Significance
The hypoplastic anemia characteristic of del(5q) myelodysplastic syndrome (MDS) arises from ribosomal protein insufficiency, resulting in erythroid-specific activation of p53. We found that suppression of p53 by cenersen, an antisense oligonucleotide, markedly improved erythroid colony formation in primary MDS specimens assessed by two-stage colony formation assay. Erythropoietic rescue significantly correlated with the magnitude of reduction in nuclear p53. In addition, in a cohort of eight lower-risk, lenalidomide-refractory del(5q) MDS patients treated with lenalidomide and dexamethasone, a glucocorticoid receptor-dependent antagonist of p53, transfusion independence was restored in five of eight patients, accompanied by in vivo expansion of erythroid precursors without clonal suppression. These results suggest inhibition of p53 may be a unique therapeutic strategy in patients with lenalidomide-resistant del(5q) MDS.
Abstract
Stabilization of p53 in erythroid precursors in response to nucleosomal stress underlies the hypoplastic anemia in myelodysplastic syndromes (MDS) with chromosome 5q deletion [del(5q)]. We investigated whether cenersen, a clinically active 20-mer antisense oligonucleotide complementary to TP53 exon10, could suppress p53 expression and restore erythropoiesis in del(5q) MDS. Cenersen treatment of ribosomal protein S-14-deficient erythroblasts significantly reduced cellular p53 and p53-up-regulated modulator of apoptosis expression compared with controls, accompanied by a significant reduction in apoptosis and increased cell proliferation. In a two-stage erythroid differentiation assay, cenersen significantly suppressed nuclear p53 in bone marrow CD34+ cells isolated from patients with del(5q) MDS, whereas erythroid burst recovery increased proportionally to the magnitude of p53 suppression without evidence of del(5q) clonal suppression (r = −0.6; P = 0.005). To explore the effect of p53 suppression on erythropoiesis in vivo, dexamethasone, a glucocorticoid receptor-dependent p53 antagonist, was added to lenalidomide treatment in eight lower-risk, transfusion-dependent, del(5q) MDS patients with acquired drug resistance. Transfusion independence was restored in five patients accompanied by expansion of erythroid precursors and decreased cellular p53 expression. We conclude that targeted suppression of p53 could support effective erythropoiesis in lenalidomide-resistant del(5q) MDS.
Myelodysplastic syndromes (MDS) with chromosome 5q deletion [del(5q)] is a pathologically and cytogenetically distinct disease subtype characterized by a refractory hypoplastic anemia (1–4). Although haploinsufficiency of several genes encoded within the commonly deleted region (CDR) have been implicated in the hematologic phenotype, allelic deletion of the ribosomal protein S-14 (RPS14) gene, which encodes a component of the 40S ribosomal subunit, is a key determinant of hypoplastic anemia (5). Ebert and coworkers, using an RNA interference screen of the CDR genes, showed that inactivation of the RPS14 gene impaired erythroblast proliferation and viability, whereas forced expression of RPS14 rescued erythropoiesis in primary del(5q) MDS specimens (6). Subsequent investigations have shown that ribosomal insufficiency disrupts ribosome integrity, which in turn triggers autologous degradation of the human homolog of the mouse double minute 2 protein (MDM2), resulting in p53 stabilization (7). Selective suppression of RPS14 in normal CD34+ cells showed that p53 activation is restricted to the erythroid lineage (6). Nevertheless, a recently reported murine model of the human 5q− syndrome generated by allelic deletion of the syntenic genes in the human CDR showed that p53 inactivation rescues the hematologic phenotype, indicating that the molecular pathogenesis of the 5q− syndrome is p53-dependent (8).
Lenalidomide is the first targeted therapy approved by the US Food and Drug Administration for the treatment of patients with lower-risk, transfusion-dependent del(5q) MDS that, in most patients, yields sustained transfusion independence and clonal suppression (2, 9). We recently reported that lenalidomide promotes p53 degradation by stabilizing MDM2, thereby fostering cell cycle reentry and subsequent G2/M arrest of del(5q) progenitors by inhibiting two haplo-deficient phosphatases: protein phosphatase 2A catalytic domain alpha and cell division cycle 25C (10). Treatment with lenalidomide significantly reduced p53 expression in bone marrow erythroid precursors of responding del(5q) MDS patients; however, on emergence of drug resistance, p53 accumulation was restored (11). Although responses to lenalidomide are relatively durable, 50% of patients acquire resistance to lenalidomide within 2–3 y (2).
Antisense oligonucleotides are a targeted therapeutic approach to suppress translation of complementary gene transcripts (12, 13). Cenersen, a 20-mer antisense phosphorothioate oligonucleotide, cleaves TP53 mRNA through an RNase H-dependent mechanism, effectively downregulating both wild-type and mutant p53 expression in vitro and in vivo. Clinical trials of patients with hematological malignancies, including acute myeloid leukemia and chronic lymphocytic leukemia, have shown increased cytotoxicity and enhanced sensitivity to conventional chemotherapies when combined with cenersen (14–17).
