Significance
Mitochondria are dynamic organelles that constantly fuse and fragment while acting as central hubs of energy production, apoptosis regulation, and Ca++ signaling, therefore emerging as potential targets of pathogens. We previously showed that the foodborne bacterial pathogen Listeria monocytogenes interferes with the dynamics and function of the host cell mitochondrial network via the bacterial toxin listeriolysin O (LLO). In this study, we analyze the effects of LLO on key players known to be involved in mitochondrial dynamics and show that the fission protein dynamin-like protein 1 (Drp1) is not essential for LLO-induced fragmentation of the mitochondrial network, whereas the endoplasmic reticulum (ER) plays an important role, suggesting a unique Drp1-independent and ER-dependent mechanism that is different from the canonical fission machinery.
Keywords: mitochondrial dynamics, live cell imaging, actin
Abstract
We recently showed that infection by Listeria monocytogenes causes mitochondrial network fragmentation through the secreted pore-forming toxin listeriolysin O (LLO). Here, we examine factors involved in canonical fusion and fission. Strikingly, LLO-induced mitochondrial fragmentation does not require the traditional fission machinery, as Drp1 oligomers are absent from fragmented mitochondria following Listeria infection or LLO treatment, as the dynamin-like protein 1 (Drp1) receptor Mff is rapidly degraded, and as fragmentation proceeds efficiently in cells with impaired Drp1 function. LLO does not cause processing of the fusion protein optic atrophy protein 1 (Opa1), despite inducing a decrease in the mitochondrial membrane potential, suggesting a unique Drp1- and Opa1-independent fission mechanism distinct from that triggered by uncouplers or the apoptosis inducer staurosporine. We show that the ER marks LLO-induced mitochondrial fragmentation sites even in the absence of functional Drp1, demonstrating that the ER activity in regulating mitochondrial fission can be induced by exogenous agents and that the ER appears to regulate fission by a mechanism independent of the canonical mitochondrial fission machinery.
Mitochondria are essential organelles that perform a multitude of functions, ranging from the production of biosynthetic intermediates and energy to innate immune signaling and cellular calcium buffering or the storage of proapoptotic components (1). To perform these diverse functions, mitochondria respond to cellular cues and display a highly variable and dynamic morphology, constantly undergoing fusion and fission. It is becoming increasingly clear that mitochondrial dynamics and function are deeply interconnected, and mitochondrial dysfunction is associated with a range of diseases.
Wild-type mitochondrial morphology and function are maintained by a balance between mitochondrial fusion and fission. Fusion allows exchange of genetic material between single mitochondria and is mediated by two large guanosine triphosphate phosphohydrolases (GTPases) embedded in the outer membrane (mitofusin 1 and 2) and an inner membrane GTPase, Opa1 (2). Deletion mutants affecting these three proteins accumulate dysfunctional mitochondria, leading to neurodegenerative phenotypes and different forms of myopathy (1, 3).
Mitochondrial fusion is balanced by fission, which is essential to ensure proper distribution of mitochondria and energy supply to daughter cells in mitosis or within a single cell. This necessity is particularly evident in neurons, where fission defects prevent efficient mitochondrial transport to synapses, the crucial sites of energy consumption (4, 5). The physiological importance of mitochondrial fission is further highlighted by its essential role in embryonic development in mice and nematodes (6–8).
Mitochondrial fission is thought to be accomplished by the dynamin-like protein Drp1, a mainly cytosolic protein that is recruited to future fission sites, where it oligomerizes to form spirals that constrict mitochondria. Mitochondrial fission is regulated at several levels: by initial ER- and actin-mediated mitochondrial constriction (9, 10), leading to the accumulation of the membrane-bound Drp1 receptor Mff and by several posttranslational modifications of Drp1, which modulate its activity (11).
Listeria monocytogenes is a foodborne pathogen capable of invading nonphagocytic cells, where it can replicate and spread. The pathogenic potential of L. monocytogenes correlates with the expression of several virulence genes (12). One of the most important virulence factors is listeriolysin O (LLO), a highly regulated secreted pore-forming toxin (reviewed in ref. 13). LLO belongs to the family of cholesterol-dependent cytolysins (CDCs), most of which are produced by extracellular bacteria such as Streptococci or Clostridia. CDCs oligomerize on cholesterol-containing membranes to form nonselective ion-permeable pores of variable sizes (14) that act in concert with bacterial phospholipases to allow bacterial escape from the phagosome. More recently, LLO has been found to have several intracellular and extracellular roles that extend beyond phagosomal escape. For example, we have shown that infection with L. monocytogenes causes fragmentation of the host mitochondrial network by action of its pore-forming toxin LLO before bacterial entry (15).
In this study, we demonstrate that LLO-induced mitochondrial fragmentation does not follow canonical pathways, because it is independent of key fusion and fission components, such as Opa1 and Drp1. We demonstrate that the ER marks mitochondrial fragmentation sites even in the absence of functional Drp1, and that the actin cytoskeleton also facilitates fragmentation. LLO-induced fragmentation is distinct from that observed upon treatment with uncouplers [such as carbonyl cyanide m-chlorophenylhydrazone (CCCP)] and apoptosis inducers (such as staurosporine), revealing a unique pathway for mitochondrial fragmentation that can be induced by an exogenous agent.
