Significance
Unlike in animals, postembryonic development in plants is highly flexible and allows them to modulate their growth patterns in response to external signals or as part of endogenous developmental programs. Differential cell elongation is a widely used developmental program used in plants to respond to external and endogenous signals. Asymmetric distribution of the plant hormone indole-acetic acid (auxin) mediated by plasma membrane localized auxin carriers is crucial for differential cell elongation. Our results identify distinct mechanisms for trafficking of auxin influx and efflux carriers from the post-Golgi compartment trans-Golgi network to the plasma membrane during differential cell elongation in which the trans-Golgi network–localized ECHIDNA protein plays a key role by acting at the level of secretory vesicles genesis.
Keywords: sorting, IAA, morphogenesis
Abstract
The plant hormone indole-acetic acid (auxin) is essential for many aspects of plant development. Auxin-mediated growth regulation typically involves the establishment of an auxin concentration gradient mediated by polarly localized auxin transporters. The localization of auxin carriers and their amount at the plasma membrane are controlled by membrane trafficking processes such as secretion, endocytosis, and recycling. In contrast to endocytosis or recycling, how the secretory pathway mediates the localization of auxin carriers is not well understood. In this study we have used the differential cell elongation process during apical hook development to elucidate the mechanisms underlying the post-Golgi trafficking of auxin carriers in Arabidopsis. We show that differential cell elongation during apical hook development is defective in Arabidopsis mutant echidna (ech). ECH protein is required for the trans-Golgi network (TGN)–mediated trafficking of the auxin influx carrier AUX1 to the plasma membrane. In contrast, ech mutation only marginally perturbs the trafficking of the highly related auxin influx carrier LIKE-AUX1-3 or the auxin efflux carrier PIN-FORMED-3, both also involved in hook development. Electron tomography reveals that the trafficking defects in ech mutant are associated with the perturbation of secretory vesicle genesis from the TGN. Our results identify differential mechanisms for the post-Golgi trafficking of de novo-synthesized auxin carriers to plasma membrane from the TGN and reveal how trafficking of auxin influx carriers mediates the control of differential cell elongation in apical hook development.
Polar auxin transport (PAT) plays a key role in plant development (1–5). PAT is mediated by plasma membrane localized auxin influx and efflux carriers of the auxin-resistant (AUX)/like-AUX (LAX), pin-formed (PIN), and ABCB families (6–12). Highly regulated tissue, cellular localization, and amount of auxin carriers at the plasma membrane (PM) provide directionality to the auxin transport and underlies the creation of auxin concentration gradient that is essential for controlling several aspects of plant development (13–18). One of the developmental programs in which auxin concentration gradient plays a central role is the formation of apical hook, a bending in the embryonic stem during early seedling germination (19). Hook formation involves differential elongation of cells on the two opposite sides of the hypocotyl. This process is mediated by the formation of an auxin maximum at the concave side of the hook, leading to the inhibition of cell elongation (20–25). A model based on mutational analysis shows that auxin carriers including polarly localized auxin efflux and influx facilitators PIN3 and AUX1/LAX3, respectively, are important for hook development (23, 24). The amount of auxin carriers at the PM is important for the regulation of auxin concentration, and this depends on the balance between secretion, endocytosis, and recycling. The analysis of PIN efflux carriers has revealed how cell wall anchoring, endocytosis, targeted degradation, and also posttranslational modifications strongly influence the location and amount of these carriers at the PM (15, 17, 26–29). In contrast, little is known about the mechanisms and molecular components underlying the deposition of auxin carriers at the PM. Post-Golgi secretion to the PM occurs via the trans-Golgi network (TGN), a post-Golgi compartment (30). The TGN is a complex tubulo-vesicular membrane network maturing from the trans-most cisternae of the Golgi apparatus to become a highly dynamic independent structure from which secretory vesicles (SVs) and CLATHRIN-coated vesicles (CCVs) originate (31–34). Although auxin carriers traffic via TGN, components and mechanisms specifically involved in trafficking to the PM of de novo-synthesized auxin carriers remain largely undefined (35, 36). Importantly, it is not known whether auxin carriers traffic through SV or CCV sites of the TGN on their way to the PM. We have used apical hook development as a model system to investigate the mechanisms that link post-Golgi trafficking of auxin carriers to the PM with control of differential cell elongation. We previously identified the transmembrane TGN-localized protein ECHIDNA (ECH) that is required for cell elongation (37). We discovered that the ech mutant is defective in hook development and is insensitive to ethylene like the aux1 mutant. These data prompted us to investigate the role of ECH and the TGN in post-Golgi trafficking of auxin carriers during hook development. Using genetic, pharmacological, and cell biological approaches, we show that distinct mechanisms/components underlie post-Golgi trafficking of influx and efflux carriers. We show that post-Golgi trafficking of de novo-synthesized AUX1 occurs via an ECH-dependent SV-based pathway, whereas that of PIN3 and LAX3 are largely independent of ECH at the TGN. Thus, these results reveal the complexity of trafficking from the TGN to PM as shown by the differential trafficking of influx carriers AUX1 versus LAX3 and the efflux carrier PIN3. Hence, our results reveal an additional layer of regulatory control to auxin transport.
Results
ECHIDNA Protein Is Required for Ethylene-Mediated Differential Cell Elongation During Apical Hook Development.