Given the role of p53 in the hypoplastic anemia of del(5q) MDS and the emerging challenge of lenalidomide resistance, we hypothesized that targeted suppression of p53 with cenersen may be an effective strategy to restore erythropoiesis. Our results show that down-regulation of p53 by cenersen enhanced erythropoiesis in del(5q) MDS without evidence of clonal suppression, thereby offering a unique treatment strategy for del(5q) MDS patients. Furthermore, in a proof-of-principle pilot study in del(5q) MDS patients with acquired lenalidomide resistance, the addition of dexamethasone, a glucocorticoid receptor-dependent p53 antagonist, to lenalidomide treatment effectively restored erythropoiesis in five of eight patients (18).
Results
Cenersen Decreases p53 and PUMA Expression in RPS14-Deficient Erythroblasts.
To model the RPS14 haploinsufficiency observed in del(5q) MDS, human bone marrow CD34+ cells were transduced with lentiviruses expressing shRNAs targeting RPS14 and then differentiated along the erythroid lineage. Cells transduced with lentiviruses expressing either of two RPS14 shRNAs (sh41 or sh44) demonstrated a significant reduction in RPS14 expression level by quantitative PCR (qRT-PCR) compared with the scramble control (P = 0.008 for sh41; P = 0.02 for sh44) (Fig. S1A). RPS14-deficient erythroblasts demonstrated impaired cell growth compared with the control (P = 0.0034) (Fig. S1B and Fig. 1A). Transfection of cells with cenersen or with the control oligo was performed 2 d after puromycin selection. Cenersen transfection was optimized to 72 h in the presence of lipofectamine through cenersen-carboxyfluorescein (FAM) (Fig. S1C). Cell growth was improved after cenersen treatment in RPS14-deficient cells compared with cells treated with the control oligonucleotide (sh41, P = 0.01 on day 5; sh44, P = 0.02, on days 2–5) (Fig. 1 B and D). There was no significant change in cell growth in the scramble control cells treated with cenersen compared to the cells treated with control oligonucleotide, except on day 1 (P = 0.03) (Fig. 1D).
Fig. 1.
Cenersen-treated, RPS14-deficient erythroblasts show improved cell growth. (A) RPS14-deficient erythroblasts (sh41 and sh44) generated from human CD34+ bone marrow cells have a significantly diminished growth capability compared with the scramble control. Scramble, open circles; sh41, asterisks; sh44, open squares. (B–D) RPS14-deficient erythroblasts showed increased cell growth when treated with cenersen compared with treatment with control oligonucleotide. Cenersen, filled circles; control oligonucleotide, open circles. Viability was assessed by trypan blue exclusion. Results are means ± SEM (n = 7). P values were calculated by two-way ANOVA (A) or Wilcoxon signed-rank test (B–D). *P < 0.05; **P < 0.01.
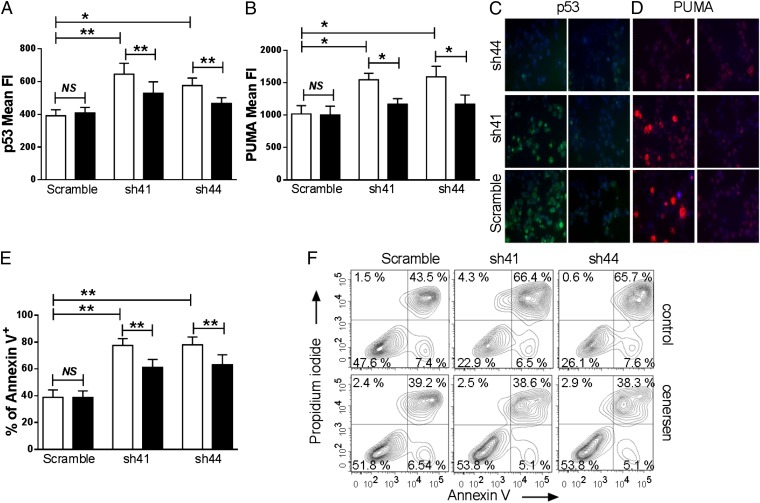
We next measured the effects of cenersen on the expression of p53 and its downstream target, p53-up-regulated modulator of apoptosis (PUMA), in RPS14-deficient erythroblasts by intracellular flow cytometry and immunofluorescence (Fig. 2 A–D). Silencing of RPS14 in human bone marrow CD34+ cells resulted in a significant up-regulation of p53 (P = 0.008 for sh41 and P = 0.03 for sh44) (Fig. 2 A and C) and PUMA (P = 0.02 for sh41and sh44) (Fig. 2 B and D). We observed a significant reduction in p53 expression levels in RPS14-deficient erythroblasts treated with cenersen versus cells treated with the control oligonucleotide (P = 0.008 for sh41 and sh44) (Fig. 2 A and C). Moreover, targeted suppression of p53 expression in RPS14-deficient erythroblasts treated with cenersen resulted in a significant decrease in PUMA expression compared with cells treated with the control oligonucleotide (P = 0.02 for sh41 and sh44) (Fig. 2 B and D). Similarly, silencing of RPS14 was associated with a significant up-regulation in the fraction of apoptotic cells (P = 0.002 for sh41 and sh44) (Fig. 2E), whereas the Annexin V-positive apoptotic cell fraction was significantly reduced in the RPS14-deficient erythroblasts transfected with cenersen compared with cells transfected with the control oligonucleotide (P = 0.002 for sh41 and sh44) (Fig. 2E). This is consistent with the increased viable, nonapoptotic (Annexin V-negative, propidium iodide-negative) cell population in cenersen-treated RPS14-deficient erythroblasts compared with the cells treated with the control oligonucleotide (Fig. 2F).
