM.5 However, antiangiogenic therapy for brain tumors potentially induces neuronal cytotoxicity, invasiveness of the tumor cells,6,7 and resistance to therapy,3–5,8,9 which diminish the benefits of current antiangiogenic therapy for GBM. Thus, to establish safe and efficient therapies that have long-term anti–tumor activity and fewer adverse effects, we need to understand the comprehensive mechanisms that govern brain tumor angiogenesis.
Most of the work in tumor angiogenesis has been focused on identifying the genetic and chemical signals that control neovascularization.3,10 Thus, most US Food and Drug Administration (FDA)–approved angiogenesis inhibitors target soluble angiogenic factors such as VEGF.5,8,9,11 These chemical signaling cascades are clearly necessary for tumor growth and vascular development; however, the insoluble extracellular matrix (ECM) and mechanical forces have equally important roles in tumor angiogenesis and progression.12–19 Cell shape controls the growth of capillary endothelial cells,20 and mechanical forces such as matrix stiffness control cell migration, angiogenesis, tumor progression, and metastasis, both in vitro and in vivo.15,21–23
Normal brain ECM is composed of hyaluronan, proteoglycans, and tenascin-C and is devoid of rigid ECM structures formed by fibrillar collagens,24 whereas ECM in GBM is associated with a large increase in components such as collagens, laminin, and fibronectin,25,26 primarily in the basement membrane of the blood vessels, which suggests that modifying these tumor-specific ECM components could be a potentially promising strategy for treatment of brain tumors.
Physical compaction is the process in which cells gather and pack together, causing associated changes in cell shape and size. Mesenchymal condensation, in which dispersed mesenchymal cells are tightly packed together, results in differentiation of these cells into specific tissue types that occur during early development of various organs (eg, tooth, cartilage, bone, muscle, tendon, kidney, and lung) in mice.27,28 During the course of mesenchymal condensation, cell shape is changed, cell size is decreased, and cell density is increased, which results in changes in mechanical and chemical signaling in the cells and dictates various cellular behaviors such as proliferation, migration, and cell fate determination that are critical for organ-specific morphogenesis. Physical compaction of the mesenchyme during early organ development increases collagen expression and governs subsequent organogenesis.29 In addition, changes in cell shape control cell growth through the Rho signaling pathway in capillary endothelial cells,20 and mechanical forces elicited by ECM stiffness, which also change cell shape and size, control angiogenesis and vascular function.22,30 Proliferating cancer cells in a confined space undergo compressive stress,16,31 and physically compacted cancer cells exhibit aggressive phenotypes.32,33 For example, rapidly growing mammary tumor cells in confined spaces become smaller and packed (ie, physically compacted),32 which affects tumor cell behavior and collagen VI expression.34 Therefore, in addition to soluble factors, mechanical forces such as physical compaction of the cells and subsequent changes in expression and structure of collagen may contribute to tumor angiogenesis and GBM progression. However, the role of physical properties of the tumor microenvironment on brain tumor angiogenesis and progression has not been well elucidated.
The present study was initiated to examine whether tumor cell compaction and associated changes in cell shape and density control collagen and angiogenic factor expression using in vitro experimental methods (ie, microcontact printing system and mechanical compressor) (Supplemental Figure S1). We have previously used these methods to examine the effects of physical compaction in normal development,29 in which we could precisely change cell size, shape, and density to mimic physical compaction in vitro. We also altered collagen expression and structure by changing the crosslinking ability of collagens and examined whether it controls brain tumor angiogenesis and tumor growth in vivo. Compaction of GBM cells changes collagen expression and structure, resulting in increased VEGF expression in vitro. Inhibition of collagen crosslinking attenuated the effects of physical compaction of GBM cells on tumor angiogenesis and progression of GBM in a mouse orthotopic brain tumor model. Because this novel approach targets tumor-specific ECM structures, it may lead to development of more stable and efficient antiangiogenic therapy for GBM and other types of hypervascular brain tumors.
Materials and Methods
Materials
Anti–collagen IV, anti–collagen VI, anti–Ki-67, anti-HIF2α, and anti–lysyl oxidase (LOX) polyclonal antibodies were purchased from Abcam (Cambridge, MA); anti-CD31 monoclonal antibody from BD Biosciences (San Jose, CA); anti-VEGFR2 polyclonal antibody from Cell Signaling Technology, Inc. (Boston, MA); anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH), anti–matrix metalloproteinase (MMP)–2, and anti–MMP-9 antibodies from Millipore Corp. (Billerica, MA); and anti–β-actin antibody, β-aminopropionitrile (BAPN), and d-penicillamine from Sigma (St. Louis, MO). U87MG, U251, and LN229 human glioblastoma cell lines (ATCC, Manassas, VA) were cultured in minimum essential medium containing 10% fetal bovine serum.35
Fabrication of Micropatterned Substrates
The microcontact printing method for producing glass substrates containing micrometer-sized ECM islands has been described previously.29,36 In brief, polydimethylsiloxane (PDMS) stamps were cast, cured, and removed from master templates, which were created using photolithographic methods. Stamps were coated with 50 μg/mL fibronectin for 1 hour and dried using compressed nitrogen. Flat and thin PDMS substrates were prepared on the cover glass and UV oxidized for 5 minutes (Ellsworth Adhesives, Germantown, WI), stamped with fibronectin, blocked with Pluronic F108 (Sigma) for 2 hours, and rinsed three times with PBS before plating various GBM cells. The cells were cultured on the fibronectin island at low (0.2 × 105 cells/cm2) or high (2.4 × 105 cells/cm2) plating density to recapitulate the physical compaction of the tumor cells in vivo (Supplemental Figure S1). After 16 hours of culture, densities and projected cell areas of the cells attached to the ECM islands were measured under a phase contrast microscope.
Mechanical Compression of GBM Cell Pellets
We fabricated a mechanical compressor (base and piston) using flexible, gas-permeable PDMS elastomer in which cell pellets can be mechanically compressed in a controlled way. One PDMS membrane was sealed to the bottom of the base, and the entire compressor rested on a fine wire mesh for support.29 The pellets of three GBM cell lines (106 cells per pellet) treated separately were placed at the base of the device. The PDMS piston was placed on top of the pellet, and a 30-g metal weight was placed over the PDMS piston to compress the pellet with constant pressure of 1 kPa for 16 to 24 hours of culture (Supplemental Figure S1).
Animal Experiments
All animal studies were reviewed and approved by the Animal Care and Use Committee of Boston Children’s Hospital. Homozygous athymic nude mice [NU(NCr)-Foxn1nu, stock No. 490; Charles River Laboratories International, Inc., Wilmington, MA) were used in the present study. For stereotactic injection into the brain in the tumor xenograft model,37 U87MG human glioblastoma cells were trypsinized and resuspended in PBS. Nude mice were anesthetized using 250 mg/kg avertin i.p. and placed on a stereotactic frame (Stoelting Co., Wood Dale, IL). Mice were immobilized using blunt ear bars, and a 1.0-cm sagittal incision was made to expose the cranium. A hole was bored 1.5 mm lateral and 2 mm anterior to the bregma using an electric drill. GBM cells, 5 × 105, in 10 μL PBS were loaded into a 25-μL syringe (Gastight; Hamilton Co., Reno, NV) with a 25-gauge blunt needle. The needle was inserted 1.5 mm beneath the pial surface, and cells were infused over 3 minutes. The needle was left in the infusion site for an additional 2 minutes before removal. Tumor angiogenesis and growth were evaluated at 12 days after implantation.
Brain Tumor Morphometry
Murine brains were fixed with 4% paraformaldehyde solution overnight at 4°C and embedded in OCT compound.29 Serial sections (20 μm thick) were cut and were analyzed via H&E staining and immunohistochemistry (IHC).22,29 Morphometric analysis was performed as described previously29 using ImageJ software version 1.46r (NIH, Bethesda, MD).
Biochemical and Molecular Biological Methods
LOX activity in the tumor homogenate was measured using a LOX activity assay kit (ABD Bioquest, Inc., Sunnyvale, CA) in which LOX substrate that releases hydrogen peroxide was detected.15,30,38,39 Hydroxyproline in brain tumor tissue or serum was measured using a hydroxyproline assay kit (BioVision, Inc., Milpitas, CA).30,40 The levels of human VEGF in the tumor cell lysate from three glioblastoma cell lines (U87MG, U251, and LN229) in vitro and the levels of human VEGF and human basic fibroblast growth factor (bFGF) in brain tumor in vivo were measured using an enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Inc., Minneapolis, MN). Immunoblotting of collagen IV, collagen VI, LOX, MMP-2, MMP-9, β-actin, and GAPDH from the tumor cell lysate of the same three GBM cell lines in vitro or brain tumor in vivo was performed as described.22 Quantitative RT-PCR (RT-qPCR) was performed using the Quantitect SYBR Green RT-PCR kit (Qiagen NV, Venlo, The Netherlands) using the ABI 7300 real-time PCR system (Applied Biosystems, Inc., Foster City, CA).22 β2-Microglobulin and cyclophilin were used as controls for overall cDNA content. The primers used are given in Table 1.