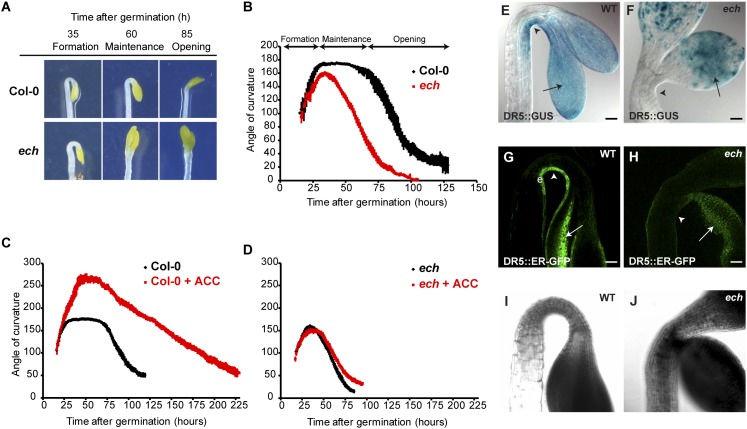
Hypocotyl and root-cell elongation defects were previously described in the ech mutant (37). We additionally found defects in apical hook development in dark-grown ech seedlings. In the WT, shortly after germination (about 15 h), during the formation phase, the hypocotyl progressively bends to establish an apical hook with an angle around 175° (ref. 21 and Fig. 1 A and B). This angle is stabilized during the maintenance phase (Fig. 1 A and B). Subsequently, about 60 h after germination, during the opening phase, a progressive opening of the hook occurs to reach a hook value around 20° (Fig. 1 A and B). In ech, the formation phase occurs at a rate similar to that of WT but the hook angle peaks at a maximum value of 160° and immediately starts to decrease, completely abolishing the maintenance phase (Fig. 1 A and B). Because the maintenance phase was previously shown to be ethylene-mediated (21, 23, 24), we investigated whether a treatment with the ethylene precursor aminocyclopropane-1-carboxylate (ACC) suppresses ech hook defect. In the WT, ACC treatment prolongs the formation phase, creating an exaggerated hook angle of around 260° (Fig. 1C). The ech mutant was insensitive to ACC treatment; no exaggerated hook was observed after ACC treatment (Fig. 1D). These results indicate that ECH is required for ethylene-mediated differential cell elongation in hook development.
Fig. 1.
ECHIDNA is involved in the apical hook maintenance phase and in the ethylene and auxin response. (A) WT and ech mutant dark-grown seedlings during distinct stages of apical hook development. (B–D) Kinematic analyses of apical hook angles show that (B) compared with WT, ech mutant is defective in maintenance phase. (C) Compared with untreated WT, 10 µM ACC treatment exaggerates the formation phase. (D) ech mutant treated with 10 µM ACC does not result in exaggerated hook. (E) In WT, DR5::GUS is detected in the concave side of apical hook (arrowheads) and in cotyledons (arrows). (G) In WT, DR5::ER-GFP tissue localization pattern is restricted to epidermal (e) cells of the concave side of the hook (arrowhead). In ech mutant, DR5::GUS (F) and DR5::ER-GFP (H) are visualized in cotyledons (arrows) but not in apical hook (arrowheads). (I and J) Transmission picture of G and H, respectively. (Scale bars in E–H, 50 µm).
Auxin Response Maxima in Hook Is Severely Attenuated in ech Mutant.
Defects in hook development and insensitivity of ech to ethylene prompted us to investigate the establishment of auxin response maxima in ech. The auxin response maxima visualized by the synthetic auxin-responsive DR5 promoter fused to reporter genes GUS (DR5::GUS) or endoplasmic reticulum-targeted GFP (DR5::ER-GFP) is constrained to the concave side of the hook in the WT from the end of the formation phase (48 h after germination) to the end of the maintenance phase (72 h after germination) (refs. 23 and 24 and Fig. 1 E, G, and I and Fig. S1 A–D and I). In contrast with the WT, severe attenuation of auxin response maxima was observed in ech already at the end of the formation phase with almost no reporter signal observed in the epidermis on the concave side of the hook at 48 h and 72 h after germination (Fig. 1 F, H, and J and Fig. S1 E–H and I).
AUX1 Is Required for Ethylene-Mediated Apical Hook Differential Growth and Genetically Interacts with ECH.