Fig. 2.
Cenersen reduces p53 and PUMA expression in RPS14-deficient erythroblasts and decreases apoptosis. Cenersen treatment resulted in significant down-regulation of p53 (A) and its downstream target PUMA (B) in RPS14-deficient erythroblasts compared with control oligonucleotide-treated cells. Cenersen, black bars; control oligonucleotide, open bars. Expression levels are shown as means ± SEM of fluorescence intensity (n = 8). Representative photomicrographs are shown of p53 (C) and PUMA (D) immunofluorescence in RPS14-deficient erythroblasts that have undergone cenersen (Right) or control oligonucleotide treatment (Left), with p53 staining shown in green, PUMA staining in red, and DAPI in blue (magnification, ×630). (E) Percentage apoptosis as assessed by Annexin V-positive staining, shown as means ± SEM (n = 10). Cenersen, black bars; control oligonucleotide, open bars. (F) Representative flow cytometry contour plots showing a significant increase in the viable, nonapoptotic (Annexin V-negative, propidium iodide-negative) cell population in RPS14-deficient erythroblasts after cenersen treatment. P values were calculated by Wilcoxon signed-rank test: *P < 0.05; **P < 0.01.
Cenersen Decreases p53 Expression in MDS Erythroid Progenitors.
Nuclear and cytoplasmic p53 expression levels were assessed by confocal microscopy and analysis of fluorescence intensity (FI) in 21 MDS patient specimens (Fig. 3). Clinical characteristics of the patients are summarized in Table S1. Cenersen treatment significantly reduced nuclear p53 expression in erythroid progenitors of del(5q) MDS patient specimens (P = 0.03), exceeding 60% decrease in four of 10 specimens. Nuclear p53 expression was also reduced in nondel(5q) MDS specimens, exceeding 60% in five of 11 specimens; however, the changes did not reach significance (P = 0.15). (Fig. 3 B and C). Mean nuclear p53 FI in del(5q) MDS was 82.2 ± 12.6 in control oligonucleotide-treated cells versus 46.6 ± 9.4 in cenersen-treated cells. Nuclear p53 FI in nondel(5q) MDS cells was 65.6 ± 9.8 with control oligonucleotide treatment and 39.0 ± 9.5 with cenersen treatment. Normal controls also showed a significant, albeit less pronounced, reduction in nuclear p53 FI (p53 FI of 69.3 ± 10.1 in control-treated versus 41.5 ± 8.6 in cenersen-treated cells; P = 0.03) (Fig. 3D). Cenersen also significantly reduced cytoplasmic p53 expression in del(5q) MDS cells (P = 0.05; p53 FI of 40.8 ± 5.6 in control versus 26.8 ± 4.5 in cenersen-treated cells), in nondel(5q) MDS cells (P = 0.005; p53 FI of 35. 5 ± 7.2 in control versus 20.4 ± 3.6 in cenersen-treated cells), and in healthy controls (P = 0.03; p53 FI of 44.7 ± 8.7 in control versus 22.8 ± 3.0 in cenersen-treated cells) (Fig. 3 B–D).
Fig. 3.
Cenersen treatment decreased p53 expression in primary MDS erythroid progenitors. (A) Representative photomicrograph of p53 immunofluorescence in del(5q) MDS progenitors, with p53 staining shown in green and DAPI in blue (magnification: Upper, ×630; Lower, ×3,780). (B–D) Nuclear and cytoplasmic p53 FI levels in del(5q) MDS (n = 10; B), nondel(5q) MDS (n = 11; C), and normal controls (n = 6; D) with cenersen and control oligonucleotide treatment. Cenersen, black bars; control oligonucleotide, open bars. Values are mean FI ± SEM. P values were calculated by Wilcoxon signed-rank test: *P < 0.05; NS, not significant.
Cenersen Promotes BFU-E Recovery in MDS in a Two-Stage Colony Formation Assay.
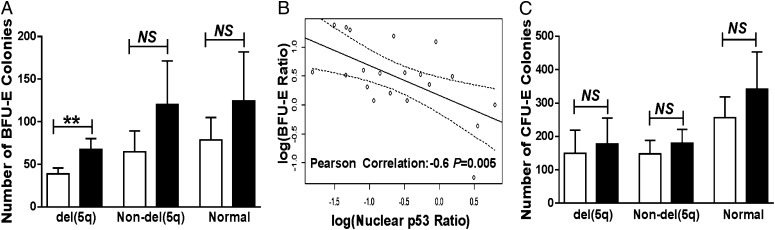
We next assessed the effect of cenersen treatment on the proliferative capacity of erythroid progenitors by performing a two-stage colony formation assay. CD34+ cells from 10 del(5q) and 11 nondel(5q) MDS patient specimens and from six healthy controls were isolated and expanded. Cells were then plated on methylcellulose to assess the effect of cenersen on proliferation and on the colony-forming capacity of erythroid progenitors. The mean burst-forming units-erythroid (BFU-E) significantly increased in del(5q) specimens (P = 0.004) but was not significantly increased in nondel(5q) MDS samples (P = 0.1) or normal controls (P = 0.3) (Fig. 4A). The magnitude of improvement in BFU-E in MDS specimens highly correlated with the magnitude of nuclear p53 suppression (Fig. 4B) (Pearson correlation coefficient r = −0.6; P = 0.005). Colony-forming units-erythroid (CFU-E) recovery was not significantly increased with cenersen treatment in del(5q) MDS (P = 0.3), nondel(5q) MDS (P = 0.08), or normal controls (P = 0.3) (Fig. 4C). Granulocyte-macrophage or mixed lineage colony forming capacity showed no consistent differences after cenersen treatment (Fig. S2).
