Abstract
Griscelli syndrome (GS), a rare autosomal recessive disorder, is characterized by partial albinism, along with immunologic abnormalities or severe neurological impairment or both. Mutations in one of two different genes on chromosome 15q can cause the different subtypes of GS. Most patients with GS display the hemophagocytic syndrome and have mutations in RAB27A, which codes for a small GTPase. Two patients with neurological involvement have mutations in MYO5A, which codes for an actin-based molecular motor. The RAB27A and MYO5A gene products interact with each other and function in vesicle trafficking. We report the molecular basis of GS in a Muslim Arab kindred whose members have extremely variable neurological involvement, along with the hemophagocytic syndrome and immunologic abnormalities. The patients have normal MYO5A genes but exhibit a homozygous 67.5-kb deletion that eliminates RAB27A mRNA and immunocytofluorescence-detectable protein. We also describe the molecular organization of RAB27A and a multiplex polymerase chain reaction assay for the founder deletion in this kindred. Finally, we propose that all patients with GS have RAB27A mutations and immunologic abnormalities that sometimes result in secondary neurological involvement. The two patients described elsewhere who have MYO5A mutations and neurological complications but no immunologic defects may not have GS but instead may have Elejalde syndrome, a condition characterized by mild hypopigmentation and severe, primary neurological abnormalities.
Griscelli syndrome (GS [MIM 214450]) is a rare autosomal recessive disorder characterized by pigmentary dilution of the skin, due to abnormal melanosomal transport, and by silvery gray hair, due to pigment clumping in hair shafts. Most patients develop the hemophagocytic syndrome, which is characterized by uncontrolled activation of T lymphocytes and macrophages and which leads to death if not treated by bone marrow transplantation (BMT). Some patients show severe neurological impairment early in life, with or without apparent immune abnormalities (Griscelli et al. 1978; Hurvitz et al. 1993; Klein et al. 1994; Menasche et al. 2000).
Recently, GS was reported to consist of two different subtypes, resulting from defects in two separate genes, MYO5A and RAB27A (Pastural et al. 2000). Myosin 5a, the product of MYO5A on chromosome 15q21 (Pastural et al. 1997), is an unconventional myosin motor protein that moves along actin filaments and functions in intracellular vesicle transport (Metha et al. 1999). Among humans with GS, one patient exhibited a missense change in MYO5A (Pastural et al. 1997), but this was later shown to be a polymorphism (Lambert et al. 2000). Both remaining reported patients who had GS with mutated MYO5A (patients P3 [Pastural et al. 1997] and P8 [Pastural et al. 2000]) exhibited primarily neurological impairment without immune defects.
A second genetic cause of GS was discovered because a large number of patients did not show a mutation in MYO5A. Linkage analysis indicated the existence of this second locus in the q21 region of chromosome 15 (Pastural et al. 2000), and physical mapping and mutation analysis identified the RAB27A gene, <1.6 cM from MYO5A (Menasche et al. 2000). The rab27a protein is a small GTPase that functions in the targeting and the fusion of transport vesicles with their appropriate acceptor membranes. Like other rab proteins, rab27a requires geranylgeranylation of two consensus C-terminal cysteine residues in order to be anchored to membranes. Truncation of the carboxy-terminal part of rab27a would render it inactive (Kinsella and Maltese 1992; Chen et al. 1997; Novick and Zerial 1997; Pfeffer et al. 2001). To date, all patients with GS with mutations in RAB27A have developed the hemophagocytic syndrome.
In accordance with the paradigm that patients with GS with neurological involvement have mutations in MYO5A and that those with the hemophagocytic syndrome have mutations in RAB27A (Pastural et al. 2000; Westbroek et al. 2001), the kindred with GS reported by Hurvitz et al. (1993) was expected to exhibit MYO5A mutations. Results of the present study show that members of this kindred have, instead, a large deletion in RAB27A. We suggest that the neurological involvement in these patients with GS occurs secondarily to the hemophagocytic syndrome and that patients with primary CNS complications and MYO5A mutations have a related disorder, namely, Elejalde syndrome.
