Significance
We provide an experimental demonstration that young infants possess abstract biological expectations about animals. Our findings represent a major breakthrough in the study of the foundations of human knowledge. In four experiments, 8-mo-old infants expected novel objects they categorized as animals to have filled insides. Thus, infants detected a violation when objects that were self-propelled and agentive were revealed to be hollow, or when an object that was self-propelled and furry rattled when shaken, as though mostly hollow. We describe possible characterizations of infants’ expectations about animals’ insides, including a characterization that emphasizes human predator–prey adaptations. We also discuss how infants’ expectation that animals have insides lays a foundation for the development of more advanced biological knowledge.
Keywords: infant cognition, conceptual development, self-propulsion, agency
Abstract
What are the developmental origins of our concept of animal? There has long been controversy concerning this question. At issue is whether biological reasoning develops from earlier forms of reasoning, such as physical and psychological reasoning, or whether from a young age children endow animals with biological properties. Here we demonstrate that 8-mo-old infants already expect novel objects they identify as animals to have insides. Infants detected a violation when an object that was self-propelled and agentive (but not an object that lacked one or both of these properties) was revealed to be hollow. Infants also detected a violation when an object that was self-propelled and furry (but not an object that lacked one or both of these properties) either was shown to be hollow or rattled (when shaken) as although mostly hollow. Young infants’ expectations about animals’ insides may serve as a foundation for the development of more advanced biological knowledge.
By the end of the preschool years, children possess considerable biological knowledge. In particular, they expect the insides of animals to be different from those of artifacts (1, 2); they realize that the insides of an animal are essential for its functioning (e.g., a dog cannot bark after its insides are removed) (3, 4); and they are beginning to understand that certain behaviors, such as eating and drinking, are necessary to maintain the continued functioning of animals and their insides (5, 6). This biological knowledge is often characterized as a vitalistic biology, in which internal organs and their workings sustain the vitality or life force of animals (7–10). A few facets of this vitalistic biology are already in place by the start of the preschool years. Thus, although 3-y-old children lack specific knowledge about the insides of animals (1, 2), they do expect these insides to differ from those of artifacts. When asked whether a pig has the same kinds of insides as a cow or a piggy bank, for example, 3-y-olds are more likely to select the cow (3). Similarly, when told that dogs have “andro” inside, 3-y-olds are more likely to project this novel property to other animals (including mammals and nonmammals) than to artifacts (11).
Does young children’s vitalistic biology have roots in infancy? Do infants possess abstract expectations about animals that could lay the foundations for the acquisition of more sophisticated biological knowledge? Below, we consider two broad hypotheses concerning this issue; we refer to them as the “nonbiological” and the “biological” hypotheses.
According to the nonbiological hypothesis, infants do not endow animals with vitalistic or biological properties: animals are simply entities that are self-propelled and agentive [for infants, these two properties are conceptually distinct (12–14); objects may be self-propelled without being agentive, and they may be agentive without being self-propelled]. Proponents of the nonbiological hypothesis differ greatly in their theoretical views on how infants come to understand self-propulsion and agency. To illustrate, consider two such views: the core-domain and image-schema views. According to the core-domain view (15), infants’ concept of self-propulsion is part of the skeletal explanatory framework that underlies core physical reasoning: when a novel object gives evidence that it is capable of autonomous motion (e.g., begins to move on its own), infants attribute to the object an internal source of energy, and they appreciate that the object may use its energy to reverse course, resist efforts to move it, and so on (16). Similarly, infants’ concept of agency is part of the skeletal explanatory framework that underlies core psychological reasoning: when a novel object provides evidence that it has autonomous control over its actions (e.g., responds contingently to events in its environment), infants attribute to the object motivational, epistemic, and other internal states, and they use these states to predict and interpret the object’s actions (17). In contrast, according to the image-schema view (18), infants’ concepts of self-propulsion and agency are formed by a perceptual-meaning-analysis mechanism that redescribes spatiotemporal information into meaningful iconic representations. Thus, self-propelled objects are those that start moving by themselves, without contact with other objects, whereas agentive objects are those that interact contingently with other objects, again without contact. In the image-schema view, infants have no notion of internal energy or internal states; these concepts are acquired later in development as enrichments of primitive spatial concepts. Despite their marked differences, however, both the core-domain and image-schema views assume that animals are, for infants, no more than self-propelled agents.
This assumption contrasts with the biological hypothesis, which admits the possibility that infants ascribe to entities that are self-propelled and agentive additional properties that are vitalistic or biological in nature (19–22). What might these biological properties be? One proposal, put forth by Gelman (19), is that infants are born with an “innards” principle: self-propelled agents have insides that make possible their behavior. According to Gelman (19), “the principle is neutral with respect to the nature of what a child or anyone may think is in the inside.” The innards principle is, of course, consistent with the findings on vitalistic biology mentioned earlier: children might at first simply expect animals to have insides, and with experience they might gradually learn how the insides of animals differ from those of artifacts (1), how the insides of one kind of animal differ from those of another kind of animal (23), and so on. In line with the innards principle, Gelman (19) found that when children age 3 and older were queried about the insides of various artifacts and animals, they sometimes said that an artifact had nothing on the inside, but they never said that an animal had nothing on the inside (see also refs. 24 and 25).
Is the nonbiological or the biological hypothesis correct? Do infants construe animals simply as self-propelled agents, or do they endow animals with additional, biological properties? One way to address these questions is to examine whether infants expect novel self-propelled agents to have insides, in accordance with the innards principle. Therefore, we used the violation-of-expectation method to test whether infants would detect a violation when a novel object that was self-propelled and agentive—but not an object that lacked one or both of these properties—was revealed to be hollow. Because there is considerable evidence that infants in the second half-year are sensitive to various cues for self-propulsion and agency (12, 26), our experiments focused on 8-mo-old infants. We reasoned that positive results would support the biological hypothesis by demonstrating that young infants immediately endow novel self-propelled agents with vitalistic, biological properties. Such results would be unique in providing an experimental demonstration that abstract biological expectations about animals are present in the first year of life.