Results and Discussion
Opa1 Is Not Processed upon LLO-Induced Mitochondrial Fragmentation.
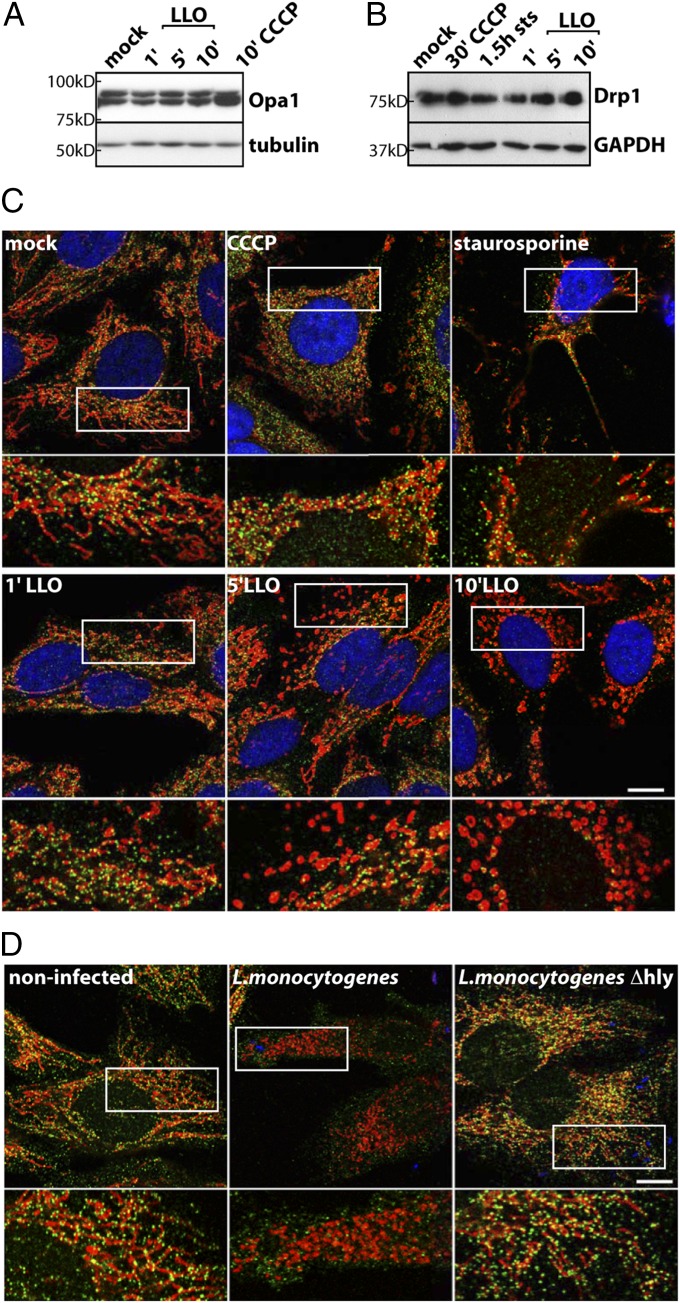
Mitochondrial fragmentation can result either from an increase in fission activity, an inhibition of fusion, or both. One possible pathway for mitochondrial fragmentation is rapid proteolytic processing of the inner membrane GTPase Opa1, as observed upon uncoupler [CCCP or trifluorocarbonyl cyanidephenylhydrazone (FCCP)]-induced mitochondrial fragmentation, which results in a fusion block and an ensuing mitochondrial fragmentation due to unopposed fission events (16–18). In contrast to CCCP, Opa1 is not processed upon LLO treatment (Fig. 1A), indicating that LLO-induced mitochondrial fragmentation is not caused by a block in inner membrane fusion. This finding is consistent with the rapid fission kinetics that we have described, which suggests an active fission mechanism (15). We therefore addressed whether LLO induces fragmentation by recruiting the key fission protein Drp1.
Fig. 1.
Analysis of Opa1 and Drp1 upon LLO-induced mitochondrial fragmentation. (A) Western blot of HeLa cells treated for 1, 5, or 10 min with 6 nM LLO or for 10 min with 10 µM CCCP and probed for Opa1, which is readily processed upon CCCP, but not LLO treatment. Tubulin was used as a loading control. (B) HeLa cells were treated for 30 min with 10 µM CCCP, for 1.5 h with 1.2 µM staurosporine (sts), or with 6 nM LLO for the indicated timepoints, and total cell lysates are analyzed by Western blotting, showing no apparent decrease in total Drp1 levels upon LLO or drug treatment. GAPDH was used as a loading control. (C) Immunofluorescence labeling of the same cells analyzed in B. Drp1 is shown in green (Drp1NT), mitochondria in red (Tom20), and nuclei in blue (DAPI). Staining reveals that mitochondrial Drp1 oligomers are lost upon LLO treatment (decrease in green puncta) but are retained upon CCCP or staurosporine treatment. (D) HeLa cells were infected for 1 h with L. monocytogenes or L.monocytogenes Δhly (LLO−) at an MOI of 50 and stained for Drp1 (green), mitochondria (CoxIV; red), and nuclei/bacteria with DAPI (blue). Wild-type L. monocytogenes induces mitochondrial fragmentation and loss of mitochondria-associated Drp1, whereas infection with the Δhly mutant does not overtly affect Drp1. For C and D, the white box indicates a 2× enlarged region, which is shown below. (Scale bars: 10 µm.)