The patient described in the present study was part of a highly consanguineous Muslim Arab family, in which Griscelli syndrome was diagnosed (Hurvitz et al. 1993). This family includes four affected children: a brother (patient IV, our proband) and a sister (patient I) in one family and their two female first cousins (patients II and III). All four individuals died in childhood, at ages 11.5 years, 10 mo, 6 years, and 2 years, respectively. All had silvery hair. Skin biopsies revealed normal numbers of melanocytes containing normally sized melanin granules; it was suggested that no melanosomes were transferred to the surrounding keratinocytes (Hurvitz et al. 1993). Patients I, II, and III presented with recurrent vomiting; patient I had an acute febrile illness, and patient III presented with lethargy. All three patients deteriorated neurologically after their initial presentation, as indicated by regression of mental and physical function. The clinical course of patient IV was characterized by periodic episodes of seizures, from the age of 1 age, and by mild cognitive delay. At the age of 2 years 10 mo, patient IV experienced an episode similar to the accelerated phase described by Griscelli et al. (1978) and recovered completely. He was well and had no recurrent infections, and his neurological status was stable until age 8 years. He was not monitored between the ages of 8 years and 10.5 years, after which he was repeatedly admitted to a local hospital because of prolonged fever, severe hepatosplenomegaly, and pancytopenia. His condition deteriorated, and he developed ascites, generalized edema, and jaundice and died at the age of 11.5 years.
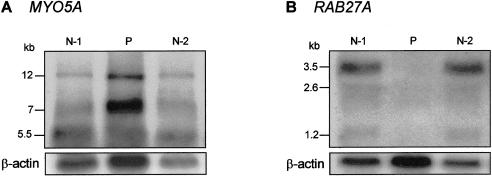
Genomic DNA and RNA were isolated from cultured skin fibroblasts of patient IV. The entire coding region of MYO5A cDNA (GenBank accession number U90942) of the patient and several normal control individuals was amplified by PCR in eight overlapping fragments (primer sequences available upon request), followed by direct sequencing. This revealed no MYO5A cDNA mutations in the patient. We then performed a northern blot, using a MYO5A tail region fragment (nt 3735–4628) as the hybridization probe. This blot showed a normal size and amount of each of the three alternatively spliced fibroblast MYO5A transcripts (12 kb, 7 kb, and 5.5 kb) in patient IV (fig. 1A). The increased intensity of the MYO5A bands in patient IV corresponds to the increased β-actin signal, indicating that more of the patient’s RNA was loaded on the gel.
Figure 1.
Northern blot analysis of MYO5A and RAB27A expression. Total RNA extracts (20 μg) of two normal individuals (N-1, N-2) and of patient IV with Griscelli syndrome (P) were separated on a formaldehyde gel, were blotted to a nylon membrane, and were hybridized with a [32P]-dCTP–labeled DNA probe to MYO5A (A) and to RAB27A (B). Both blots were also labeled with a β-actin probe (lower panels), which served as a control for the amount of loaded RNA. The patient has abundant MYO5A RNA and no RAB27A RNA.
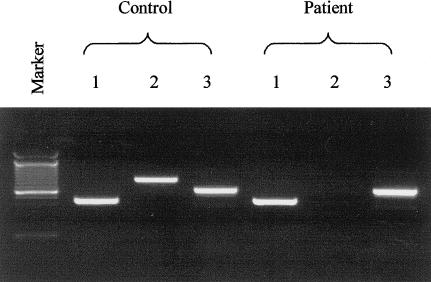
Next, we attempted to amplify RAB27A, using cDNA from patient IV and from normal control subjects as template (forward primer 5′-TGAAGCATTGGTAACTCCAG-3′ and reverse primer 5′-TCTCACTGTGCCATGTATCAA-3′). No PCR product was amplified for the patient (fig. 2). Subsequent northern blot analysis with a RAB27A cDNA hybridization probe (GenBank accession number U38654; nt 155–990) showed no expression of the 3.5-kb major transcript of RAB27A in fibroblasts from the patient (fig. 1B).
Figure 2.
cDNA amplification of RAB27A and the adjacent genes, LOC51187 and PIGB. PCR products of portions of LOC51187 (lane 1), RAB27A (lane 2), and PIGB (lane 3) obtained by use of cDNA from an unaffected control subject (left) and patient IV (right). The patient has normal amplification of LOC51187 and PIGB but no signal for RAB27A.