Although no prior experiment had examined whether infants expect animals to have insides, previous findings with 14-mo-olds indicated that, when shown novel objects with eyes and visible insides, infants do notice these insides. Thus, infants assigned perceptually different objects to the same category if they possessed similar insides (27); infants readily formed an association between a transparent object’s self-propelled motion and the presence of an internal part (28); and infants also readily associated a transparent object’s particular style of self-propelled motion with the color of its internal part (28). Broadly construed, these findings suggested that infants attend to the insides of animals. Building on these findings, we asked in four experiments whether 8-mo-old infants would expect novel objects they identified as animals to have insides. All of the experiments followed the same general design. During the familiarization phase, infants were introduced to two novel objects; across conditions, we varied whether or not the objects were capable of self-propulsion and agency. During the test phase, the objects were rotated (Exps. 1–3) or shaken (Exp. 4) to assess infants’ expectations about their insides.
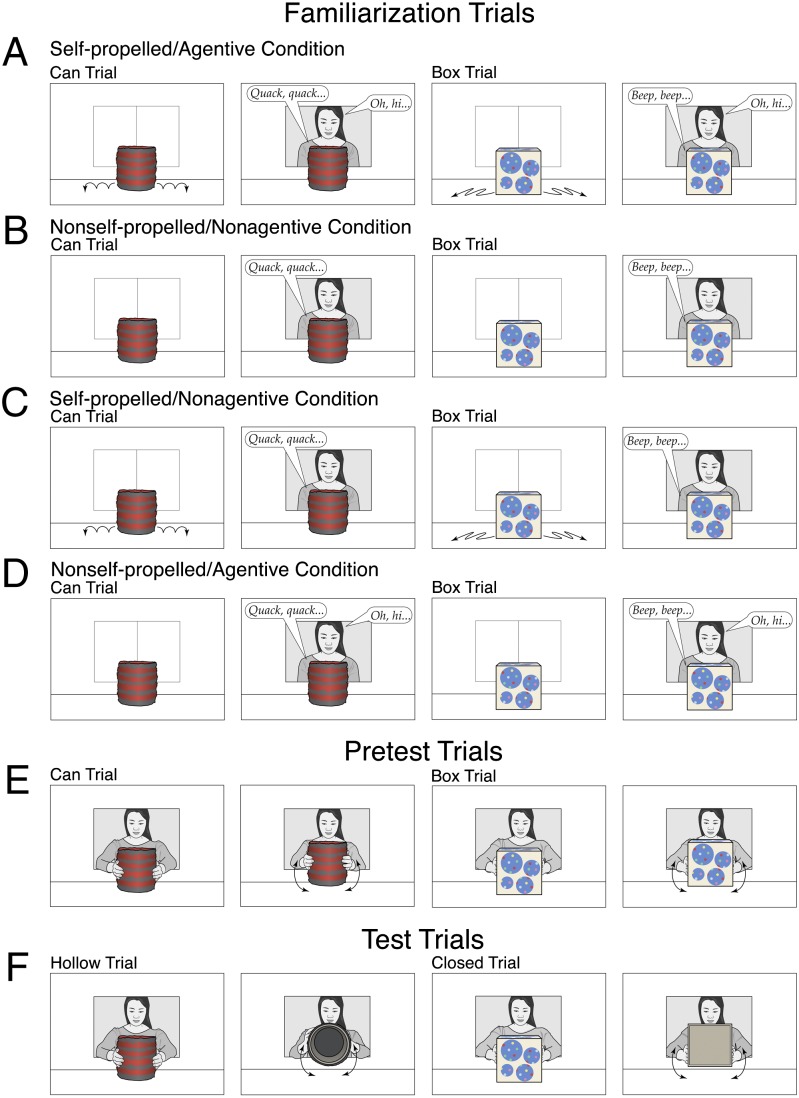
In Exp. 1, 8-mo-old infants from English-speaking families (n = 36) were assigned to a self-propelled/agentive condition or a nonself-propelled/nonagentive condition. Infants watched live events involving two novel objects: a large can covered with alternating stripes of red and gray yarn and a large box covered with beige paper and varying round patches of blue cloth with multicolored dots. All infants received two familiarization trials, two pretest trials, and two test trials, one with the can and one with the box; half of the infants received the can trial first in each pair of trials, and half received the box trial first. Only the familiarization trials differed between the two conditions. Each familiarization trial had an initial phase and a final phase; looking times during the two phases were computed separately. At the beginning of the (76 s) initial phase of the can trial in the self-propelled/agentive condition (Fig. 1A), the can rested at the center of the apparatus floor. To start, the can moved in a slight bouncing manner back and forth across the floor and then returned to its original position [this displacement lasted about 16 s and served to establish that the can was self-propelled (16)]. Next, a female experimenter opened a window in the back wall of the apparatus; the can then initiated a “conversation” by quacking at the experimenter, who responded contingently in English [this exchange lasted about 49 s and served to demonstrate that the can was agentive (17)]. Finally, the experimenter left, closing her window behind her. During the final phase of the trial, the can rested at the center of the apparatus, and infants watched this paused scene until the trial ended. The box familiarization trial was identical except that the box moved in a slight zigzag manner and beeped at the experimenter. Infants in the nonself-propelled/nonagentive condition (Fig. 1B) received similar familiarization trials except that the can and box remained stationary [thus providing no evidence that they were self-propelled (16)], and the experimenter remained silent in response to the can’s quacks or the box’s beeps [thus providing no evidence that they were agentive (14)].
Fig. 1.
Schematic drawing of the events shown in Exps. 1 and 2. In Exp. 1, the can and box were either self-propelled and agentive (A) or neither self-propelled nor agentive (B). In Exp. 2, the can and box were self-propelled and agentive (A), self-propelled but nonagentive (C), or nonself-propelled but agentive (D). Whether the can trial or the box trial was shown first in the familiarization (A–D), pretest (E), and test (F) trials was counterbalanced across infants in each condition; whether the can or the box was hollow in the test trials was also counterbalanced across infants in each condition.