Table 1.
RT-qPCR Primers Sequences
| Primer | Forward | Reverse |
|---|---|---|
| Human VEGF | 5′-TCGAGTACATCTTCAAGCCATCCTGTGTGC-3′ | 5′-CCTATGTGCTGGCCTTGGTGAGGTTTGAT-3′ |
| Human collagen IV | 5′-GGACTACCTGGAACAAAAGGG-3′ | 5′-GCCAAGTATCTCACCTGGATCA-3′ |
| Human collagen VI | 5′-ACAGTGACGAGGTGGAGATCA-3′ | 5′-GATAGCGCAGTCGGTGTAGG-3′ |
| Human LOX | 5′-CGGCGGAGGAAAACTGTCT-3′ | 5′-TCGGCTGGGTAAGAAATCTG-3′ |
| Human β2-microglobulin | 5′-GAATGGAGAGAGAATTGAAAAAGTGGAGCA-3′ | 5′-CAATCCAAATGCGGCATCTTCAAAC-3′ |
| Mouse collagen IV | 5′-CTGGCACAAAAGGGACGAG-3′ | 5′-ACGTGGCCGAGAATTTCACC-3′ |
| Mouse collagen VI | 5′-CTGCTGCTACAAGCCTGCT-3′ | 5′-CCCCATAAGGTTTCAGCCTCA-3′ |
| Mouse cyclophilin | 5′-CAGACGCCACTGTCGCTTT-3′ | 5′-TGTCTTTGGAACTTTGTCTGCAA-3′ |
Statistical Analysis
Error bars (SEM) and P values were determined from the results of at least three independent experiments. The unpaired t-test was used for analysis of statistical significance.
Results
Cell Compaction Increases Expression of Collagens and VEGF in GBM Cells
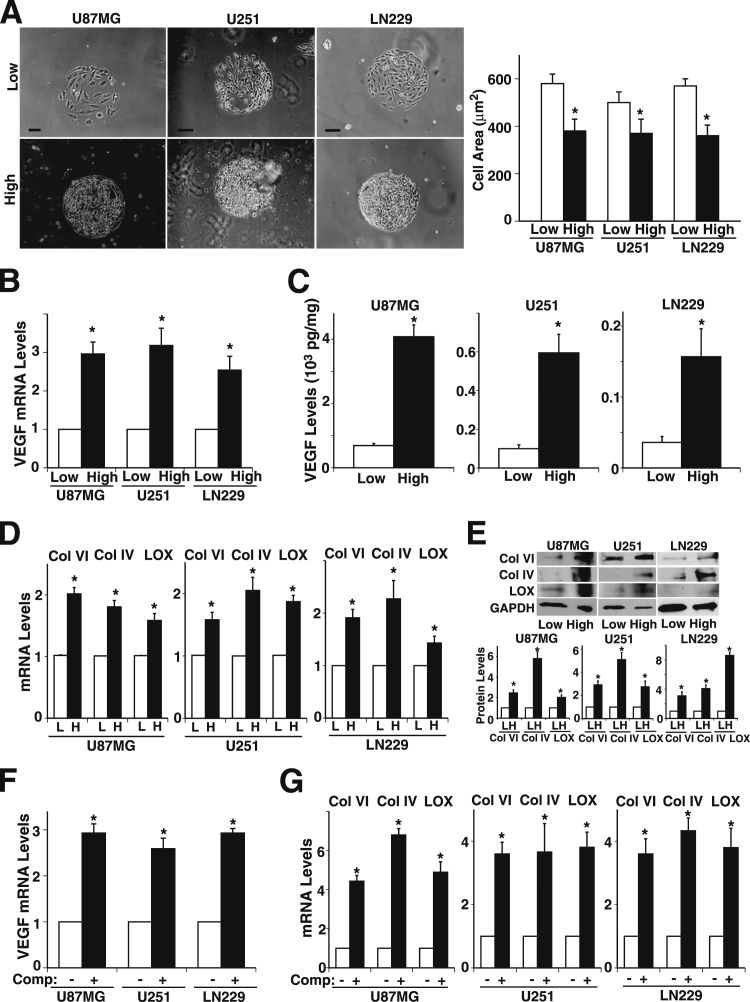
Physical compaction of tissues and subsequent changes in cell shape and ECM deposition result in cell fate switching in vitro and in vivo by modulating specific gene expression.29 Physical compaction of rapidly growing cancer cells in confined spaces also has a key role in tumor progression in vivo.32,34 Because angiogenesis is an important step in tumor growth and metastasis in GBM3,4 and is controlled by mechanical forces,22,41 we first examined whether tumor cell compaction regulates expression of angiogenic factors and ECMs in U87MG, U251, and LN229 GBM cell lines. To explore whether changes in cell shape, size, and density by physical cell compaction contribute to angiogenic factor expression, we precisely controlled cell size and density using microengineered adhesive islands29,36 coated with the ECM protein fibronectin, which is present in glioblastomas in vivo42 (Supplemental Figure S1). U87MG, U251, and LN229 cells cultured for 16 hours on large circular 500-μm diameter fibronectin islands at a high plating density of 2.4 × 105 cells/cm2 induced cell compaction and resulted in reduction in cell size by 34%, 26%, and 36%, respectively, compared with cells plated at a low plating density of 0.2 × 105 cells/cm2 in 2D culture (Figure 1A). Expression of the angiogenic factor VEGF in U87MG, U251, or LN229 cells was 2.9-, 3.1-, and 2.5-fold higher, respectively, for mRNA (Figure 1B), and 5.9-, 5.9-, and 4.3-fold higher, respectively, for protein levels compared with cells plated at the low plating density (Figure 1C). Collagen VI is specifically up-regulated and deposited around individual mesenchymal cells within the condensed mesenchyme during the early stage of tooth development.29 Collagen VI expression is also increased during mammary tumor progression.34 Thus, we examined whether expression of collagen VI is also changed in glioblastoma cells in a cell density–dependent manner. Consistent with previous findings in early tooth development,29 the collagen VI mRNA levels in U87MG, U251, or LN229 cells plated at high density were 2-, 1.5-, and 1.9-fold higher, respectively (Figure 1D). Collagen IV, one of the major forms identified in brain tumor samples, and collagen crosslinker LOX mRNA levels were also significantly higher than those plated at low density (Figure 1D). Immunoblotting and IHC confirmed that protein expression of collagens and LOX was higher in glioblastoma cells plated at high density (Figure 1E and Supplemental Figure S2). The cell density–dependent increase in the levels of collagens, LOX, and VEGF observed in the three GBM cell lines U87MG, U251, and LN229 suggests that cell compaction–dependent changes in GBM cell size and density control collagen expression and VEGF production in vitro. To further explore whether physical compaction of the cells induces angiogenic factor expression in three dimensional culture, we made pellets of the glioblastoma cell lines and mechanically compressed them between two blocks of PDMS polymer for 16 hours using constant pressure of 1 kPa 29 (Supplemental Figure S1). VEGF expression was up-regulated in the compressed glioblastoma cell pellets compared with unloaded controls (Figure 1F). Mechanical compression of the glioblastoma cell pellets also up-regulated mRNA expression of collagens VI and IV and LOX (Figure 1G), confirming that mechanical forces that cause cell compaction change collagen and angiogenic factor expression in vitro.
Figure 1.
Cell density and mechanical compression control collagen, lysyl oxidase (LOX) and angiogenic factor expression in glioblastoma cells in vitro. A–D: Glioblastoma cell lines U87MG, U251, and LN229 were cultured for 16 hours on microfabricated, 500-μm diameter fibronectin islands at low (0.2 × 105 cells/cm2) or high (2.4 × 105 cells/cm2) plating cell density in vitro. A: Phase contrast micrographs. Scale bar = 100 μm. Projected cell areas were measured under each condition. B: VEGF mRNA levels. C: VEGF protein levels in three glioblastoma cell lines cultured for 16 hours on microfabricated circular fibronectin islands at low or high density. D: mRNA levels of collagen (Col) VI and IV and LOX. E: Representative immunoblots show Col VI and IV, LOX, and GAPDH protein levels in three glioblastoma cell lines cultured for 16 hours on microfabricated circular fibronectin islands at low (L) or high (H) density. Quantitative results of protein levels of Col VI and IV and LOX were normalized to GAPDH protein levels. mRNA levels of VEGF (F) and Col VI and IV and LOX (G) in control versus compressed (comp.) glioblastoma cells. ∗P < 0.05. Data are given as means ± SEM of at least three independent experiments.