Mitochondrial Drp1 Oligomers Dissociate upon Infection or LLO Treatment.
Fission-inducing agents such as uncouplers or the apoptosis inducer staurosporine appear to act by recruiting Drp1 to fragmenting mitochondria (Fig. 1C; refs. 19–21). In contrast, LLO treatment and L. monocytogenes infection were characterized by a decrease in Drp1 puncta associated with mitochondria (Fig. 1 C and D and Fig. S1A). This phenomenon was unique to LLO and L. monocytogenes infection because a number of other fission-inducing agents (the protonophore FCCP, the potassium ionophore valinomycin, and the detergent digitonin) did not show a decrease in mitochondria-associated Drp1 (Fig. S1B). Interestingly, potassium accumulation into mitochondria was invoked by Dimmer et al. to explain Drp1-independent fragmentation upon silencing of the inner membrane protein LETM1, because the potassium ionophore nigericin could restore tubular morphology of mitochondria in these cells (22). LLO may cause an intramitochondrial potassium imbalance that could impinge on mitochondrial morphology, although it does not explain the dissociation of Drp1 from mitochondria. Western blot analysis of cell extracts showed no decrease in total Drp1 levels in LLO-treated (Fig. 1B and Fig. S1C) cells. Use of two different antibodies against endogenous Drp1 on fixed samples (Fig. S1A) and live cell imaging of overexpressed Drp1-GFP (23) (Fig. S1D) confirmed a strong decrease in mitochondria-associated Drp1 staining upon LLO treatment.
LLO Effect on the Drp1 Receptors Fis1 and Mff.
Drp1 requires several membrane-anchored receptor proteins to bind to the mitochondrial surface. We thus investigated whether the LLO-induced decrease in mitochondria-associated Drp1 staining was due to modulation of its receptors Fis1 (24–26) and Mff (27) at the mitochondrial outer membrane.
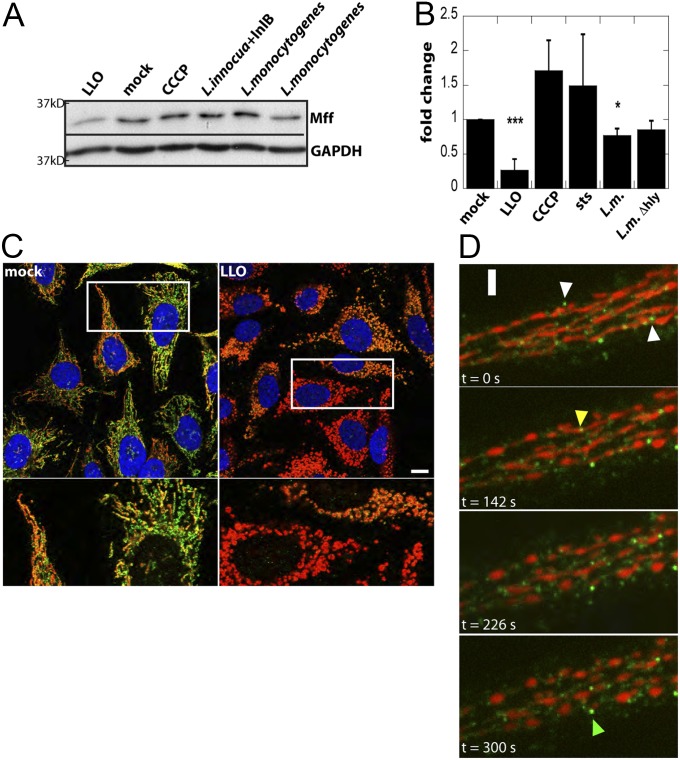
Upon LLO treatment or infection with L. monocytogenes, there was no detectable decrease in the association of Fis1 with mitochondria by immunofluorescence or total Fis1 protein levels by Western blotting (Fig. S2 A and B), suggesting that loss of mitochondrial Drp1 does not depend on a decrease in Fis1. In contrast, LLO treatment and L. monocytogenes infection induced a decrease in Mff protein as observed in total cell extracts by Western blotting and at the single-cell level by immunofluorescence and live-cell imaging (Fig. 2). The level of Mff mRNA was unaltered, suggesting posttranscriptional modulation of Mff (Fig. S2C). Mff did not decrease upon uncoupling (Fig. 2 A and B) nor when LLO treatment was performed in the absence of extracellular calcium (Fig. S2D). However, ionophore-induced calcium influx was not sufficient to induce Mff degradation (Fig. S2D), suggesting that calcium influx alone is not sufficient to cause Mff degradation upon LLO treatment. LLO-induced degradation of Mff appeared proteasome independent, because it was not prevented by treatment with MG132 and lactacystin (Fig. S2E), consistent with previous reports suggesting that LLO induces protein degradation through an as-yet-unknown pathway (28, 29). Timelapse studies of Mff-GFP in HeLa cells treated with LLO indicate that Mff degradation begins almost immediately after LLO addition (Fig. 2D and Fig. S2F) and before mitochondrial fragmentation. Indeed, LLO induced mitochondrial fragmentation in Mff −/− HCT116 cells (Fig. S2G). Given that Mff serves as one of the primary receptors for anchoring Drp1 at mitochondria, this result indicates Drp1 may be lost from mitochondria before fragmentation, further suggesting that Drp1 is not required at fragmentation sites.
