Abstract
Urocortin 2 (Ucn2), a peptide of the corticotropin-releasing factor (CRF) family, binds with high affinity to type 2 CRF receptors (CRFR2) on cardiomyocytes and confers protection against ischemia/reperfusion. The mechanisms by which the Ucn2-CRFR2 axis mitigates against ischemia/reperfusion injury remain incompletely delineated. Activation of AMP-activated protein kinase (AMPK) also limits cardiac damage during ischemia/reperfusion. AMPK is classically activated by alterations in cellular energetics; however, hormones, cytokines, and additional autocrine/paracrine factors also modulate its activity. We examined the effects of both the endogenous cardiac Ucn2 autocrine/paracrine pathway and Ucn2 treatment on AMPK regulation. Ucn2 treatment increased AMPK activation and downstream acetyl-CoA carboxylase phosphorylation and glucose uptake in isolated heart muscles. These actions were blocked by the CRFR2 antagonist anti–sauvagine-30 and by a PKCε translocation-inhibitor peptide (εV1-2). Hypoxia-induced AMPK activation was also blunted in heart muscles by preincubation with either anti–sauvagine-30, a neutralizing anti-Ucn2 antibody, or εV1-2. Treatment with Ucn2 in vivo augmented ischemic AMPK activation and reduced myocardial injury and cardiac contractile dysfunction after regional ischemia/reperfusion in mice. Ucn2 also directly activated AMPK in ex vivo-perfused mouse hearts and diminished injury and contractile dysfunction during ischemia/reperfusion. Thus, both Ucn2 treatment and the endogenous cardiac Ucn2 autocrine/paracrine pathway activate AMPK signaling pathway, via a PKCε-dependent mechanism, defining a Ucn2-CRFR2-PKCε-AMPK pathway that mitigates against ischemia/reperfusion injury.
Keywords: metabolism, cardiac function
Urocortin (Ucn) is a peptide that is closely related to the neuroendocrine hypothalamic hormone, corticotrophin-releasing factor (CRF). Ucn was originally identified in the rat midbrain (1) and has an appetite-suppressing effect (2). The actions of Ucn and CRF are mediated by binding to two distinct receptors, type 1 CRF receptor (CRFR1) and CRFR2. The CRF family also includes two related peptides, Ucn2 and Ucn3. Ucn2 and the CRFR2 receptor are expressed in the heart (3) and CRFR2 binds Ucn2 with 40-fold higher avidity than CRF (3).
Ucn2 has diverse cardiovascular effects that are the focus of recent interest because it protects the myocardium against ischemic injury (4). The molecular mechanisms by which Ucn2 protect the heart are incompletely understood, but may include activation of the protein kinase Cε (PKCε) (5), phosphatidylinositol 3-kinase (PI3K)/Akt (6), and p42/p44 mitogen-activated protein kinase (7) signaling pathways. Ucn2 also activates mitochondrial ATP-sensitive potassium channels (8) and prevents the opening of the mitochondrial permeability transition pore (8).
Activation of PKCε protects the heart against ischemic injury (9) and plays an important role in the cardioprotective effect of Ucn2 (5). Signaling pathways have substantial cross-talk, and pharmacologic inhibitor studies suggest a possible association between activation of PKCε and the energy-stress kinase AMP-activated protein kinase (AMPK) (10). AMPK is activated by changes in cellular energetics, but its activity is also modulated by other factors, including adiponectin, leptin, and macrophage migration inhibitory factor (11). Activated AMPK has important physiologic effects during ischemia, so that inactivation of AMPK in mouse hearts interferes with energy generation and increases their susceptibility to injury during ischemia/reperfusion (12).
CRF family peptides, including Ucn, also play a role in modulating energy homeostasis (13). Ucn2 binds with high affinity to the CRFR2 in cardiomyocytes (14). Thus, we hypothesized that an endogenous cardiac Ucn2 autocrine/paracrine pathway might modulate AMPK activation during ischemia. We also postulated that pharmacologic treatment with Ucn2 might augment the intrinsic response of the AMPK pathway during ischemia, contributing to its cardioprotective effect.
Results
Ucn2 Treatment Stimulates AMPK Activation and Glucose Uptake.