Fig. 4.
Cenersen treatment promotes erythroid colony-forming capacity. (A) BFU-E numbers were significantly increased in del(5q) MDS specimens (n = 10). This increase was not significant in nondel(5q) MDS (n = 11) or control (n = 6) specimens. (B) There is a significant correlation between the magnitude of BFU-E improvement and magnitude of nuclear p53 suppression in MDS erythroid progenitors treated with cenersen (Pearson correlation factor r = −0.6; P = 0.005). (C) Cenersen treatment increased the number of CFU-E in del(5q) (n = 9), nondel(5q) (n = 10), and control samples (n = 5). Results are means ± SEM. P values were calculated by Wilcoxon signed-rank test: **P < 0.01; NS, not significant.
Cenersen Does Not Suppress the del(5q) Clone.
To determine whether cenersen was cytotoxic to or suppressed the del(5q) clonal population, we performed FISH analysis after cenersen treatment in the del(5q) CD34+ cells. We found no significant changes in the number of clonal cells in either cenersen or control oligonucleotide-treated cells (P = 0.72; cenersen, 56.5 ± 12.5% vs. control oligonucleotide, 49.8 ± 11.3%; n = 9), indicating no evidence of del(5q) clonal suppression with cenersen treatment.
TP53 Amplification and Sequence.
Cenersen is complementary to the exon 10 coding sequence of the TP53 gene transcript. We sequenced p53 exon 10 in 13 MDS samples to determine whether mutations in this exon might disrupt cenersen binding to TP53 transcripts and account for reduced response to cenersen treatment. None of the samples tested harbored a mutation in exon 10 of TP53. In addition, we sequenced the entire DNA binding domain of TP53 (exons 3–9) in 11 specimens (Table S2). Most of the samples had previously characterized polymorphisms in exon 3. Mutations were found in only two patient specimens: one patient with intermediate 1 risk disease (Table S1; patient 6) with a P152L mutation in exon 4 and one patient with intermediate 2 risk disease (patient 7) with a L265P mutation in exon 7. Both patients had del(5q): patient 6 associated with add(17) by metaphase karyotyping and patient 7 with a complex karyotype. In both mutant TP53 patient specimens, cenersen significantly increased the number of BFU-E (P < 0.0001 and P = 0.0006, respectively) and CFU-E (P = 0.0002 and P = 0.01, respectively), with a corresponding reduction in nuclear and cytoplasmic p53 expression (P < 0.0001).
Lenalidomide and Dexamethasone Treatment Restores Effective Erythropoiesis in Vivo.
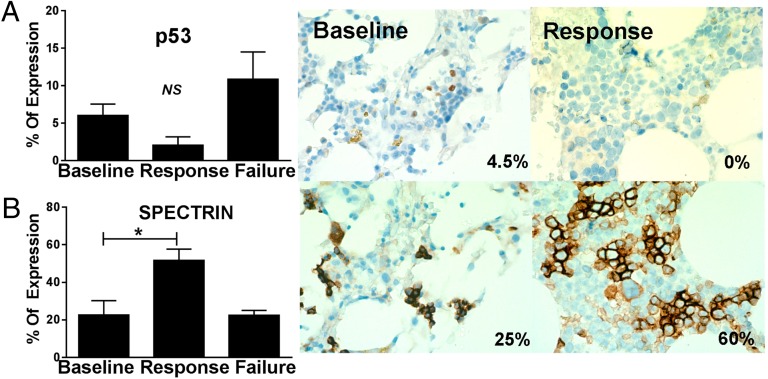
A pilot study to determine whether p53 suppression could restore hematopoiesis in lenalidomide-resistant patients was performed in a cohort of eight patients treated with lenalidomide and dexamethasone. The dexamethasone/glucocorticoid receptor is a transcriptional antagonist of p53 and the transcription factor GATA-1 that promotes in vitro expansion of immature erythroblasts (18). All eight patients were transfusion-dependent and achieved variable durations of sustained TI in response to lenalidomide treatment, but subsequently developed drug resistance with the return of red blood cell transfusion dependence. All patients had International Prognostic Scoring System intermediate 1 risk MDS with less than 5% myeloblasts: four had isolated del(5q), three had del(5q) plus one additional abnormality, and one had del(5q) plus two additional abnormalities. Patients continued on their current dose of lenalidomide (5–10 mg/d), with dexamethasone added at a dose of 20 mg orally weekly. Among the eight treated patients, five achieved transfusion independence with the combination treatment, with durations of response ranging from 4.4 to more than 15 mo, with the latter patient currently continuing on treatment. Cytogenetic analysis after achievement of transfusion independence showed no consistent changes in the proportion of del(5q) metaphases (Table S3). Cellular expression levels of p53 and the early erythroid marker spectrin were assessed by immunohistochemistry in bone marrow biopsies before and after combination therapy. We found that relative expression of p53 (percentage of positive cells × relative intensity) was reduced 65% after response to the combination treatment (Fig. 5A) (response = 2.08 ± 1.1% vs. baseline = 6.04 ± 1.5%; P = 0.11), accompanied by a corresponding expansion of erythroid precursors, as evidenced by an increase in the number of spectrin-positive cells (Fig. 5B) (response = 51.7 ± 6.0% vs. baseline = 22.7 ± 7.5%; P = 0.04). Biopsies available from 2 patients at the time of combination treatment failure showed that cellular p53 increased (10.9 ± 3.6%) and that expression levels exceeded those at baseline (Fig. 5A). In nonresponders, we observed no decrease in p53 expression or expansion of erythroid progenitors.