Fig. 2.
LLO treatment and L. monocytogenes infection cause a decrease in the Drp1 receptor Mff. (A) HeLa cells were treated for 10 min with 6 nM LLO, 30 min with 10 µM CCCP, or infected at an MOI of 50 with the indicated strains for 1 h. Representative Western blot of Mff and GAPDH (loading control) on total cell lysates shows a decrease in Mff levels upon LLO treatment or infection with wild-type L. monocytogenes. (B) Quantification of Western blots from >3 independent experiments (***P < 0.001; *P < 0.05). (C) Immunofluorescence of HeLa cells treated for 10 min with LLO and stained for Mff (green), mitochondria (Tom20; red), and DNA (DAPI; blue). Although there is heterogeneity in staining of endogenous Mff, there is a significant decrease in Mff staining upon LLO treatment. White box indicates a 4× enlarged region that is shown below. (D) Heterogeneous behavior of mitochondria-associated Mff puncta observed by live cell imaging. Timelapse spinning disk confocal images of HeLa cells expressing Mff-GFP (green) and TagBFP-mito (red). Data were collected every 2 s 2 nM LLO was added at time t = 50 s White arrowheads mark Mff-GFP puncta that are rapidly lost from mitochondria. Yellow arrowhead marks an Mff dot that disappears before fragmentation and green arrowhead marks one that remains associated with mitochondria over the time course. (Scale bars: C, 10 μm; D, 2.6 μm.)
LLO-Induced Mitochondrial Fragmentation Is Drp1 Independent.
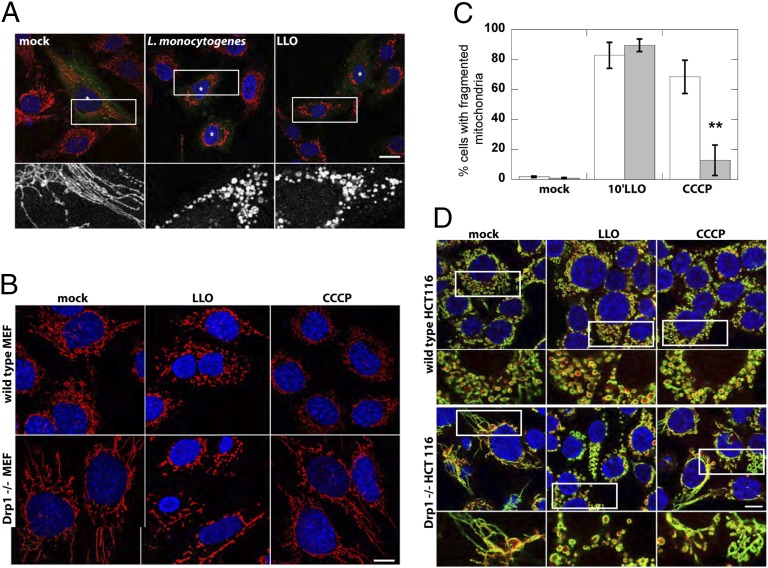
Drp1 is described as the key and essential factor mediating mitochondrial fission. Given that we observed a decrease rather than an augmentation of the Drp1 signal at mitochondria upon infection or LLO treatment, we tested whether Drp1 is essential for LLO-induced mitochondrial fragmentation by using dominant-negative K38A Drp1 in HeLa cells, Drp1−/− mouse embryonic fibroblasts (MEFs) (6), and HCT116 Drp1−/− cells. Strikingly, there was no difference in mitochondrial fragmentation compared with controls in cells transfected with K38A Drp1, as well as both Drp1 knockout cell lines (Fig. 3), indicating that LLO- and L. monocytogenes-induced mitochondrial fragmentation is truly Drp1 independent. In contrast, CCCP-induced mitochondrial fragmentation was substantially reduced in the Drp1 knockout cell lines. Staining of fixed cells, and live cell imaging with fluorescent protein-tagged markers, with both matrix and outer-membrane markers confirmed complete abscission of mitochondria upon LLO treatment (Fig. 3C and Fig. S3 A and B). Furthermore, upon incubation of wild-type and knockout cells with LLO for different amounts of time, similar percentages of cells with fragmented mitochondria were scored, indicating that the loss of Drp1 did not delay the kinetics of LLO-induced mitochondrial fragmentation (Fig. S3C).
Fig. 3.