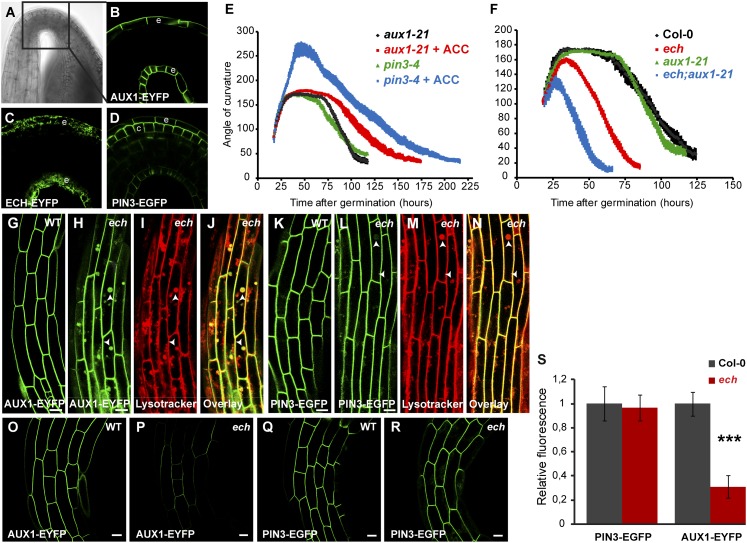
The formation of the auxin response maximum on the concave side of the hook is mediated by the coordinated action of auxin carriers including auxin influx carrier AUX1 and the auxin efflux carrier PIN3 (23, 24). Around 50 h after germination, AUX1–YFP tissue localization (Fig. 2 A and B) is restricted to the epidermal cell layer, whereas PIN3 tissue localization is restricted to the epidermal and cortical cell layer of the hook in the WT (Fig. 2 A and D). Notably, ECH–YFP localization is also present in the epidermis of the hook, resembling the AUX1–YFP tissue localization pattern (Fig. 2 A and C). Moreover, the hook development of the aux1-21 mutant is insensitive to ACC (23) as in ech (Fig. 2E). Unlike aux1-21, the pin3-4 mutant responds to ACC treatment similarly to the WT (Fig. 2E). These results strongly suggest that ECH and AUX1, but not PIN3, act in a common pathway in which ethylene is an upstream regulator. To test this possibility we constructed a double mutant between ech and aux1-21. The double mutant ech;aux1-21 showed an enhancement of the hook development defects compared with the single mutants ech or aux1-21 (Fig. 2F). The synergistic effect of ECH and AUX1 on apical hook development is consistent with the notion that ECH and AUX1 act in the same pathway but ECH presumably has additional targets as well.
Fig. 2.
The auxin influx carrier AUX1 genetically interacts with ECH and is mislocalized in the ech mutant. (A–D) In WT hook region (black box in transmission picture in A) AUX1-YFP (B) and ECH–YFP (C) are localized in the epidermal (e) cell layer whereas PIN3–GFP (D) is localized in the cortical (c) and epidermal (e) cell layers. (E) Kinematic analyses of hook angles of aux1-21 and pin3-4 mutants seedlings untreated or treated with 10 µM ACC. (F) Kinematic analyses of hook angles of WT, aux1-21, and ech single mutants and ech;aux1-21 double mutant. (G–N) As compared with WT (G and K), ech hook epidermal cells accumulate AUX1–YFP (H) in intracellular spherical compartments that colabel with Lysotracker Red (I and J; arrowheads in H–J), whereas PIN3–GFP (L) displays only a faint signal in these Lysotracker Red-positive compartments (M and N; arrowheads in L–N). (O–R) Confocal pictures of AUX1–YFP (O and P) and PIN3–GFP (Q and R) fluorescence in apical hook epidermal cells acquired under the same acquisition settings between WT (O and Q) and ech (P and R). (S) Plasma membrane fluorescence intensities quantification from experiments in O–R. (Scale bars, 5 µm in G–N, and 10 µm in O–R.)
ECHIDNA Is Required for the Targeting of AUX1 to the Plasma Membrane.
The epidermal expression of ECH and AUX1–YFP and the genetic interaction between them led us to analyze the subcellular distribution of AUX1–YFP in ech and WT background. In WT hook epidermal cells, AUX1 is predominantly located at the PM with almost no intracellular signal detected, whereas strong intracellular localization of AUX1-YFP is observed in ech (Fig. 2 G and H). Costaining of intracellular spherical AUX1–YFP structures in ech with the low-pH-associated fluorescent dye Lysotracker Red reveals their acidic nature (Fig. 2 H–J). In contrast with AUX1–YFP, PIN3–GFP localization in ech is nearly identical to that in the WT with only a minor fraction localizing intracellularly (Fig. 2 K–N). Furthermore the AUX1–YFP fluorescence at the PM in the ech is reduced to about 30% of the WT in the hook and in the hypocotyl (Fig. 2 O, P, and S and Fig. S2). By contrast, PIN3 fluorescence at the PM in the ech was not significantly reduced compared with the WT (Fig. 2 Q, R, and S). All together, our results suggest that post-Golgi trafficking of AUX1 and PIN3 via TGN is differentially mediated, with AUX1 trafficking to PM requiring ECH function.
ECHIDNA-Mediated Trafficking Is Involved in Sorting of the Auxin Influx Carrier AUX1 from the TGN.