Fig. 5.
Lenalidomide plus dexamethasone treatment suppresses p53 expression and promotes erythroid expansion. Bone marrow biopsies of treated patients were stained with p53 (A) and spectrin (B), with percentage of positive staining shown. Representative micrographs of a single responder are shown at both baseline (Left) and time of response (Right). (Upper) p53 staining was decreased between baseline and the time of response. (Lower) Spectrin staining increased from baseline to the time of response (magnification: ×600). Results are means ± SEM. P values were calculated by t-test: *P < 0.05; NS, not significant.
Discussion
p53 is a critical determinant of primitive erythroid fate through control of proliferative potential, and its action as a reciprocal antagonist of the erythroid-specific transcription factor GATA-1, which is indispensable for erythroid differentiation and survival (19). As a consequence, sustained stabilization of p53 in erythroid precursors as a result of impaired ribosome biogenesis, as occurs in del(5q) MDS, is a critical effector of the hypoplastic anemia. Our results provide evidence that antisense strategies targeting suppression of p53 may rescue erythroid precursors and restore effective erythropoiesis in del(5q) MDS. To evaluate the gene-specific effects of RPS14 haploinsufficiency in isolation, we performed lentiviral transduction of bone marrow CD34+ cells from normal donors, using lentiviruses expressing shRNAs targeting RPS14. Cenersen treatment of RPS14-deficient erythroblasts significantly reduced expression of both p53 and its downstream transcriptional target, the proapoptotic protein PUMA. Moreover, down-regulation of p53 in RPS14-deficient erythroblasts fostered cell cycle reentry, thereby enabling cell proliferation and enhancing colony-forming capacity with a corresponding reduction in apoptosis (Figs. 1 and 2). Similarly, in del(5q) MDS primary specimens, cenersen effectively reduced expression of nuclear p53 accompanied by a pronounced increase in BFU-E recovery compared with control oligonucleotide treatment (Fig. 4). Importantly, there was no discernible difference in response to cenersen in specimens from del(5q) patients who were either lenalidomide-naive or had failed lenalidomide treatment, nor in the two specimens harboring an exon 4 or 7 TP53 gene mutation. The magnitude of p53 suppression and improvement in erythroid colony-forming capacity was greater in del(5q) MDS compared with nondel(5q) MDS or specimens from healthy volunteers, perhaps accounting for the greater improvement in erythroid burst recovery. Notably, the magnitude of improvement in BFU-E recovery significantly correlated with the extent of reduction in nuclear p53 florescence intensity (P = 0.005), confirming the critical role of p53 in the control of erythroid potential (Fig. 4B). We found no mutations in exon 10 of the TP53 gene that could account for differences in sensitivity to cenersen.
To explore the clinical potential of this approach, we performed a pilot study evaluating hematologic response and p53 expression in bone marrow erythroid precursors after pulsed treatment with weekly dexamethasone in del(5q) patients who acquired resistance to lenalidomide treatment. The glucocorticoid receptor acts as a ligand-dependent transcription factor and antagonist of both p53 and GATA-1 to promote expansion of immature erythroblasts (18). In inherited disorders of ribosomal insufficiency such as Diamond-Blackfan anemia, corticosteroids remain the cornerstone of treatment to improve erythropoiesis. We previously reported that p53 expression is up-regulated in del(5q) erythroid precursors upon the development of resistance to lenalidomide treatment (11). We reasoned that the addition of dexamethasone may suppress p53 in vivo, thereby restoring lenalidomide responsiveness. Indeed, red blood cell transfusion independence was restored in five of the eight patients treated with the combination, lasting from 4 to more than 15 mo. Responses occurred rapidly, with improvement in transfusion needs evident within weeks of initiating treatment. Of particular importance, only one patient had a decrease in the proportion of del(5q) metaphases after response to the addition of dexamethasone, whereas the percentage of del(5q) metaphases increased in 2 patients, suggesting that effective erythropoiesis was restored within the MDS clone. Results of the immunohistochemical staining of sequential trephine biopsies support this notion. Cellular p53 expression in erythroid precursors decreased in responding patients, accompanied by expansion of erythroid precursors, as evidenced by an increasing number of spectrin-positive cells. On treatment failure, levels of p53 again increased (Fig. 5).
