Significance
If treatments with small-molecule trkB agonists, especially systemically, proved effective in enhancing axon regeneration in injured peripheral nerves, such a result could form the basis for the development of a unique treatment strategy for peripheral nerve injuries.
Abstract
Treatments with two-small molecule tropomyosin receptor kinase B (trkB) ligands, 7,8 dihydroxyflavone (7,8 DHF) and deoxygedunin, were evaluated for their ability to promote the regeneration of cut axons in injured peripheral nerves in mice in which sensory and motor axons are marked by YFP. Peripheral nerves were cut and repaired with grafts from strain-matched, nonfluorescent donors and secured in place with fibrin glue. Lengths of profiles of regenerating YFP+ axons were measured 2 wk later from confocal images. Axon regeneration was enhanced when the fibrin glue contained dilutions of 500-nM solution of either small-molecule trkB agonist. In mice in which the neurotrophin receptor trkB is knocked out selectively in neurons, axon regeneration is very weak, and topical treatment with 7,8 DHF had no effect on axon regeneration. Similar treatments with deoxygedunin had only a modest effect. In conditional BDNF knockout mice, topical treatments with either 7,8 DHF or deoxygedunin resulted in a reversal of the poor regeneration found in controls and produced significant enhancement of regeneration. In WT mice treated with 2 wk of daily i.p. injections of either 7,8 DHF or deoxygedunin (5 mg/kg), regenerating axon profiles were nearly twice as long as in controls. Restoration of direct muscle responses evoked by sciatic nerve stimulation to pretransection levels over an 8-wk survival period was found only in the treated mice. Treatments with either small-molecule trkB agonist enhanced axon regeneration and muscle reinnervation after peripheral nerve injuries.
Despite the capacity for axons in injured peripheral nerves to regenerate and reinnervate targets in the periphery, functional recovery after peripheral nerve injury is poor (1, 2). A major factor contributing to these poor functional outcomes is the need for regenerating axons to elongate in a pathway in the distal segment of a cut nerve to reach their targets (3). The regenerating axons enter this pathway over a protracted period (4), and once in the pathway, their elongation is dependent upon a favorable balance of growth-promoting and growth-inhibiting molecules (5). The availability of growth-promoting molecules can be limiting. Their production by supporting (Schwann) cells in the distal nerve segment becomes compromised over time, which has a negative impact on functional recovery when repair of damaged nerves is delayed or when regeneration has to take place over long distances, as it often does in humans (6, 7).
One of the key growth-promoting molecules in the pathway used by regenerating axons in peripheral nerves is the neurotrophin brain derived neurotrophic factor (BDNF). In the pathway, it is produced by transformed Schwann cells and stimulates neurite elongation by binding to tropomyosin receptor kinase B (trkB) receptors on the regenerating axons (8, 9). Axon regeneration in cut nerves is severely compromised if the availability of BDNF to regenerating axons is reduced, such as by treatments with function-blocking antibodies (10) or by genetic manipulation of BDNF expression in Schwann cells (9). Local treatment of repaired nerves with recombinant human BDNF (rhBDNF) promotes enhanced early regeneration in mice (9, 11).
On the basis of these findings, one might expect that administration of rhBDNF might form an effective treatment strategy to promote the regeneration of axons in cut nerves. Unfortunately, rhBDNF is a relatively large molecule (13 Kd), and it does not cross the blood–brain or blood–nerve barriers effectively (12). In addition, its biological half-life is relatively short (1–3 h) (13, 14). When it has been used experimentally, it has been given in very large doses (15), which is both expensive and runs the risk of untoward side effects or toxicities (16).
Because of these limitations, small molecules that mimic the action of BDNF have been sought. Two molecules have emerged from a screen for their cell survival promotion and cell-signaling properties. Both 7,8 dihydroxyflavone (7,8 DHF) (17) and deoxygedunin (18) have been shown to function as trkB agonists in vitro. Both molecules have been used to promote BDNF-dependent activities in vivo (18–20). Because they are small enough to pass the blood–brain (or blood–nerve) barrier, they have been used systemically, even by oral administration (18). One or both of these molecules have been applied to the study of a wide range of different neurological model systems, including learning (21), memory loss (22), excitotoxic stress (23), depression (24, 25), fear conditioning (19), neuromuscular synaptic transmission (26), and age-related synaptic plasticity (27). If treatments with these molecules, especially systemically, proved effective in enhancing axon regeneration in injured peripheral nerves, such a result could form the basis for the development of a novel treatment strategy for peripheral nerve injuries. The objective of this study was to evaluate the effectiveness of treating injured peripheral nerves with these small molecules on axon regeneration. We show here that treatments with 7,8 DHF and deoxygedunin promote enhanced axon regeneration in cut peripheral nerves, and systemic treatments result in more extensive muscle reinnervation. We also show that this enhancement is produced by signaling through neuronal trkB receptors. A preliminary report of some of these data has been made.†
Results
Topical Treatments with 7,8 DHF and Deoxygedunin Enhance Axon Regeneration.