Next, all infants received the can and box pretest trials (Fig. 1E), which served to introduce the actions performed in the test trials. In each trial, the experimenter lifted the can or box with both hands, tilted it right and left twice, returned it to the apparatus floor, and then repeated this entire (12 s) sequence until the trial ended. Finally, all infants received the can and box test trials (Fig. 1F). These trials were identical to the pretest trials except that, before tilting the can or box from side to side, the experimenter rotated it to reveal its bottom to the infant. When the objects were rotated, infants could see that one was hollow, like an inverted bowl (hollow trial), whereas the other one was closed, like a block (closed trial). For half the infants in each condition, the can was hollow and the box was closed; for the other infants, the reverse was true. Preliminary analyses of the test data in this report revealed no interactions of condition and trial with infants’ sex; the data were therefore collapsed across this factor in subsequent analyses.
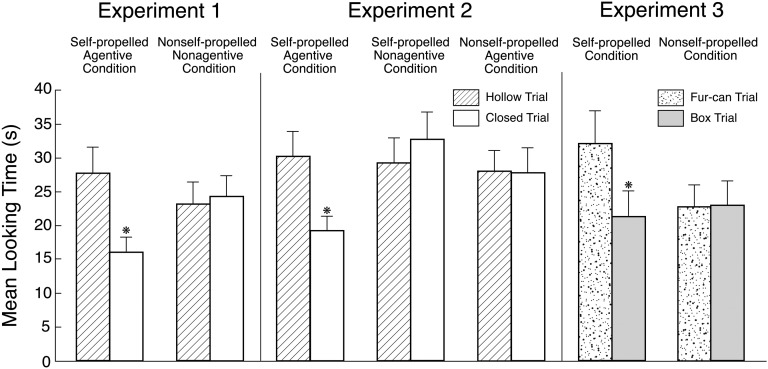
Infants’ looking times during the test trials (Fig. 2) were analyzed by means of an ANOVA with condition and order as between-subject factors and trial as a within-subject factor. The Condition X Trial interaction was significant, F(1, 32) = 6.11, P = 0.019 [no such interaction was found in an analysis of the final phases of the familiarization trials or in an analysis of the pretest trials, both Fs (1, 32) < 1]. Planned comparisons revealed that in the self-propelled/agentive condition, infants looked reliably longer during the hollow than the closed trial, F(1, 32) = 10.08, P = 0.003; 14 of 18 infants showed this pattern. In contrast, in the nonself-propelled/nonagentive condition, infants looked about equally during the two trials, F(1, 32) < 1; 7 of 18 infants looked longer at the hollow event. Thus, infants detected a violation when the can and box were shown to be hollow, but only if they were self-propelled agents; if the objects were neither self-propelled nor agentive, infants held no expectations about whether they should have insides.
Fig. 2.
Mean looking times of infants in Exps. 1–3 during the test trials as a function of condition and trial. Errors bars represent SEs, and an asterisk denotes a significant difference between the trials within a condition (P < 0.05 or better).
Exp. 2 investigated the specificity of infants’ expectations about what objects should have insides. According to the innards principle, infants should expect novel objects that are both self-propelled and agentive to have insides, but they should hold no expectation for objects that are only self-propelled or only agentive. To evaluate this prediction, additional 8-mo-old infants from English-speaking families (n = 54) were assigned to one of three conditions. The self-propelled/agentive condition was identical to that in Exp. 1 (Fig. 1A). The other two conditions were similar, except that in the familiarization trials, either the experimenter remained silent [self-propelled/nonagentive condition (Fig. 1C)], or the can and box remained stationary [nonself-propelled/agentive condition (Fig. 1D)].
Analysis of infants’ looking times during the test trials (Fig. 2) revealed a significant Condition X Trial interaction, F(2, 48) = 3.64, P = 0.034 [no such interaction was found in an analysis of the final phases of the familiarization trials, F(2, 48) < 1, or in an analysis of the pretest trials, F(2, 48) = 1.28, P = 0.289]. In the self-propelled/agentive condition, as before, infants looked reliably longer during the hollow than the closed trial, F(1, 48) = 7.82, P = 0.007; 14 of 18 infants showed this pattern. In contrast, infants looked about equally during the two trials in both the self-propelled/nonagentive and nonself-propelled/agentive conditions, both Fs < 1; 8 of 18 infants in self-propelled/nonagentive condition and 9 of 18 infants in the nonself-propelled/agentive condition looked longer during the hollow trial. Thus, infants expected the can and box to have insides only if they were self-propelled and agentive; if they lacked either property, infants held no expectations about their insides.
The results of Exps. 1 and 2 indicated that when a novel object gives evidence that it is capable of both autonomous motion and control, young infants identify it as an animal and immediately expect it to have insides, in accordance with the innards principle. These results provided direct support for the biological hypothesis by demonstrating that young infants possess abstract biological expectations about animals. Exp. 3 sought to provide converging evidence for these conclusions: it examined whether young infants would also expect novel animals identified via learned predictive cues to have insides.
Proponents of both the nonbiological and biological hypotheses assume that, with experience, infants learn to use details of surface appearance and form as cues that novel objects are animals (this cue-learning process enables infants to rapidly identify novel animals without having to wait for evidence of autonomous motion and control). For example, previous research indicates that by 7 mo of age, infants already use fur on a self-propelled object as a cue that the object is an animal (29). When a ball and a furry object with a face moved together in close contact, infants attributed the source of the motion to the furry object; when the two objects later rested stationary side by side, infants looked reliably longer at the furry object as though they anticipated that it would move again. However, no such effect was found when an experimenter moved the ball and the furry object together with her hand. (In a survey we conducted of parents of 35 6- to 9-mo-old infants, 83% reported that their infant had touched a furry animal at least once, and 60% reported that their infant had regular contact with one or more furry animals. These results support the notion that infants in the second half-year have opportunities to identify fur as a predictive cue for animals). Building on these results, we asked in Exp. 3 whether 8-mo-old infants would expect an object that was furry and self-propelled, but not an object that lacked one or both of these properties, to have insides.