Genomic DNA of the patient and several control individuals was then PCR amplified for each exon of the RAB27A gene (GenBank accession number AF443871), using primer sequences for exons 2–6 as reported by Tolmachova et al. (1999). For amplification of the exon 1 region, we used forward primer 5′-GTGCCATATGACAAACAGCTG-3′ and reverse primer 5′-CTCCGTGGTCACTGAAAATGC-3′. All six exons of RAB27A (Tolmachova et al. 1999) were successfully amplified by use of control gDNA. However, amplification of the patient’s genomic DNA yielded product only for exon 6, while exons 1–5 could not be amplified.
Amplification of the patient’s cDNA for genes neighboring RAB27A (i.e., PIGB and LOC51187) yielded the expected bands (fig. 2). For PIGB (GenBank accession number NM_004855) amplification, the forward primer was 5′-CGAAAGAGAAAGTCTACCTTGTAC-3′, and the reverse primer was 5′-CAAAGGTGTCCACAGAATGACAGC-3′. For LOC51187 (GenBank accession number XM_046136) amplification, the forward primer was 5′-CATCTATCCTGGACACGGCATG-3′, and the reverse primer was 5′-GAGCATCTTCCATGTCCACATC-3′. These results pointed to a large deletion extending from its 5′ breakpoint in the region between PIGB and exon 1 of RAB27A (∼49-kb) to its 3′ breakpoint in intron 5 of the RAB27A gene (∼18 kb).
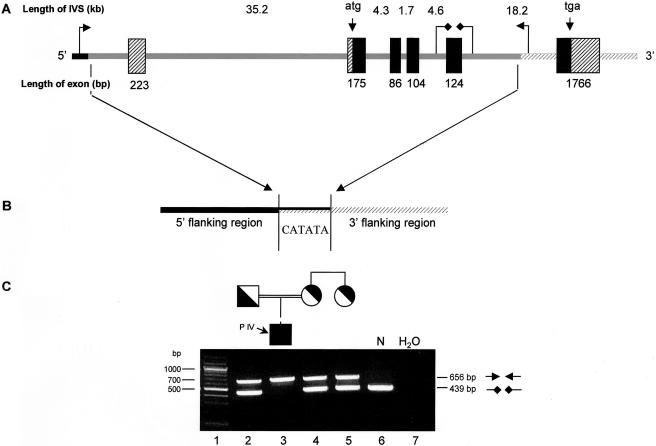
To identify the exact deletion breakpoints, we first determined the organization of the RAB27A gene, including the complete intronic sequences, which were previously unreported. Two overlapping BACs (GenBank accession numbers AC011912 and AC018926) covered the entire RAB27A region. Using these BACs and the intron/exon boundaries reported by Tolmachova et al. (1999), we identified the complete RAB27A gDNA (submitted to GenBank as accession number AF443871), which spans ∼86.6 kb of DNA. We then designed PCR primers to amplify short fragments (∼200–300 bp) at intervals of ∼3 kb throughout the region of the predicted RAB27A deletion, and the breakpoint locations were narrowed until we were able to amplify a fragment encompassing the breakpoints. The sequencing of this fragment revealed a PCR product that lacked 67,536 bp, including all of exons 1–5 and the predicted gene-promoter region. A 6-bp sequence repeat on each end of the deletion exists only once in the affected individual (fig. 3).
Figure 3.
Structure of the RAB27A deletion and multiplex PCR amplification assay for its presence or absence. A, Schematic basis of the 67,536-bp deletion. The deletion (shaded line) contains exons 1–5 (black and crossed boxes), as well as the predicted promoter region, and is flanked by normal sequence. Intron sizes and locations of the start and stop codons are shown above the gene, and exon sizes are shown below. B, The deletion breakpoint occurs somewhere within the 6-bp region shared at either end of the deletion. PCR amplification across the deletion yields a 656-bp fragment. Two primers within the deleted area (diamond-headed arrows) are used to amplify the DNA of alleles lacking the deletion; the product is a 439-bp fragment. C, Multiplex PCR in the affected family. PCR amplification products indicate the presence (656-bp fragment) or absence (439-bp fragment) of the 67,536-bp deletion. Lane 1, molecular weight markers; lane 2, father of patient IV, heterozygous for the deletion; lane 3, patient IV, homozygous for the deletion; lane 4, mother of patient IV, heterozygous for the deletion; lane 5, aunt of patient IV, heterozygous for the deletion; lane 6, normal control, lacking the deletion; lane 7, no-DNA control.
