Significance
Patients with thyroid disease often report sensitivity to environmental temperature, feeling too hot or too cold in hyper- and hypothyroidism, respectively. Textbook knowledge attributes this to the role of thyroid hormone in basal metabolism. In this study, we show that a mouse model for hypothyroidism lacks proper control of tail vasoconstriction at room temperature, which causes inappropriate heat loss. This in turn activates heat-generating brown adipose tissue to maintain body temperature. Our study therefore demonstrates that changes in vascular control may in fact significantly contribute to the temperature hypersensitivity observed in patients with thyroid disorders and reveal a unique connection between vascular and metabolic effects of thyroid hormone.
Keywords: adipose tissue, tail temperature
Abstract
Thyroid hormone is a major regulator of thermogenesis, acting both in peripheral organs and on central autonomic pathways. Mice heterozygous for a point mutation in thyroid hormone receptor α1 display increased thermogenesis as a consequence of high sympathetic brown fat stimulation. Surprisingly, despite the hypermetabolism, their body temperature is not elevated. Here we show, using isolated tail arteries, that defective thyroid hormone receptor α1 signaling impairs acetylcholine-mediated vascular relaxation as well as phenylephrine-induced vasoconstriction. Using infrared thermography on conscious animals, we demonstrate that these defects severely interfere with appropriate peripheral heat conservation and dissipation, which in turn leads to compensatory alterations in brown fat activity. Consequently, when the vasoconstrictive defect in mice heterozygous for a point mutation in thyroid hormone receptor α1 was reversed with the selective α1-adrenergic agonist midodrine, the inappropriate heat loss over their tail surface was reduced, normalizing brown fat activity and energy expenditure. Our analyses demonstrate that thyroid hormone plays a key role in vascular heat conservation and dissipation processes, adding a unique aspect to its well-documented functions in thermoregulation. The data thus facilitate understanding of temperature hypersensitivity in patients with thyroid disorders. Moreover, the previously unrecognized connection between cardiovascular regulation and metabolic activity revealed in this study challenges the interpretation of several experimental paradigms and questions some of the currently derived hypotheses on the role of thyroid hormone in thermogenesis.
Thyroid hormone affects energy metabolism, body temperature, and cardiovascular function (1, 2). This is evident in hypo- and hyperthyroid patients, who display metabolic and cardiovascular problems as well as inadequacies in heat and cold tolerance (3, 4). The latter effects are thought to be a consequence of a shift in obligatory thermogenesis, because thyroid hormone affects basal metabolic rate through the regulation of genes controlling cellular metabolism and mitochondrial function. In addition, facultative thermogenesis, brought about by shivering and brown adipose tissue (BAT) activation (5, 6), is also modulated by thyroid hormone. The recent discovery of BAT in adult humans, where previously thought to exist only in rodents and neonates (7), suggests that the role of BAT in thermoregulation and energy expenditure may be underestimated. Recently, a new role of thyroid hormone in facultative thermogenesis has emerged, controlling BAT via the central nervous system (8, 9). The importance of central thyroid hormone receptor α1 (TRα1) signaling for BAT activity was further supported by findings in mice heterozygous for a R384C mutation in TRα1 (TRα1+m), which display a strong hypermetabolism as the result of a central overactivation of BAT (10). However, the finding was unexpected, as the specific mutation causes a 10-fold reduced affinity to 3,3′,5-triiodothyronine (T3), and lower brown fat thermogenesis would be expected from impaired TRα1 signaling (11). Even more surprisingly, when TRα1+m mice were treated with supraphysiological doses of T3 to reactivate the mutant TRα1 (12, 13), their BAT activity returned to normal levels, although T3 is known to stimulate thermogenesis. This puzzling phenotype of TRα1+m animals therefore prompted us to investigate their thermoregulation in greater detail.
Here we demonstrate that TRα1+m mice display lower nocturnal body temperature despite BAT hyperactivity. Studying the tail as an important thermoregulatory effector organ (14), we reveal a defective vascular control of heat dissipation and conservation in vivo and identify possible vascular mechanisms affected by the impaired TRα1 signaling in ex vivo studies. Our findings show that the BAT hyperactivity observed in TRα1+m mice is a consequence of impaired vasoconstriction causing increased heat loss. The results describe a previously unknown role for TRα1 in vascular control of thermoregulation, thus constituting a unique link between thyroid hormone signaling and facultative thermogenesis. Moreover, they provide a possible explanation for the exaggerated thermosensitivity observed in patients with defects in thyroid hormone metabolism.
Results
Decreased Body Temperature in TRα1+m Mice, Despite Increased BAT Activity.
As TRα1+m mice display profound hypermetabolism (10), it was hypothesized that the animals would exhibit an increased body temperature. However, when core body temperature was analyzed in freely moving animals using a radiotelemetry system, an interaction was found between wild-type and TRα1+m mice over a 24-h period (Fig. 1A), with significantly lower body temperature in TRα1+m mice in the dark phase than in wild-type controls (Fig. 1B). The unexpected decrease in body temperature in TRα1+m mice manifests despite overactivation of BAT: Infrared thermography revealed that TRα1+m mice had a significantly higher interscapular BAT temperature than wild-type mice at room temperature (Fig. 1 C and D) in the presence of normal skin heat conductivity, capacity, and diffusivity (Table S1). The absolute metabolic rate, as assessed by oxygen consumption, was similar in wild-type mice and TRα1+m mutants despite their different body weights (Fig. 1E and Fig. S1A) and comparable locomotor activities (15, 16). This translated to disproportionally high relative energy expenditure for the small size of the TRα1+m mice when normalized to body weight (Fig. 1F, two-way ANOVA, day vs. night, P < 0.0001; WT vs. TRα1+m, P < 0.005; interaction, P < 0.0001), concurring with the previously demonstrated BAT hyperactivity in TRα1+m mice (10). Interestingly, tail temperature was also significantly increased in the infrared pictures of TRα1+m mice (Fig. 1D), pointing to elevated heat dissipation over the tail surface.