This study provides proof of principle that strategies that suppress p53 in erythroid precursors can restore effective erythropoiesis in del(5q) MDS and possibly overcome clinical resistance to lenalidomide. This unique approach to rescuing clonal erythroid precursors and restoring differentiation potential contrasts with that of lenalidomide monotherapy, which suppresses the del(5q) clone. Nevertheless, we cannot exclude the possibility that alternate pharmacologic effects of dexamethasone or a specific interaction with lenalidomide may have contributed to the improvement in erythropoiesis (20). Although sustained suppression of p53 could have potentially deleterious consequences on the risk of neoplasia, our findings of hematologic response to weekly treatment with dexamethasone suggest that nonsustained approaches to suppress p53 may be sufficient to promote erythropoiesis. Moreover, transient pharmacological inhibition of p53 has been shown not to increase the incidence of cancer in a murine carcinogenicity model (21). Recent studies indicate that TP53 gene mutations are demonstrable in ∼20% of del(5q) MDS patients, expand over time, and are associated with higher risk of disease progression and lower frequency of cytogenetic response to lenalidomide (22–24). Given that cenersen binds to exon 10 of the TP53 gene transcript (i.e., outside of mutation hot spots in the DNA-binding domain), cenersen suppresses both wild-type and mutant p53, and therefore could possibly favorably modify risk of disease progression. In fact, in a patient with chromosome 17p13 deletion in all metaphases with attendant loss of heterozygosity at the TP53 locus before treatment, del(5q) cells persisted after response to dexamethasone despite loss of the chromosome 17p abnormality, suggesting selective suppression of the TP53 mutant clone. Similar strategies may also be applicable to heritable forms of ribosomopathies, such as Diamond-Blackfan anemia, thus meriting further investigation.
Materials and Methods
Isolation of CD34+ Primary Cells.
Bone marrow aspirates from 21 MDS patients were obtained from individuals who had provided written informed consent on institutional review board -approved research protocols. Bone marrow CD34+ cells were cultured for 2–10 d, as previously described (6). For details, see SI Materials and Methods. Healthy donors were acquired from Lonza Walkersville Inc., purified CD34+ bone marrow cells from 10 controls were bought for generation of RPS14-deficient erythroblasts, and three bone marrow aspirates were purchased for cenersen treatment. The control group included three individuals who assisted in the clinic and revealed normocellular bone marrow.
Generation of RPS14-Deficient Erythroblasts.
Healthy donor CD34+ cells were cultured for 48 h and then transduced with lentivirus, as previously described (25). For details see SI Materials and Methods. After transduction, cells were grown for 48 h before treatment.
Real-Time Quantitative PCR.
RPS14 expression was validated using real-time qRT-PCR. The β2-microglobulin gene was used to normalize for differences in input cDNA. Predeveloped TaqMan assays were used (Assays-on-Demand, Applied Biosystems), and reactions were run on a LightCycler 480 Real-Time PCR System (Roche Diagnostics). Each sample was run in triplicate, and the expression ratios were calculated using the ΔΔCT method.
Cenersen Treatment.
Cells were transfected with 40 nM cenersen (5′-CCCTGCTCCCCCCTGGCTCC-3′; Aezea, Eleos Inc.) or control oligonucleotide with cenersen-reversed sequence (5′-CCTCGGTCCCCCCTCGTCCC-3′, TriLink Biotechnologies) for 72 h, using Lipofectamine RNAiMAX (Life Technologies Corporation), according to manufacturer’s protocol. See SI Materials and Methods for further details.
Cell Growth Assay of RPS14-Deficient Erythroblasts.
Cells after lentiviral transduction and cenersen transfection were seeded into 96-well plates (20,000 cells/0.2 mL). Viable cell counts were determined by trypan blue exclusion for 5 consecutive days postlentiviral transduction and postcenersen transfection. Medium was replenished every second day to maintain the same volume.
Flow Cytometry.
For p53 and PUMA staining, cells were harvested, washed in PBS, and stained with the Fixable Viability dye eFluor 780 (eBioscience) for 30 min. After PBS washing, cells were fixed and permeabilized using the FOXP3 Fix/Perm Buffer Set, according to the manufacturer’s instructions (eBioscience). Cells were incubated with anti-p53-Alexa Fluor 488 (Cell Signaling Technology) and anti-PUMA (Abcam), washed twice, and resuspended in Perm buffer before analysis. Goat anti-rabbit IgG-PE (Santa Cruz Biotechnology, Inc.) was used with anti-PUMA antibody. For apoptosis assays, cells were resuspended in Annexin V buffer and stained with Annexin V-FITC for 20 min in the dark, according to manufacturer’s protocol (BD Bioscience). Propidium iodide, 1 µg/mL, was added to the cells immediately before analysis. Flow cytometry was performed on a BD LSRII flow cytometer (BD Bioscience), and the data were analyzed using FlowJo software version 7.6.4 (Tree Star, Inc.).
p53 and PUMA Immunofluorescence.
For RPS14-deficient erythroblasts, cells were cytospun, fixed with 4% (vol/vol) paraformaldehyde in PBS for 10 min, and then washed with PBS. The cells were permeabilized for 10 min with 0.5% Triton X-100 in PBS, blocked for 1 h with 3% BSA/0.1%Triton X-100 (vol/vol) in PBS, and incubated overnight with anti-p53-Alexa Fluor 488 (Santa Cruz Biotechnology, Inc.) and anti-PUMA antibody (EP512Y; Novus Biologicals). Cells were then washed with PBS and incubated with Alexa Fluor 555 goat anti-rabbit IgG (Life Technologies) for 1 h at room temperature. After they were washed with PBS, cells were mounted in ProLong Gold Antifade Reagent with 4,6 diamino-2-phenylindole (DAPI) (Life Technologies Corporation). p53 and PUMA expression were assessed by a Zeiss AxioSkop fluorescent microscope (Zeiss) equipped with a digital camera (Hamamatsu).