LOX Controls Glioblastoma Progression in Vivo
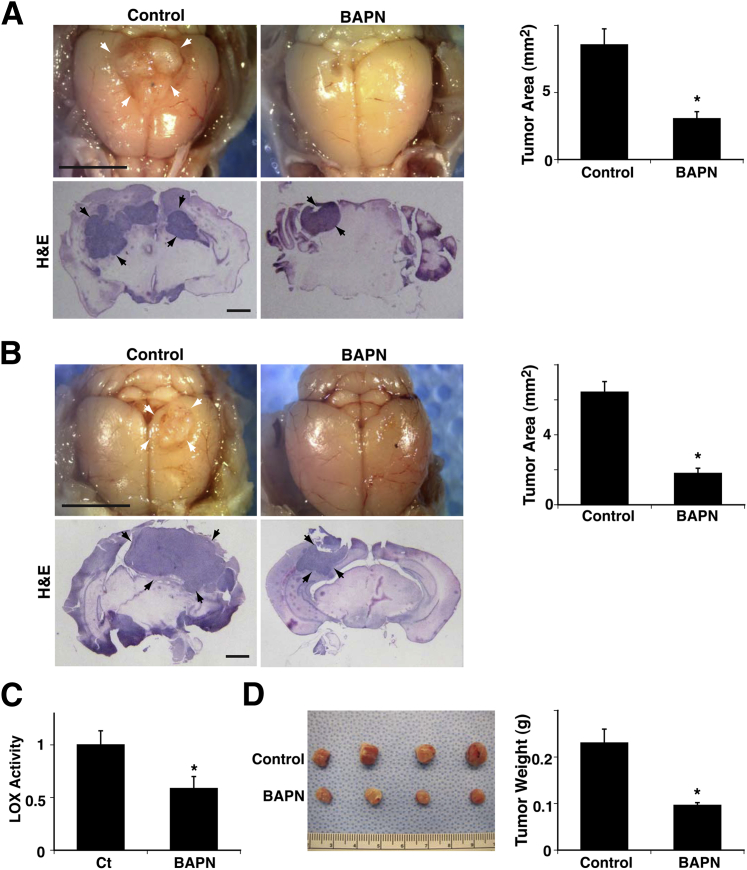
Breast cancer progression is accompanied by changing ECM structure and mechanics that are controlled by the activity of the ECM crosslinking enzyme LOX.15,19,43 Inhibition of LOX activity represses the malignant behavior of mammary epithelial cells, therefore impeding cancer progression in vivo.15,19,43 Because ECM structure and stiffness control angiogenesis in vivo22 and LOX expression is increased in a cell compaction–dependent manner in GBM cells in vitro (Figure 1, D, E, and G), we next examined whether inhibition of LOX activity represses brain tumor growth and angiogenesis in vivo using an orthotopic mouse brain tumor model in which U87MG cells were stereotactically injected into the mouse brain cortex.37 To manipulate LOX activity in the brain, mice were given 3 mg/kg LOX inhibitor BAPN in drinking water15,30,44 beginning on the day of tumor implantation. BAPN prevents crosslinking of collagens,45,46 major tumor ECM components in GBM,25,26 and has been extensively used in vitro and in vivo to evaluate the role of LOX in cell proliferation,47 embryonic development,48 and tumor cell reversion15 and invasion.49 Consistent with previous reports of breast cancer,15,43,49 BAPN decreased brain tumor size by 65% compared with brain tumors in untreated mice at 12 days after implantation (Figure 2A). We also evaluated the effects of BAPN on established orthotopic brain tumors in vivo by starting BAPN therapy 4 days after tumor implantation. BAPN therapy decreased LOX activity in tumors by 42% as measured using a LOX activity assay (Figure 2C).15,44 It decreased tumor size by 72%, and weight by 58%, compared with tumors in untreated animals (Figure 2, B and D), which suggests that LOX inhibition could be a potential strategy for treatment of GBM.
Figure 2.
Lysyl oxidase (LOX) controls glioblastoma growth in mouse brain. A: Representative micrographs show brain tumors (arrows) growing in mouse brain for 12 days after implantation of U87MG cells. Treatment with β-aminopropionitrile (BAPN) was started on the day of tumor implantation. Light micrographs show brain tumor growing in brains of control and BAPN-treated mice. Quantitation shows size of tumor growing in mouse brain (n = 8). B: Representative micrographs show brain tumors (arrows) growing in mouse brain for 12 days after implantation of U87MG cells. Treatment with BAPN was started 4 days after tumor implantation. Light micrographs show brain tumor growing in brains of control or BAPN-treated mice. Quantitation shows size of tumor growing in mouse brain (n = 8). C: Relative LOX activity was measured in mouse brain tumors treated with BAPN (n = 8). D: Micrographs show representative brain tumors harvested 12 days after implantation of U87MG cells. Weight of brain tumors treated with BAPN was measured (n = 8). ∗P < 0.05. Error bars represent SEM. Scale bar = 5 mm (A and B, top panels); 1 mm (A and B, bottom panels).
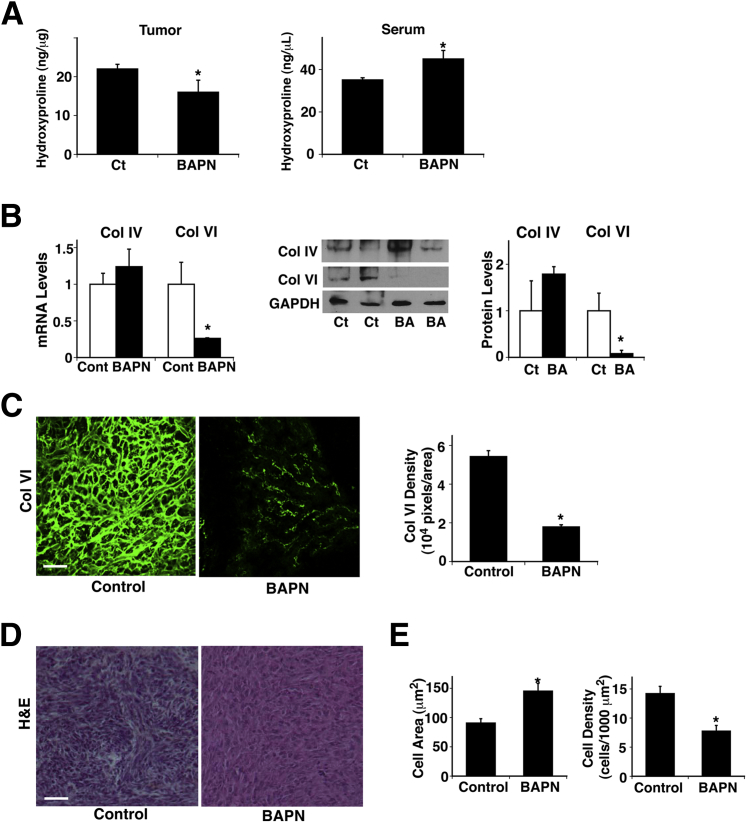
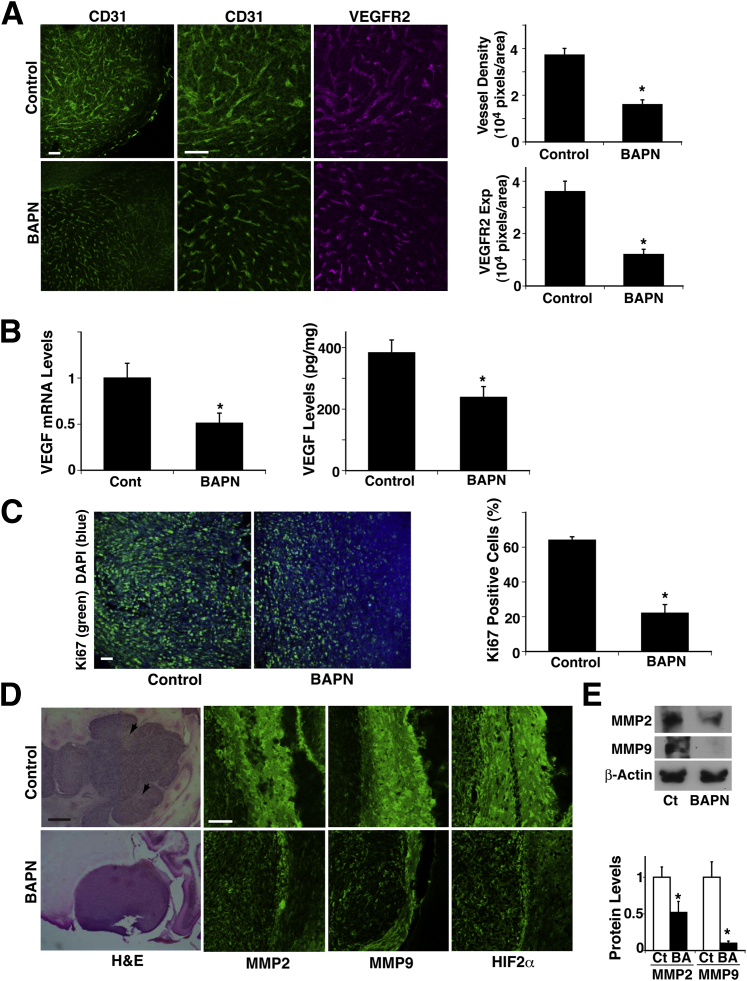
Because cell compaction, a prominent feature of tumors, increased collagen expression in GBM cells in vitro (Figure 1), we next examined whether BAPN alters collagen expression and/or turnover by measuring the amount of 4-hydroxyproline, a common nonproteinogenic amino acid found in collagen and elastin in mammals. Hydroxyproline in tissue hydrolysates is a direct measure of the amount of collagen present in the tissue,40 and conditions that increase collagen turnover can result in elevated serum hydroxyproline concentration.50 BAPN decreased the hydroxyproline level in the tumor by 27%, whereas it increased serum concentration by 28% (Figure 3A), which suggests that BAPN decreased collagen volume in the tumor and increased the rate of turnover in addition to altering collagen crosslinking ability. When we examined the expression of collagen IV and VI, the major types of collagen in brain tumors, BAPN did not significantly decrease collagen IV expression but decreased collagen VI expression by 75% and 93%, respectively, at mRNA and protein levels (Figure 3B). We further confirmed the results using IHC. In control untreated tumors, thick and tortuous collagen VI fibers were distributed over the tumors, whereas in BAPN-treated tumors, collagen VI fibers were thinner and the density was lower by 67% (Figure 3C). Cell size was 60% larger, and cell density was lower by 45% in BAPN-treated tumors compared with untreated control tumors (Figure 3, D and E), suggesting