Fig. 1.
Temperature regulation in TRα1+m mice. (A) Core body temperature was measured using radiotelemetry, revealing a significant interaction over a 24-h period in TRα1+m mice (solid circles) compared with wild-type controls (open circles; two-way ANOVA, P < 0.01). (B) This translated to significantly lower mean TRα1+m body temperature in the dark phase (two-way ANOVA with Bonferroni post hoc test, **P < 0.01; ns, P > 0.05). (C and D) Infrared thermography was used to quantify heat generation from the interscapular brown fat depot (*P < 0.05) and heat dissipation from the tail (*P < 0.05). (E and F) Wild-type and TRα1+m mice were analyzed in metabolic cages for oxygen consumption, showing hypermetabolism in the mutants (absolute values in E and relative to body weight in F, two-way ANOVA with Bonferroni post hoc test, *P < 0.05, ***P < 0.001). The number of animals is given in parentheses in the respective experiments.
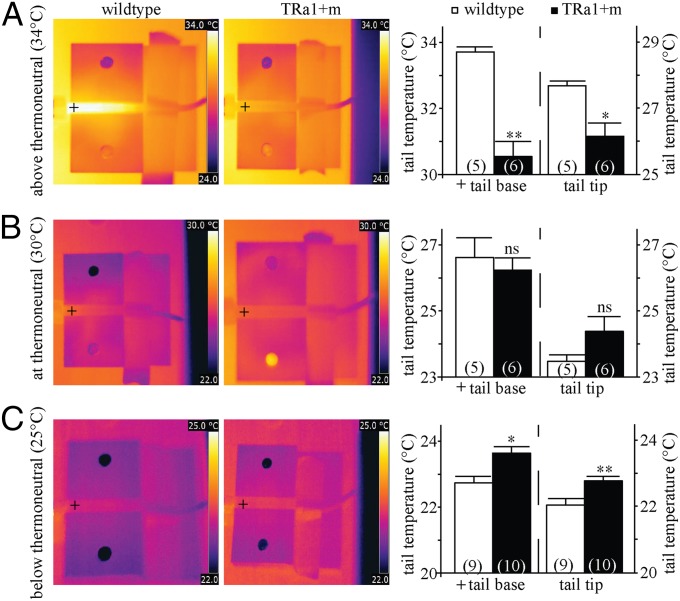
As tail heat dissipation constitutes an important yet often overlooked thermoregulatory mechanism in small rodents (14), a more detailed analysis was performed at different environmental temperatures. The results revealed that despite normal vascularization (Fig. S1C), tail thermoregulation was strongly impaired in the TRα1+m mice: We observed a significantly lower tail base and tip temperature in TRα1+m mice at 34 °C compared with wild type (Fig. 2A), indicating reduced heat dissipation above thermoneutrality. Conversely, a significant increase in tail base and tip temperature was noted in TRα1+m mice at 25 °C (Fig. 2C), indicative of increased heat dissipation below thermoneutrality. No significant differences were observed within the thermoneutral range at 30 °C (Fig. 2B).
Fig. 2.
Heat dissipation from TRα1+m tails. (A–C) Infrared thermography was used to measure heat dissipation from the tail at environmental temperatures above (A), at (B), and below thermoneutrality (C). Crosshairs indicate where the temperature for the tail base was taken. TRα1+m tail temperature (black bar) was found to be significantly higher below thermoneutrality (C) (*P < 0.05, **P < 0.01) and significantly lower above thermoneutrality (A) (*P < 0.05, **P < 0.01) compared with wild type (white bars). There was no significant difference within the thermoneutral range (B) (ns, P > 0.05). The number of animals is given in parentheses in the respective experiments.
Impaired TRα1+m Tail Vasculature Responsiveness ex Vivo.
To clarify the role of TRα1 in vasodilation and constriction, an ex vivo study on isolated tail arteries was done. An initial analysis revealed similar arterial diameter, as well as comparable passive, active, and maximal tension properties in wild-type and TRα1+m mice, which decreased upon T3 treatment in both groups (Table S2). Similar effects were seen in the small mesenteric arteries (Table S3), which were used as a control to identify defects related specifically to thermoregulation.
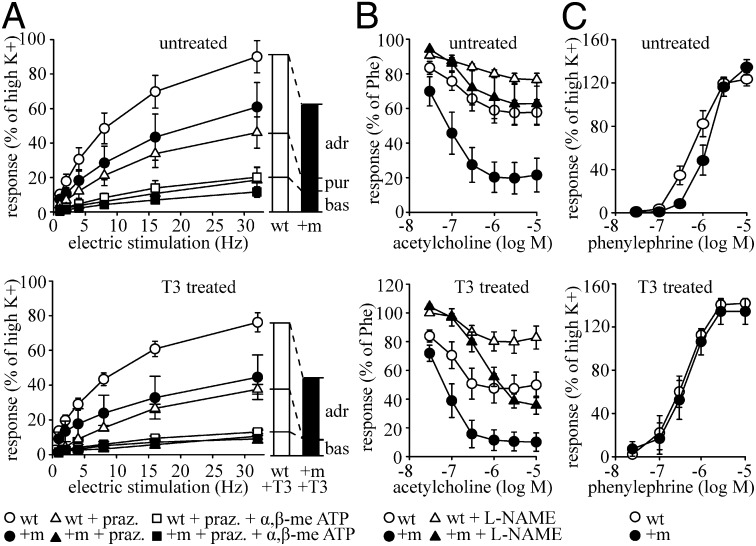
To assess the vascular contractility in detail, the responses of isolated tail arteries to electric field stimulation were measured (Fig. 3A). This procedure stimulates the surrounding nerve terminals to release neurotransmitters, which in turn act on the vasculature. The resulting contraction is therefore not a direct consequence of an electric stimulation of the smooth muscle, but caused indirectly by forced neurotransmitter release. When stimulated at 32 Hz, the contractile responses of wild-type tail arteries were comparable to those at the high K+ stimulation, whereas TRα1+m mutant tail arteries displayed overall reduced contraction. Using the α1-adrenergic antagonist prazosin and the purinergic blocker α,β-methylene ATP, we revealed that ∼50% of the contractions in wild-type and TRα1+m mice result from adrenergic stimulation (two-way ANOVA, effect of prazosin, P < 0.001; WT vs. TRα1+m, P < 0.01), whereas another 20% are caused by purinergic input (two-way ANOVA, effect of α,β-methylene ATP, P < 0.05; WT vs. TRα1+m, P < 0.01). The similar relative contribution of adrenergic signaling to the full contraction in TRα1+m mice thus indicates an absence of agonist-mediated receptor refractoriness and points to a tissue-autonomous defect rather than changes in the autonomic tone (17–19). This is further supported by similar serum catecholamine levels in wild-type and TRα1+m mice (15), as well as unaltered mRNA expression of the underlying receptors (Fig. S2F).