To detect the presence or absence of the 67.5-kb deletion (fig. 3), a multiplex PCR amplification assay was designed (fig. 3). We used two primer sets in a standard PCR amplification with an annealing temperature of 58°C. The primer set flanking the deletion resulted in a 656-bp fragment (forward primer 5′-GGACAGTGTATGCTTCCTTTCG-3′ and reverse primer 5′-GGGAGTCCTGCAATTGAATTCAG-3′), and the primer set within the deleted area yielded a 439-bp fragment (forward primer 5′-CTGATGCTTTGAAAGCATTATG-3′ and reverse primer 5′-GCATTGTCAATGGATCCTAC-3′).
Multiplex PCR screening of patient IV’s parents and an aunt who had two affected children (patients I and II) confirmed that all three were carriers of the 67.5-kb deletion (fig. 3). To screen for the presence of the 67.5-kb deletion in the Palestinian population, 86 anonymous samples of Palestinian origin were obtained from the National Laboratory for the Genetics of Israeli Populations at Tel Aviv University. None of these samples revealed the deletion.
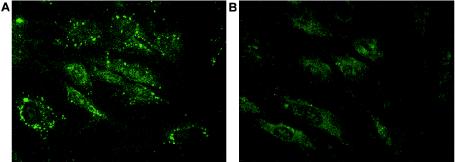
Immunocytofluorescence studies were performed to show the presence and distribution of the RAB27A protein in the fibroblasts of patient IV and normal unaffected control subjects (fig. 4). Fibroblasts were grown on glass coverslips and were fixed for 10 min in 2% formaldehyde in PBS. The cells were then incubated for 1 h with mouse monoclonal antibodies to RAB27A (Transduction Laboratories) that were diluted in PBS, 0.2% saponin (wt/vol), and 0.1% BSA (wt/vol) and were washed with PBS. The cells were then incubated for 45 min with FITC-conjugated donkey anti-mouse secondary antibodies (Jackson Immunoresearch), also diluted in PBS/saponin/BSA. After the cells were washed a second time in PBS, coverslips were mounted onto glass slides with Fluoromont G (Southern Biotechnology Associates). Microscopy was performed on a Zeiss Axioscop-20 with a FITC filter (Carl Zeiss, Inc.) Normal fibroblasts showed a distinct punctated distribution of the rab27a protein throughout the cell, similar to the distribution pattern in melanocytes reported by Hume et al. (2001). In contrast, there was no visible rab27a signal in fibroblasts from the patient (fig. 4).
Figure 4.
Intracellular rab27a distribution detected by indirect immunocytofluorescence. Normal (A) and patient (B) fibroblasts were grown on coverslips and were fixed and permeabilized before incubation with rab27a antibodies. FITC-conjugated secondary antibodies were used for fluorescent labeling. Rab27a shows a distinct punctated distribution throughout the normal fibroblasts. The patient’s fibroblasts, lacking rab27a, show only background fluorescence.
Three different rare, autosomal recessive disorders are characterized by hypopigmentation and silvery hair in infancy or early childhood (table 1). Chediak-Higashi syndrome (CHS [MIM 214500]) presents with variable hypopigmentation of the skin, hair, and eyes, a bleeding diathesis due to platelet-storage-pool deficiency, slowly progressive neurological dysfunction, and severe immunodeficiency (Introne et al. 1999; Ward et al. 2000). CHS cells show giant lysosomes, melanosomes, lytic granules, and azurophilic granules (Kjeldsen et al. 1998; Ward et al. 2000). Platelet-dense granules are absent or reduced in number (Rendu et al. 1983). The gene responsible for CHS, LYST, encodes a cytoplasmic protein of ∼430 kD (Barbosa et al. 1996; Nagle et al. 1996). Expression studies of LYST suggest a role in membrane fusion or fission events (Perou et al. 1997), and the large size of the LYST protein and its cytoplasmic location also argue for a role in vesicle transport. The beige mouse is the murine counterpart of CHS (Barbosa et al. 1996; Perou et al. 1996).
Table 1.