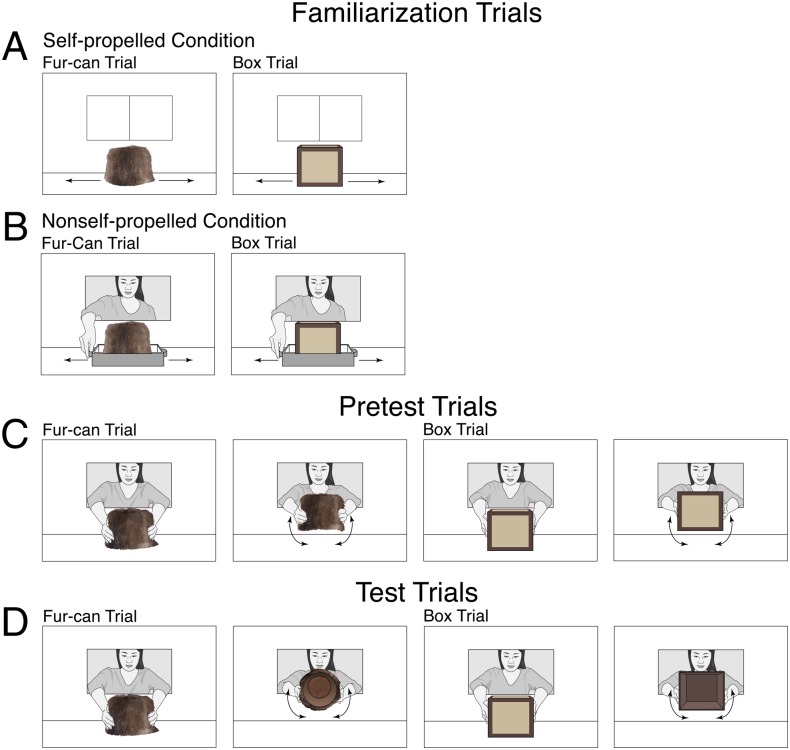
Infants (n = 36) were assigned to a self-propelled or a nonself-propelled condition and watched events involving a new can that was covered with brown beaver fur and a new box that was covered with tan paper and edged with brown tape (Fig. 3). During the (32 s) initial phase of each familiarization trial in the self-propelled condition, the fur-can or box moved smoothly back and forth across the apparatus floor to demonstrate that it was self-propelled; during the final phase, the object paused at the center of the apparatus until the trial ended. The familiarization trials in the nonself-propelled condition were identical except that the fur-can and box rested on a tray, and the experimenter reached through a window in the back wall of the apparatus to move the tray back and forth. Next, all infants received pretest and test trials identical to those in Exp. 1, except that the fur-can (fur-can trial) and box (box trial) were both revealed to be hollow.
Fig. 3.
Schematic drawing of the events shown in Exp. 3. Whether the fur-can trial or the box trial was shown first in the familiarization (A and B), pretest (C), and test (D) trials was counterbalanced across infants in each condition.
Analysis of infants’ looking times during the test trials (Fig. 2) yielded a significant Condition X Trial interaction, F(1, 32) = 6.18, P = 0.018 [no such interaction was found in an analysis of the final phases of the familiarization trials or in an analysis of the pretest trials, both Fs (1, 32) < 1]. In the self-propelled condition, infants looked reliably longer during the fur-can than the box trial, F(1, 32) = 12.00, P = 0.002; 16 of 18 infants showed this pattern. In contrast, infants in the nonself-propelled condition looked about equally during the two trials, F(1, 32) < 1; 9 of 18 infants looked longer during the fur-can trial. Thus, infants expected the self-propelled fur-can to have insides, but they held no expectations about the insides of the nonself-propelled fur-can or about those of the box, whether it was self-propelled or not. These results also provide additional evidence that by 8 mo, infants use fur on a self-propelled object as a cue that it is an animal.
In Exps. 1–3, infants detected a violation when an object they had identified as an animal was rotated to reveal that it had no insides. To provide converging evidence for these results, in Exp. 4 we used a different manipulation to assess 8-mo-olds’ expectations about insides: instead of rotating the fur-can and box from Exp. 3, the experimenter shook each object to demonstrate that it rattled, as though the shaking caused a few parts to bounce inside the object’s largely hollow interior. If infants expected the self-propelled fur-can to have insides, they should detect a violation when it produced a rattling noise when shaken, as though it was mostly hollow inside. (To check our manipulation, we presented 20 adults with the rattling fur-can and the rattling box, and we asked them to estimate based on the sounds they heard how full each object was inside. On average, subjects guessed that the objects were 28% full, supporting our claim that the rattling sounds conveyed that the objects were largely hollow.)