Fig. 3.
Ex vivo characterization of tail arteries. (A) Responses to electrical field stimulation at different frequencies in the isolated tail artery of untreated (Upper) and T3-treated (Lower) wild-type (WT, open symbol) and TRα1+m (+m, solid symbol) mice were recorded in a wire myograph in relation to the initial high-K+ activations. The frequency–tension responses were determined in the absence of blockers (circles), in the presence of prazosin (praz., triangles), and in the presence of prazosin and α,β-methylene-ATP (squares). Untreated, n = 5–6; T3 treated, n = 5. adr, adrenergic component; bas, baseline; pur, purinergic component. (B) Dose–response relationship to acetylcholine in the tail artery from untreated (Upper) and T3-treated (Lower) wild-type and TRα1+m mice. Tension values at each dose of acetylcholine were obtained in the absence (circles) and presence of l-NAME (triangles) in relation to the initial phenylephrine (Phe)-induced tension. Untreated, n = 4; T3 treated; n = 5. (C) Dose–response relationship to phenylephrine in the tail artery from untreated (Upper) and T3-treated (Lower) wild-type and TRα1+m mice. Tension values are related to the tension recorded during the initial high-K+ activations. Untreated, n = 5–6; T3 treated, n = 5.
To test whether reactivation of TRα1 signaling would improve the contractility of the tail arteries, TRα1+m mice were treated for 12 d with supraphysiological doses of T3. However, no effect was observed on the overall contractility upon electric field stimulation (Fig. 3A, Lower, two-way ANOVA, effect of prazosin, P < 0.001; WT vs. TRα1+m, P < 0.001); only the purinergic component of the contraction was additionally impaired (two-way ANOVA, effect of α,β-methylene ATP, P < 0.001; WT vs. TRα1+m, P < 0.001; interaction, P < 0.001). This effect was specific to the tail artery and not observed in control mesenteric arteries (Fig. S2A).
When we analyzed the responses to the main regulators of vasoconstriction and vasodilation, a higher sensitivity to acetylcholine was observed in the tail arteries of TRα1+m mice precontracted with phenylephrine compared with controls (Fig. 3B). A large component of this response appeared to depend on nitric oxide signaling, as a strong effect was observed upon inhibition of nitric oxide synthase with l-nitroarginine methyl ester (l-NAME). As expected from previous studies (20, 21), T3 promoted the NO-dependent relaxation in wild-type mice (Fig. 3B, Lower, open circles and triangles); however, the increased sensitivity to acetylcholine persisted in T3-treated TRα1+m mice, whereas its dependency on NO signaling was reduced (Fig. 3B, Lower, solid circles and triangles). A similar observation was made in small mesenteric arteries, which suggests that this is an overall vascular defect caused by impaired TRα1 signaling (Fig. S2B).
Because a thyroid hormone receptor mutation can interfere with its nongenomic regulation of NO signaling through phosphoinositide 3-kinase (PI3-kinase) (22), we measured the expression of catalase and cyclin G2, two well-defined PI3-kinase target genes (23). No alterations were found in tail or small mesenteric artery (Fig. S2G), which together with the normal thyroid hormone levels of TRα1+m animals (12) suggests unimpaired nongenomic TRα1 signaling in the mutants.
We then tested the contractile response to phenylephrine and identified a significantly reduced sensitivity in TRα1+m tail arteries (Fig. 3C, mean log EC50 values: controls, −6.2 ± 0.1; TRα1+m, −5.9 ± 0.1; log M, P < 0.05), which normalized after T3 treatment (Fig. 3C, Lower, mean log EC50 values: controls, −6.5 ± 0.2; TRα1+m, −6.4 ± 0.1; log M, P = 0.81). This effect was not observed in the small mesenteric arteries (Fig. S2C), suggesting a specific role for TRα1 in the tail vasculature. In concurrence with the decreased sensitivity to phenylephrine and a similar contractile capacity at full stimulation ex vivo, TRα1+m animals initially performed poorly in adjusting tail heat loss in vivo when acutely exposed to cold, but reached a similar end point to that of wild-type mice (Fig. S2 D and E).
Restoration of Tail and BAT Function in Vivo After T3 Treatment.