For MDS erythroid progenitors, cells were cytospun, fixed with prewarmed Cytofix (BD Biosciences) for 10 min at 37 °C, and stored at −20 °C. Samples were permeabilized for 5 min with 0.5% Triton X-100 in PBS, blocked for 4 h with 3% BSA/5% normal goat serum/0.1%Triton X-100 (vol/vol) in PBS, and incubated overnight with anti-p53-Alexa Fluor 488 (Santa Cruz Biotechnology, Inc.). Cells were covered with ProLong Gold Antifade Reagent with DAPI. p53 expression was assessed by confocal microscopy using a Leica TCS SP5 AOBS Laser Scanning Confocal microscope (Leica Microsystems). Images were analyzed with Definiens Developer version 1.5 (Definiens AG). Nuclear and cytoplasmic regions were segmented using an auto-threshold segmentation algorithm on the DAPI and Alexa Fluor 488 stains, and mean FI values for p53 staining were extracted from these regions.
TP53 Sequencing.
TP53 sequencing in 13 MDS patients and 5 controls is detailed in SI Materials and Methods.
Two-Stage Erythroid Colony Formation Assay.
For the two-stage colony formation assay, CD34+ cells were isolated and expanded as described earlier. Four to five replicates were performed per patient and per condition with 50,000 cells/mL in MethoCult (Stemcell Technologies) supplemented with 3 U/mL erythropoietin and 1% (vol/vol) penicillin-streptomycin. CFU-E were counted on day 7. BFU-E, colony-forming units-granulocyte-macrophage, and colony-forming units-erythroid-macrophage-megakaryocyte were counted on day 14.
Fluorescence in Situ Hybridization.
Cytospin preparations were fixed with 100% (vol/vol) ethanol for 5 min, dried at 65 °C for 4 h, and then stored at −20 °C. Cells were treated with 0.005% (wt/vol) pepsin solution for 10 min, followed by dehydration with 70%, 85%, and 100% (vol/vol) ethanol for 2 min each. Hybridization was performed with 10 µL of the EGR1/D5S23, D5S721 probe (Cytocell), a coverslip was placed, and the slides were sealed with rubber cement. The specimens were subjected to denaturation at 75 °C for 3 min and hybridized at 37 °C for 16 h. The slides were washed in 0.4× saline-sodium citrate at pH 7.2 and then counterstained with DAPI. Results were analyzed on a Leica DM 5000B fluorescent microscope. At least 100 cells were scored for each experimental group.
Immunohistochemical Staining.
Paraffin-embedded bone marrow biopsy sections (4 µm thick) from lenalidomide-resistant MDS patients were evaluated for p53 and spectrin expression before and after combined treatment with lenalidomide and dexamethasone, as previously described (11). For details, see SI Materials and Methods. All slides were reviewed by a hematopathologist (L.Z.). Five hundred cells per sample were counted.
Dexamethasone/Lenalidomide Treatment.
A pilot study with eight del(5q)MDS patients, (IPSS: INT-1) who were transfusion-dependent after development of lenalidomide resistance, were treated with dexamethasone 20 mg weekly and lenalidomide 5–10 mg/d, depending on the current adjusted dose, for at least 16 wk. Four of these patients were part of the in vitro studies, and four of them were exclusively used in this trial. The primary endpoint was transfusion independence according to International Working Group criteria (26). Secondary assessments included cytogenetic response and cellular immunohistochemical staining for p53 and spectrin in bone marrow biopsies from patients at baseline, at time of transfusion independence response, and at treatment failure.
Statistical Analyses.
Values are expressed as means ± SE. Comparisons between control oligonucleotide and cenersen-treated groups were performed with Wilcoxon signed-rank test. Comparison between bone marrow biopsies of treated patients was determined by Student t test. Growth comparisons of RPS14-deficient erythroblasts (sh41, sh42) and the scramble control over time were tested with two-way ANOVA. Pearson correlation coefficient was used to relate p53 expression and colony formation capacity in MDS patients. P values < 0.05 are considered statistically significant.
Supplementary Material
Acknowledgments
We thank Rasa Hamilton (H. Lee Moffitt Cancer Center and Research Institute) for editorial assistance. Financial support was provided by Leukaemia & Lymphoma Research of the United Kingdom (to J.B.), by a grant from Eleos, Inc. (to to A.F.L.), and by the National Cancer Institute/National Institutes of Health Grant 5R01 CA-13107604
Footnotes
Conflict of interest statement: L.J.S. has equity ownership in Smith Holdings, which owns rights to the drug involved in this study. F.J.D. has employment, equity ownership, and membership on the board of directors or advisory committees in Eleos, Inc. R.A.W. is a consultant for Novartis, Celgene Corporation, Janssen, and Alexion. A.F.L. is a consultant for Celgene Corporation.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311055110/-/DCSupplemental.