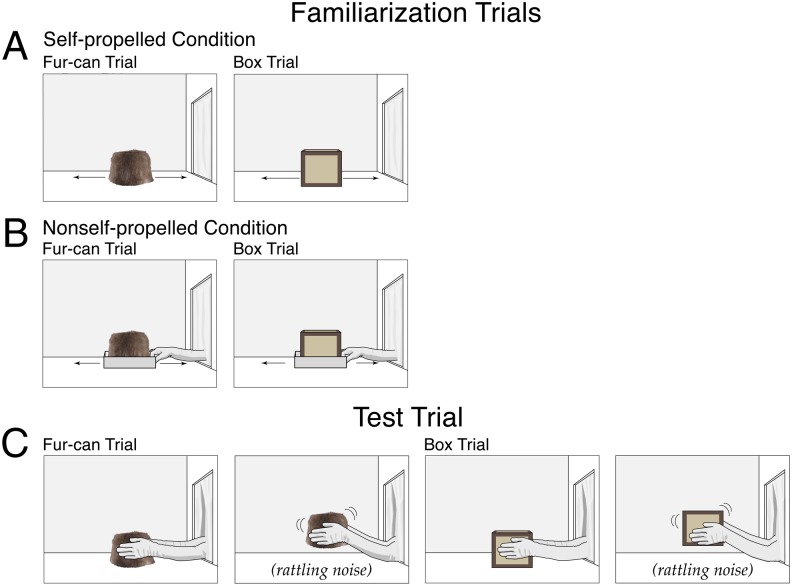
Infants (n = 51) were assigned to a self-propelled, a nonself-propelled, or a silent-control condition (Fig. 4). In the self-propelled condition, infants received the same fur-can and box familiarization trials as in the self-propelled condition of Exp. 3 for two pairs of trials. Next, infants received either a fur-can or a box test trial. During the (25 s) initial phase of each trial, the experimenter’s gloved hands (which reached through a curtained window in the right wall of the apparatus) first grasped the fur-can or box. Next, the hands lifted the object, shook it (causing it to rattle), and returned it to the apparatus floor; this sequence was repeated two more times, and then the hands rested on either side of the object. During the final phase, infants watched this paused scene until the trial ended (pilot data indicated that infants tended to look continuously if the rattling persisted, so this nonrepeating procedure was used instead). The nonself-propelled condition was identical, except that in the familiarization trials the fur-can and box rested on a tray and the experimenter’s right gloved hand moved the tray back and forth on the apparatus floor. Finally, because infants in the self-propelled condition might look longer when the fur-can rattled not because they expected it to have insides but because they had never seen an animal being shaken before, a silent-control condition was also included. This condition was identical to the self-propelled condition except that in the test trials the objects produced no noise when shaken. In the self-propelled and nonself-propelled conditions, nine infants received a fur-can test trial and eight infants received a box test trial; in the silent-control condition, these numbers were reversed.
Fig. 4.
Schematic drawing of the events shown in Exp. 4. Whether the fur-can trial or the box trial was shown first in the familiarization trials (A and B) was counterbalanced across infants in each condition. In the test trial (C), infants saw either the fur-can or the box trial. The silent-control condition (not shown) was identical to the self-propelled condition except that in the test trial the fur-can or box produced no noise when shaken.
Analyses of infants’ looking times during the final phase of the test trial (Fig. 5) yielded a significant Condition X Trial interaction, F(2, 45) = 4.85, P = 0.012 [no such interaction was found in an analysis of infants’ averaged looking times during the final phases of the fur-can and box familiarization trials, F(2, 45) < 1]. In the self-propelled condition, infants looked reliably longer if shown the fur-can as opposed to the box trial, F(1, 45) = 16.08, P = 0.0002. In contrast, in the nonself-propelled and silent-control conditions, infants looked about equally during either trial, both Fs (1, 45) < 1. Thus, infants detected a violation when the fur-can produced a rattling noise when shaken, but only if it was self-propelled. These results provided converging evidence that infants identify self-propelled furry objects as animals and expect their insides to be filled as opposed to hollow.
Fig. 5.
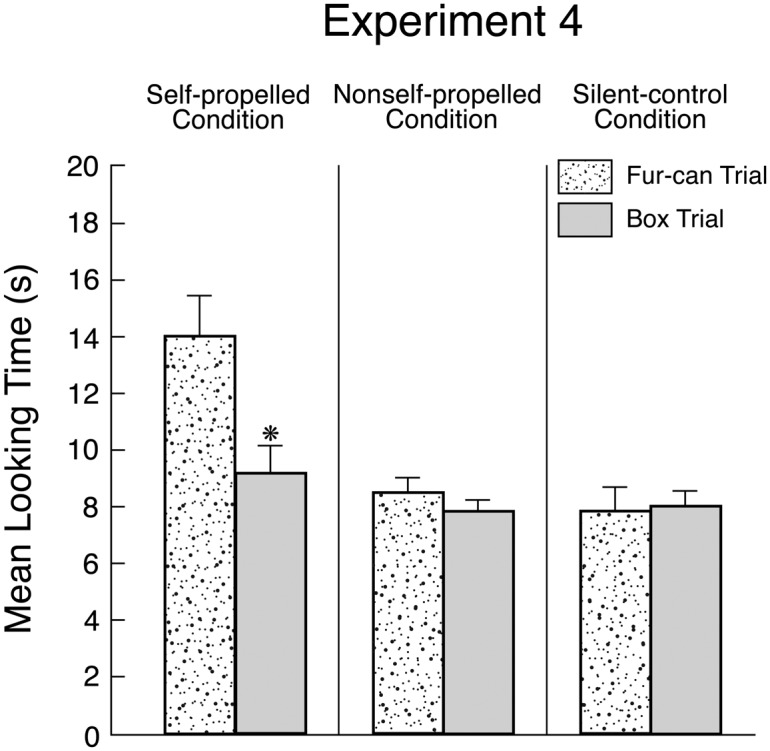
Mean looking times of infants in Exp. 4 during the final phase of the test trial as a function of condition and trial. Errors bars represent SEs, and an asterisk denotes a significant difference between the trials within a condition (P < 0.05 or better).
The present experiments indicate that 8-mo-old infants expect a novel object they identify as an animal to have insides, in accordance with the innards principle. This identification may come about because the object gives evidence of autonomous motion and control, or because it presents cues with learned predictive validity for distinguishing animals from other objects. In either case, upon identifying the novel object as an animal, infants immediately expect it to have insides: they detect a violation if the object either is shown to be hollow or rattles when shaken as although mostly hollow. Taken together, these results provide strong support for the biological hypothesis that infants endow animals with vitalistic, biological properties.
At least two broad questions remain for future research. First, what general expectations do infants possess about animals’ insides? For example, would infants expect the insides of a novel object that was self-propelled and agentive to differ from those of an object that lacked these properties? Moreover, would infants regard an animal’s insides as essential for its functioning? If infants witnessed the removal of the insides of a novel self-propelled agent, would they expect it to no longer be capable of autonomous motion and control?