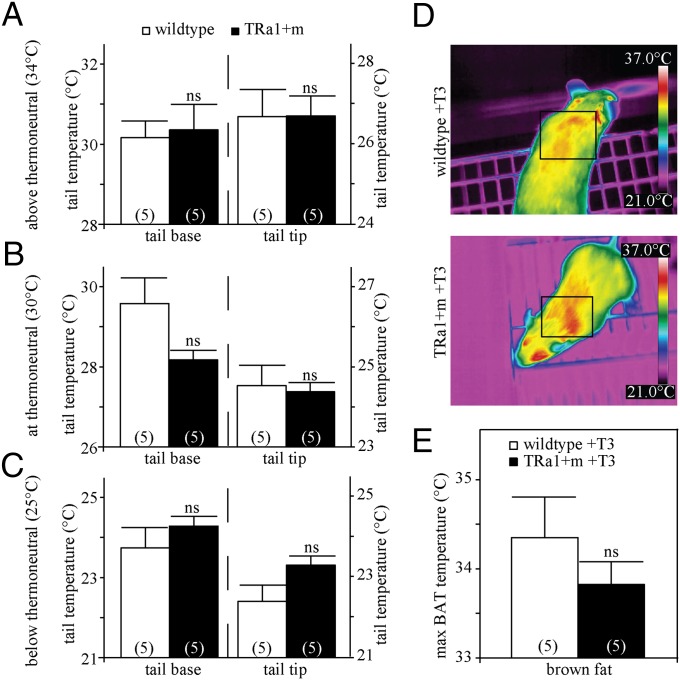
Because the contractile sensitivity to adrenergic stimulation was normalized in T3-treated TRα1+m mice, we tested whether their defective tail vasoconstriction at room temperature would also improve with T3 in vivo. When treated with T3 for 14 d, the TRα1+m animals no longer displayed any significant differences in tail base and tip temperatures (Fig. 4 A–C), suggesting improved thermoregulatory function of the tail vasculature. Most remarkably, BAT hyperactivity was also ameliorated in T3-treated TRα1+m mice as demonstrated by infrared thermography (Fig. 4D, two-way ANOVA, effect of T3 treatment, P < 0.0001; interaction, P < 0.05), concurring with the previously reported normalization of metabolic rate and BAT histology in T3-treated TRα1+m mice (10).
Fig. 4.
Tail temperature regulation in TRα1+m mice after 14 d of T3 treatment. (A–C) Infrared thermography was used to measure heat dissipation from the tail, showing no significant difference above thermoneutrality (A) (two-way ANOVA with Bonferroni post hoc test for 34 °C, tail base, WT vs. TRα1+m before T3, P < 0.001; WT vs. TRα1+m after T3, ns, P = 0.99; tail tip, WT vs. TRα1+m before T3, P = 0.06; WT vs. TRα1+m after T3, ns, P = 0.99), at thermoneutrality (B) (all ns, P > 0.05), and below thermoneutrality (C) (tail base, WT vs. TRα1+m before T3, P < 0.05; WT vs. TRα1+m after T3, ns, P = 0.72; tail tip, WT vs. TRα1+m before T3, P < 0.05; WT vs. TRα1+m after T3, ns, P = 0.13) for tail base and tail tip of wild-type (white bars) and TRα1+m mice (black bars). (D and E) Likewise there was no significant difference in BAT temperature between WT and TRα1+m mice after T3 treatment (ns, P = 0.53). The number of animals is given in parentheses in the respective experiments.
Midodrine Restores Tail and BAT Function at Room Temperature.
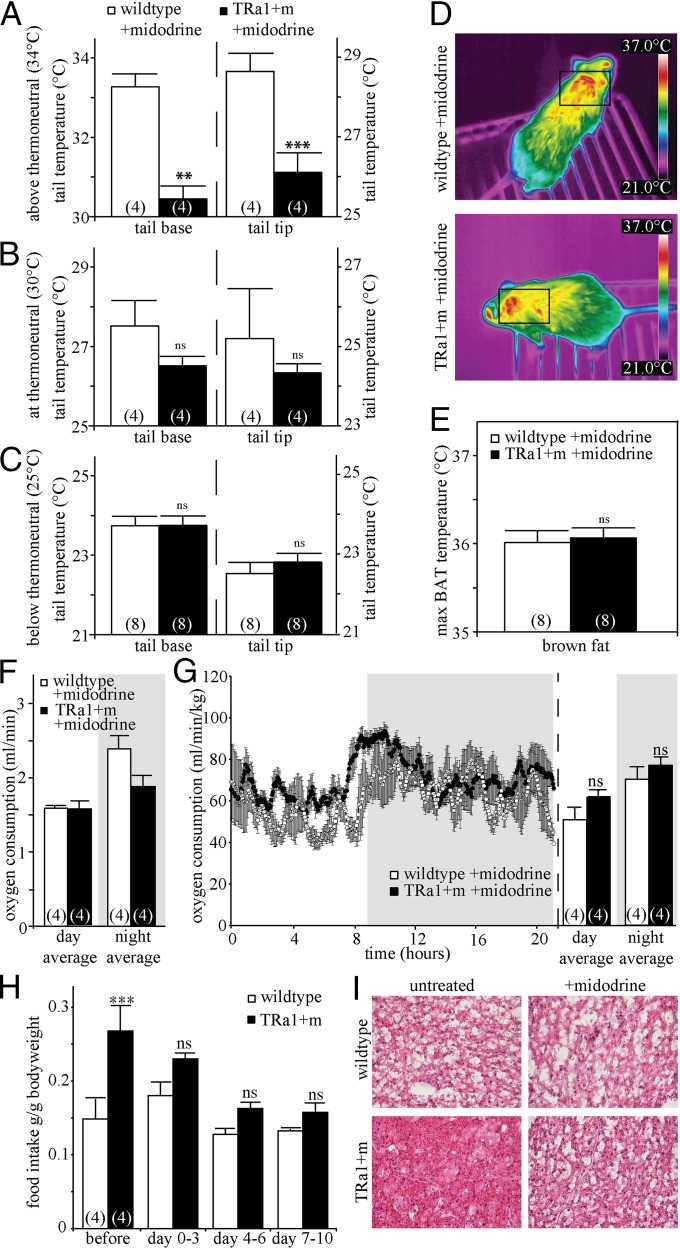
To test whether BAT hyperactivity in TRα1+m mice was a secondary consequence to increased heat loss caused by impaired vasoconstriction, mice were treated with the α1-adrenergic agonist midodrine, which specifically causes peripheral vasoconstriction. After 6 d of midodrine treatment, TRα1+m mice no longer showed a significant difference in tail base temperature compared with wild-type mice at room temperature (Fig. 5C), indicating a normalized vasoconstriction and normalized tail heat loss. As expected, the tail temperature difference at 34 °C remained between wild-type and TRα1+m mice (Fig. 5A), as the vasodilation defect is not normalized by midodrine. When BAT temperature was analyzed in the midodrine-treated TRα1+m mice at room temperature, it had returned to wild-type levels (Fig. 5 D and E). This was accompanied by reduced oxygen consumption (Fig. 5F and Fig. S1A), which was now proportionally normal to the weight of the TRα1+m animals (Fig. 5G), as well as normalized BAT histology (Fig. 5I) and uncoupling protein 1 (UCP1) mRNA expression (Fig. S2H). Moreover, the hyperphagia of TRα1+m mice, which is a secondary consequence of their hypermetabolism (10), was also reversed during the treatment (Fig. 5H and Fig. S1B), whereas the weight of the animals was not affected by the treatment. Importantly, wild-type BAT temperature and energy expenditure were not altered by the treatment, clearly illustrating that midodrine itself has no effect on BAT thermogenesis (compare with Fig. 1, two-way ANOVA with Bonferroni post hoc test, BAT temperature WT before midodrine vs. WT after midodrine, P = 0.99; oxygen consumption WT before midodrine vs. WT after midodrine, P = 0.86). The data therefore demonstrate that the BAT hyperactivity of TRα1+m mice is caused by impairments in tail vasoconstriction, which are reversible by midodrine. That defective vasoconstriction can in fact ignite facultative thermogenesis was further supported by elevated BAT activity observed in wild-type mice treated for 6 d with the α-adrenergic antagonist prazosin to enforce tail vasodilation (Fig. S1 D–F).