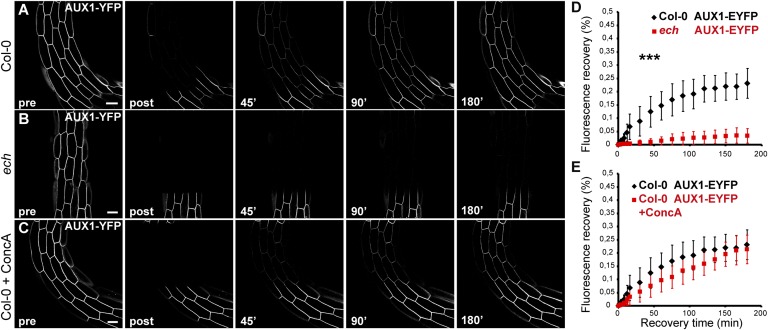
The reduction in the levels of AUX1–YFP at the PM in the ech led us to investigate whether trafficking of AUX1 to the PM is defective in ech. Therefore, we performed full-cell photobleaching by confocal microscopy on apical hook epidermal cells and quantified the fluorescence recovery after photobleaching (FRAP) of AUX1 and PIN3 at the PM. Under these conditions, the recovery of fluorescence at the PM reflects the trafficking of neosynthesized proteins to the PM. In the WT, AUX1–YFP fluorescence recovery at the PM did not differ significantly from that of PIN3–GFP (Fig. 3A and Fig. S3 A and F). These results show that neosynthesized AUX1 and PIN3 trafficked to the PM at a similar rate. More strikingly, fluorescence recovery of AUX1–YFP in ech was drastically reduced to a level where almost no recovery was observed at the PM within 3 h after photobleaching (Fig. 3 A, B, and D). We also performed FRAP analyses on a 5-µm section of the PM in WT and ech expressing AUX1–YFP upon pretreatment with the protein synthesis inhibitor cyclohexamide to investigate potential PM recycling and lateral diffusion of AUX1 without interference from de novo protein synthesis. Our results clearly show that under these experimental conditions recovery at the PM does not differ in ech compared with WT (Fig. S4). In contrast with AUX1 trafficking to PM in fully bleached cells, PIN3–GFP fluorescence recovery at the PM, although statistically distinct from the WT, was only marginally affected in ech (Fig. S3 A, B, and G). We also investigated whether ECH is required for the trafficking to PM of the auxin influx carrier LAX3, another member of the AUX/LAX family that is also involved in hook development. Because LAX3 is not expressed in the hook itself, we performed FRAP on the cells of the lower part of the hypocotyl where LAX3–YFP expression is detected. Our results show that LAX3–YFP recovery at the PM is only marginally reduced in ech compared with the WT (Fig. S3 D, E, and I). Overall, these results demonstrate that ECH is predominantly involved in deposition of de novo-synthesized AUX1 at the PM, whereas its contribution to PIN3 or LAX3 trafficking to PM is minor.
Fig. 3.
FRAP-monitored deposition of AUX1 to the plasma membrane in the ech mutant background or upon concA. Apical hook epidermal cells (14 cells from n = 7 individual seedlings) expressing AUX1–YFP (A–C) were imaged before photobleaching (pre), after photobleaching (post), and during recovery after photobleaching at indicated time (over 180 min) in WT (A), ech mutant (B), and upon pretreatment with 10 µM concA for 1.5 h before photobleaching (C). (D) Recovery of AUX1–YFP in WT (A and D) is highly different from recovery of AUX1–YFP in the ech mutant (B and D). (E) ConcA pretreatment does not result in statistical difference in recovery curve of WT seedlings expressing AUX1–YFP (A, C, and E). (All scale bars, 5 µm.)
TGN-Mediated Trafficking of AUX1 and PIN3 to the Plasma Membrane Is Independent of V-ATPases.
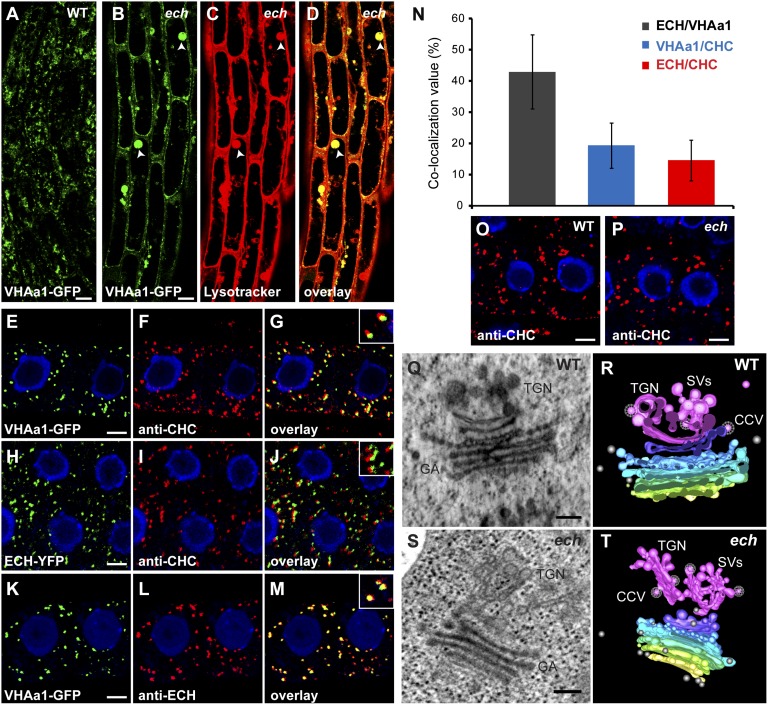
It has been suggested that the cell elongation defects in ech could be due to the mislocalization of VHA-a1, a V-ATPase that is a key component of the TGN required for secretion and endocytosis (37, 38). Hence, we investigated whether the defects in apical hook development in the ech could be due to the altered TGN function resulting from the mislocalization of VHA-a1. As in roots, we observed a mislocalization of VHA-a1 in ech hook cells (Fig. 4 A and B). Colabeling of VHA-a1–GFP with Lysotracker Red revealed that VHA-a1, similarly to AUX1, is strongly mislocalized to acidic spherical structures in ech hook (Fig. 4 B–D). To further investigate whether VHA-a1 was involved in hook development, we used concanamycin A (concA), a specific inhibitor of V-ATPases (39). Three-day-old WT dark-grown seedlings treated with 0.5 µM concA displayed a markedly reduced hypocotyl growth (62% reduction of the hypocotyl length compared with the nontreated samples) (Fig. S5A), indicating that the inhibitor was functioning. When hook development was analyzed, 0.5 µM concA treatment did affect its development, but the effect was modest compared with ech (Fig. S5B). Following concA treatment the maintenance phase is slightly delayed (∼10 h after) and slightly shortened (by ∼10 h) compared with the untreated control (Fig. S5B). All together, these results indicate that although VHA-a1 is required for apical hook development, its overall contribution to this process is minor and its mislocalization is not the major factor for defects in hook development in ech. We also investigated whether inhibition of VHA-a1 affects the trafficking of AUX1 and PIN3 to the PM. FRAP analysis after pharmacological interference with V-ATPases using 10 µM concA pretreatment for 90 min before photobleaching does not significantly affect fluorescence recovery of either AUX1–YFP or PIN3–GFP at the PM (Fig. 3 A, C, and E and Fig. S3 A, C, and H). To examine that the concA pretreatment is functional, we analyzed the distribution of VHA-a1–GFP-labeled compartments in the apical hook region in the presence of concA. Compared with untreated samples, in which VHA-a1 compartments are homogeneously distributed within the cell, 10 µM concA pretreatment for 90 min results in agglomeration of VHA-a1 signal (Fig. S5 C and D). Furthermore, we did not detect obvious mislocalization of either AUX1 or PIN3 upon 10 µM concA pretreatment for 90 min (Fig. S5 E–H). These results indicate that AUX1 and PIN3 trafficking to the PM is independent of VHA-a1 function.