that inhibition of collagen crosslinking may feed back and further suppress tumor cell compaction. In addition, blood vessels detected by the vessel markers CD31 and VEGFR2 in the implanted tumors were thinner, and density was lower by 66% in BAPN-treated tumors compared with control untreated tumors (Figure 4A). VEGF mRNA and protein levels as measured by RT-qPCR and ELISA were also decreased, by 49% and 37%, respectively, in BAPN-treated tumors (Figure 4B). Tumor cell proliferation detected via Ki-67 staining confirmed that BAPN decreased tumor cell proliferation by 65% (Figure 4C). These findings suggest that inhibition of LOX activity disrupts collagen expression and structure and hence represses tumor angiogenesis and growth.
Figure 3.
Lysyl oxidase (LOX) controls collagen expression in brain tumor. A: Levels of hydroxyproline were measured in brain tumors and serum in mice treated with β-aminopropionitrile (BAPN) (n = 7). BAPN treatment was started 4 days after tumor implantation. B: mRNA levels of collagens (Col) IV and VI were measured in mouse brain tumors treated with BAPN (n = 7). Immunoblots show protein levels of Col IV and VI and GAPDH in mouse brain tumors treated with BAPN (BA). Quantitation of protein levels of Col IV and VI was normalized to GAPDH protein levels. C: Immunofluorescence micrographs show Col VI expression and distribution in brain tumors in control (Ct) and BAPN-treated mice. Col VI density was measured in brain tumors in control versus BAPN-treated mice (n = 8). D: H&E staining of brain tumors in control and BAPN-treated mice. E: Cell area and cell density were measured in brain tumors in control and BAPN-treated mice (n = 8). ∗P < 0.05. Error bars represent SEM. Scale bar = 75 μm (C and D).
Figure 4.
Lysyl oxidase (LOX) controls angiogenesis in brain tumors. A: Immunofluorescence micrographs show CD31-stained blood vessels (green) and VEGFR2 expression (magenta) in brain tumors from control (Ct) or β-aminopropionitrile (BAPN)-treated mice. BAPN treatment started 4 days after tumor implantation. Vessel density and VEGFR2 expression (Exp) were measured in brain tumors from control and BAPN-treated mice (n = 8). B: mRNA levels of VEGF were measured in mouse brain tumors treated with BAPN (n = 8). Protein levels of VEGF was measured via ELISA in mouse brain tumors treated with BAPN (n = 7). C: Immunofluorescence micrographs show Ki-67 (green) and DAPI (blue) staining in brain tumors from control or BAPN-treated mice. Percent Ki-67–positive cells was measured in brain tumors from control and BAPN-treated mice (n = 8). D: Light micrographs show tumors growing in brains of control and BAPN-treated mice. Arrowheads show necrotic areas. Immunofluorescence micrographs show staining of invasion-related proteins MMP-2 and MMP-9 and hypoxia-related protein HIF2α in brain tumors from control and BAPN-treated mice. E: Representative immunoblots show MMP-2, MMP-9, and β-actin protein levels in brain tumors from control or BAPN (BA)–treated mice. Quantitation of protein levels of MMP-2 and MMP-9 was normalized to β-actin protein levels (n = 6). ∗P < 0.05. Data are given as means ± SEM. Scale bars: 75 μm [A, C, and D (MMP2, MMP9, and HIF2α columns)]; 0.5 mm (D, H&E column).
It has become clear that the FDA-approved anti-VEGF therapy can promote infiltration or invasion of GBM cells.6,51 Therefore, we also examined the effect of BAPN on the invasive phenotype of the tumors. Control untreated tumors had undefined edges, and multiple satellite tumors were present around the principal mass, whereas BAPN-treated tumors had well-delineated margins and no satellite tumors (Figure 4D). We also examined the levels of invasion-related proteins such as MMP-2 and MMP-9 in control and BAPN-treated tumors. IHC (Figure 4D) and immunoblotting (Figure 4E) revealed that the levels of MMP-2 and MMP-9 in the edge of the tumors were lower in BAPN-treated tumors than in control untreated tumors. Control untreated tumors also demonstrated a number of necrotic regions, whereas BAPN-treated tumors exhibited few necrotic regions. Because necrotic regions are potentially caused by hypoxia, we also evaluated the hypoxic phenotype via IHC using the hypoxia marker HIF-2α in a mouse orthotopic tumor model. Consistent with the results for necrosis, the levels of HIF2α in the tumors were lower in BAPN-treated tumors than in control untreated tumors (Figure 4D). These results suggest that although LOX inhibition by BAPN decreases the levels of VEGF in tumors, it does not promote glioma invasion and necrosis.