Fig. 5.
Normalized phenotype in TRα1+m mice after treatment with the α1-adrenergic agonist midodrine. (A–C) Infrared thermography was used to measure heat dissipation from the tail above thermoneutrality (A) (two-way ANOVA with Bonferroni post hoc test for 34 °C, tail base, WT vs. TRα1+m before midodrine, P < 0.001; WT vs. TRα1+m after midodrine, **P < 0.01; tail tip, WT vs. TRα1+m before midodrine, P < 0.001; WT vs. TRα1+m after midodrine, ***P < 0.001), at thermoneutrality (B) (all ns, P > 0.05), and below thermoneutrality (C) (tail base, WT vs. TRα1+m before midodrine, P < 0.05; WT vs. TRα1+m after midodrine, ns, P = 0.99; tail tip, WT vs. TRα1+m before midodrine, P = 0.06; WT vs. TRα1+m after midodrine, ns, P = 0.74) for tail base and tail tip of wild-type (white bars) and TRα1+m mice (black bars). (D–G) There was no significant difference between WT and TRα1+m mice after midodrine treatment in BAT temperature (D and E) (ns, P = 0.99) or oxygen consumption (absolute values in F and relative to body weight in G) (day, WT vs. TRα1+m before midodrine, P < 0.01; WT vs. TRα1+m after midodrine, ns, P = 0.14; night, WT vs. TRα1+m before midodrine, P < 0.01; WT vs. TRα1+m after midodrine, ns, P = 0.81). (H and I) The treatment also reversed the hyperphagia of TRα1+m mice (H) (two-way ANOVA for repeated measurements with Bonferroni post hoc test, WT vs. TRα1+m pretreatment, ***P < 0.001; other days, ns, P > 0.05) and largely normalized brown fat histology (I). The number of animals is given in parentheses in the respective experiments.
Discussion
Our data identify a unique role of TRα1 signaling in the regulation of body temperature and solve the apparent paradox of unexpected increased brown fat thermogenesis in an animal model reflecting receptor-mediated hypothyroidism (10). We confirm the previously described BAT hyperactivity in TRα1+m mice, using infrared thermography, but also demonstrate that this is likely a secondary consequence of impaired vascular regulation: The relative insensitivity of the tail artery to the adrenergic constriction signal leads to increased heat loss over the surface (illustration in Fig. S3), thus lowering body temperature and causing a compensatory increase in brown fat thermogenesis. The relative normalization of body temperature during daytime is also explained by this mechanism, as the inactive mice are curled up in their nest and the surface is less exposed, thus reducing heat dissipation.
Given the overall importance of thyroid hormone in the control of cardiovascular function (1), it was not surprising that TRα1+m mice displayed intrinsic vascular defects. Several other studies previously demonstrated that arterial compliance is strongly affected by thyroid hormone (24–26) and that TRα1 is important for vascular smooth muscle function (27), whereas capillary density is controlled by TRβ (28). However, using the temporal reactivation of TRα1 signaling in our animal model, we obtained interesting unique insight: Although the promoting effects of T3 on NO-mediated relaxation of vasculature in wild-type mice were observed as expected (20, 21), nonreversible alterations in NO-mediated relaxation and the purinergic component of contraction were surprisingly noted in the TRα1+m mice. These irreversible defects were not a consequence of impaired nongenomic TRα1 signaling in the mutants, because no differences were observed in the expression of the PI3-kinase target genes catalase and cyclin G2; however, whether they could indicate an important role for TRα1 during vascular development remains to be elucidated.