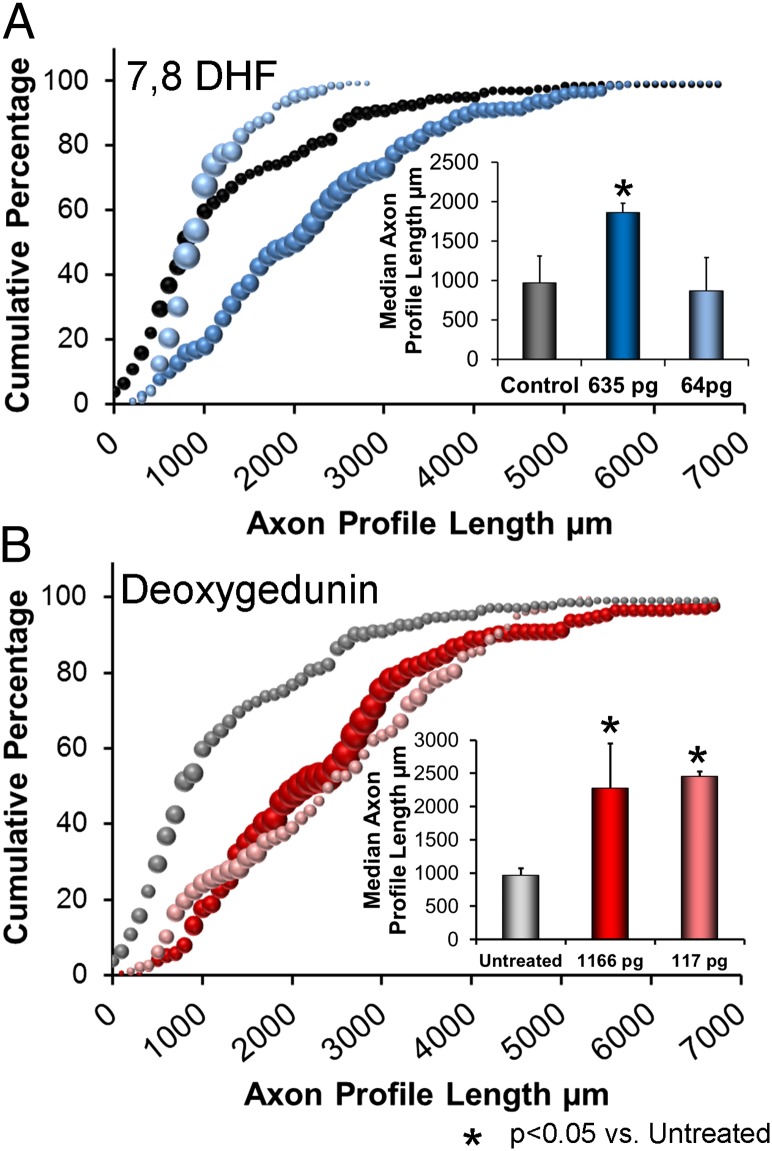
Topical treatment with very small quantities (635 pg or 64 pg) of 7,8 DHF resulted in a marked and dose-dependent enhancement of axon regeneration. At the larger of the two doses applied, the distribution of regenerating axon profile lengths was shifted markedly to the right of the distribution in untreated animals (Fig. 1A). The average median axon profile length in this group was nearly twice that observed in untreated animals (Fig. 1A, Inset), and this increase was statistically significant [Fisher’s least significant differences (LSD), P < 0.001]. In contrast, at the lower dose of 7,8 DHF, no shift in the distributions of axon profile lengths relative to untreated mice was noted, and no significant increase in average median length was observed (P = 0.895).
Fig. 1.
(A) Distributions of axon profile lengths measured in optical sections through nerve grafts used to repair cut mouse nerves are shown as cumulative histograms. Each symbol represents the average of four nerves, and the size of the symbol is proportional to the SEM for that axon profile length. (Inset) Average median axon profile lengths (+SEM) for the three groups (n = four nerves for each). (B) Distributions of axon profile lengths measured in optical sections through nerve grafts, as in A. (Inset) Average median axon profile lengths (+SEM) for the three groups (n = four nerves for each).
A similar analysis was conducted using two different dilutions of a 500-nM solution of deoxygedunin in the manufacture of the fibrin glue. These results are summarized in Fig. 1B. At both concentrations of deoxygedunin, the distributions of axon profile lengths measured in grafts used to repair the cut nerves were shifted to the right of the distribution for untreated mice (Fig. 1B). Average median axon profile length was more than twice that of untreated mice for both doses (Fig. 1B, Inset). These differences were statistically significant (LSD, P < 0.004 for the larger dose and P < 10−9 for the lower dose).
Effects of 7,8 DHF Require Neuronal trkB Signaling.
It is well established that 7,8 DHF can bind to the trkB receptor and activate downstream signaling elements in a manner similar to that produce by BDNF (17). One might then hypothesize that the effect of topical treatments just described might be due to this same trkB activation. To test this hypothesis, we repeated the experiments using topical treatments with 7,8 DHF in mice in which the trkB receptor was knocked out.
We generated neuron-specific trkB knockout (SLICK::trkBf/f) mice, in which axons containing YFP are null for trkB (SI Materials and Methods). Cut peripheral nerves were repaired either with grafts from strain-matched WT mice, in which transformed Schwann cells in the grafts are expected to produce normal amounts of BDNF, or from mice in which the gene for BDNF is knocked out selectively in Schwann cells (CNTF-Cre::BDNFf/f) (9). Both of these groups were considered controls, because neither was treated with either 7,8 DHF or deoxygedunin.