LLO and L. monocytogenes induce mitochondrial-fragmentation in Drp1−/− cells. (A) Infection of HeLa cells transfected with K38A-HA Drp1 with L. monocytogenes for 1 h or treatment with 6 nM LLO for 10 min induces mitochondrial fragmentation. Immunofluorescence images show mitochondria (CoxIV; red) and K38A-HA Drp1 (anti-HA; green). Asterisk marks transfected cells, as identified by immufluorescence. The white box indicates the portion of the image enlarged 4× and shown below. (Scale bars: 20 μm.) (B) Immunofluorescence of mitochondria (CoxIV; red) and nuclei (DAPI; blue) upon treatment of WT or Drp1−/− MEFs with either 6 nM LLO (10 min) or 10 µM CCCP (30 min). (C) The percentage of cells displaying a fragmented mitochondrial network upon CCCP or LLO treatment was quantified in five or six randomly chosen fields of view (n > 150 cells) per experiment. Pooled data from three independent experiments is shown (**P < 0.003, one-tailed Student t test). (D) Immunofluorescence of mitochondrial matrix (cytochrome c; red), mitochondrial outer membrane (Tom20; green), and nuclei (DAPI; blue) upon treatment of WT or Drp1−/− HCT116 cells with either 6 nM LLO (10 min) or 10 µM CCCP (30 min). White box indicates 2× enlarged region shown below. (Scale bars: 10 μm.)
The Drp1 dependence of mitochondrial fission induced by uncouplers or activators of apoptosis is a matter of debate. For example, it has been demonstrated that transfection of K38A Drp1 does not block uncoupling-induced formation of disk-shaped mitochondria (30), and deletion of Drp1 does not affect the number of cells with fragmented mitochondria upon induction of apoptosis (8). However, several other groups found that overexpression of K38A Drp1 or Drp1 depletion prevented or diminished apoptosis- or CCCP-induced mitochondrial fragmentation (6, 31–33). It has been suggested that this discrepancy may arise because fragmentation depends both on the cell type and the physiological cue used to induce apoptosis (6). In contrast, we see no measureable perturbation of fragmentation upon knock out of Drp1 or expression of dominant-negative Drp1, indicating that fragmentation induced by LLO is truly independent of functional Drp1. Moreover, LLO-induced fragmentation is not associated with cytochrome c release and activation of canonical apoptosis (15), suggesting that this mechanism of fragmentation is distinct from the one that activates uncouplers or apoptosis inducers. Opening of the mitochondrial transition pore (MTP) may be invoked to explain LLO-induced mitochondrial fragmentation. We do not favor this hypothesis because of previous experiments confirming that the mitochondrial inner membrane remains intact upon LLO treatment, as respiration can resume (15). Furthermore, we could not inhibit the fragmentation phenotype with the MTP inhibitor cyclosporine A (15).
The ER Marks Sites of Mitochondrial Fragmentation Even in the Absence of Functional Drp1.
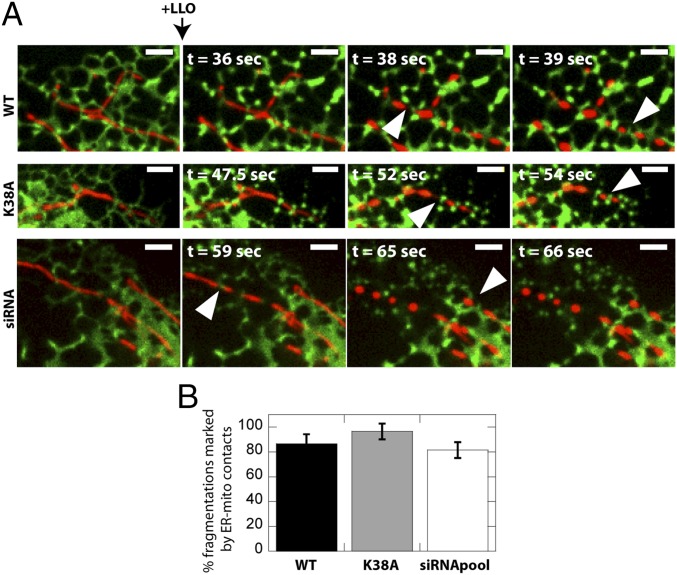
It has recently been shown that mitochondrial fission occurs at contact sites with the ER, and that mitochondria are constricted at such contact sites (9). Although the mechanism of how the ER regulates fission was not identified, it was suggested that ER proteins may participate in fission and/or that mitochondrial constriction by the ER facilitates recruitment of the mitochondrial fission machinery (i.e., Drp1). We thus explored whether LLO-induced and Drp1-independent mitochondrial fragmentation was marked by ER-mitochondrial contact sites. Timelapse studies of U2OS epithelial cells expressing the ER marker Sec61β-GFP (34) upon treatment with LLO revealed that the ER marked sites of mitochondrial fragmentation in 87 ± 8% of events (Fig. 4). Transfection of U2OS cells with K38A Drp1 or siRNA to knockdown Drp1 did not prevent LLO-induced mitochondrial fragmentation. Intriguingly, there was no change in the percent of fragmentations marked by ER-mitochondria contact sites (96 ± 6% for K38A; 82 ± 6% for siRNA), demonstrating that the ER plays an active role in regulating mitochondria fission even in the absence of Drp1.