In contrast to the irreversible defects in relaxation, the contractility of TRα1+m tail arteries was normalized by T3 treatment, suggesting that it is a consequence of acutely disturbed TRα1 signaling. Our results concur with the previously reported reduced sensitivity to α1-adrenergic stimulation in isolated aortic rings of hypothyroid rats (29). Although the maximum contractile response upon high K+ was still lowered by T3, as previously also described for coronary arteries (27), the adrenergic sensitivity of the tail vasculature in the physiological range was restored by T3, which reversed the inappropriate heat dissipation. This finding provided us with a unique opportunity to test the hypothesis that an impaired vasoconstriction and consequently increased heat loss over the tail surface are the primary cause for the brown fat hyperactivity. Treatment with the selective α1-adrenoreceptor agonist midodrine, which does not pass the blood–brain barrier (30, 31), specifically normalized the tail artery constriction in the TRα1+m mice and reversed the excessive heat loss. Evidently, this eliminated the need for additional facultative brown fat thermogenesis in the mutant mice and subsequently normalized BAT temperature, UCP1 mRNA expression, histology, and oxygen consumption. Moreover, food intake also returned to wild-type levels, demonstrating that the hyperphagia of the TRα1+m animals is not a cause, but a secondary consequence of their hypermetabolism. That this hypermetabolism is paradoxically observed in an animal model of “receptor-mediated hypothyroidism” and not in classic hypothyroid rodents is likely explained by the intact TRβ signaling in the TRα1+m mice, which seems sufficient to partially maintain the compensatory thermogenesis.
Similar to the effects of the midodrine treatment, adrenergic sensitivity and brown fat hyperactivity were also restored by T3 treatment, confirming the importance of acute TRα1 signaling in this process. However, the interpretation of the T3 effects is complicated by the overall decrease in BAT temperature in both T3-treated groups, most likely due to the parallel increase in obligatory thermogenesis caused by the treatment (32). The complex interplay between heat dissipation and obligatory and facultative thermogenesis observed in TRα1+m mice underlines the requirement for a complete analysis to understand and evaluate experimental paradigms. Particularly in the thyroid field this is highly desirable, as the hormone exerts strong peripheral and central effects on thermogenesis and cardiovascular function (8, 9, 15). Although less sensitive than calorimetric analyses combined with BAT histology, infrared thermography is becoming increasingly accepted for the quantification of heat generation and dissipation in vivo (33–36), measuring all aspects of thermoregulation in a continuous noninvasive manner in the freely moving animal. This technology will therefore be beneficial in the reevaluation of previous paradigms to dissect direct brown fat actions from secondary effects induced by vascular alterations.
In addition to the implications for rodent models, our data are also of direct relevance for the understanding of human conditions: Patients with hypo- and hyperthyroid conditions often suffer from cold and heat intolerance, respectively (3). Although textbook knowledge attributes these symptoms to the alterations in basal metabolic rate, our results from TRα1+m mice suggest that defective heat conservation and dissipation processes contribute to the phenomenon. This underestimated connection between vascular function and energy expenditure may furthermore explain the metabolic problems in humans with pathological changes in TH signaling, for instance in nonthyroidal illness like sepsis or cancer (37) or in the recently discovered patients with a mutation in TRα1 (38, 39). However, because humans often successfully create an artificial thermoneutral environment through clothing—thus reducing the need for facultative thermogenesis—the metabolic consequences of impaired vascular function are likely less pronounced than in rodents.
Materials and Methods
Animal Husbandry.
Animal care procedures were conducted according to the guidelines of the European Community Council directives (86/609/EEC). Permission was obtained from the local ethical committee for all procedures performed. Adult male mice were kept under a 12-h light:12-h dark lighting regime at an ambient temperature of 21 ± 1 °C with standard chow and water ad libitum and were 4–6 mo old at the time of studies. For T3 treatment, wild-type and TRα1+m mice were provided with T3 in drinking water (0.5 μg/mL), leading to a 10-fold increase of serum T3 after 14 d (12). For midodrine treatment, wild-type and mutant mice were provided with the α1-adrenergic receptor agonist midodrine in drinking water for 6 d (equivalent dose of 1 mg⋅kg−1⋅d−1), according to previous studies and literature (30, 31). Body weight and food intake were measured daily throughout. For histology, BAT was dissected, frozen in isopentane immersed in liquid nitrogen, cut in 20-μm sections on a kryostat, and stained with hematoxylin and eosin. For the prazosin treatment, wild-type mice were provided with the α1-adrenergic receptor antagonist prazosin in drinking water for 6 d (equivalent dose of 8 mg⋅kg−1⋅d−1), according to previous studies and literature (40).
Core Body Temperature and Metabolic Cages.
Implantable radio transmitters (Mini Mitter Respironics) were used to determine core body temperature and locomotor activity of freely moving wild-type and TRα1+m mice. The mice were anesthetized using isoflurane, and transmitters were implanted into the peritoneal cavity. The animals were allowed to recover for 1 wk before baseline set recording for several days, and then core body temperature and locomotor activity were recorded over a 24-h period. Oxygen consumption was measured using metabolic cages (INCA metabolic system; Somedic) as described previously (10).
Infrared Thermography.
BAT temperature was measured at room temperature, using an infrared camera (FLIR Systems) according to previous publications (33–35, 41). Several infrared pictures were taken of the same animal during the light phase, while it was allowed to move freely on a cage lid. Infrared analysis software (FLIR Systems) was used to determine maximum temperature in the interscapular area across all pictures from the same animal. Temperatures of tail base and tip were measured on three separate days by infrared camera after the mouse had been allowed to acclimate in a restraint on a temperature-controlled platform for 10 min.
Thermal Hot Bridge Analyses.
Skin samples including fur of wild-type and TRα1+m mice were dissected from the interscapular area covering the brown fat and analyzed in a transient hot bridge instrument (Linseis System THB; Gammadata Instruments). A Kapton sensor type A was used to heat the sample, and the resulting temperature change was measured to determine heat conductivity and diffusivity. Heat capacity was calculated subsequently.
Contractile Response Studies.