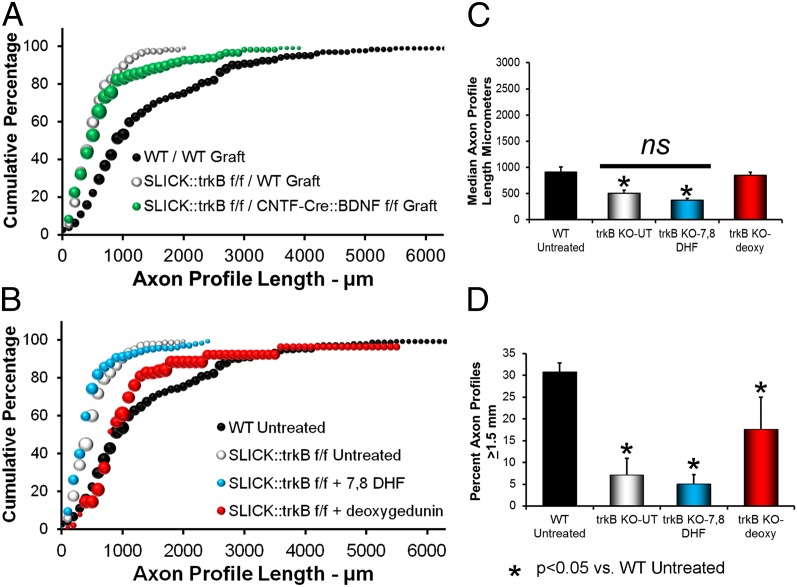
Whether the graft used to repair the cut nerves contained BDNF or was null for the gene for BDNF, when used to repair cut nerves in conditional trkB knockout mice, axon regeneration was impaired dramatically. The distributions of axon profile lengths measured in these grafts were both shifted markedly to the left of WT mice whose nerves had been repaired with grafts from WT donors (Fig. 2A), and median lengths of axon profiles measured in grafts from WT mice were significantly (LSD, P < 0.005) shorter than in controls (Fig. 2C). Thus, as suggested by others (28), successful axon regeneration in cut peripheral nerves requires functioning trkB receptors in the regenerating axons.
Fig. 2.
(A) Distribution of axon profile lengths in neuron-specific trkB knockout mice (SLICK::trkBf/f) whose cut nerves had been repaired either with a nerve graft from a WT mouse or a segment of the same nerve obtained from a mouse in which the gene for BDNF had been knocked out in Schwann cells (CNTF-Cre::BDNFf/f). (B) Distribution of axon profile lengths in SLICK::trkBf/f whose cut nerves had been repaired with a nerve graft from a WT mouse and were treated topically with 7,8 DHF or deoxygedunin. (C) Average median axon profile lengths (+SEM) for the groups shown in B. (D) Average (+SEM) percentage of axon profiles that had elongated at least 1.5 mm during the 2-wk survival period in the four groups of mice in B.
If these SLICK::trkBf/f mice whose cut nerves were repaired with grafts from WT donors were treated topically with the larger dose of 7,8 DHF at the time of repair of the cut nerves, no significant effect on axon regeneration was observed. The distribution of axon profile lengths was shifted markedly to the left of that of controls (Fig. 2B, blue symbols) and was nearly identical to that derived from untreated SLICK::trkBf/f mice (Fig. 2B, white symbols). Average median axon profile lengths were significantly smaller (P < 0.029) than found in WT mice whose nerves had been repaired with grafts from WT donors and not significantly different (P = 0.67) from untreated SLICK::trkBf/f mice. Thus, the enhancement of axon regeneration in cut peripheral nerves produced by topical treatment with 7,8 DHF depends on trkB in the regenerating axons.
When we performed the same experiments but applied deoxygedunin in the fibrin glue used to repair the cut nerve, a slightly different outcome was obtained. The distribution of shorter axon profile lengths in SLICK::trkBf/f mice whose cut nerves were repaired with grafts from WT donors and were treated topically with the larger dose of deoxygedunin at the time of repair of the cut nerves (Fig. 2B, red symbols) was nearly identical to that of the WT controls (Fig. 2B, black symbols). Median axon profile lengths were not significantly different (LSD, P = 0.599) from those of untreated WT mice (Fig. 2C). However, median axon profile lengths were significantly shorter than found in WT mice treated with either dose of deoxygedunin (P < 0.05), suggesting that the effects of treatment with this small molecule were attenuated in trkB knockout mice. The distribution of longer axon profile lengths was not similar to that of WT controls. We determined the proportion of axon profiles that had extended to at least 1.5 mm during the 2-wk survival period as an arbitrary measure of long regenerating axons. This proportion was significantly smaller than that found in untreated WT mice (P < 0.044) or WT mice treated with deoxygedunin (P < 0.007) and not significantly different from that in untreated SLICK::trkBf/f mice (P = 0.126) or the proportion found in SLICK::trkBf/f mice treated topically with 7,8 DHF (P = 0.339) (Fig. 2D).
Topical Treatment with 7,8 DHF or Deoxygedunin Enhances Axon Regeneration in the Absence of Native BDNF.
Even though the effectiveness of topical 7,8 DHF treatment requires signaling through neuronal trkB receptors, it is not yet clear whether this signaling is direct or indirect. Effective treatments could simply result in stimulation of BNDF release by cells in the host mouse and not involve direct effects of 7,8 DHF on the trkB receptors. We investigated this possibility using conditional BDNF knockout mice. Using the Cre-lox system, we generated mice in which the gene for BDNF is knocked out systemically in adults after treatment with the synthetic estrogen tamoxifen (Tam-Cre::BDNFf/f). Some of these mice were back-crossed onto the thy-1-YFP-H strain used in the experiments described above, resulting in systemic BDNF knockout mice that also contained YFP+ axons in their peripheral nerves (thy-1-YFP-H::Tam-Cre::BDNFf/f). Nerves in thy-1-YFP-H::Tam-Cre::BDNFf/f mice were cut and repaired with grafts from systemic BDNF knockout mice (Tam-Cre::BDNFf/f). These mice were either untreated or treated with topical 7,8 DHF or topical deoxygedunin at a dose that was effective in WT mice.