Second, how should we conceptualize infants’ expectations about animals’ insides? There are at least three possibilities. One is that these early expectations are part of a skeletal explanatory framework that underlies core biological reasoning (21). In this view, infants would possess a naïve theory of biology as well as naïve theories of physics and psychology, although their naïve theory of biology might be less rich. Another possibility is that infants’ expectations about insides reflect general biases or modes of construal that are not exclusively tailored for biological phenomena (30, 31). For example, abstract biases for teleology and essentialism, perhaps with sparse conceptual constraints, might lead infants to posit various internal features to explain objects’ capacity for self-propulsion (internal energy), for agency (internal states), and for both self-propulsion and agency (innards). Finally, a third possibility is that infants’ expectations about insides arise from a quite different source: the cognitive systems that humans evolved to deal with predators and prey and, more generally, to understand animals as a food source (32). As Barrett (32) noted, “Predators are things that systematically try to kill you and eat you. Prey are things you try to capture and eat.” From this perspective, it seems plausible that the human mind would have evolved an abstract expectation that animals have filled insides. Damaging the insides of a predator or prey brings about its demise, and consuming these insides provides valuable nutrients. Why would an expectation of filled insides apply to entities that are both self-propelled and agentive, but not to entities that are only self-propelled or only agentive? It could be that in the evolution of predator-prey adaptations, the systems for detecting self-propulsion came first, and those for detecting agency were integrated later as they became available; understanding animals as self-propelled agents would have presented significant advantages for predator evasion and prey capture.
Whichever possibility turns out to be correct, there can be no doubt that infants’ expectations about animals are highly primitive and that considerable conceptual elaboration and change must occur for young children to develop a more advanced understanding of biology. Nevertheless, the present research fits well with several developmental results. If infants construe animals as self-propelled agents with biological properties, then it makes sense that (i) young children initially have difficulty constructing a category of living things that includes plants as well as animals (33, 34); (ii) young children who are taught that plants engage in self-propelled, agentive motion immediately infer that plants are living things (35); and (iii) school-aged children and adults who see computer-animated blobs engage in self-propelled, agentive motion describe them as alive and attribute to them various biological properties (36). All of these results suggest that key components of the interpretive framework that guides infants’ expectations about animals persist throughout life.
Methods
Participants.
Participants were 177 full-term infants (91 male, range: 6 mo, 17 d to 9 mo, 14 d). Mean ages were 8 mo, 4 d (Exp. 1), 8 mo, 1 d (Exp. 2), 7 mo, 24 d (Exp. 3), and 7 mo, 15 d (Exp. 4). Another 34 infants were tested but excluded because they looked the maximum time allowed in both test trials (16 infants); because they were fussy (6 infants), distracted (2 infants), drowsy (1 infant), inattentive (1 infant), or overly active (1 infant); because the difference in their looking times during the two test trials was over 2.5 SDs from the condition mean (2 infants); because they showed a marked bias during the familiarization trials for one object over the other (2 infants); or because they peeked under one or both objects during the pretest trials (2 infants) or stood too tall to see inside the rotated objects in the test trials (1 infant). Written informed consent was obtained from each infant's parent prior to the test session, and all protocols were approved by the University of Illinois IRB.
Apparatus and Stimuli.
The apparatus consisted of a brightly lit display booth (183 cm high × 100 cm wide × 57 cm deep) with a large opening (55 cm × 94 cm) in its front wall; between trials a supervisor lowered a curtain in front of this opening. Inside the apparatus the side walls were painted white, and the back wall (made of foam core) and floor were covered with colored adhesive paper. The experimenter was a female native English speaker. In Exps. 1–3, she wore a green shirt and sat at a window (34 cm × 48 cm, Exps. 1–2; 25 cm × 48 cm, Exp. 3) in the back wall of the apparatus; this back window could be closed with two identical doors. In Exp. 4 the experimenter wore long silver gloves and reached through a window (51 cm × 38 cm and filled with a fringed curtain) in the right wall of the apparatus. In all experiments, a large screen behind the experimenter hid the testing room. In Exps. 1 and 2 the can (18 cm × 17 cm in diameter) was wrapped with red and gray yarn in alternating stripes; the can had a removable gray felt bottom and its interior was lined with beige felt. The box (18 cm × 18 cm × 18 cm) was covered with beige adhesive paper and decorated with varying round patches of blue cloth with multicolored dots; the box had a removable beige felt bottom, and its interior was lined with brown felt. In the familiarization trials, a long flat handle was attached to the bottom of the can or box and protruded through a narrow slit at the bottom of the back wall. In the self-propelled conditions, the experimenter used the handle to move the can (in a slight bouncing manner) and the box (in a slight zigzag manner) along the apparatus floor, between predetermined marks. In the familiarization trials, the can or box also held a small speaker; its wire was tied to the handle and was connected, behind the apparatus wall, to an MP3 player. The can produced varying synthesized quacking sounds and the box produced varying beeping sounds; these sounds were prerecorded on the MP3 player and played through the speaker in the can or box. In the agentive conditions, a small reminder card with the written conversation script was attached to the back of the can or box for the experimenter to follow; in the nonagentive conditions, the can and box produced the same sounds but the experimenter remained silent. In Exps. 3 and 4, the fur-can (about 15 cm × 22 cm in diameter) consisted of a brown beaver fur hat that was placed over an upright cylinder; in the fur-can pretest and test trials of Exp. 3, the upright cylinder was replaced by an inverted cylinder lined with tan felt. The box (15 cm × 18 cm × 18 cm) was covered with tan packing paper and edged with brown tape; in the box pretest and test trials of Exp. 3, an exact copy of the box was used that had no bottom and was lined with brown felt. In the familiarization trials of the self-propelled conditions in Exps. 3 and 4, the fur-can or box was again attached to a long flat handle; behind the wall the experimenter used the handle to move the object smoothly back and forth between predetermined marks. In the familiarization trials of the nonself-propelled conditions, the fur-can or box rested either on a pink tray (5 cm × 29 cm × 23 cm) with handles that was moved by the experimenter from the back window (Exp. 3) or on a yellow tray (5 cm × 23 cm × 29 cm) without handles that was moved by the experimenter’s right gloved hand from the right window (Exp. 4); in each experiment, the experimenter moved the tray in such a way that the fur-can and box traveled the same distance as in the corresponding self-propelled condition. In the test trials with rattling sounds, a small plastic bag filled with 22 1-cm metal bells was partly affixed to the interior bottom surface of the fur-can or box; when the object was shaken briskly up and down, the bag bounced against the rigid bottom of the object, producing rattling sounds. During each test session, one camera captured an image of the events and another camera captured an image of the infant. The two images were combined, projected onto a television set located behind the apparatus, and monitored by the supervisor to confirm that the trials followed the prescribed scripts. Recorded sessions were also checked off-line for observer and experimenter accuracy.