To determine whether Ucn2 treatment stimulates cardiac AMPK signaling, we initially incubated rat heart muscles with rat Ucn2 (100 nmol/L) for 10 min. Immunoblots of muscle homogenates demonstrated that Ucn2 stimulated the phosphorylation of the activating Thr172 site on the AMPK α subunit (Fig. 1A) as well its direct downstream target acetyl-CoA carboxylase (ACC) on Ser79 (Fig. 1A). Ucn2 also stimulated heart muscle glucose uptake (Fig. 1B), another well-established metabolic consequence of AMPK activation (15). Heart muscles expressed CRFR2 (Fig. 1A). To determine the mechanism by which Ucn2 treatment stimulates heart AMPK, we used the CRFR2 antagonist anti–sauvagine-30 (a-SVG-30), an N-terminally truncated version of Ucn2 (14). Preincubation with a-SVG-30 abolished Ucn2-stimulated AMPK and downstream ACC phosphorylation (Fig. 1C). In addition, a-SVG-30 also eliminated Ucn2-stimulated glucose transport (Fig. 1D).
Fig. 1.
Effects of Ucn2 treatment and the endogenous cardiac Ucn pathway on AMPK signaling. (A) Rat heart left ventricular papillary muscles were incubated with or without Ucn2 (100 nmol/L) for 10 min and AMPK and downstream ACC phosphorylation were assessed. Representative immunoblots of phospho-AMPK Thr172 (p-AMPK), total AMPKα (AMPKα), phospho-ACC Ser79 (p-ACC), total ACC, and CRFR2 in heart homogenates. (B) The heart muscles were incubated with or without Ucn2 (100 nmol/L) for 10 min before measuring 2-deoxy-[1-3H] glucose uptake. (C and D) Heart muscles were preincubated with CRFR2 antagonist (a-SVG-30, 100 nmol/L) for 15 min, before incubation with or without Ucn2 (100 nmol/L) for 10 min. (E and F) Heart muscles were preincubated with a-SVG-30 (100 nmol/L) or Ucn2 Ab (0.2 μg/mL) for 15 min, then incubated in normoxic (control) or hypoxic (hypoxia) buffer for 30 min. (C and E) Representative immunoblots show p-AMPK, AMPKα, p-ACC, and total ACC in heart muscle homogenates. (D and F) Glucose uptake measured by 2-deoxy-[1-3H] glucose. Values are means ± SE for three to four experiments. *P < 0.01 vs. control, †P < 0.01 vs. Ucn + vehicle, §P < 0.05 vs. hypoxia + vehicle. (G) Heart muscles were preincubated with or without compound C (10 μmol/L) for 15 min and then incubated with or without Ucn2 (100 nmol/L) in oxygenated buffer for 10 min, or incubated in oxygenated (control) or hypoxic (hypoxia) buffer for 30 min, or incubated with or without insulin (10 U/mL) in oxygenated buffer for 30 min, before measuring 2-[1-3H]deoxyglucose uptake. Values are means ± SE for four experiments. *P < 0.01 vs. control, §P < 0.05 vs. control, †P < 0.01 vs. Ucn or hypoxia alone.
Endogenous Ucn2 Regulates AMPK Activation and Glucose Uptake During Hypoxia.
Ucn2 is highly expressed in the heart (7) and we hypothesized that endogenous secreted Ucn2 might activate AMPK in an autocrine/paracrine fashion. Thus, we pretreated heart muscles with a-SVG-30 or neutralizing Ucn2 antibody (Ucn2 Ab) before 30 min of hypoxia. Both a-SVG-30 and Ucn2 Ab partially inhibited hypoxic AMPK activation as well as downstream ACC phosphorylation (Fig. 1E) and glucose uptake (Fig. 1F). These results indicate that endogenous cardiac Ucn2 plays a role in modulating hypoxia-stimulated AMPK pathway activation.
Additional signaling pathways potentially might contribute to the stimulation of glucose uptake by Ucn2. Thus, to examine the role of AMPK in mediating Ucn2-stimulated glucose uptake, we assessed whether the AMPK inhibitor, compound C (16), attenuated the effects of Ucn2 on glucose uptake. We found that compound C blocked the stimulation of glucose uptake by exogenous Ucn2 (Fig. 1G). In addition, compound C inhibited hypoxia-stimulated glucose uptake, which is known to be mediated by AMPK (12) (Fig. 1G). Although compound C has off-target effects, it did not affect insulin-stimulated glucose uptake, which is known to be PI3K-dependent (17) (Fig. 1G). These data indicate that Ucn2-stimulated glucose uptake is mediated primarily through AMPK signaling.
Endogenous Ucn2 Release During Ischemia.