Syndromes That Involve Silvery Hair [Note]
| Characteristic | GS | ES | Chediak-Higashi |
| Inheritance | Autosomal recessive | Autosomal recessive | Autosomal recessive |
| Hair color | Silvery/metallic | Silvery/metallic | Silvery/metallic |
| Age at onset | Infancy/early childhood | Infancy/early childhood | Infancy/early childhood |
| Skin pigment dilution | + | + | + |
| Eye pigment dilution | +/− | +/− | + |
| Accelerated phase | +++ | − | ++ |
| Immune defects | ++ | − | +++ |
| Giant granules | − | − | ++ |
| Neurological involvement | + | +++ | + |
| No. of patients reported | >40 | >10 | ∼200 |
| Gene | RAB27A | MYO5A? | LYST |
| Mouse model | dilute | ashen | beige |
| Bleeding diathesis | − | − | + |
| Treatment | BMT | − | BMT |
Note.— + = present; − = absent; +/− = may or may not be present; ++ = a significant feature of disease; and +++ = the primary characteristic of disease.
Elejalde syndrome (ES [MIM 256710]), also called “neuroectodermal melanolysosomal disease,” is characterized by silvery hair, pigment abnormalities, and severe dysfunction of the CNS, with seizures, severe hypotonia, and mental retardation (Elejalde et al. 1979; Sanal et al. 2000). Patients with ES do not manifest the hemophagocytic syndrome or immunologic defects. Large granules of melanin are unevenly distributed in hair shafts, and abnormal melanosomes may occur in melanocytes, along with inclusion bodies in fibroblasts (Duran-McKinster et al. 2000). No gene has been reported to be associated with ES, nor has any mouse model been identified.
Patients with GS exhibit the accelerated phase and immunologic dysfunction seen in CHS but not in ES. In addition, some patients with GS have neurological involvement that shows extremely variable expression and severity. Two genes, RAB27A and MYO5A, which are separated by only ∼3 mB on chromosome 15, have been reported to cause GS. In mice, Myo5a mutations correspond with the dilute phenotype (Mercer et al. 1991), whereas Rab27a mutations cause the ashen phenotype (Wilson et al. 2000). These two mouse models, along with leaden (which has mutations in melanophilin [Matesic et al. 2001]), provide a unique system for studying vesicle transport in mammals. All three mice have a lightened coat color because of defects in pigment granule transport (Swank et al. 1998), and all three are suppressed by the semidominant dilute-suppressor (dsu) (Moore et al. 1988). Genetic evidence indicates that the gene products of dilute, ashen, and leaden function in the same or overlapping pathways, and molecular and cell-biological studies show that they interact in effecting melanosome trafficking in the distal region of melanocyte dendrites (Wu et al. 2001; Hume et al. 2002; Provance et al. 2002).
It has been reported—and accepted by the medical community—that patients who have GS with immunologic defects have RAB27A mutations and that those with neurological involvement have MYO5A mutations (Pastural et al. 2000; Westbroek et al. 2001). We present an alternative explanation. Specifically, we propose that all patients with RAB27A mutations have GS and that neurological complications in these individuals occur secondarily to lymphocytic and histiocytic infiltration of the brain (Gogus et al. 1995). Patients with MYO5A mutations, then, have ES with primary neurological involvement, which occurs early in life and in the absence of any signs of the hemophagocytic syndrome.
Several lines of evidence support this thesis. First, the original report of MYO5A mutations in GS provides information on only two patients. The first of these, designated patient 2, had an R1246C substitution which later proved to be a polymorphism (Lambert et al. 2000). The second, designated patient 3, was homozygous for a nonsense mutation in MYO5A, an observation that predicted a truncated protein lacking 1,087 amino acids. However, this patient was described by the authors as having “a severe neurological disorder, similar to the neuroectodermal melanolysosomal disease described by Elejalde et al.” (Pastural et al. 1997, p. 290). There was no history of the hemophagocytic syndrome or of immunologic defects in patient 3, who most likely had ES, not GS.
Second, the initial linkage studies that identified MYO5A as a candidate GS-causing gene were misleading because of the extremely close proximity of RAB27A to MYO5A on chromosome 15. In fact, the majority of the patients with GS who were used to identify MYO5A as a GS-causing gene actually had RAB27A mutations. At the same time, patients with MYO5A mutations would not disrupt the linkage analysis, so their diagnosis of GS was not questioned. However, these individuals may, in fact, have had ES.