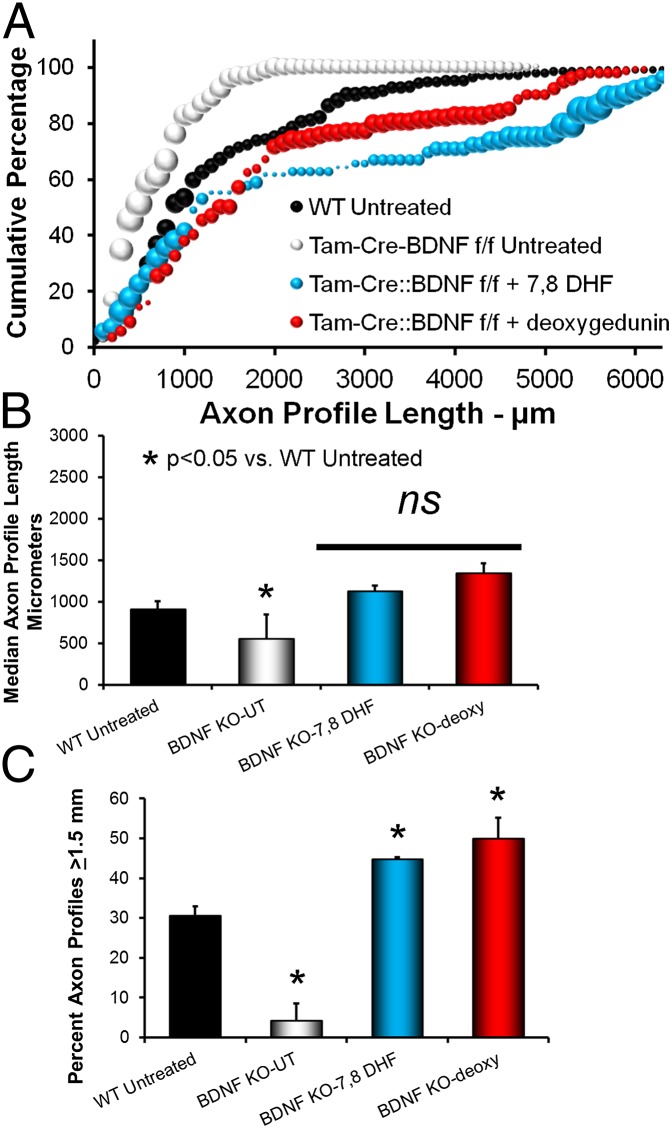
Axon regeneration in cut nerves of systemic BDNF knockout mice was very poor. The distribution of axon profile lengths was shifted to the left of that of WT mice whose nerves had been repaired with grafts from WT donors (Fig. 3A, white symbols), and average median axon profile lengths were significantly (LSD, P < 0.017) smaller (Fig. 3B). In contrast, if cut nerves in these mice were treated with 7,8 DHF the distribution of axon profile lengths was shifted to the right of that of WT mice whose nerves had been repaired with grafts from WT donors (Fig. 3A, blue symbols), especially at longer axon profile lengths (Fig. 3C). Average median axon profile length was increased significantly relative to untreated BDNF knockout mice (LSD, P < 0.03) but not relative to WT untreated controls (P = 0.53) (Fig. 3B). The proportion of axon profile lengths at least 1.5 mm long was increased significantly in BDNF knockout mice treated topically with 7,8 DHF relative to untreated BDNF knockout mice (P < 10−6) and relative to untreated WT mice (P < 0.01) (Fig. 3C), with nearly half of all regenerating axons reaching this arbitrary benchmark. Thus, treatment with 7,8 DHF reversed the effects of knocking out the BDNF gene and promoted enhanced regeneration of cut axons.
Fig. 3.
(A) Distribution of axon profile lengths in systemic BDNF knockout mice (Tam-Cre::BDNFf/f) whose cut nerves had been repaired with a nerve graft from a systemic BDNF knockout mouse and left untreated, were treated topically with 7,8 DHF, or were treated topically with deoxygedunin. (B) Average median axon profile lengths (+SEM) for the four groups shown in A. (C) Average (+SEM) percentage of axon profiles that had elongated at least 1.5 mm during the 2-wk survival period in the four groups of mice in A.
Effects of treatments of BDNF knockout mice with deoxygedunin were similar. A shift in the distribution of axon profile lengths relative to untreated WT controls was found (Fig. 3A, red symbols). Median axon profile lengths were significantly longer in BDNF knockout mice treated with deoxygedunin than in untreated BDNF knockout mice (LSD, P < 0.040) and not significantly different from that in WT controls (P = 0.19) (Fig. 3B). The proportions of all axon profile lengths ≥1.5 mm were significantly larger in deoxygedunin-treated BDNF knockout mice than in either untreated BDNF knockout mice (LSD, P < 0.00084) or WT controls (P < 0.03) (Fig. 3C). Thus, treatments with deoxygedunin reversed the effects of knocking out the BDNF gene and promoted enhanced regeneration of cut axons.
Systemic Treatment with 7,8 DHF Enhances Axon Regeneration and Muscle Reinnervation After Nerve Transection.