d-Penicillamine Inhibits GBM Progression in Vivo
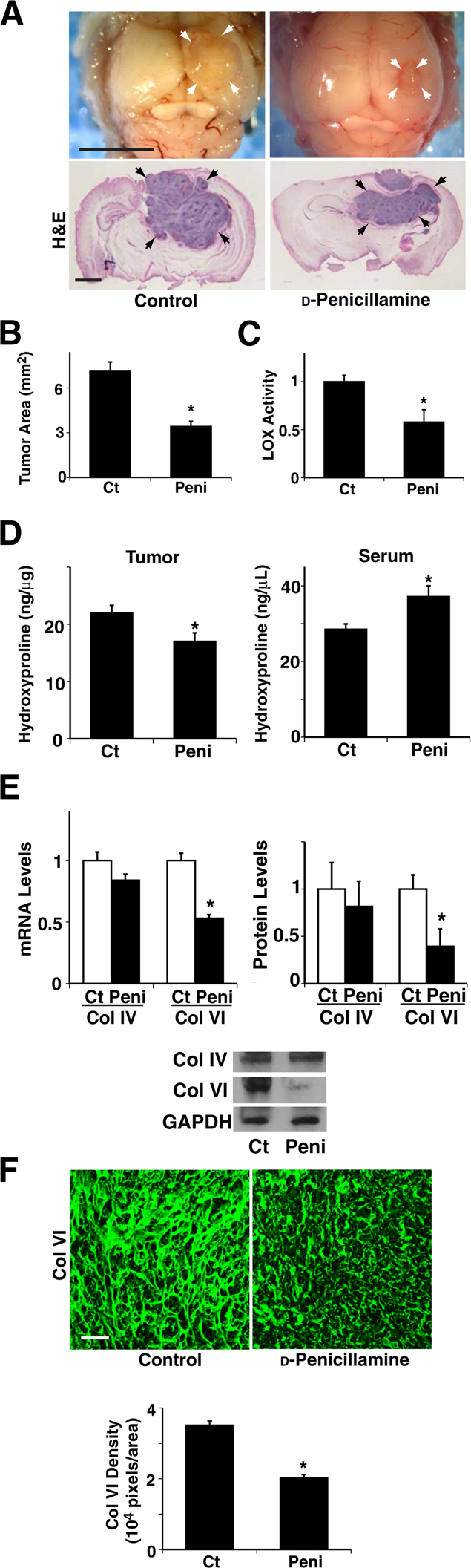
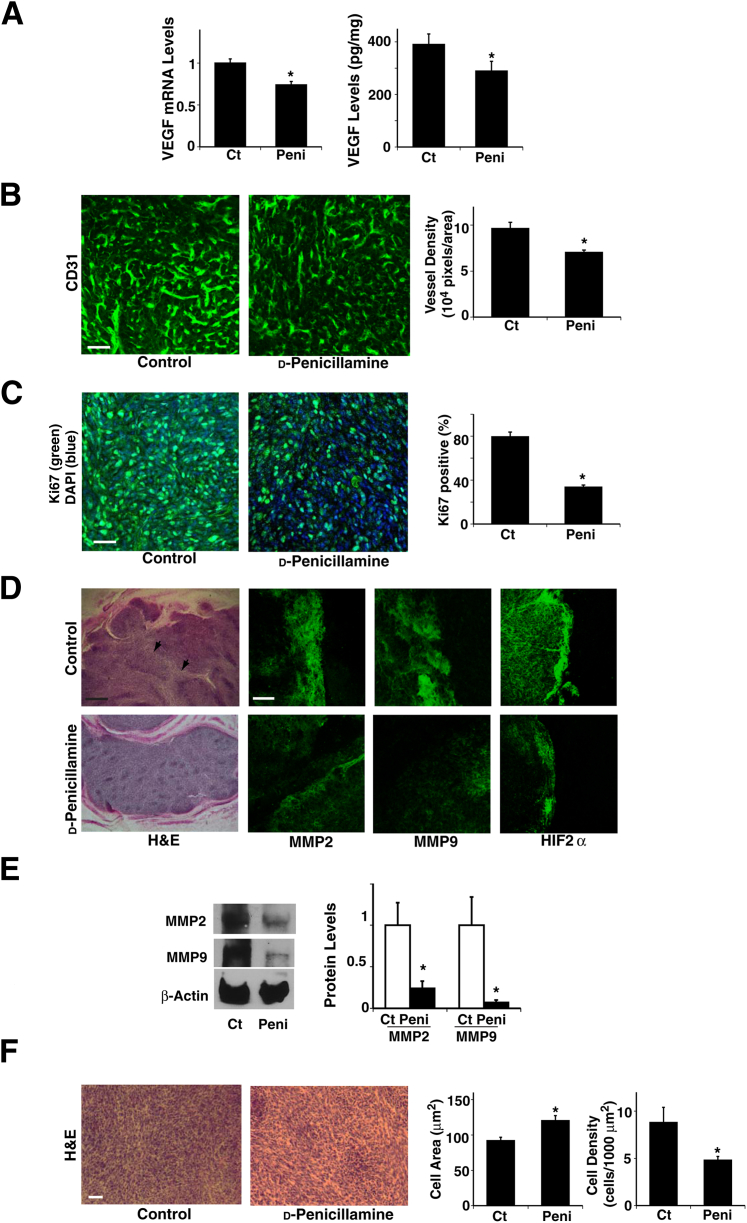
Our findings suggest that collagen structure and remodeling have an important role in brain tumor progression, and the LOX inhibitor BAPN seems to inhibit brain tumor growth and angiogenesis (Figure 2, 3, and 4). However, because of its nonspecific chemical effects and toxicity,52 BAPN has not been approved for clinical use. LOX is a copper-dependent enzyme, and copper is essential for the functional activity of this enzyme, including extracellular processing of collagens and elastin.53 d-Penicillamine, an FDA-approved agent used to treat intracerebral copper overload in Wilson’s disease,54 inhibits glioma growth and tumor angiogenesis in experimental animal models.55 Consistent with a previous report, when we treated orthotopically implanted glioblastoma tumors with d-penicillamine, beginning 4 days after implantation, tumor size was significantly smaller in treated mice than in untreated control mice (Figure 5, A and B), and LOX activity was reduced by 41% in tumors treated with d-penicillamine (Figure 5C). When we examined collagen expression and degradation using the hydroxyproline assay, d-penicillamine decreased the tumor hydroxyproline level by 22%, whereas it increased serum hydroxyproline concentration by 30% (Figure 5D), which suggests that d-penicillamine also decreased collagen expression and increased its turnover, as observed in BAPN-treated tumors (Figure 3A). In addition, RT-qPCR and immunoblotting revealed that d-penicillamine decreased collagen VI mRNA and protein expression by 47% and 61%, respectively, but did not change collagen IV levels significantly (Figure 5E). IHC also confirmed that d-penicillamine decreased collagen VI density and thickness by 42% in treated tumors compared with untreated tumors (Figure 5F), which suggests that d-penicillamine modulates collagen structure in the tumor primarily by altering collagen VI crosslinking ability and expression levels. Insofar as tumor angiogenesis, d-penicillamine decreased tumor VEGF mRNA and protein levels by 26% and 25%, respectively (Figure 6A), and blood vessel density by 26% (Figure 6B). d-Penicillamine diminished tumor cell proliferation by 57%, as detected via Ki-67 (Figure 6C). These results suggest that collagen expression and structure modulated by LOX have key roles in tumor angiogenesis and progression in GBM in which tumor cells are compacted. We also examined the effect of d-penicillamine on the invasive phenotype of the tumors. Similar to the BAPN-treated tumors (Figure 4D), d-penicillamine-treated tumors exhibited well-delineated margins without satellite tumors (Figure 6D), and the levels of MMP-2 and MMP-9 were lower in tumors treated using d-penicillamine than in control untreated tumors (Figure 6, D and E). In addition, compared with untreated tumors, those treated with d-penicillamine exhibited a decreased necrotic and HIF2α-positive hypoxic phenotype (Figure 6D). Furthermore, cell size was 30% larger and cell density was 45% lower in d-penicillamine–treated tumors compared with untreated control tumors (Figure 6F), similar to the tumors treated with BAPN, which suggests that d-penicillamine–induced inhibition of collagen crosslinking also seems to feed back and suppress tumor cell compaction.
Figure 5.
d-Penicillamine controls collagen expression and structure in mouse brain tumors. A: Representative micrographs show tumors (arrows) growing in brains of control (Ct) or d-penicillamine–treated mice. d-Penicillamine treatment was started 4 days after tumor implantation. H&E staining of brain tumors from control or d-penicillamine–treated mice. B: Size of tumors growing in brains of control or d-penicillamine (peni)–treated mice (n = 8). C: Lysyl oxidase (LOX) activity of tumors growing in brains of control or d-penicillamine–treated mice (n = 8). D: Hydroxyproline levels were measured in brain tumors and serum from control or d-penicillamine–treated mice (n = 7). E: Left: mRNA levels of collagen (Col) IV and VI in brain tumors from control or d-penicillamine–treated mice (n = 8). Right: Quantitation of protein levels of Col IV and VI was normalized to GADPH protein levels (n = 6). Representative immunoblots show protein levels of Col IV, Col VI, and GAPDH in brain tumors from control and d-penicillamine–treated mice. F: Immunofluorescence micrographs show Col VI structure and distribution in brain tumors from control or d-penicillamine–treated mice. Col VI density in brain tumors was measured from control or d-penicillamine–treated mice (n = 8). ∗P < 0.05. Data are given as means ± SEM. Scale bars: 5 mm (A, top panels); 1 mm (A, bottom panels); 75 μm (F).
Figure 6.
d-Penicillamine controls angiogenesis and tumor growth in mouse brain tumors. A: VEGF mRNA (n = 8) and protein (n = 8) levels were measured in brain tumors from control (Ct) or d-penicillamine (peni)–treated mice. B: Immunofluorescence micrographs show CD31-stained blood vessels in brain tumors from control or d-penicillamine–treated mice. Vessel density was measured in brain tumors from control or d-penicillamine–treated mice (n = 8). C: Immunofluorescence micrographs show Ki-67 (green) and DAPI (blue) staining in brain tumors from control or d-penicillamine–treated mice. Percent Ki-67–positive cells was measured in brain tumors from control or d-Penicillamine–treated mice (n = 8). D: Light micrographs (H&E stained) show brain tumor growing in control or d-penicillamine–treated mice. Arrowheads show necrotic areas. Immunofluorescence micrographs show staining of invasion-related proteins MMP-2 and MMP-9 and hypoxia-related protein HIF2α in brain tumors from control or d-penicillamine–treated mice. E: Immunoblots show MMP-2, MMP-9, and β-actin protein levels in brain tumors from control or d-penicillamine–treated mice. Quantitation of protein levels of MMP-2 and MMP-9 was normalized to β-actin protein levels (n = 6). F: H&E staining of brain tumors in control or d-penicillamine–treated mice. Cell area and cell density were measured in brain tumors from control or d-penicillamine–treated mice (n = 8). ∗P < 0.05. Error bars represent SEM. Scale bars: 75 μm [B, C, D (MMP2, MMP9, and HIF2α columns), and F]; 0.5 mm (D, H&E column).