Procedure.
Infants sat on a parent’s lap in front of the apparatus; parents were instructed to remain silent and to close their eyes during the test trials. Two hidden observers helped monitor infants’ looking behavior; unless otherwise noted, the primary observers’ responses were used in the analyses. In Exps. 1–3, the primary observer left the testing room during the familiarization trials to be naïve during the pretest and test trials about infants’ condition and trial order. Interobserver agreement during the test trials averaged 95% per trial per infant. In Exp. 4, observers could use available sounds to determine which test trials were shown; therefore, all final phases of the test trials were recoded frame-by-frame by two independent coders from edited silent videos. The two coders agreed on 97% of coded frames (trials with agreement below 90% were resolved through discussion). In Exps. 1–3 infants were attentive during the initial phases of the two familiarization trials and looked, on average, for 85% of each initial phase. The final phase of each familiarization trial ended when infants (i) looked away for 2 consecutive seconds after having looked for at least 2 cumulative seconds or (ii) looked for a maximum of 60 cumulative seconds. Each pretest and test trial ended when infants (i) looked away for 1 consecutive second after having looked for at least 5 cumulative seconds or (ii) looked for a maximum of 50 cumulative seconds; the 5-s minimum value ensured that infants had the opportunity to observe the rotated objects in the test trials. In Exp. 4, infants received two pairs of familiarization trials and one test trial, and each trial had an initial and a final phase. Across conditions, infants looked, on average, for 82% of each initial phase. The final phase of each trial ended when infants (i) looked away for 1 consecutive second after having looked for at least 5 (familiarization) or 6 (test) cumulative seconds, or (ii) looked for a maximum of 20 cumulative seconds. Shorter maximum values were used in Exp. 4 because infants received two pairs (instead of one pair) of familiarization trials and because the test trial used a nonrepeating procedure with a final paused scene.
Additional Results.
Because in each experiment the two test trials involved different objects, we conducted additional analyses to confirm that the results did not reflect baseline preferences for either object. For Exps. 1 and 2, we examined the test data using ANOVAs with condition, order, and hollow object (can or box) as between-subject factors and trial as a within-subject factor. In each experiment, the Condition X Trial interaction was again significant [Exp. 1: F(1, 28) = 8.29, P = 0.008; Exp. 2: F(2, 42) = 3.68, P = 0.034], but the Condition X Trial X hollow object interaction was not [Exp. 1: F(1, 28) < 1; Exp. 2: F(2, 42) = 1.27, P = 0.292], indicating that infants responded similarly whether the can or box served as the hollow object in the test trials. In Exp. 3, the objects used in the test trials could not be counterbalanced because the design called for one furry and one nonfurry object. We therefore conducted an ANCOVA using as the four covariates infants’ looking times at the fur-can and box in the familiarization and pretest trials. The Condition X Trial interaction remained significant, F(1, 28) = 6.48, P = 0.017, indicating that infants’ responses during the test trials did not simply reflect baseline preferences for the fur-can or the box. In Exp. 4, which also used the fur-can and box, we performed a similar ANCOVA using as the two covariates infants’ averaged looking times in the fur-can and box familiarization trials (recall that infants received two pairs of familiarization trials and no pretest trials). The Condition X Trial interaction remained significant, F(2, 43) = 4.10, P = 0.024, again indicating that infants’ responses to the test events did not simply reflect baseline preferences for either test object.
Acknowledgments
We thank the families who participated in the research, which was supported by National Institute of Child Health and Human Development Grant HD021104 (to R.B.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 15857.
References
- 1.Gottfried GM, Gelman SA. Developing domain-specific causal-explanatory frameworks: The role of insides and immanence. Cogn Dev. 2005;20(1):137–158. [Google Scholar]
- 2.Simons DJ, Keil FC. An abstract to concrete shift in the development of biological thought: The insides story. Cognition. 1995;56(2):129–163. doi: 10.1016/0010-0277(94)00660-d. [DOI] [PubMed] [Google Scholar]
- 3.Gelman SA, Wellman HM. Insides and essences: Early understandings of the non-obvious. Cognition. 1991;38(3):213–244. doi: 10.1016/0010-0277(91)90007-q. [DOI] [PubMed] [Google Scholar]
- 4.Gottfried GM, Gelman SA, Schultz J. Children’s understanding of the brain: From early essentialism to biological theory. Cogn Dev. 1999;14(1):147–174. [Google Scholar]
- 5.Inagaki K, Hatano G. Young children’s understanding of the mind-body distinction. Child Dev. 1993;64(5):1534–1549. [PubMed] [Google Scholar]
- 6.Morris SC, Taplin JE, Gelman SA. Vitalism in naive biological thinking. Dev Psychol. 2000;36(5):582–595. doi: 10.1037/0012-1649.36.5.582. [DOI] [PubMed] [Google Scholar]
- 7.Carey S. Science education as conceptual change. J Appl Dev Psychol. 2000;21(1):13–19. [Google Scholar]
- 8.Gutheil G, Vera A, Keil FC. Do houseflies think? Patterns of induction and biological beliefs in development. Cognition. 1998;66(1):33–49. doi: 10.1016/s0010-0277(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 9.Hatano G, Inagaki K. Young children’s naive theory of biology. Cognition. 1994;50(1-3):171–188. doi: 10.1016/0010-0277(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki K, Hatano G. Vitalistic causality in young children’s naive biology. Trends Cogn Sci. 2004;8(8):356–362. doi: 10.1016/j.tics.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann P, Waxman SR, Medin DL. Anthropocentrism is not the first step in children’s reasoning about the natural world. Proc Natl Acad Sci USA. 2010;107(22):9979–9984. doi: 10.1073/pnas.1004440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baillargeon R, Wu D (2009) in The Origins of Object Knowledge, eds Hood B, Santos L (Oxford Univ Press, Oxford, United Kingdom), pp 285–352.