Fig. 4.
ECHIDNA acts at SV/VHAa1 sites rather than at CHC sites of TGN. (A–D) In apical hook epidermal cells, compared with the WT (A) VHA-a1–GFP is mislocalized to vacuolar-like structures (arrowheads) in ech mutant (B), which colocalized strongly (arrowheads) with Lysotracker Red (C and D). (E–N) In roots, VHA-a1–GFP-labeled structures (E) or ECH–YFP-positive compartments (H) are often associated with structures recognized by anti-CHC (F and I) but rarely colocalize (G and J; see the magnification in the top right corner box). (K–M) VHA-a1–GFP compartments (K) and anti–ECH-positive structures (L) are found to strongly colocalize together (M; see the magnification in the top right corner box). (N) Quantification histogram of colocalization analyzed in E–M. (O and P) Immunolocalization of anti–CHC-labeled compartments in the WT (O) and the ech mutant (P). (Q–T) Electron tomography of WT (Q and R) and ech mutant (S and T) roots. (Q and S) Still images of WT (Q) and ech mutant (S). GA, Golgi apparatus. (R and T) Models of WT (R) and ech mutant (T) tomograms. The cis-citernae of the Golgi apparatus is labeled in yellow, medial-citernae are labeled in gradient from green to blue, the trans-citernae is labeled in purple, and the TGN is highlighted in pink. SVs, in pink, are budding from the TGN. CCVs are represented with a white meshwork over the vesicle. Free vesicles are labeled in gray. (Scale bars, 5 µm in A–M, 5 μm in O and P, and 200 nm in Q–T.)
ECHIDNA Resides Predominantly with SVs at the TGN.
The TGN is a complex structure from which both SVs and CCVs originate (31–34). Therefore, we investigated whether ECH-mediated trafficking at TGN of de novo-synthesized AUX1 proteins involves SVs or CCVs. Electron tomography of Arabidopsis roots indicates that VHA-a1–GFP resides on TGN sites that are rich in SVs (34). Whereas ECH-positive structures that colocalize with VHA-a1 can also correspond to SV sites on the TGN, it is not known whether ECH or VHA-a1 can also localize to CCV sites of TGN. To address this, we used a stringent confocal morphology-based quantitative approach to evaluate the colocalization percentage of VHA-a1- and ECH-positive structures with CLATHRIN HEAVY CHAIN (CHC)-positive structures in roots. Our results revealed that only a small fraction of VHA-a1 and ECH-labeled structures (19% and 14%, respectively) colocalized with CHC-positive structures (Fig. 4 E–J and N). Although ECH- or VHA-a1-labeled compartments are often found in close proximity to CHC-labeled compartments, these rarely colocalize with one another (Fig. 4 E–J and N). Interestingly, 42% of ECH-positive compartments colocalized with VHA-a1–GFP-positive structures (Fig. 4 K–N). These results suggest that ECH resides at the SV sites rather than the CCV sites of TGN. In agreement with this result, no perturbation of CHC-positive compartments is observed in ech roots, in contrast with the mislocalization of VHA-a1 (ref. 37 and Fig. 4 O and P). To further investigate whether ECH plays a role in SV morphology we performed high-resolution 3D electron tomography of the Golgi apparatus/TGN structures in the WT and ech roots. In WT, the TGN forms a complex network of budding secretory vesicles (ref. 34 and Fig. 4 Q and R and Movie S1). From this network, a fraction of associated vesicles display a characteristic clathrin-like protein coat, whereas other vesicles display features characteristic of SV because these do not exhibit a protein coat and are larger and more electron-dense (Fig. 4 Q and R and Movie S1). In contrast, in ech the TGN appears more tubular with fewer SVs than in the WT (5.75 ± 0.3 SVs per TGN in ech versus 9.76 ± 0.5 SVs per TGN in WT; Fig. 4 S and T, Fig. S6, and Movie S2), whereas clathrin-coated vesicles are not affected (0.55 ± 0.05 CCVs per TGN in ech versus 0.7 ± 0.06 CCVs per TGN in WT; Fig. 4 S and T, Fig. S6, and Movie S2). The TGN in ech is also stained less intensely, which could be indicative of reduced secretory cargo (Fig. 4 S and T and Movie S2). Thus, these results show that the loss of ECH attenuates the genesis of SVs at the TGN.