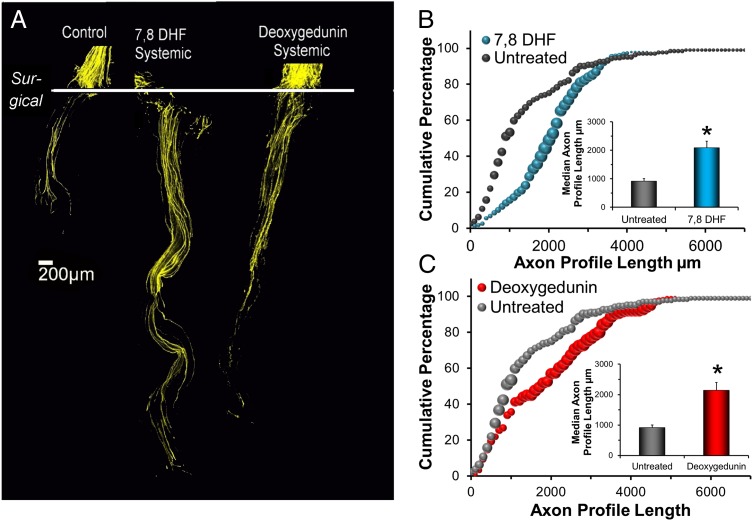
We treated thy-1-YFP-H mice with daily i.p. injections of either 7,8 DHF or deoxygedunin (5 mg/kg) for 2 wk after transection and repair of their common fibular or tibial nerves. Images of nerves harvested from these mice are shown in Fig. 4A. For both 7,8 DHF and deoxygedunin treatments, regenerating axons profiles were strikingly longer than found in untreated controls. The distributions of YFP+ axon profile lengths measured in the grafts used to repair cut nerves were shifted markedly to the right of the distributions found in untreated WT mice whose nerves had been repaired with grafts from WT donors (Fig. 4 B and C). These shifts were reflected in a marked increase in average median axon profile lengths (Fig. 4 B and C, Insets). For both small-molecule trkB agonists, systemic treatments resulted in axon profiles that were more than twice as long as in untreated controls. These increases were statistically significant (7,8 DHF, P < 0.000026; deoxygedunin, P < 0.001). Thus, systemic treatments with either small-molecule trkB agonist were as effective as topical application in enhancing the elongation of regenerating axons.
Fig. 4.
(A) Branches of the sciatic nerve of thy-1-YFP-H mice were cut and repaired with grafts of the same nerve obtained from WT, non-YFP-expressing littermates and were treated daily by i.p. injections of either 7,8 DHF or deoxygedunin or were untreated (Control). Each image is a compilation of stitched images from single 10-μm-thick optical sections through the graft. The proximal cut nerve segments are aligned at the top of each panel. The horizontal line marks the site of surgical repair. (B and C) Distributions of axon profile lengths in these mice. Data from systemically treated mice are compared with similar data from untreated mice, the same data as shown in Figs. 1–3. (Insets) Average median axon profile lengths (+SEM).
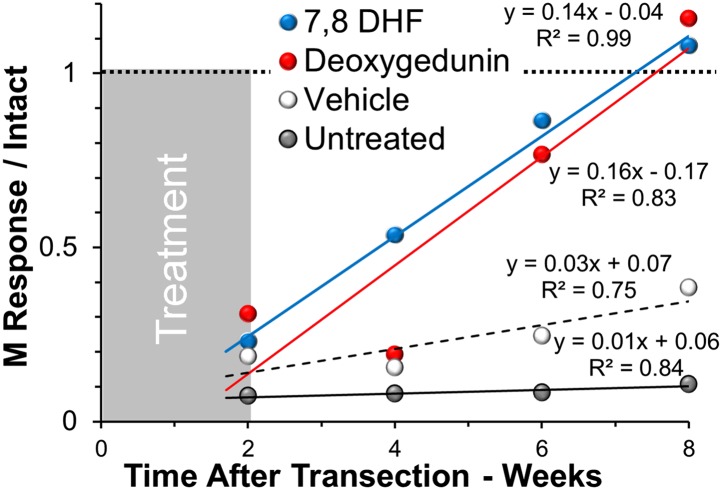
To evaluate a functional outcome of small-molecule trkB agonist treatment, direct muscle (M) responses were evoked from the gastrocnemius muscles in response to supramaximal stimulation of the sciatic nerve proximal to the original injury site in the same mice at the end of the treatment period (2 wk after nerve transection) and again at 4, 6, and 8 wk after nerve transection. Two weeks after sciatic nerve transection and repair, small amplitude M responses were noted in all animals studied (Fig. 5). No significant differences were found between mice in the different treatment groups. In untreated control mice, only very modest increases in evoked potential amplitude were noted during the remainder of the study period. A similar outcome was found in mice treated with vehicle. Slopes of the regression lines for these two groups were not significantly different (P = 0.19). In contrast, in animals treated systemically with either 7,8 DHF or deoxygedunin, a marked increase in M response amplitude was noted over time. By 8 wk after injury, M response amplitudes in muscles from treated animals had been restored to pretransection levels, and their amplitudes were nearly 10 times greater than in untreated mice and more than threefold larger than found in vehicle-treated mice. Slopes of the regression lines for mice treated with either 7,8 DHF or deoxygedunin were significantly greater than those for untreated mice (P < 0.0002 and P < 0.004) and vehicle-treated mice (P < 0.02 and P < 0.04). Thus, the effects of brief treatment with two trkB agonists promoted not only enhanced axon regeneration but increased muscle reinnervation that continued for at least 6 weeks after the end of treatment.
Fig. 5.