Discussion
In addition to genetic and chemical signals that control tumor angiogenesis,3,4,10 insoluble ECM and mechanical forces have equally important roles in tumor progression.12–19 We found that growth of glioblastoma cells in confined spaces induces physical compaction of the cells and increases collagen expression and changes in its structure, which results in induction of tumor angiogenesis and GBM progression in murine orthotopic brain tumors. Inhibition of collagen crosslinking and expression using BAPN or d-penicillamine suppressed angiogenesis and GBM progression in the mouse model. Inhibition of collagen crosslinking and expression also seems to feed back to suppress tumor cell compaction and further inhibit angiogenesis and GBM progression. In contrast to normal brain, in which the ECM is composed primarily of hyaluronan, proteoglycans, and tenascin-C but lacks much collagen,24 GBM tumors consist of fibrillar collagens, primarily in the basement membrane of the blood vessels.25,26 Thus, targeting the process of collagen remodeling, which is specific for tumors in the brain, could be a novel and efficient strategy for treatment of GBM. Multicellular drug resistance is associated with cellular compaction in ovarian cancer cells and mammary tumor cells.33,56,57 Tumor cell compaction is also involved in resistance to radiotherapy in colon cancer.58 Thus, modifiers of tumor cell compaction or subsequent collagen remodeling could also reduce drug resistance in various other cancers. During early tooth development, physically compacted mesenchymal cells synthesize and deposit collagens and induce subsequent organ development, which is controlled by two distinct growth factors, semaphorin and fibroblast growth factor 8.29 Although GBM cell compaction increased expression of collagens, their crosslinker LOX, and the angiogenic factor VEGF, the mechanism by which tumor cell compaction and up-regulation of these proangiogenic molecules occurs remains unknown.
Consistent with other reports of mammary tumor progression,15 the LOX inhibitor BAPN inhibited LOX activity in the tumor, disrupted collagen structure, and repressed GBM progression in a mouse brain tumor model. LOX can change the expression or activities of various chemical factors that may directly influence tumor angiogenesis (eg, transforming growth factor-β, platelet-derived growth factor-BB, and VEGF)59–61 or alter ECM structure in multiple ways and hence contribute to GBM progression. However, LOX inhibitors such as BAPN and d-penicillamine did not inhibit endothelial cell differentiation when analyzed using an endothelial cell tube formation assay in vitro (data not shown), which suggests that the direct physical effects of LOX on ECM remodeling may have an essential role in tumor cell behavior and angiogenesis in vivo.
LOX inhibition may render collagen more susceptible to degradation.62 Indeed, when we measured the amount of hydroxyproline in brain tumor tissue and serum, BAPN and d-penicillamine decreased the levels of hydroxyproline in tumor but increased the concentrations in serum, which suggests that in addition to changing the structure of ECM, BAPN and d-penicillamine decreased the amount of collagen and increased the rate of turnover. However, treatment with BAPN or d-penicillamine clearly changed collagen structure, fiber density, and thickness in the tumors, and therefore these treatments seem to affect tumor growth, at least in part, by inhibiting collagen crosslinking ability. Changes in ECM structure may also feed back to alter LOX activity or expression, ECM expression levels, ECM turnover rate, and intracellular signaling activity, all of which remodel tumor ECM structure in different ways in a spatiotemporal manner and modify angiogenesis and vascular function (permeability), which can further alter tumor cell behavior. Furthermore, inhibition of LOX activity seems to feed back to inhibit tumor cell compaction and suppress angiogenesis and tumor growth. Thus, complex feedback systems control ECM structure and tumor growth.
Although cell compaction increased both collagen IV and VI levels in vitro, LOX inhibition decreased collagen VI levels but not collagen IV levels in vivo. This may be because our in vitro studies used brain tumor cells cultured on top of one type of ECM component, whereas the whole tumor microenvironment contains various cell types grown in three-dimensional ECMs composed of many kinds of insoluble ECM components and bound growth factors. Tumor epithelial, stromal, or endothelial cells may secrete soluble factors and ECM proteins differently in response to changes in cell compaction and LOX manipulation.
Although the FDA-approved anti-VEGF therapy seems to be effective in normalizing abnormal tumor vasculature, leading to an enhanced response to radiation and chemotherapy, anti-VEGF therapy can promote expression of proangiogenic and proinvasive factors and eventually stimulate glioma infiltration or invasion and become resistant to the therapy.6,51 For example, hepatocyte growth factor–Met signaling in GBM cells, which is negatively regulated by VEGF-A,63 enhances growth and invasion of the cells in response to bevacizumab treatment.64 Consequently, FDA-approved VEGF-A neutralizing antibody bevacizumab (Avastin) does not improve overall survival in patients with GBM.65 Although manipulation of LOX activity using BAPN or d-penicillamine decreased VEGF levels in the tumor, unlike anti-VEGF therapy, it did not promote tumor cell invasion and/or the hypoxic or necrotic phenotype in a murine orthotopic tumor model, which suggests that ECM-targeting strategies may attenuate the adverse effects of current antiangiogenic therapy. Given that angiogenesis is in part controlled by ECM structure and mechanics and that tumor cell compaction is involved in drug resistance during cancer therapy, modifying ECM structure associated with tumor cell compaction in combination with conventional antiangiogenic therapy (such as bevacizumab) and/or radiotherapy could improve response, decrease toxic adverse effects, and prevent resistance by changing the tumor microenvironment.
We used d-penicillamine as a more clinically relevant approach to inhibit LOX activity and disrupt collagen structure. In addition to VEGF, copper has important roles in angiogenesis66 and is involved in tumor angiogenesis by changing the levels of FGFs; the release of bFGF is inhibited by chelating copper in tumors.67,68 bFGF increases over time in patients with recurrent glioblastoma during treatment with VEGFR inhibitor or anti-VEGF therapy.6,69 However, when we analyzed whether the levels of bFGF are also altered in d-penicillamine–treated implanted tumors using RT-qPCR and ELISA, mRNA and protein levels of bFGF were not changed in d-penicillamine–treated tumors (data not shown). The copper chelating effects of d-penicillamine that decrease bFGF levels and anti-VEGF effects that increase bFGF levels may leave bFGF levels unchanged in the tumor. Our results suggest that d-penicillamine decreased collagen expression and disrupted collagen structure in tumors and inhibited brain tumor growth in an experimental murine orthotopic tumor model. However, this drug has not improved survival in patients with GBM.55 Modification of the treatment strategy such as time course, dosages, route of administration, or combination with other collagen modifiers may improve the efficiency of the approach.55,70,71
In summary, tumor cell compaction changes collagen structure and expression and controls VEGF-mediated tumor angiogenesis and GBM growth. Our findings may lead to new and better understanding of the pathogenesis of GBM, which will open opportunities for development of new therapeutic interventions for GBM and other hypervascular brain tumors.
Acknowledgments
We thank Donald Ingber for technical assistance and helpful discussion.
T.M. and A.M. conceived and designed the experiments; T.M., E.J., A.J., and A.M. performed the experiments; T.M. and A.M. analyzed the data; T.M., D.P., M.W.K., and A.M. contributed reagents/materials/analysis tools; and A.M., T.M., and M.W.K. prepared the manuscript.
Footnotes
Supported by funds from the American Heart Association (A.M.), a William Randolph Hearst Award (A.M.), the American Brain Tumor Association (A.M.), a Children’s Hospital Boston Faculty Career Development Fellowship (T.M.), the Stop and Shop Pediatric Brain Tumor Fund (M.W.K.), the C.J. Buckley Pediatric Brain Tumor Fund (M.W.K), grant R01 CA148633 from the National Cancer Institute, NIH (D.P.), and grant W81XWH-05-1-0115 from Department of Defense.
T.M. and A.M. contributed equally to this work.
Supplemental Data
Cell compaction–dependent brain tumor angiogenesis and tumor progression. A: Schema of brain tumor angiogenesis and tumor progression. Rapidly growing brain tumor in a confined space results in physical compaction of tumor cells (cells gather and pack together and cause associated changes in cell shape and size), which stimulates vascular endothelial growth factor (VEGF) expression and activates tumor angiogenesis in the brain. B and C: Illustrations of experimental systems mimicking cell compaction–dependent brain tumor progression. B: Microcontact printing. Brain tumor cells are cultured on the ECM islands at low or high plating density to recapitulate physical compaction of tumor cells in vivo. C: Mechanical compression. Pellets of brain tumor cells are physically compressed (1 kPa) for 16 to 24 hours between two pieces of PDMS polymer overlaid with a metal weight to mimic three-dimensional cell compaction.