Third, the only other patient (P8) found to have mutations in MYO5A had a clinical presentation consistent with ES and not with GS (Pastural et al. 2000). The child had profound CNS deficits but no immunologic problems and no evidence of hemophagocytic syndrome.
Fourth, the expression patterns of RAB27A and MYO5A are consistent with the clinical phenotypes of GS and ES, respectively. RAB27A is not expressed in brain tissue, in keeping with the lack of primary neurological abnormalities in GS. In addition, rab27a-deficient T cells exhibit reduced cytotoxity and cytolytic granule exocytosis required for immune homeostasis (Menasche et al. 2000; Stinchcombe et al. 2001), consistent with the occurrence of the hemophagocytic syndrome of GS. In contrast, some splice products of MYO5A are expressed in brain tissue (Huang et al. 1998; Lambert et al. 1998), and those that are expressed in brain tissue may not interact with rab27a (Wu et al. 2002).
Finally, the molecular analysis of patient IV and his family is consistent with our proposal. Because of his neurological involvement, patient IV was expected to have mutations in MYO5A. However, the neurological symptoms of the affected kindred varied extensively in age at onset and rate of progression. Furthermore, the hemophagocytic syndrome antedated the neurological complications, so histiocytic and lymphocytic invasion could have caused the CNS involvement. Indeed, the MYO5A gene was normal in sequence and expression in our patient, who instead exhibited a large homozygous deletion in RAB27A. We eliminated the possibility of a contiguous gene syndrome by showing normal expression of the flanking genes, PIGB and LOC51187, and we conclude that our patient has genuine GS due to a RAB27A mutation.
The identification of the breakpoints of the large deletion present in the family of patient IV has provided a useful tool for genetic counseling of this kindred. In addition, we are now able to screen for the mutation among unaffected individuals. That the mutation was absent in 86 anonymous Palestinian individuals is not surprising. Although a low carrier frequency for the mutation would produce this result, it can also be explained by the marriage practices of the isolated kindreds that constitute the Palestinian Arab world. High rates of consanguinity and the rarity of interfamily marriage prevent the spread of genetic variants through the population, causing unequal distribution of many autosomal recessive disorders (Zlotogora 1997; Zlotogora et al. 2000).
As more patients with GS or ES are studied at the molecular level, the clinical and genetic distinctions between these two entities will be resolved. Because prevention of the hemophagocytic syndrome by BMT may be lifesaving for patients with GS and an unnecessary risk for patients with ES, the distinction is critical. We suggest that patients with MYO5A defects not be assigned the diagnosis of GS at this time.
Acknowledgment
Y.A. is a Howard Hughes Medical Institute Physician Postdoctoral Fellow.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for RAB27A gDNA [accession number AF443871], RAB27A cDNA [accession number U38654], MYO5A cDNA [accession number U90942], PIGB cDNA [accession number NM_004855], and LOC51187 cDNA [accession number XM_046136])
- Map Viewer, http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/map_search (for RAB27A, MYO5A, PIGB, and LOC51187 location on chromosome 15)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for GS [MIM 214450], ES [MIM 256710], and Chediak-Higashi syndrome [MIM 214500])
References
- Barbosa MD, Nguyen QA, Tchernev VT, Ashley JA, Detter JC, Blaydes SM, Brandt SJ, Chotai D, Hodgman C, Solari RC, Lovett M, Kingsmore SF (1996) Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature 382:262–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Guo J, Miki T, Tachibana M, Gahl WA (1997) Molecular cloning and characterization of rab27a and rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem Mol Med 60:27–37 [DOI] [PubMed] [Google Scholar]