Fig. 4.
The ER marks sites of mitochondrial fragmentation independent of Drp1. (A) U2OS Sec61β-GFP cells were treated with 2 nM LLO and examined live by confocal microscopy. Representative images of mitochondria (DsRed2-mito; red) and ER (Sec61β-GFP; green) are shown. Arrowheads indicate mitochondrial fragmentation sites marked by ER-mitochondria contact sites. Experiments were repeated a minimum of three times. (Scale bars: 2.6 μm.) (B) Quantification of mitochondrial fragmentations marked by ER-mitochondrial contact sites. Analysis was carried out as described in Materials and Methods.
Actin Is Involved in LLO-Induced Mitochondrial Fragmentation.
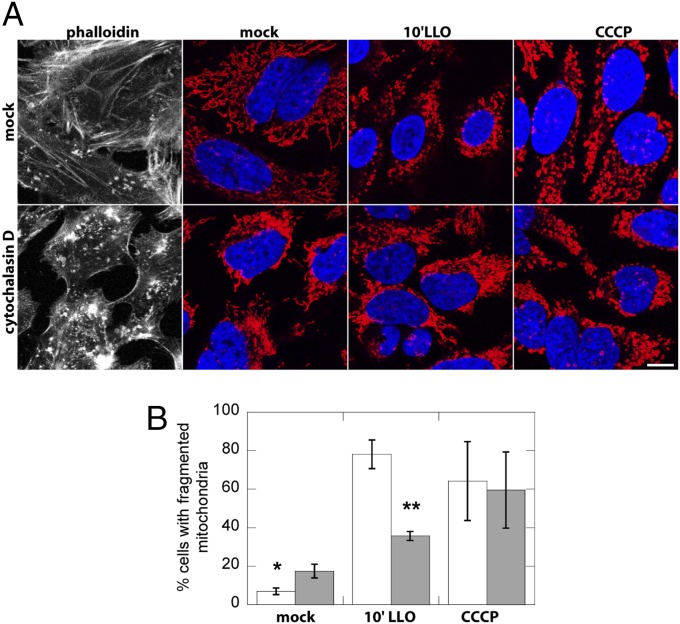
Actin has been shown to play a role in drug-induced mitochondrial fission in mammalian cells, although the effect appears to be stimulus dependent because oligomycin and cyclosporine A, but not CCCP, depended on F-actin for efficient mitochondrial network fragmentation (30). We tested whether the action of LLO on mitochondria required the presence of F-actin and found that actin depolymerization by cytochalasin D reduces the number of cells that display a fragmented mitochondrial network upon LLO treatment (Fig. 5). Given that Drp1 is dispensable for LLO-induced mitochondrial fragmentation, we hypothesize that, in addition to contributing to Drp1 recruitment to mitochondria as observed by ref. 30, actin may also facilitate mitochondrial fragmentation by preconstricting or physically stretching mitochondria independently of Drp1.
Fig. 5.
Actin contribution to LLO-induced mitochondrial fragmentation. (A) Immunofluorescence images of HeLa cells mock-treated or preincubated with 2.5 µM cytochalasin D for 30 min to depolymerize actin and then treated for 10 min with 6 nM LLO or for 30 min with 10 µM CCCP, showing phallodin staining of actin in the grayscale images, mitochondria (red; CoxIV), and nuclei (blue; DAPI). Actin depolymerization caused partial perinuclear aggregation of mitochondria, but mitochondria retained a tubular morphology. (B) Pooled data from three independent experiments (n > 150 cells per experiment, randomly chosen fields of view) showing that cytoD treatment decreases the number of cells with fragmented mitochondria upon LLO treatment. *P < 0.01, **P < 0.001 (one-tailed Student t test).
In summary, we have shown that in contrast to uncouplers and apoptosis-inducing agents, mitochondrial fragmentation caused by Listeria via its toxin LLO proceeds in a Drp1-independent manner.
LLO-induced mitochondrial fragmentation concurs with degradation of the Drp1-receptor Mff, causing a loss of Drp1 at mitochondria. Drp1 has been proposed to be a multifunctional protein that contributes to peroxisomal and possibly also to ER fission (35, 36), as well as possessing a putative GTPase-independent role in cell death (37). Its modulation by bacterial infection might therefore represent a means to act on several organelles and cellular functions in a coordinated fashion.
The data presented in this study suggests that actin and the ER play an active role in regulating LLO-induced mitochondrial fission. Recently, the ER-localized formin INF2 has been proposed as a link between the ER and actin at mitochondrial constriction sites (10), but it remains to be clarified whether this protein plays a role in LLO-induced mitochondrial fragmentation. Although LLO acts on mitochondrial dynamics before bacterial invasion and we could not detect overt LLO accumulation on intracellular structures by confocal imaging (15), we cannot exclude that a fraction of LLO inserts into mitochondria-associated membranes enriched in cholesterol (38), where it could modulate mitochondria-ER contact sites or their protein components, such as Mfn2 (39). Upon LLO treatment, ER-mediated mitochondrial fission occurs even in the absence of Drp1, indicating that either ER proteins may contribute to mitochondrial abscission or that the ER recruits another as-yet-unidentified mitochondrial fission machinery that exists in mammalian cells.