Cell density controls collagen expression in glioblastoma (GBM) cells in vitro. Immunofluorescence (IF) micrographs show expression and distribution of collagen (Col) IV and Col VI (green) and nuclear staining using DAPI (blue) in U87MG cells cultured for 16 hours on microfabricated circular fibronectin islands at low or high density. Scale bar = 75 μm.
References
- 1.Zong H., Verhaak R.G., Canoll P. The cellular origin for malignant glioma and prospects for clinical advancements. Expert Rev Mol Diagn. 2012;12:383–394. doi: 10.1586/erm.12.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behin A., Hoang-Xuan K., Carpentier A.F., Delattre J.Y. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 3.Bergers G., Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim W.Y., Lee H.Y. Brain angiogenesis in developmental and pathological processes: mechanism and therapeutic intervention in brain tumors. FEBS J. 2009;276:4653–4664. doi: 10.1111/j.1742-4658.2009.07177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reardon D.A., Turner S., Peters K.B., Desjardins A., Gururangan S., Sampson J.H., McLendon R.E., Herndon J.E., 2nd, Jones L.W., Kirkpatrick J.P., Friedman A.H., Vredenburgh J.J., Bigner D.D., Friedman H.S. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw. 2011;9:414–427. doi: 10.6004/jnccn.2011.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucio-Eterovic A.K., Piao Y., de Groot J.F. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:4589–4599. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 7.Verhoeff J.J., van Tellingen O., Claes A., Stalpers L.J., van Linde M.E., Richel D.J., Leenders W.P., van Furth W.R. Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer. 2009;9:444. doi: 10.1186/1471-2407-9-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vredenburgh J.J., Desjardins A., Herndon J.E., 2nd, Dowell J.M., Reardon D.A., Quinn J.A., Rich J.N., Sathornsumetee S., Gururangan S., Wagner M., Bigner D.D., Friedman A.H., Friedman H.S. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 9.Pope W.B., Lai A., Nghiemphu P., Mischel P., Cloughesy T.F. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 11.Goel S., Duda D.G., Xu L., Munn L.L., Boucher Y., Fukumura D., Jain R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S., Ingber D.E. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Mammoto A., Ingber D.E. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009;21:864–870. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Discher D., Dong C., Fredberg J.J., Guilak F., Ingber D., Janmey P., Kamm R.D., Schmid-Schönbein G.W., Weinbaum S. Biomechanics: cell research and applications for the next decade. Ann Biomed Eng. 2009;37:847–859. doi: 10.1007/s10439-009-9661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levental K.R., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J.T., Fong S.F., Csiszar K., Giaccia A., Weninger W., Yamauchi M., Gasser D.L., Weaver V.M. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng G., Tse J., Jain R.K., Munn L.L. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS One. 2009;4:e4632. doi: 10.1371/journal.pone.0004632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butcher D.T., Alliston T., Weaver V.M. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann M., Guschel M., Bernd A., Bereiter-Hahn J., Kaufmann R., Tandi C., Wiig H., Kippenberger S. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8:89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paszek M.J., Zahir N., Johnson K.R., Lakins J.N., Rozenberg G.I., Gefen A., Reinhart-King C.A., Margulies S.S., Dembo M., Boettiger D., Hammer D.A., Weaver V.M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Mammoto A., Huang S., Moore K., Oh P., Ingber D.E. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- 21.Lo C.M., Wang H.B., Dembo M., Wang Y.L. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammoto A., Connor K.M., Mammoto T., Yung C.W., Huh D., Aderman C.M., Mostoslavsky G., Smith L.E., Ingber D.E. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H., Mouw J.K., Weaver V.M. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 2011;21:47–56. doi: 10.1016/j.tcb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellail A.C., Hunter S.B., Brat D.J., Tan C., Van Meir E.G. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Huijbers I.J., Iravani M., Popov S., Robertson D., Al-Sarraj S., Jones C., Isacke C.M. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One. 2010;5:e9808. doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamecnik J. The extracellular space and matrix of gliomas. Acta Neuropathol. 2005;110:435–442. doi: 10.1007/s00401-005-1078-5. [DOI] [PubMed] [Google Scholar]
- 27.Smith M.M., Hall B.K. Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol Rev Camb Philos Soc. 1990;65:277–373. doi: 10.1111/j.1469-185x.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 28.Hall B.K., Miyake T. The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anat Embryol (Berl) 1992;186:107–124. doi: 10.1007/BF00174948. [DOI] [PubMed] [Google Scholar]
- 29.Mammoto T., Mammoto A., Torisawa Y.S., Tat T., Gibbs A., Derda R., Mannix R., de Bruijn M., Yung C.W., Huh D., Ingber D.E. Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev Cell. 2011;21:758–769. doi: 10.1016/j.devcel.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mammoto A., Mammoto T., Kanapathipillai M., Wing Yung C., Jiang E., Jiang A., Lofgren K., Gee E.P.S., Ingber D.E. Control of lung vascular permeability and endotoxin-induced pulmonary oedema by changes in extracellular matrix mechanics. Nature Commun. 2013;4:1759. doi: 10.1038/ncomms2774. [DOI] [PubMed] [Google Scholar]
- 31.Helmlinger G., Netti P.A., Lichtenbeld H.C., Melder R.J., Jain R.K. Solid stress inhibits the growth of multicellular tumor spheroids. Nature Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- 32.Tse J.M., Cheng G., Tyrrell J.A., Wilcox-Adelman S.A., Boucher Y., Jain R.K., Munn L.L. Mechanical compression drives cancer cells toward invasive phenotype. Proc Natl Acad Sci U S A. 2012;109:911–916. doi: 10.1073/pnas.1118910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sodek K.L., Ringuette M.J., Brown T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int J Cancer. 2009;124:2060–2070. doi: 10.1002/ijc.24188. [DOI] [PubMed] [Google Scholar]
- 34.Iyengar P., Espina V., Williams T.W., Lin Y., Berry D., Jelicks L.A., Lee H., Temple K., Graves R., Pollard J., Chopra N., Russell R.G., Sasisekharan R., Trock B.J., Lippman M., Calvert V.S., Petricoin E.F., 3rd, Liotta L., Dadachova E., Pestell R.G., Lisanti M.P., Bonaldo P., Scherer P.E. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu A., Mammoto A., Italiano J.E., Jr., Pravda E., Dudley A.C., Ingber D.E., Klagsbrun M. ABL2/ARG tyrosine kinase mediates SEMA3F-induced RhoA inactivation and cytoskeleton collapse in human glioma cells. J Biol Chem. 2008;283:27230–27238. doi: 10.1074/jbc.M804520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mammoto A., Sero J.E., Mammoto T., Ingber D.E. Methods for studying mechanical control of angiogenesis by the cytoskeleton and extracellular matrix. Methods Enzymol. 2008;443:227–259. doi: 10.1016/S0076-6879(08)02012-0. [DOI] [PubMed] [Google Scholar]
- 37.Rubin J.B., Kung A.L., Klein R.S., Chan J.A., Sun Y., Schmidt K., Kieran M.W., Luster A.D., Segal R.A. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erler J.T., Bennewith K.L., Nicolau M., Dornhofer N., Kong C., Le Q.T., Chi J.T., Jeffrey S.S., Giaccia A.J. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 39.Fogelgren B., Polgár N., Szauter K.M., Ujfaludi Z., Laczkó R., Fong K.S., Csiszar K. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem. 2005;280:24690–24697. doi: 10.1074/jbc.M412979200. [DOI] [PubMed] [Google Scholar]
- 40.Atabai K., Jame S., Azhar N., Kuo A., Lam M., McKleroy W., Dehart G., Rahman S., Xia D.D., Melton A.C., Wolters P., Emson C.L., Turner S.M., Werb Z., Sheppard D. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119:3713–3722. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mammoto A., Mammoto T., Ingber D.E. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci. 2012;125:3061–3073. doi: 10.1242/jcs.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan L., Siegel M., Choi K., Khosla C., Miller C.R., Jackson E.N., Piwnica-Worms D., Rich K.M. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene. 2007;26:2563–2573. doi: 10.1038/sj.onc.1210048. [DOI] [PubMed] [Google Scholar]