- Duran-McKinster C, Rodriguez-Jurado R, Ridaura C, de la Luz Orozco-Covarrubias M, Tamayo L, Ruiz-Maldonando R (1999) Elejalde syndrome—a melanolysosomal neurocutaneous syndrome: clinical and morphological findings in 7 patients. Arch Dermatol 135:182–186 [DOI] [PubMed] [Google Scholar]
- Elejalde BR, Holguin J, Valencia A, Gilbert EF, Molina J, Marin G, Arango LA (1979) Mutations affecting pigmentation in man. I. Neuroectodermal melanolysosomal disease. Am J Med Genet 3:65–80 [DOI] [PubMed] [Google Scholar]
- Gogus S, Topcu M, Kucukali T, Akcoren Z, Berkel I, Ersoy F, Gunay M, Saatci I (1995) Griscelli syndrome: report of three cases. Pediatr Pathol Lab Med 15:309–319 [DOI] [PubMed] [Google Scholar]
- Griscelli C, Durandy A, Guy-Grand D, Daguillard F, Herzog C, Prunicras M (1978) A syndrome associating partial albinism and immunodeficiency. Am J Med 65:691–702 [DOI] [PubMed] [Google Scholar]
- Huang JD, Mermall V, Strobel MC, Russell LB, Mooseker MS, Copeland NG, Jenkins NA (1998) Molecular genetic dissection of mouse unconventional myosin-VA: tail region mutations. Genetics 148:1963–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume AN, Collinson LM, Hopkins CR, Strom M, Barral DC, Bossi G, Griffiths GM, Seabra MC (2002) The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic 3:193–202 [DOI] [PubMed] [Google Scholar]
- Hume AN, Collinson LM, Rapak A, Gomes AQ, Hopkins CR, Seabra MC (2001) Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol 152:795–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvitz H, Gillis R, Klaus S, Klar A, Gross-Kieselstein F, Okon E (1993) A kindred with Griscelli disease: spectrum of neurological involvement. Eur J Pediatr 152:402–405 [DOI] [PubMed] [Google Scholar]
- Introne W, Boissy RE, Gahl WA (1999) Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab 68:283–303 [DOI] [PubMed] [Google Scholar]
- Kinsella BT, Maltese WA (1992) rab GTP-binding proteins with three different carboxyl-terminal cysteine motifs are modified in vivo by 20-carbon isoprenoids. J Biol Chem 267:3940–3945 [PubMed] [Google Scholar]
- Kjeldsen L, Calafat J, Borregaard N (1998) Giant granules of neutrophils in Chediak-Higashi syndrome are derived from azurophil granules but not from specific and gelatinase granules. J Leukoc Biol 64:72–77 [DOI] [PubMed] [Google Scholar]
- Klein C, Philippe N, Le Deist F, Fraitag S, Prost C, Durandy A, Fischer A, Griscelli C (1994) Partial albinism with immunodeficiency (Griscelli syndrome). J Pediatr 125:886–895 [DOI] [PubMed] [Google Scholar]
- Lambert J, Naeyaert JM, Callens T, De Paepe A, Messiaen L (1998) Human myosin V gene produces different transcripts in a cell type-specific manner. Biochem Biophys Res Commun 252:329–333 [DOI] [PubMed] [Google Scholar]
- Lambert J, Naeyaert JM, De Paepe A, Van Coster R, Ferster A, Song M, Messiaen L (2000) Arg-Cys substitution at codon 1246 of the human myosin Va gene is not associated with Griscelli syndrome. J Invest Dermatol 114:731–733 [DOI] [PubMed] [Google Scholar]
- Matesic LE, Yip R, Reuss AE, Swing DA, O'Sullivan TN, Fletcher CF, Copeland NG, Jenkins NA (2001) Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc Natl Acad Sci USA 98:10238–10243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G (2000) Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25:173–176 [DOI] [PubMed] [Google Scholar]
- Mercer JA, Seperack PK, Strobel MC, Copeland NG, Jenkins NA (1991) Novel myosin heavy chain encoded by murine dilute coat color locus. Nature 349:709–713 [DOI] [PubMed] [Google Scholar]
- Metha AD, Rock RS, Rief M, Spudich JA, Mooseker MS, Cheney RE (1999) Myosin-V is a processive actin-based motor. Nature 400:590–593 [DOI] [PubMed] [Google Scholar]
- Moore KJ, Swing DA, Rinchik EM, Mucenski ML, Buchberg AM, Copeland NG, Jenkins NA (1988) The murine dilute suppressor gene dsu suppresses the coat-color phenotype of three pigment mutations that alter melanocyte morphology, d, ash and ln. Genetics 119:933–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle DL, Karim MA, Woolf EA, Holmgren L, Bork P, Misumi DJ, McGrail SH, Dussault BJ Jr, Perou CM, Boissy RE, Duyk GM, Spritz RA, Moore KJ (1996) Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat Genet 14:307–311 [DOI] [PubMed] [Google Scholar]