Average M response amplitudes recorded from the gastrocnemius muscles of mice treated systemically with 7,8 DHF, deoxygedunin, or vehicle, or untreated are shown at different times after nerve transection and repair. All data are expressed as a proportion of the maximal M response amplitude recorded from intact muscles. Slopes of linear least-square regression lines and correlation coefficients for each are shown. The gray area to the left of the graph is used to indicate the period of treatment. The horizontal dashed line at 1 represents the M response amplitude recorded before nerve transection.
Treatments with Small-Molecule trkB Agonists Affects Numbers of Regenerating Axon Profiles.
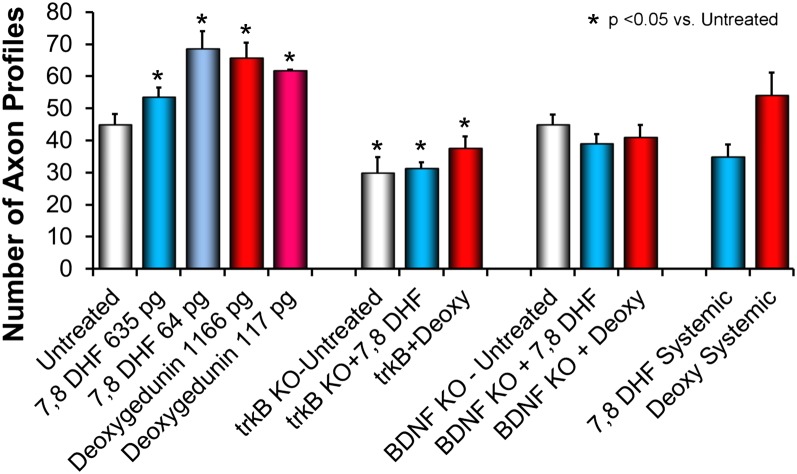
The mean (+SEM) numbers of axon profiles encountered in grafts studied in each of the treatment groups described above are shown in Fig. 6. The mean number of regenerating axon profiles was increased in nerve grafts treated locally with either 7,8 DHF or deoxygedunin, relative to untreated controls. No significant difference was found among these four groups of mice. In contrast, the number of regenerating axon profiles was reduced significantly, relative to untreated controls, in all mice in which the gene for trkB had been knocked out in neurons. No significant difference was found among these three groups of mice. No significant difference in axon profile number was found, relative to untreated controls, either in BDNF knockout mice or in WT mice treated systemically with trkB ligands. All of these results are summarized in Table 1.
Fig. 6.
Mean (±SEM) numbers of regenerating YFP+ axon profiles measured in nerve grafts in the different treatment groups.
Table 1.
Summary of results
| Treatment | Untreated (topical)/ DMSO (systemic) | 7,8 DHF | Deoxygedunin | |||
| 635 pg | 64 pg | 1,166 pg | 167 pg | |||
| Topical | ||||||
| WT mice | ||||||
| Axon profile length | + | NS | + | + | ||
| Axon profile number | + | + | + | + | ||
| trkB KO | ||||||
| Axon profile length | − | − | − | |||
| Axon profile number | − | − | − | |||
| BDNF KO | ||||||
| Axon profile length | − | + | + | |||
| Axon profile number | NS | NS | NS | |||
| Systemic | ||||||
| WT mice | ||||||
| Axon profile length | NS | + | + | |||
| Axon profile number | NS | NS | ||||
| M response | NS | + | + | |||
+, increased relative to untreated WT controls; −, decreased relative to untreated WT controls; NS, not significantly different from untreated WT controls.
Discussion
Over the past three decades, refinements of technique and materials for the surgical repair of cut nerves have been impressive, but functional recovery after nerve injuries remains poor (3). The slowness and incompleteness of axon regeneration is often blamed for these poor functional outcomes (28). In considering the cell biology of axon regeneration, BDNF has emerged as an important molecule that promotes the elongation of regenerating axons, and some methods have been developed to stimulate axon regeneration that rely on the increased production of BDNF and/or its receptor, trkB (9, 29, 30). However, the potential for treatments of injured nerves with recombinant human BDNF is limited because it is a large molecule that will not cross the blood–brain or blood–nerve barriers well, because its biological half-life is short and because the high doses that might be required would increase the likelihood of toxicity or untoward side effects.
Using a cell-based screening method, Ye and colleagues identified two small-molecule trkB agonists, 7,8 DHF and deoxygedunin, from a library of natural products (17, 18). In experiments in vitro, they showed that both molecules bind to the trkB receptor with high affinity, produce appropriate tyrosine phosphorylation, and activate the same cascade of downstream signaling components as BDNF. When used in vivo these molecules have been shown to have effects similar to those of BDNF in a number of different model neuronal systems (Introduction). In all of these experiments, the doses of trkB agonists have been very small, and no observed toxic or side effects have yet been reported. Thus, 7,8 DHF and deoxygedunin are attractive as alternatives to rhBDNF to promote enhanced axon regeneration in cut peripheral nerves,
The main finding of this study is that both 7,8 DHF and deoxygedunin are potent promoters of axon regeneration in cut peripheral nerves. Topical application of either molecule to the site of surgical repair of cut nerves results in the elongation of regenerating axons during the first 2 wk after injury to at least twice the length found in control mice. The number of regenerating axon profiles encountered in these mice also was increased significantly. Although we cannot differentiate, on the basis of axon profile counts, whether this increase was the result of participation of more neurons in the regeneration process or simply a pronounced sprouting of regenerating axons, the effects of these topical treatments must be considered robust. The magnitude of this enhancement of axon regeneration was similar to that observed in mice, either with topical treatment using rhBDNF or neurotrophin-4/5 (11), or with brief electrical stimulation (31) or treadmill exercise (30), all of which share a common mechanism of increasing trkB signaling in the regenerating axons. Even though establishing complete dose–response relationships for these two molecules will have to await future study, it is important to note that very small doses of local application of small-molecule trkB agonists, in the picogram to low nanogram range, were sufficient to produce a robust and repeatable enhancement.