Given that among the exogenous agents that induce mitochondrial fragmentation LLO is unique in its ability to act rapidly, without activating canonical apoptosis, and in a Drp1-independent manner, we suggest it could serve as an unprecedented tool to understand the regulation and function of mitochondrial dynamics proteins. Indeed, efforts in this field should aim toward the identification of the molecule(s) that facilitate mitochondrial fragmentation upon LLO treatment in the absence of Drp1 and the signaling cascade that activates such fragmentation.
Materials and Methods
Reagent references, detailed experimental protocols, image and statistical analysis, and nonstandard abbreviations used in this study are provided in SI Materials and Methods.
Bacterial Infections.
For infection experiments, bacteria were grown overnight in brain-heart infusion medium (Difco; BD Biosciences) at 37 °C, subcultured at a 1:10 dilution until an optical density of 0.8–1 was reached, washed three times in medium without serum, and added to cells for 1 h at a multiplicity of infection (MOI) of 50. The strains used were as follows: wild-type L. monocytogenes (EGD, BUG600), L. monocytogenes Δhly (EGD, BUG2132), and L. innocua overexpressing InlB (BUG1642).
Cell Culture, Drug Treatments, and Transfection.
HeLa CCL2 cells were cultured according to American Type Culture Collection guidelines. MEF (kind gift from K. Mihara, Kyushu University, Japan) and U2OS Sec61β-GFP cells (kind gift from Yoko Shibata and Tom Rapoport, Harvard Medical School, Boston; for the Sec61β-GFP plasmid, see ref. 34) were maintained in DMEM supplemented with 10% (vol/vol) FCS. HCT116 cells were maintained in McCoys 5A medium supplemented with 10% FCS, nonessential amino acids, and gluatamine. All cells were maintained under 10% (vol/vol) CO2. Media/additives were purchased from Invitrogen. Drugs were purchased from Sigma-Aldrich and incubated in full medium unless otherwise stated. Drugs were incubated at the following concentrations and times: 10 µM CCCP for 30 min, 0.1 µM valinomycin for 30 min, 1.2 µM staurosporine for 90 min, 0.05% (wt/vol) digitonin for 5 min, 20 µM MG132 and 20 µM lactacystine for 90 min, 2.5µM cytochalasin D for 30 min (HeLa) and 1 µM for 20 min (MEF), and 2–6 nM LLO for 10 min (in absence of FCS). DNA transfection (3.5-cm wells) was performed with FuGENE HD (Roche) according to the manufacturer’s instructions. The following plasmids were used for live-cell imaging: pDsRed2-mito (Clontech; 0.2 μg), Mff-GFP (ref. 9; 0.125 μg), mCherry-Drp1 (ref. 9; 0.1 μg), Drp1-GFP (ref. 23; 0.5 µg), BFP-mito (ref. 9; 0.5 μg), OM-GFP (“Mito-GFP”, ref. 40; 1 μg), and K38A-HA Drp1 (ref. 23; 4 μg). siRNA transfection was performed with Oligofectamine (Invitrogen) according to the manufacturer’s instructions, using Drp1-specific sequences described in ref. 15.
After treatment, cells were either analyzed with a spinning disk microscope for live-cell imaging or processed for immunofluorescence with the antibodies indicated in the respective figure legends (see SI Materials and Methods for antibody catalog numbers and for detailed live cell imaging immunofluorescence procedures).
Quantitative RT-PCR.
Total RNA was extracted using the RNeasy kit (Qiagen) and treated with TurboDNase (Ambion). cDNA was synthetized from 500 ng of RNA with the RT2 cDNA synthesis kit (Qiagen). Standard quantitative RT-PCR was performed with the EvaGreen kit (Bio-Rad) on a Bio-Rad CFX machine, and gene expression was calculated by the 2^(-ΔΔ Ct) method. Two independent primer sets were used for MFF. Data represent the mean of two biological replicates and three technical replicates.
Western Blotting.
Cells were lysed with 2× Laemmli loading buffer (124 mM Tris⋅HCl at pH 6.8, 4% (vol/vol) SDS, 20% (wt/vol) glycerol, 0.02% bromophenol blue, and 0.03% DTT), and genomic DNA was digested with Benzonase (Novagen). Proteins were separated by SDS/PAGE (Bio-Rad), transferred by wet transfer to a nitrocellulose membrane (GE Healthcare) that was blocked in 10% (wt/vol) milk; antibodies were incubated in milk at dilutions indicated by the manufacturers. Quantification of Western blots was accomplished by using the Gel Analyzer plugin in ImageJ. The Mff signal was normalized to GAPDH signal, and ≥3 independent experiments were analyzed.