- 13.Csibra G. Goal attribution to inanimate agents by 6.5-month-old infants. Cognition. 2008;107(2):705–717. doi: 10.1016/j.cognition.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu YA, Johnson SC. Infants’ attribution of a goal to a morphologically unfamiliar agent. Dev Sci. 2004;7(4):425–430. doi: 10.1111/j.1467-7687.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 15.Carey S. Conceptual differences between children and adults. Mind Lang. 1988;3(3):167–181. [Google Scholar]
- 16.Luo Y, Kaufman L, Baillargeon R. Young infants’ reasoning about physical events involving inert and self-propelled objects. Cognit Psychol. 2009;58(4):441–486. doi: 10.1016/j.cogpsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson SC, Shimizu YA, Ok S-J. Actors and actions: The role of agent behavior in infants’ attribution of goals. Cogn Dev. 2007;22(3):310–322. doi: 10.1016/j.cogdev.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandler JM. On the spatial foundations of the conceptual system and its enrichment. Cogn Sci. 2012;36(3):421–451. doi: 10.1111/j.1551-6709.2012.01241.x. [DOI] [PubMed] [Google Scholar]
- 19.Gelman R. First principles organize attention to and learning about relevant data: Number and the animate-inanimate distinction as examples. Cogn Sci. 1990;14(1):79–106. [Google Scholar]
- 20.Keil FC. Biology and beyond: Domain specificity in a broader developmental context. Hum Dev. 2007;50(1):31–38. [Google Scholar]
- 21. Opfer JE, Gelman SA (2010) in The Wiley-Blackwell Handbook of Childhood Cognitive Development, ed Goswami U (Blackwell, Oxford, United Kingdom), pp 213–238. 2nd Ed.
- 22. Waxman S (2005) in Categorization Inside and Outside the Laboratory: Essays in Honor of Douglas L. Medin, eds Ahn WKE, Goldstone RL, Love BC, Markman AB, Wolff PE (American Psychological Association, Washington, DC)
- 23.Waxman S, Medin D, Ross N. Folkbiological reasoning from a cross-cultural developmental perspective: Early essentialist notions are shaped by cultural beliefs. Dev Psychol. 2007;43(2):294–308. doi: 10.1037/0012-1649.43.2.294. [DOI] [PubMed] [Google Scholar]
- 24. Gelman R (2002) in Representation, Memory, and Development: Essays in Honor of Jean Mandler, eds Stein N, Bauer P, Rabinowitz M (Lawrence Erlbaum Associates, Mahwah, NJ), pp 75–87.
- 25. Gelman R, Durgin F, Kaufman L (1995) in Causal Cognition: A Multi-Disciplinary Debate, eds Sperber D, Premack D, Premack AJ (Clarendon, Oxford), pp 130–184.
- 26. Baillargeon R, et al. (in press) in APA Handbook of Personality and Social Psychology: Vol.1. Attitudes and Social Cognition, series eds Shaver P, Mikulincer M, vol. eds Borgida E, Bargh J (American Psychological Association, Washington, DC)
- 27.Welder AN, Graham SA. Infants’ categorization of novel objects with more or less obvious features. Cognit Psychol. 2006;52(1):57–91. doi: 10.1016/j.cogpsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Newman GE, Herrmann P, Wynn K, Keil FC. Biases towards internal features in infants’ reasoning about objects. Cognition. 2008;107(2):420–432. doi: 10.1016/j.cognition.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Träuble B, Pauen S. Infants’ reasoning about ambiguous motion events: The role of spatiotemporal and dispositional status information. Cogn Dev. 2011;26(1):1–15. [Google Scholar]
- 30. Keil FC (1992) in Modularity and Constraints in Language and Cognition, ed Keil FC (Lawrence Erlbaum Associates, Hillsdale, NJ), pp 103–137.
- 31. Gelman SA (2009) in Neurobiology of “Umwelt”: How Living Beings Perceive the World, Research and Perspectives in Neurosciences, eds Berthoz A, Christen Y (Springer, Berlin, Heidelberg), pp 7–16.
- 32. Barrett HC (2005) in The Handbook of Evolutionary Psychology, ed Buss DM (John Wiley & Sons, Hoboken, NJ), pp 200–223.
- 33. Carey S (1985) in Conceptual Change in Childhood (MIT Press, Cambridge, MA)
- 34.Leddon EM, Waxman SR, Medin DL. Unmasking “alive”: Children’s appreciation of a concept linking all living things. J Cogn Dev. 2008;9(4):461–473. doi: 10.1080/15248370802678463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opfer JE, Siegler RS. Revisiting preschoolers’ living things concept: A microgenetic analysis of conceptual change in basic biology. Cognit Psychol. 2004;49(4):301–332. doi: 10.1016/j.cogpsych.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Opfer JE. Identifying living and sentient kinds from dynamic information: The case of goal-directed versus aimless autonomous movement in conceptual change. Cognition. 2002;86(2):97–122. doi: 10.1016/s0010-0277(02)00171-3. [DOI] [PubMed] [Google Scholar]