The most straightforward explanation for these effects would be that the small-molecule trkB agonists enhance axon regeneration in cut peripheral nerves by binding to neuronal trkB receptors. In this manner they would mimic the effects of endogenous BDNF released by transformed Schwann cells in the pathway used by regenerating axons (8, 9). However, other possible mechanisms might exist. When used in vivo, the small molecules could promote the synthesis and release of endogenous trkB ligands, or they could bind to receptors other than trkB to exert their effect.
We believe that in our experiments using knockout mice we have provided a compelling test of the cellular basis for the effects of the small-molecule trkB agonists. Axon regeneration in systemic BDNF knockout mice is very poor, but topical treatments with both small-molecule trkB agonists not only reversed the effect of knocking out the BDNF gene, but they resulted in significant enhancement of regeneration. No significant effect was found on the number of regenerating axon profiles observed. This enhancement did not depend on the availability of endogenous BDNF. Similarly, neither 7,8 DHF nor deoxygedunin was terribly effective in promoting axon regeneration in neuron-specific trkB knockout mice. Axon regeneration is very poor in these mice, and although a modest reduction of this effect was found after topical treatment with deoxygedunin, no significant enhancement of regeneration was noted. Significantly fewer regenerating axon profiles were encountered in trkB knockout mice, and treatments with small-molecule trkB agonists did not affect axon profile number. It is possible that deoxygedunin, which is more potent in this application than 7,8, DHF (compare lower doses in Fig. 1 A and B), could promote axon regeneration, albeit weakly, by some other mechanism than binding to the trkB receptor. Whether signaling of deoxygedunin through other receptors, either directly or indirectly in some sort of paracrine manner, explains its quite limited effect in selective trkB knockout mice is not clear at this time. More experimental results using this molecule are needed. The simplest explanation of the results of these experiments remains that both 7,8 DHF and deoxygedunin act primarily by binding to trkB receptors on regenerating neurites and promote their elongation.
In addition to these findings, we show here that systemic treatment with either small-molecule agonist is equally effective in promoting axon regeneration in cut nerves. Daily i.p. injection of relatively small amounts of either 7,8 DHF or deoxygedunin were as effective in promoting axon regeneration as topical application at the time of nerve repair. The increase in axon profile length is not accompanied by an increase in axon profile number, suggesting that the systemic treatments promoted axon elongation, but neither recruited more axons to regenerate nor promoted regenerative sprouting. Using functional outcome measures similar to those used clinically to evaluate nerve regeneration and muscle reinnervation, we found that brief systemic treatments with either small-molecule agonist produced striking effects on the restoration of muscle innervation that persisted for several weeks after the end of the treatments. Although it is clear that further studies are required, including studies in larger animal models, to explore the optimal conditions for treatments that might capitalize the translational potential of these molecules, we believe that the results presented above are an important and logical starting point for an effective treatment of peripheral nerve injuries.
Materials and Methods
Animals and Surgical Methods.
All experiments were approved by the Institutional Animal Care and Use Committee of Emory University and were in accordance with the Principles of the Use of Animals in Research of the Society for Neuroscience. For all anatomical experiments, one or both of the two terminal branches of the sciatic nerve was cut, under isoflurane anesthesia, and repaired by attaching a 10- to 12-mm-long segment of the same nerve, harvested from a strain-matched donor mouse, to the proximal segment of the nerve using fibrin glue (32). We chose not to attach the grafts to the distal nerve segments for two reasons. First, we wanted to eliminate the influence of distal nerve segment and peripheral target tissues from our study. We believe that this was especially important when we used knockout mice. Second, we have shown previously (9) that axon regeneration into these grafts is not affected by connection of the grafts to the distal segments of the cut. The host mice in anatomical experiments were animals in which YFP is expressed in a subset of axons in peripheral nerves under the control of the thy-1 promoter (33). We have shown that YFP is found in both motor and sensory axons in these mice (5, 31), and we assume that the sample of axons that express YFP are representative of all of the axons in the nerves. More details of the generation of knockout mice are given in SI Materials and Methods. For WT (C57B6) mice used in electrophysiological experiments, the sciatic nerve was cut in the midthigh and repaired by simple end-to-end anastomosis using plain fibrin glue. A total of 67 nerves from 46 mice of both sexes was studied.
Drug Treatments.
Deoxygedunin was obtained from Gaia Chemical Company (catalog no. L4250); 7,8 DHF was obtained from Tokyo Chemical Industry Co. (catalog no. D1916). In most experiments, 7,8 DHF or deoxygedunin was administered topically, in the fibrin glue used to secure the cut nerves to grafts. For control nerves nothing was added to the fibronectin component of the glue. For systemic treatments, nerves were cut and repaired, as described above, and the animals were treated with either 7,8 DHF, deoxygedunin, or a comparable volume of vehicle (10% DMSO) or were left untreated. Compounds were administered via daily i.p. injection, beginning at the time of surgery and extending for 2 wk. A single dose of each molecule was studied (5 mg/kg) because reports in the literature of in vivo use of these compounds reported these doses to be effective (19).
Tissue Harvesting and Analysis.