Discussion
In contrast to animals, plants display a highly flexible postembryonic development in which auxin-mediated differential cell elongation is used to modulate growth patterns, as exemplified by apical hook development. Differential accumulation of auxin mediated by polar, plasma membrane-localized auxin carriers is crucial for differential cell elongation during hook development (23, 24). We have used ech, a mutant in Arabidopsis, to reveal distinct mechanisms for post-Golgi trafficking of de novo-synthesized auxin influx and efflux carriers to the PM essential for differential cell elongation during apical hook development.
ECHIDNA Is Required for Ethylene-Mediated Control of Apical Hook and Genetically Interacts with AUX1.
The plant hormone ethylene plays a key role in hook development, and the maintenance phase of hook development requires ethylene action; this is completely abolished in ethylene-insensitive mutants (e.g., ein2) (40). Our results show that the maintenance phase of apical hook is severely attenuated in ech, as described for ethylene-insensitive mutants. Also, in contrast to the WT, ech fails to form exaggerated hook in response to ACC, a precursor of ethylene. Taken together, these data establish ECH, a TGN-localized protein, as a unique component required for ethylene-mediated control of hook development. Downstream of ethylene, differential cell elongation requires the formation of auxin response maxima on the concave side of the apical hook (22–25). Consistent with this, we observed a strong perturbation of auxin response maxima in ech on the concave side of the hook already at the end of the formation phase and during the maintenance phase. The auxin influx carrier AUX1, acting redundantly with LAX3, and the auxin efflux carrier PIN3 were shown to be critical for hook development (23, 24). Moreover, in agreement with previous data, in our conditions we observed that, like ech, the aux1-21 mutant, but not pin3-4 mutant, is insensitive to an ACC treatment. These results indicate that ECH and AUX1, rather than PIN3, could act in a common pathway. Interestingly, the kinematic analysis of hook development revealed a synergistic effect of ech when combined with the aux1-21 mutation on hook development compared with either of the single mutants. These results strongly suggest that whereas AUX1 could be one of the components of the ECH-mediated pathway, this pathway also involves additional elements.
ECHIDNA Is Required for Post-Golgi Trafficking of AUX1 from the TGN to the PM.
Genetic analysis indicated that AUX1, but not PIN3, requires ECH for hook development. In agreement with this, our quantification of AUX1 and PIN3 intensities at the PM shows that AUX1, but not PIN3, is less abundant at the PM during the maintenance phase in ech. Moreover, unlike PIN3, a strong intracellular AUX1 signal was observed in ech. Strikingly, FRAP results revealed that the trafficking of de novo-synthesized AUX1 to PM via TGN requires ECH. Our experiments involving FRAP on a small portion of PM (Fig. S4) suggest that lateral mobility of AUX1 at the PM may not require ECH. However, these experiments do not allow us to fully rule out the possibility that ECH is not involved in PM recycling of AUX1. In contrast to AUX1, ECH seems only marginally involved in the deposition of PIN3 and LAX3 at the PM from the TGN. Recently, several proteins of the RAB family have been shown to be involved in the trafficking of auxin carriers via the TGN, including BEX5/RABA1b and RABA1c (35, 36). However, in contrast with ECH, these proteins affect transport of both AUX1 and PIN proteins equally. Moreover, whether these RABs are involved in recycling to PM or deposition of de novo-synthesized auxin carriers at the PM via TGN is not entirely clear. Thus, our results reveal a potential mechanism underlying a differential post-Golgi trafficking of auxin influx carriers AUX1 and LAX3 and the auxin efflux carrier PIN3 from the TGN in which ECH acts predominantly in AUX1trafficking.
Post-Golgi Trafficking of AUX1 and PIN3 Is Independent of V-ATPases During Apical Hook Development.
ECH strongly colocalizes with VHA-a1 at the TGN (37). VHA-a1 is a key component of the TGN and, like ECH, has been previously shown to be involved in hypocotyl cell elongation (38, 39, 41). Moreover, inhibition of V-ATPases with the specific inhibitor concA was previously reported to inhibit the secretion of newly synthesized plasma membrane-localized steroid receptor BRI1 (38). Importantly, our results show a strong mislocalization of VHA-a1–GFP in ech apical hook. We have previously suggested that growth and other cell biological defects in ech could be explained at least partly by mislocalization of VHA-a1 (37). However, this study provides two lines of evidence that VHA-a1 mislocalization is not likely to constitute a major underlying factor for defects in ech hook development. First, our results show that the effect of concA on hook development remains modest as compared with the ech phenotype. Second, our FRAP results revealed that concA does not significantly affect the delivery of either AUX1 or PIN3 to the PM, suggesting that the trafficking of these auxin carriers to PM, although dependent on ECH, is independent of V-ATPases, including VHA-a1.
ECH Predominantly Localizes to and Affects SV Formation at the TGN.