- Novick P, Zerial M (1997) The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol 9:496–504 [DOI] [PubMed] [Google Scholar]
- Pastural E, Barrat FJ, Dufourcq-Lagelouse R, Certain S, Sanal O, Jabado N, Seger R, Griscelli C, Fischer A, de Saint Basile G (1997) Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat Genet 16:289–292 [DOI] [PubMed] [Google Scholar]
- Pastural E, Ersoy F, Yalman N, Wulffraat N, Grillo E, Ozkinay F, Tezcan I, Gedikoglu G, Philippe N, Fischer A, de Saint Basile G (2000) Two genes are responsible for Griscelli syndrome at the same 15q21 locus. Genomics 63:299–306 [DOI] [PubMed] [Google Scholar]
- Perou CM, Leslie JD, Green W, Li L, Ward DM, Kaplan J (1997) The Beige/Chediak-Higashi syndrome gene encodes a widely expressed cytosolic protein. J Biol Chem 272:29790–29794 [DOI] [PubMed] [Google Scholar]
- Perou CM, Moore KJ, Nagle DL, Misumi DJ, Woolf EA, McGrail SH, Holmgren L, Brody TH, Dussault BJ Jr, Monroe CA, Duyk GM, Pryor RJ, Li L, Justice MJ, Kaplan J (1996) Identification of the murine beige gene by YAC complementation and positional cloning. Nat Genet 13:303–308 [DOI] [PubMed] [Google Scholar]
- Pfeffer SR (2001) Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol 11:487–491 [DOI] [PubMed] [Google Scholar]
- Provance DW, James TL, Mercer JA (2002) Melanophilin, the product of the leaden locus, is required for targeting of myosin-Va to melanosomes. Traffic 3:124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendu F, Breton-Gorius J, Lebret M, Klebanoff C, Buriot D, Griscelli C, Levy-Toledano S, Caen JP (1983) Evidence that abnormal platelet functions in human Chediak-Higashi syndrome are the result of a lack of dense bodies. Am J Pathol 111:307–314 [PMC free article] [PubMed] [Google Scholar]
- Sanal O, Yel L, Kucukali T, Gilbert-Barnes E, Tardieu M, Texcan I, Ersoy F, Metin A, de Saint Basile G (2000) An allelic variant of Griscelli disease: presentation with severe hypotonia, mental-motor retardation, and hypopigmentation consistent with Elejalde syndrome (neuroectodermal melanolysosomal disorder). J Neurol 247:570–572 [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM (2001) Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol 152:825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank RT, Novak EK, McGarry MP, Rusiniak ME, Feng L (1998) Mouse models of Hermansky-Pudlak syndrome: a review. Pigment Cell Res 1:60–80 [DOI] [PubMed] [Google Scholar]
- Tolmachova T, Ramalho JS, Anant JS, Schultz RA, Huxley CM, Seabra MC (1999) Cloning, mapping and characterization of the human RAB27A gene. Gene 239:109–116 [DOI] [PubMed] [Google Scholar]
- Ward D, Griffiths GM, Stinchcombe JC, Kaplan J (2000) Analysis of the lysosomal storage disease Chediak-Higashi syndrome. Traffic 11:816–822 [DOI] [PubMed] [Google Scholar]
- Westbroek W, Lambert J, Naeyaert JM (2001) The dilute locus and Griscelli syndrome: gateways towards a better understanding of melanosome transport. Pigment Cell Res 14:320–327 [DOI] [PubMed] [Google Scholar]
- Wilson SM, Yip R, Swing DA, O'Sullivan TN, Zhang Y, Novak EK, Swank RT, Russell LB, Copeland NG, Jenkins NA (2000) A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci USA 97:7933–7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Rao K, Bowers MB, Copeland NG, Jenkins NA, Hammer JA 3d (2001) Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J Cell Sci 114:1091–1100 [DOI] [PubMed] [Google Scholar]
- Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA 3d (2002) Identification of an organelle receptor for myosin-Va. Nat Cell Biol 4:271–278 [DOI] [PubMed] [Google Scholar]
- Zlotogora J (1997) Autosomal recessive diseases among Palestinian Arabs. J Med Genet 34:765–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotogora J, Shalev S, Habiballah H, Barjes S (2000) Genetic disorders among Palestinian Arabs: 3. Autosomal recessive disorders in a single village. Am J Med Genet 92:343–345 [DOI] [PubMed] [Google Scholar]