Methods used are similar to those described in our previous articles (5, 9). The lengths of YFP+ axon profiles were measured in confocal images of grafts harvested from killed mice, from their growth cones to the surgical repair site, using ImageJ software. Significance of differences in average median axon profile lengths between groups was evaluated using ANOVA. Because the omnibus test of significance from this ANOVA was significant (F12,41 = 11.59204, P < 10−6), post hoc paired comparisons (Fisher’s LSD) were performed. A similar analysis of differences in the mean number of YFP+ regenerating axons in each group was conducted. The results of ANOVA were significant (F12,41 = 6.00042, P < 0.000007), so that a post hoc paired testing was conducted. Significance was set at P < 0.05 in all cases.
For electrophysiological studies, evoked M responses were generated in the gastrocnemius muscles in response to stimulation of the sciatic nerve with a percutaneously placed needle electrode, as it emerges from the pelvis. Changes in average rectified voltages in a defined M response time window in the different treatment groups were compared over an 8-wk posttransection time period using multiple linear regression methods.
Supplementary Material
Acknowledgments
We thank Dr. Luis Parada for the gift of the floxed trkB mouse, Dr. James McNamara for his help in making these mice available to us, and Dr. Michael Sendtner for the gift of the floxed BDNF and CNTF-Cre mice used to generate the different BDNF knockout mice. This work was completed while supported by US Public Health Service Grant NS067012 (to A.W.E.). This research project was supported in part by the Emory University Integrated Cellular Imaging Microscopy Core.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
†English AW, Liu K, Wilhelm JC, Ye K (2011) Effects of treatments with small molecule trkB agonists on axon regeneration in cut peripheral nerves (Society for Neuroscience Abstracts, Washington, DC).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303646110/-/DCSupplemental.
References
- 1.Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18(7):397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Scholz T, et al. Peripheral nerve injuries: An international survey of current treatments and future perspectives. J Reconstr Microsurg. 2009;25(6):339–344. doi: 10.1055/s-0029-1215529. [DOI] [PubMed] [Google Scholar]
- 3.Brushart TM. Nerve Repair. New York: Oxford Univ Press; 2011. p. 463. [Google Scholar]
- 4.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20(7):2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groves ML, et al. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp Neurol. 2005;195(2):278–292. doi: 10.1016/j.expneurol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Höke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002;173(1):77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- 7.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14(1-2):67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 8.Funakoshi H, et al. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123(2):455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilhelm JC, et al. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci. 2012;32(14):5002–5009. doi: 10.1523/JNEUROSCI.1411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000;12(12):4171–4180. [PubMed] [Google Scholar]
- 11.English AW, Meador W, Carrasco DI. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005;21(10):2624–2634. doi: 10.1111/j.1460-9568.2005.04124.x. [DOI] [PubMed] [Google Scholar]
- 12.Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res. 1996;36(2):280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- 13.Kishino A, et al. Analysis of effects and pharmacokinetics of subcutaneously administered BDNF. Neuroreport. 2001;12(5):1067–1072. doi: 10.1097/00001756-200104170-00040. [DOI] [PubMed] [Google Scholar]
- 14.Soderquist RG, et al. PEGylation of brain-derived neurotrophic factor for preserved biological activity and enhanced spinal cord distribution. J Biomed Mater Res A. 2009;91(3):719–729. doi: 10.1002/jbm.a.32254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munson JB, Shelton DL, McMahon SB. Adult mammalian sensory and motor neurons: Roles of endogenous neurotrophins and rescue by exogenous neurotrophins after axotomy. J Neurosci. 1997;17(1):470–476. doi: 10.1523/JNEUROSCI.17-01-00470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci. 2002;5(Suppl):1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- 17.Jang SW, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang SW, et al. Deoxygedunin, a natural product with potent neurotrophic activity in mice. PLoS ONE. 2010;5(7):e11528. doi: 10.1371/journal.pone.0011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andero R, Ressler KJ. Fear extinction and BDNF: Translating animal models of PTSD to the clinic. Genes Brain Behav. 2012;11(5):503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, et al. A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect. J Med Chem. 2010;53:8274–8286. doi: 10.1021/jm101206p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi DC, Gourley SL, Ressler KJ. Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transcult Psychiatry. 2012;2:e205. doi: 10.1038/tp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devi L, Ohno M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37(2):434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta VK, You Y, Li JC, Klistorner A, Graham SL. Protective effects of 7,8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J Mol Neurosci. 2013;49(1):96–104. doi: 10.1007/s12031-012-9899-x. [DOI] [PubMed] [Google Scholar]
- 24.Blugeot A, et al. Vulnerability to depression: From brain neuroplasticity to identification of biomarkers. J Neurosci. 2011;31(36):12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, et al. Optimization of a small tropomyosin-related kinase B (TrkB) agonist 7,8-dihydroxyflavone active in mouse models of depression. J Med Chem. 2012;55(19):8524–8537. doi: 10.1021/jm301099x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve. 2012;45(2):274–276. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng Y, et al. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31(49):17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26(2):E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- 29.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12(12):4381–4390. [PubMed] [Google Scholar]
- 30.Sabatier MJ, Redmon N, Schwartz G, English AW. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211(2):489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.English AW, Schwartz G, Meador W, Sabatier MJ, Mulligan A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol. 2007;67(2):158–172. doi: 10.1002/dneu.20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacGillivray TE. Fibrin sealants and glues. J Card Surg. 2003;18(6):480–485. doi: 10.1046/j.0886-0440.2003.02073.x. [DOI] [PubMed] [Google Scholar]
- 33.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.