Supplementary Material
Acknowledgments
We thank Dr. K. Mihara for Drp1 knockout MEFs, Drs. Yoko Shibata and Tom Rapoport for U2OS-Sec61βGFP cells, and Drs. Gia Voeltz and A. van der Bliek for constructs; Dr. Tham Tho-Nam for excellent technical support and the Plate-Forme Imagerie Dynamique staff at Institut Pasteur for support; Dr. Ascel Samba-Louaka for critical reading of the manuscript; and Drs. Janet Shaw and Aurélien Roux for insightful discussions. This work received financial support from the Institut Pasteur, Institut National de la Santé et de la Recherche Médicale Unité 604, Institut National de la Recherche Agronomique Unité Sous Contrat 2020, Fondation Louis Jeantet, European Research Council Advanced Grant 233348 MODELIST, and Agence Nationale pour la Recherche (ERANET Pathogenomics Grant LISTRESS and Grant Blanc MITOPATHO). F.S. was supported by postdoctoral fellowships from European Molecular Biology Organization and the Fondation pour la Recherche Médicale. C.W. and R.Y. are supported in part by the Intramural Research Program of the National Institutes of Neurological Disorders and Stroke, National Institutes of Health. P.C. is a Howard Hughes Medical Institute Senior International Research Scholar.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315784110/-/DCSupplemental.
References
- 1.Nunnari J, Suomalainen A. Mitochondria: In sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141(2):280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan DC. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 4.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118(Pt 23):5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Ishihara N, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11(8):958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 7.Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4(5):815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi J, et al. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186(6):805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman JR, et al. ER tubules mark sites of mitochondrial division. Science. 2011;334(6054):358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339(6118):464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833(5):1256–1268. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Cossart P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc Natl Acad Sci USA. 2011;108(49):19484–19491. doi: 10.1073/pnas.1112371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamon MA, Ribet D, Stavru F, Cossart P. Listeriolysin O: The Swiss army knife of Listeria. (Translated from eng) Trends Microbiol. 2012;20(8):360–368. doi: 10.1016/j.tim.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Rosado CJ, et al. The MACPF/CDC family of pore-forming toxins. Cell Microbiol. 2008;10(9):1765–1774. doi: 10.1111/j.1462-5822.2008.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stavru F, Bouillaud F, Sartori A, Ricquier D, Cossart P. Listeria monocytogenes transiently alters mitochondrial dynamics during infection. Proc Natl Acad Sci USA. 2011;108(9):3612–3617. doi: 10.1073/pnas.1100126108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25(13):2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duvezin-Caubet S, et al. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem. 2006;281(49):37972–37979. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- 18.Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178(5):757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank S, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1(4):515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 20.Cereghetti GM, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA. 2008;105(41):15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cereghetti GM, Costa V, Scorrano L. Inhibition of Drp1-dependent mitochondrial fragmentation and apoptosis by a polypeptide antagonist of calcineurin. Cell Death Differ. 2010;17(11):1785–1794. doi: 10.1038/cdd.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimmer KS, et al. LETM1, deleted in Wolf-Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum Mol Genet. 2008;17(2):201–214. doi: 10.1093/hmg/ddm297. [DOI] [PubMed] [Google Scholar]
- 23.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12(8):2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151(2):367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23(15):5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu T, Fox RJ, Burwell LS, Yoon Y. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. J Cell Sci. 2005;118(Pt 18):4141–4151. doi: 10.1242/jcs.02537. [DOI] [PubMed] [Google Scholar]
- 27.Otera H, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191(6):1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribet D, et al. Listeria monocytogenes impairs SUMOylation for efficient infection. Nature. 2010;464(7292):1192–1195. doi: 10.1038/nature08963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samba-Louaka A, Stavru F, Cossart P. Role for telomerase in Listeria monocytogenes infection. Infect Immun. 2012;80(12):4257–4263. doi: 10.1128/IAI.00614-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol. 2005;15(7):678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 31.Legros F, Lombès A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13(12):4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishihara N, Jofuku A, Eura Y, Mihara K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem Biophys Res Commun. 2003;301(4):891–898. doi: 10.1016/s0006-291x(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 33.Malka F, et al. Separate fusion of outer and inner mitochondrial membranes. EMBO Rep. 2005;6(9):853–859. doi: 10.1038/sj.embor.7400488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata Y, et al. The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. J Biol Chem. 2008;283(27):18892–18904. doi: 10.1074/jbc.M800986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch A, et al. Dynamin-like protein 1 is involved in peroxisomal fission. J Biol Chem. 2003;278(10):8597–8605. doi: 10.1074/jbc.M211761200. [DOI] [PubMed] [Google Scholar]
- 36.Pitts KR, Yoon Y, Krueger EW, McNiven MA. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell. 1999;10(12):4403–4417. doi: 10.1091/mbc.10.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bras M, et al. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol. 2007;27(20):7073–7088. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Area-Gomez E, et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31(21):4106–4123. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 40.Stavru F, Nautrup-Pedersen G, Cordes VC, Görlich D. Nuclear pore complex assembly and maintenance in POM121- and gp210-deficient cells. J Cell Biol. 2006;173(4):477–483. doi: 10.1083/jcb.200601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.