The TGN is a complex network of tubulo-vesicular membranes with distinct TGN subdomains (34, 42, 43). Diverse microscopy methods have further shown that SVs and CCVs arise from distinct subdomains of the TGN (31–34). Therefore, an important question was whether ECH acts at the SVs or CCVs, or both, in post-Golgi trafficking. Transmission electron tomography has revealed that VHA-a1 resides at SV sites of TGN (34). Our quantitative morphologically based method revealed that VHA-a1 and ECH colocalize only marginally with CHC-labeled structures, whereas a higher percentage of colocalization is observed between ECH- and VHA-a1-positive structures. These results suggest that ECH associates with VHA-a1/SV sites rather than CHC/CCV sites at the TGN. In agreement with ECH localization data and trafficking defects, electron tomography and quantification of SVs revealed that whereas SVs are significantly reduced CCVs are unaffected in ech. The EM tomography data suggest a role for ECH in SV genesis and explain the TGN-to-PM trafficking defects in ech. Our data are indicative of differential trafficking routes taken by auxin carriers, with AUX1 but neither LAX3 nor PIN3 trafficking via SVs to reach the PM. AUX1 and LAX3 are highly similar at the amino acid sequence level. However, LAX3 is unable to functionally replace AUX1 (44). Our data showing that AUX1 and LAX3 could traffic via distinct pathways at the TGN provide another explanation for why the LAX proteins cannot rescue AUX1 function despite high similarity at the sequence level as well as why LAX3 is mistargeted even when expressed under the AUX1 promoter. It is not clear how proteins such as AUX1 and PIN3 that localize differentially at the PM can be trafficked via the same compartment. Our data suggest that although ECH and VHA-a1 localized to the same subdomain of the TGN, and although both are involved in post-Golgi trafficking pathway, ech mutation affects trafficking of de novo-synthesized AUX1, whereas inhibition of VHA-a1 has little effect on that process. This result suggests that even within the VHA-a1 subdomain of the TGN there could be further differentiation, with trafficking of some cargo depending on ECH and of other on VHA-a1. Thus, our results reveal the complexity and differentiation of the post-Golgi trafficking pathway at the TGN and add a previously uncovered level of complexity in addition to that for the endocytosis pathway to regulate polar auxin transport.
Experimental Procedures
Plant Material and Growth Conditions.
The Arabidopsis thaliana ecotype and mutants used are described in SI Experimental Procedures. Plant growth conditions were as described (37). Seeds were sown on ½ Murashige and Skoog (MS) agar medium plates (0.8% plant agar, 1% sucrose, and 2.5 mM Mes, pH 5.8 with KOH) and left at 4 °C for 2 d, followed by 6 h of white light treatment. For time-lapse analysis of apical hook development, plates were subsequently placed vertically in a dark room at 22 °C where the only source of light was a far-infrared light. Further details are provided in SI Experimental Procedures. For other experiments, plates were wrapped in aluminum foil after the 6-h white light treatment and left in the growth chamber at 22 °C for the indicated time. For experiments performed in roots, seeds were sown on ½ MS agar medium plates, left at 4 °C for 2 d, and then grown in light for 5 d before experiments.
Inhibitor Treatments.
Concanamycin A (Sigma-Aldrich) was added from a 10 mM stock in DMSO; 1-aminocyclopropane-1-carboxylate (ACC; Sigma-Aldrich) was added from a 10 mM stock in DMSO. Further details are provided in SI Experimental Procedures.
Histochemical Visualization of GUS activity.
Three-day-old dark-grown seedlings were used. Detailed procedures are described in SI Experimental Procedures. Seedlings were mounted in glycerol and imaged using differential interference contrast on a Zeiss Axioplan 2 microscope equipped with an AxioCam and Axiovision software.
Lysotracker Red Staining, Immunocytochemistry, and Confocal Laser-Scanning Microscopy.
Lysotracker Red was used at 1 µM final concentration (SI Experimental Procedures gives details). Immunocytochemistry on roots was performed using 5-d-old Arabidopsis light-grown seedlings as previously described (37); SI Experimental Procedures gives details. All confocal laser-scanning microscopy experiments were carried out using a Carl Zeiss LSM780 with a 40× lens (C-Apochromat 40×/1.2 W Corr M27).
Quantitative Analyses of Plasma Membrane Intensity and Colocalization.
Quantification of AUX1–YFP and PIN3–GFP fluorescence intensities at the plasma membrane of apical hook epidermal cells was performed using strictly identical confocal acquisition parameters between the WT Columbia-0 and ech. Colocalization quantification was performed using geometrical object-based method as described previously (43). Further details are provided in SI Experimental Procedures.
Cryofixation and Transmission Electron Tomography.
Detailed procedures for high-pressure freezing, freeze substitution, sectioning, poststaining, imaging, and quantification are given in SI Experimental Procedures.
FRAP Experiments.
The bleaching mode of the Carl Zeiss LSM780 software was used for the bleaching by placing a frame over a cell of interest and direct neighboring cells to monitor exclusively the deposition rate to the plasma membrane through the secretion pathway. Further protocol description is provided in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Malcolm Bennett, Karin Schumacher, and Klaus Palme for sharing published research materials that were important for this study. We also thank Urs Fischer for helpful comments on the paper. This work was supported by grants from the Knut and Alice Wallenberg Foundation and the Swedish University of Agricultural Sciences (Excellence) (to R.P.B.) and a Natural Sciences and Engineering Research Council of Canada Discovery grant (to L.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309057110/-/DCSupplemental.