- 43.Kanapathipillai M., Mammoto A., Mammoto T., Kang J.H., Jiang E., Ghosh K., Korin N., Gibbs A., Mannix R., Ingber D. Inhibition of mammary tumor growth using lysyl oxidase-targeting nanoparticles to modify extracellular matrix. Nano Lett. 2012;12:3213–3217. doi: 10.1021/nl301206p. [DOI] [PubMed] [Google Scholar]
- 44.Fornieri C., Baccarani-Contri M., Quaglino D., Jr., Pasquali-Ronchetti I. Lysyl oxidase activity and elastin/glycosaminoglycan interactions in growing chick and rat aortas. J Cell Biol. 1987;105:1463–1469. doi: 10.1083/jcb.105.3.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kagan H.M., Trackman P.C. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–210. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- 46.Kagan H.M., Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 47.Li W., Nugent M.A., Zhao Y., Chau A.N., Li S.J., Chou I.N., Liu G., Kagan H.M. Lysyl oxidase oxidizes basic fibroblast growth factor and inactivates its mitogenic potential. J Cell Biochem. 2003;88:152–164. doi: 10.1002/jcb.10304. [DOI] [PubMed] [Google Scholar]
- 48.Tchaparian E.H., Uriu-Adams J.Y., Keen C.L., Mitchell A.E., Rucker R.B. Lysyl oxidase and P-ATPase-7A expression during embryonic development in the rat. Arch Biochem Biophys. 2000;379:71–77. doi: 10.1006/abbi.2000.1842. [DOI] [PubMed] [Google Scholar]
- 49.Kirschmann D.A., Seftor E.A., Fong S.F., Nieva D.R., Sullivan C.M., Edwards E.M., Sommer P., Csiszar K., Hendrix M.J. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–4483. [PubMed] [Google Scholar]
- 50.Miller L.F., Judge M.D., Schanbacher B.D. Intramuscular collagen and serum hydroxyproline as related to implanted testosterone, dihydrotestosterone and estradiol-17 beta in growing wethers. J Anim Sci. 1990;68:1044–1048. doi: 10.2527/1990.6841044x. [DOI] [PubMed] [Google Scholar]
- 51.Norden A.D., Young G.S., Setayesh K., Muzikansky A., Klufas R., Ross G.L., Ciampa A.S., Ebbeling L.G., Levy B., Drappatz J., Kesari S., Wen P.Y. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 52.Trackman P.C., Kagan H.M. Nonpeptidyl amine inhibitors are substrates of lysyl oxidase. J Biol Chem. 1979;254:7831–7836. [PubMed] [Google Scholar]
- 53.Kosonen T., Uriu-Hare J.Y., Clegg M.S., Keen C.L., Rucker R.B. Incorporation of copper into lysyl oxidase. Biochem J. 1997;327(Pt 1):283–289. doi: 10.1042/bj3270283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ala A., Walker A.P., Ashkan K., Dooley J.S., Schilsky M.L. Wilson’s disease. Lancet. 2007;369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 55.Brem S., Grossman S.A., Carson K.A., New P., Phuphanich S., Alavi J.B., Mikkelsen T., Fisher J.D., New Approaches to Brain Tumor Therapy CNS Consortium Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro Oncol. 2005;7:246–253. doi: 10.1215/S1152851704000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guttilla I.K., Phoenix K.N., Hong X., Tirnauer J.S., Claffey K.P., White B.A. Prolonged mammosphere culture of MCF-7 cells induces an EMT and repression of the estrogen receptor by microRNAs. Breast Cancer Res Treat. 2012;132:75–85. doi: 10.1007/s10549-011-1534-y. [DOI] [PubMed] [Google Scholar]
- 57.Croix B.S., Rak J.W., Kapitain S., Sheehan C., Graham C.H., Kerbel R.S. Reversal by hyaluronidase of adhesion-dependent multicellular drug resistance in mammary carcinoma cells. J Natl Cancer Inst. 1996;88:1285–1296. doi: 10.1093/jnci/88.18.1285. [DOI] [PubMed] [Google Scholar]
- 58.Ferrante A., Rainaldi G., Indovina P., Indovina P.L., Santini M.T. Increased cell compaction can augment the resistance of HT-29 human colon adenocarcinoma spheroids to ionizing radiation. Int J Oncol. 2006;28:111–118. [PubMed] [Google Scholar]
- 59.Taylor M.A., Amin J.D., Kirschmann D.A., Schiemann W.P. Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-beta signaling in breast cancer cells. Neoplasia. 2011;13 doi: 10.1593/neo.101086. 406–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giampuzzi M., Oleggini R., Di Donato A. Altered adhesion features and signal transduction in NRK-49F cells transformed by down-regulation of lysyl oxidase. Biochim Biophys Acta. 2003;1647:239–244. doi: 10.1016/s1570-9639(03)00058-x. [DOI] [PubMed] [Google Scholar]
- 61.Baker A.M., Bird D., Welti J.C., Gourlaouen M., Lang G., Murray G.I., Reynolds A.R., Cox T.R., Erler J.T. Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumor angiogenesis. Cancer Res. 2013;73:583–594. doi: 10.1158/0008-5472.CAN-12-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen C.Z., Raghunath M. Focus on collagen: in vitro systems to study fibrogenesis and antifibrosis state of the art. Fibrogenesis Tissue Repair. 2009;2:7. doi: 10.1186/1755-1536-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu K.V., Chang J.P., Parachoniak C.A., Pandika M.M., Aghi M.K., Meyronet D., Isachenko N., Fouse S.D., Phillips J.J., Cheresh D.A., Park M., Bergers G. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jahangiri A., De Lay M., Miller L.M., Carbonell W.S., Hu Y.L., Lu K., Tom M.W., Paquette J., Tokuyasu T.A., Tsao S., Marshall R., Perry A., Bjorgan K.M., Chaumeil M.M., Ronen S.M., Bergers G., Aghi M.K. Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res. 2013;19:1773–1783. doi: 10.1158/1078-0432.CCR-12-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ellis L.M., Reardon D.A. Cancer: the nuances of therapy. Nature. 2009;458:290–292. doi: 10.1038/458290a. [DOI] [PubMed] [Google Scholar]
- 66.Brewer G.J. Copper control as an antiangiogenic anticancer therapy: lessons from treating Wilson’s disease. Exp Biol Med (Maywood) 2001;226:665–673. doi: 10.1177/153537020222600712. [DOI] [PubMed] [Google Scholar]
- 67.Di Serio C., Doria L., Pellerito S., Prudovsky I., Micucci I., Massi D., Landriscina M., Marchionni N., Masotti G., Tarantini F. The release of fibroblast growth factor-1 from melanoma cells requires copper ions and is mediated by phosphatidylinositol 3-kinase/Akt intracellular signaling pathway. Cancer Lett. 2008;267:67–74. doi: 10.1016/j.canlet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Pan Q., Kleer C.G., van Golen K.L., Irani J., Bottema K.M., Bias C., De Carvalho M., Mesri E.A., Robins D.M., Dick R.D., Brewer G.J., Merajver S.D. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 69.Batchelor T.T., Sorensen A.G., di Tomaso E., Zhang W.T., Duda D.G., Cohen K.S., Kozak K.R., Cahill D.P., Chen P.J., Zhu M., Ancukiewicz M., Mrugala M.M., Plotkin S., Drappatz J., Louis D.N., Ivy P., Scadden D.T., Benner T., Loeffler J.S., Wen P.Y., Jain R.K. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brem S., Tsanaclis A.M., Zagzag D. Anticopper treatment inhibits pseudopodial protrusion and the invasive spread of 9L gliosarcoma cells in the rat brain. Neurosurgery. 1990;26:391–396. doi: 10.1097/00006123-199003000-00003. [DOI] [PubMed] [Google Scholar]
- 71.Brem S.S., Zagzag D., Tsanaclis A.M., Gately S., Elkouby M.P., Brien S.E. Inhibition of angiogenesis and tumor growth in the brain: suppression of endothelial cell turnover by penicillamine and the depletion of copper, an angiogenic cofactor. Am J Pathol. 1990;137:1121–1142. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell compaction–dependent brain tumor angiogenesis and tumor progression. A: Schema of brain tumor angiogenesis and tumor progression. Rapidly growing brain tumor in a confined space results in physical compaction of tumor cells (cells gather and pack together and cause associated changes in cell shape and size), which stimulates vascular endothelial growth factor (VEGF) expression and activates tumor angiogenesis in the brain. B and C: Illustrations of experimental systems mimicking cell compaction–dependent brain tumor progression. B: Microcontact printing. Brain tumor cells are cultured on the ECM islands at low or high plating density to recapitulate physical compaction of tumor cells in vivo. C: Mechanical compression. Pellets of brain tumor cells are physically compressed (1 kPa) for 16 to 24 hours between two pieces of PDMS polymer overlaid with a metal weight to mimic three-dimensional cell compaction.
Cell density controls collagen expression in glioblastoma (GBM) cells in vitro. Immunofluorescence (IF) micrographs show expression and distribution of collagen (Col) IV and Col VI (green) and nuclear staining using DAPI (blue) in U87MG cells cultured for 16 hours on microfabricated circular fibronectin islands at low or high density. Scale bar = 75 μm.