Tail artery and small mesenteric arteries were excised in cold Krebs–Ringer solution (123 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgCl2, 20 mM NaHCO3, 2.5 mM CaCl2, 5.5 mM glucose). The arteries (with endothelium intact) were cut into rings (∼2 mm) and mounted in a wire myograph (610M; DMT), using stainless steel wires (40 µm diameter). The baths were filled with Krebs–Ringer solution and gassed with 95% (vol/vol) O2/5% (vol/vol) CO2 to give pH 7.4 at 37 °C. Vessel circumference (∼250–300 µm) was adjusted near optimal for active force and the vessels were allowed to equilibrate for 15–30 min. Vessels were activated with high K+ two to three times at 5-min time intervals to obtain initial contractile responses used to normalize tension of subsequent contractions. Vessels from the two genotypes were examined in parallel, and their responses to electrical field stimulation and challenges with contractile and relaxant agonists were recorded. Electrical field stimulation was performed via electrodes placed in the mounting jaws of the myograph (DMT) at 2-min intervals, using 0.3-ms pulses during 3-s trains, using the optimal voltage for eliciting contractile responses (42). Frequency–response curves were determined from 1 to 32 Hz. The maximal tension responses at each stimulation frequency were recorded (i) in the absence of blockers, (ii) after adding the α-adrenergic receptor blocker prazosin (1 µM), and (iii) after adding the purinoceptor antagonist α,β-methylene ATP (1 µM) in the presence of prazosin. Thereafter, a final high-K+ activation was performed to confirm the viability of the preparations. For dose–response curves, the α1-receptor agonist phenylephrine (from 30 nM to 10 μM) was added cumulatively at 5-min intervals. After washout the vessels were exposed to 300 µM NG-nitro-l-arginine methyl ester hydrochloride (l-NAME, a nitric oxide synthase inhibitor). The arteries were then precontracted with 10 µM phenylephrine and after 5 min, acetylcholine (from 30 nM to 10 µM) was added cumulatively at 5-min intervals. The relaxation was recorded and expressed relative to the initial phenylephrine (10 µM)-induced tension. The force (f) and phenylephrine concentration (c) data for each vessel were fitted by a hyperbolic equation f = m*ch/(ch+EC50h), using SigmaPlot 8.02 for Windows (Systat Software), where m is the extrapolated maximal force, h is a factor determining the steepness of the relation, and EC50 is the concentration giving half-maximal force. One animal was excluded from the analysis due to low-tension responses.
Quantitative Real-Time PCR.
RNA was isolated from snap-frozen tissues, using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. cDNA was generated using reverse transcription with Oligo-dT Primers (Invitrogen) and used for real-time PCR with the ABI 7300/7000 (Applied Biosystems). A standard curve was used to correct for PCR efficiency, and the results were normalized using HPRT as a reference gene. A melting curve was recorded to confirm the specificity of the reaction.
Tail Vascularization.
The vascularization was analyzed from fresh-frozen 40-μm tail cross sections (same distance from the tail as the tail base temperature recordings), using the FITC-labeled lectin from Bandeireae Simplicifolia as described elsewhere (28). Corresponding quadrants of the tail cross sections from wild-type and TRα1+m mice were documented with the Spot5.0 Software under a Nikon Eclipse E800 microscope, and the vascular density was analyzed with ImageJ (National Institutes of Health software).
Statistical Analysis.
Values are represented as mean ± SEM. Statistical testing was performed using an unpaired two-tailed Student’s t test or a two-way ANOVA where indicated. A P value of less than 0.05 was considered significant, and the respective levels of significance and group sizes are stated in Figs. 1–5.
Supplementary Material
Acknowledgments
We thank the staff of the CMB animal facility, in particular Emelie Brinkhead and Bettina Semsch, as well as Kristina Nordström and Cecilia Lövdahl for their technical assistance, and Dr. Henriette Kirchner for supporting the metabolic data analysis. Parts of the experiments were performed at the Karolinska Institute’s core facility for Physiological Gene Function. We are grateful for financial support from the Swedish Research Council (A.W., B.V., A.A., and J.M.), The Swedish Cancer Society and Söderbergsstifelse (B.V.), the Karolinska Institute’s Foundation (A.W. and J.M.), and Lillian Sagens och Curt Ericssons Stiftelse (J.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310300110/-/DCSupplemental.