References
- 1.Okada K, Ueda J, Komaki M-K, Bell C-J, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3(7):677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabatini S, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99(5):463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 3.Benková E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115(5):591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 4.Friml J, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426(6963):147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 5.Grieneisen V-A, Xu J, Marée A-F, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449(7165):1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- 6.Bennett M-J, et al. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science. 1996;273(5277):948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- 7.Gälweiler L, et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282(5397):2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 8.Luschnig C, Gaxiola R-A, Grisafi P, Fink G-R. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12(14):2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noh B, Murphy A-S, Spalding E-P. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 2001;13(11):2441–2454. doi: 10.1105/tpc.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrásek J, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312(5775):914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Hammes U-Z, Taylor C-G, Schachtman D-P, Nielsen E. High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol. 2006;16(11):1123–1127. doi: 10.1016/j.cub.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Mravec J, et al. Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development. 2008;135(20):3345–3354. doi: 10.1242/dev.021071. [DOI] [PubMed] [Google Scholar]
- 13.Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413(6854):425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 14.Wisniewska J, et al. Polar PIN localization directs auxin flow in plants. Science. 2006;312(5775):883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 15.Friml J, et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306(5697):862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- 16.Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell. 2006;18(11):3171–3181. doi: 10.1105/tpc.106.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michniewicz M, et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130(6):1044–1056. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Dhonukshe P, et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature. 2008;456(7224):962–966. doi: 10.1038/nature07409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Harpham N-V-J, et al. The effect of ethylene on the growth and development of WT and mutant Arabidopsis thaliana (L.) Heynh. Ann Bot (Lond) 1991;68:55–61. [Google Scholar]
- 20.Lehman A, Black R, Ecker J-R. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85(2):183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- 21.Raz V, Ecker J-R. Regulation of differential growth in the apical hook of Arabidopsis. Development. 1999;126(16):3661–3668. doi: 10.1242/dev.126.16.3661. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Johnson P, Stepanova A, Alonso J-M, Ecker J-R. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell. 2004;7(2):193–204. doi: 10.1016/j.devcel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbussche F, et al. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development. 2010;137(4):597–606. doi: 10.1242/dev.040790. [DOI] [PubMed] [Google Scholar]
- 24.Zádníková P, et al. Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development. 2010;137(4):607–617. doi: 10.1242/dev.041277. [DOI] [PubMed] [Google Scholar]
- 25.Gallego-Bartolomé J, et al. Hierarchy of hormone action controlling apical hook development in Arabidopsis. Plant J. 2011;67(4):622–634. doi: 10.1111/j.1365-313X.2011.04621.x. [DOI] [PubMed] [Google Scholar]
- 26.Abas L, et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006;8(3):249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- 27.Kleine-Vehn J, et al. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci USA. 2008;105(46):17812–17817. doi: 10.1073/pnas.0808073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paciorek T, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435(7046):1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 29.Feraru E, et al. PIN polarity maintenance by the cell wall in Arabidopsis. Curr Biol. 2011;21(4):338–343. doi: 10.1016/j.cub.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths G, Simons K. The trans Golgi network: Sorting at the exit site of the Golgi complex. Science. 1986;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- 31.Hillmer S, Freundt H, Robinson D-G. The partially coated reticulum and its relationship to the Golgi apparatus in higher plant cells. Eur J Cell Biol. 1988;47:206–212. [Google Scholar]
- 32.Staehelin L-A, Giddings T-H, Jr, Kiss J-Z, Sack F-D. Macromolecular differentiation of Golgi stacks in root tips of Arabidopsis and Nicotiana seedlings as visualized in high pressure frozen and freeze-substituted samples. Protoplasma. 1990;157(1–3):75–91. doi: 10.1007/BF01322640. [DOI] [PubMed] [Google Scholar]
- 33.Viotti C, et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell. 2010;22(4):1344–1357. doi: 10.1105/tpc.109.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang B-H, Nielsen E, Preuss M-L, Mastronarde D, Staehelin L-A. Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic. 2011;12(3):313–329. doi: 10.1111/j.1600-0854.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- 35.Feraru E, et al. BEX5/RabA1b regulates trans-Golgi network-to-plasma membrane protein trafficking in Arabidopsis. Plant Cell. 2012;24(7):3074–3086. doi: 10.1105/tpc.112.098152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi X, Zheng H. Arabidopsis TRAPPII is functionally linked to Rab-A, but not Rab-D in polar protein trafficking in trans-Golgi network. Plant Signal Behav. 2011;6(11):1679–1683. doi: 10.4161/psb.6.11.17915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gendre D, et al. Conserved Arabidopsis ECHIDNA protein mediates trans-Golgi-network trafficking and cell elongation. Proc Natl Acad Sci USA. 2011;108(19):8048–8053. doi: 10.1073/pnas.1018371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dettmer J, Hong-Hermesdorf A, Stierhof Y-D, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18(3):715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brüx A, et al. Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth Inhibition in Arabidopsis. Plant Cell. 2008;20(4):1088–1100. doi: 10.1105/tpc.108.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guzmán P, Ecker J-R. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2(6):513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von der Fecht-Bartenbach J, et al. Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J. 2007;50(3):466–474. doi: 10.1111/j.1365-313X.2007.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chow C-M, Neto H, Foucart C, Moore I. Rab-A2 and Rab-A3 GTPases define a trans-golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate. Plant Cell. 2008;20(1):101–123. doi: 10.1105/tpc.107.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boutté Y, et al. Endocytosis restricts Arabidopsis KNOLLE syntaxin to the cell division plane during late cytokinesis. EMBO J. 2010;29(3):546–558. doi: 10.1038/emboj.2009.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Péret B, et al. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell. 2012;24(7):2874–2885. doi: 10.1105/tpc.112.097766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.