References
- 1.Nimer SD. Myelodysplastic syndromes. Blood. 2008;111(10):4841–4851. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- 2.List A, et al. Myelodysplastic Syndrome-003 Study Investigators Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 3.Giagounidis AA, et al. Clinical, morphological, cytogenetic, and prognostic features of patients with myelodysplastic syndromes and del(5q) including band q31. Leukemia. 2004;18(1):113–119. doi: 10.1038/sj.leu.2403189. [DOI] [PubMed] [Google Scholar]
- 4.Giagounidis AA, Germing U, Aul C. Biological and prognostic significance of chromosome 5q deletions in myeloid malignancies. Clin Cancer Res. 2006;12(1):5–10. doi: 10.1158/1078-0432.CCR-05-1437. [DOI] [PubMed] [Google Scholar]
- 5.Boultwood J, et al. Narrowing and genomic annotation of the commonly deleted region of the 5q- syndrome. Blood. 2002;99(12):4638–4641. doi: 10.1182/blood.v99.12.4638. [DOI] [PubMed] [Google Scholar]
- 6.Dutt S, et al. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011;117(9):2567–2576. doi: 10.1182/blood-2010-07-295238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell. 2003;3(6):577–587. doi: 10.1016/s1535-6108(03)00134-x. [DOI] [PubMed] [Google Scholar]
- 8.Barlow JL, et al. New insights into 5q- syndrome as a ribosomopathy. Cell Cycle. 2010;9(21):4286–4293. doi: 10.4161/cc.9.21.13742. [DOI] [PubMed] [Google Scholar]
- 9.List AF. New therapeutics for myelodysplastic syndromes. Leuk Res. 2012;36(12):1470–1474. doi: 10.1016/j.leukres.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Wei S, et al. A critical role for phosphatase haplodeficiency in the selective suppression of deletion 5q MDS by lenalidomide. Proc Natl Acad Sci USA. 2009;106(31):12974–12979. doi: 10.1073/pnas.0811267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei S, et al. Lenalidomide promotes p53 degradation by inhibiting MDM2 auto-ubiquitination in myelodysplastic syndrome with chromosome 5q deletion. Oncogene. 2013;32(9):1110–1120. doi: 10.1038/onc.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dias N, Stein CA. Potential roles of antisense oligonucleotides in cancer therapy. The example of Bcl-2 antisense oligonucleotides. Eur J Pharm Biopharm. 2002;54(3):263–269. doi: 10.1016/s0939-6411(02)00060-7. [DOI] [PubMed] [Google Scholar]
- 13.Bennett CF, Swayze EE. RNA targeting therapeutics: Molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 14.Sharp JG, et al. Oligonucleotide enhanced cytotoxicity of Idarubicin for lymphoma cells. Leuk Lymphoma. 2001;42(3):417–427. doi: 10.3109/10428190109064599. [DOI] [PubMed] [Google Scholar]
- 15.Bishop MR, et al. Phase I trial of an antisense oligonucleotide OL(1)p53 in hematologic malignancies. J Clin Oncol. 1996;14(4):1320–1326. doi: 10.1200/JCO.1996.14.4.1320. [DOI] [PubMed] [Google Scholar]
- 16.Cortes J, et al. Phase 2 randomized study of p53 antisense oligonucleotide (cenersen) plus idarubicin with or without cytarabine in refractory and relapsed acute myeloid leukemia. Cancer. 2012;118(2):418–427. doi: 10.1002/cncr.26292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanasa MC, et al. Phase II study of cenersen, an antisense inhibitor of p53, in combination with fludarabine, cyclophosphamide and rituximab for high-risk chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(2):218–224. doi: 10.3109/10428194.2011.610012. [DOI] [PubMed] [Google Scholar]
- 18.Ganguli G, Back J, Sengupta S, Wasylyk B. The p53 tumour suppressor inhibits glucocorticoid-induced proliferation of erythroid progenitors. EMBO Rep. 2002;3(6):569–574. doi: 10.1093/embo-reports/kvf114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trainor CD, Mas C, Archambault P, Di Lello P, Omichinski JG. GATA-1 associates with and inhibits p53. Blood. 2009;114(1):165–173. doi: 10.1182/blood-2008-10-180489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narla A, et al. Dexamethasone and lenalidomide have distinct functional effects on erythropoiesis. Blood. 2011;118(8):2296–2304. doi: 10.1182/blood-2010-11-318543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonova KI, et al. A small molecule inhibitor of p53 stimulates amplification of hematopoietic stem cells but does not promote tumor development in mice. Cell Cycle. 2010;9(7):1434–1443. doi: 10.4161/cc.9.7.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jädersten M, et al. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol. 2011;29(15):1971–1979. doi: 10.1200/JCO.2010.31.8576. [DOI] [PubMed] [Google Scholar]
- 23.Kulasekararaj AG, et al. TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5, and correlate with adverse prognosis. Br J Haematol. 2013;160(5):660–672. doi: 10.1111/bjh.12203. [DOI] [PubMed] [Google Scholar]
- 24.Bally CAL, et al. Incidence and Prognostic Value of TP53 Mutations in Lower Risk MDS with Del 5q. Blood. 2012;120:A1706. [Google Scholar]
- 25.Yip BH, et al. Effects of L-leucine in 5q- syndrome and other RPS14-deficient erythroblasts. Leukemia. 2012;26(9):2154–2158. doi: 10.1038/leu.2012.82. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.