References
- 1.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344(7):501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 2.Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev. 2010;15(2):125–132. doi: 10.1007/s10741-008-9125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva JE. The thermogenic effect of thyroid hormone and its clinical implications. Ann Intern Med. 2003;139(3):205–213. [PubMed] [Google Scholar]
- 4.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12(4):287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 5.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214(Pt 2):242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 6.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enerbäck S. Human brown adipose tissue. Cell Metab. 2010;11(4):248–252. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Warner A, Mittag J. Thyroid hormone and the central control of homeostasis. J Mol Endocrinol. 2012;49(1):R29–R35. doi: 10.1530/JME-12-0068. [DOI] [PubMed] [Google Scholar]
- 9.López M, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16(9):1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjögren M, et al. Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor alpha1. EMBO J. 2007;26(21):4535–4545. doi: 10.1038/sj.emboj.7601882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro MO, et al. Thyroid hormone—sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform—specific. J Clin Invest. 2001;108(1):97–105. doi: 10.1172/JCI12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinnikov A, et al. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor alpha1. EMBO J. 2002;21(19):5079–5087. doi: 10.1093/emboj/cdf523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vennström B, Mittag J, Wallis K. Severe psychomotor and metabolic damages caused by a mutant thyroid hormone receptor alpha 1 in mice: Can patients with a similar mutation be found and treated? Acta Paediatr. 2008;97(12):1605–1610. doi: 10.1111/j.1651-2227.2008.01031.x. [DOI] [PubMed] [Google Scholar]
- 14.Gordon CJ. Temperature Regulation in Laboratory Rodents. Cambridge, UK: Cambridge Univ Press; 1993. [Google Scholar]
- 15.Mittag J, Davis B, Vujovic M, Arner A, Vennström B. Adaptations of the autonomous nervous system controlling heart rate are impaired by a mutant thyroid hormone receptor-alpha1. Endocrinology. 2010;151(5):2388–2395. doi: 10.1210/en.2009-1201. [DOI] [PubMed] [Google Scholar]
- 16.Wallis K, et al. Locomotor deficiencies and aberrant development of subtype-specific GABAergic interneurons caused by an unliganded thyroid hormone receptor alpha1. J Neurosci. 2008;28(8):1904–1915. doi: 10.1523/JNEUROSCI.5163-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodde OE, Bruck H, Leineweber K. Cardiac adrenoceptors: Physiological and pathophysiological relevance. J Pharmacol Sci. 2006;100(5):323–337. doi: 10.1254/jphs.crj06001x. [DOI] [PubMed] [Google Scholar]
- 18.Grote L, Kraiczi H, Hedner J. Reduced alpha- and beta(2)-adrenergic vascular response in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1480–1487. doi: 10.1164/ajrccm.162.4.9912028. [DOI] [PubMed] [Google Scholar]
- 19.Toews ML, Prinster SC, Schulte NA. Regulation of alpha-1B adrenergic receptor localization, trafficking, function, and stability. Life Sci. 2003;74(2–3):379–389. doi: 10.1016/j.lfs.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Carrillo-Sepúlveda MA, et al. Thyroid hormone stimulates NO production via activation of the PI3K/Akt pathway in vascular myocytes. Cardiovasc Res. 2010;85(3):560–570. doi: 10.1093/cvr/cvp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiroi Y, et al. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci USA. 2006;103(38):14104–14109. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuya F, Lu C, Guigon CJ, Cheng SY. Nongenomic activation of phosphatidylinositol 3-kinase signaling by thyroid hormone receptors. Steroids. 2009;74(7):628–634. doi: 10.1016/j.steroids.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oellerich MF, Potente M. FOXOs and sirtuins in vascular growth, maintenance, and aging. Circ Res. 2012;110(9):1238–1251. doi: 10.1161/CIRCRESAHA.111.246488. [DOI] [PubMed] [Google Scholar]
- 24.Taddei S, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: Beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab. 2003;88(8):3731–3737. doi: 10.1210/jc.2003-030039. [DOI] [PubMed] [Google Scholar]
- 25.Dagre AG, et al. Arterial stiffness is increased in subjects with hypothyroidism. Int J Cardiol. 2005;103(1):1–6. doi: 10.1016/j.ijcard.2004.05.068. [DOI] [PubMed] [Google Scholar]
- 26.McAllister RM, Albarracin I, Jasperse JL, Price EM. Thyroid status and endothelium-dependent vasodilation in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R284–R291. doi: 10.1152/ajpregu.00061.2003. [DOI] [PubMed] [Google Scholar]
- 27.Makino A, Wang H, Scott BT, Yuan JX, Dillmann WH. Thyroid hormone receptor-α and vascular function. Am J Physiol Cell Physiol. 2012;302(9):C1346–C1352. doi: 10.1152/ajpcell.00292.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makino A, et al. Thyroid hormone receptor-beta is associated with coronary angiogenesis during pathological cardiac hypertrophy. Endocrinology. 2009;150(4):2008–2015. doi: 10.1210/en.2008-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pantos C, et al. Decreased vascular reactivity to alpha1 adrenergic stimulation in the presence of hypothyroid state: A part of an adaptive response? Int Angiol. 2006;25(2):216–220. [PubMed] [Google Scholar]
- 30.Kaufmann H, Brannan T, Krakoff L, Yahr MD, Mandeli J. Treatment of orthostatic hypotension due to autonomic failure with a peripheral alpha-adrenergic agonist (midodrine) Neurology. 1988;38(6):951–956. doi: 10.1212/wnl.38.6.951. [DOI] [PubMed] [Google Scholar]
- 31.Virno M, Pecori-Giraldi J, Pellegrino N, Palombaro S, Garofalo G. Effects of midodrine on intraocular pressure and on the permeability of the blood-aqueous barrier in the rabbit. Eur Rev Med Pharmacol Sci. 1987;9(1):107–110. [PubMed] [Google Scholar]
- 32.Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5(6):481–492. doi: 10.1089/thy.1995.5.481. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann SM, et al. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J Clin Invest. 2007;117(11):3271–3282. doi: 10.1172/JCI31929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SJ, et al. A functional role for the p62-ERK1 axis in the control of energy homeostasis and adipogenesis. EMBO Rep. 2010;11(3):226–232. doi: 10.1038/embor.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller TD, et al. p62 links β-adrenergic input to mitochondrial function and thermogenesis. J Clin Invest. 2013;123(1):469–478. doi: 10.1172/JCI64209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whittle AJ, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149(4):871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: An update. J Endocrinol. 2010;205(1):1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 38.van Mullem A, et al. Clinical phenotype and mutant TRα1. N Engl J Med. 2012;366(15):1451–1453. doi: 10.1056/NEJMc1113940. [DOI] [PubMed] [Google Scholar]
- 39.Bochukova E, et al. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med. 2012;366(3):243–249. doi: 10.1056/NEJMoa1110296. [DOI] [PubMed] [Google Scholar]
- 40.Williams JL, Cartland D, Hussain A, Egginton S. A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. J Physiol. 2006;570(Pt 3):445–454. doi: 10.1113/jphysiol.2005.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittag J, et al. Thyroid hormone is required for hypothalamic neurons regulating cardiovascular functions. J Clin Invest. 2013;123(1):509–516. doi: 10.1172/JCI65252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borovik A, Golubinskaya V, Tarasova O, Aalkjaer C, Nilsson H. Phase resetting of arterial vasomotion by burst stimulation of perivascular nerves. J Vasc Res. 2005;42(2):165–173. doi: 10.1159/000084405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.