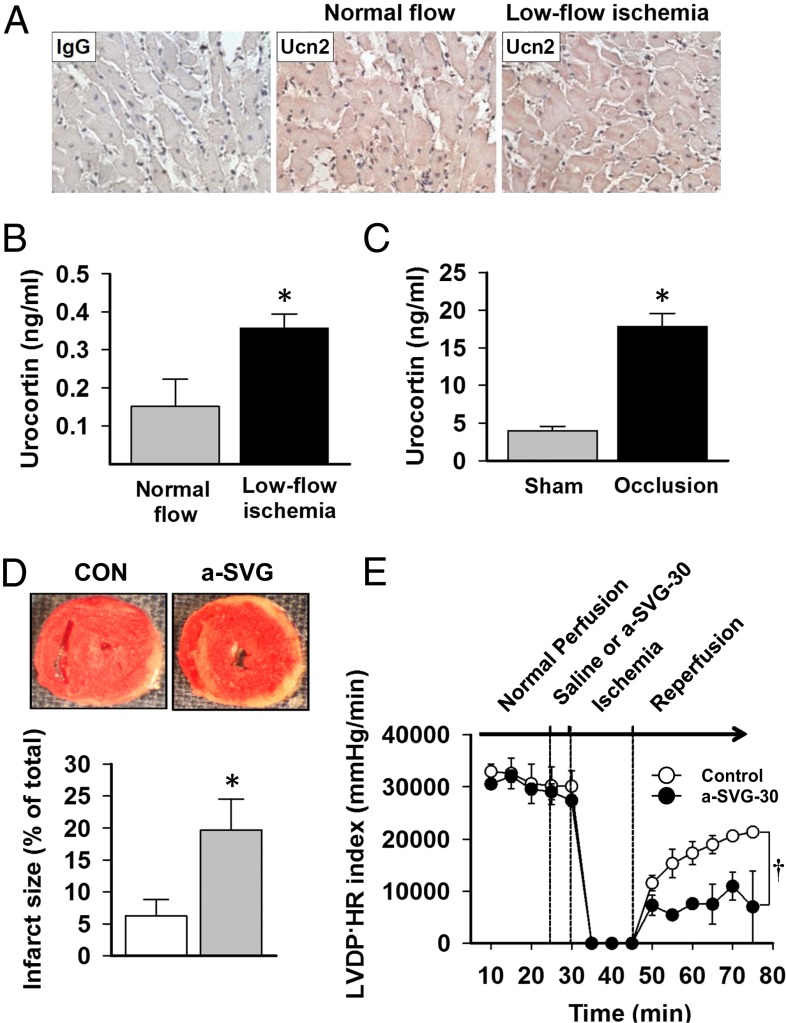
We next assessed whether Ucn2 is released from the heart during ischemia. Immunostaining heart sections showed depletion of Ucn2 after ischemia (Fig. 2A), and Ucn2 concentrations increased in the venous effluents of ex vivo-isolated perfused hearts during postischemic reperfusion (Fig. 2B). These studies indicate that endogenous Ucn2 was released during ischemia and are consistent with previous results in isolated cardiomyocytes during hypoxia (18). We also measured serum Ucn2 concentrations during in vivo regional ischemia. Although not specifically indicative of a cardiac origin, serum Ucn2 levels increased after short durations of ischemia (Fig. 2C).
Fig. 2.
Heart Ucn2 release and cardioprotective actions of endogenous cardiac Ucn2 during ischemia. (A) Immunohistochemical staining of mouse heart left ventricular sections with nonimmune IgG (Left) and Ucn2 Ab (Right). Concentration of Ucn2 (B) in coronary effluent during normal perfusion (4 mL/min) or low-flow ischemia (1.2 mL/min) in isolated ex vivo mouse hearts and (C) plasma after 15 min of in vivo regional ischemia induced by left coronary occlusion and 5 min of reperfusion or sham thoracotomy in anesthetized mice. (D) Infarct size was assessed by TTC staining of ex vivo-isolated mouse hearts treated with a-SVG-30 or saline for 5 min before 15 min of no flow ischemia and 30 min of reperfusion. (E) The recovery of LV contractile function in these hearts after ischemia determined by the product of LV developed pressure (LVDP) and heart rate (HR). Values are means ± SEM for four independent experiments. *P < 0.01 vs. normal flow or sham operation, †P < 0.05 vs. control. CON, saline.
To determine whether endogenously secreted cardiac Ucn2 has functional autocrine/paracrine effects in the ischemic heart, we also perfused isolated hearts ex vivo with a-SVG-30 before ischemia/reperfusion. Treatment with a-SVG-30 increased necrosis (Fig. 2D) and cardiac contractile dysfunction (Fig. 2E) after ischemia, indicating that activation of the endogenous urocortin-CRFR2 pathway has protective physiologic effects during ischemia/reperfusion.
PKCε Mediates Ucn2 Stimulation of AMPK.
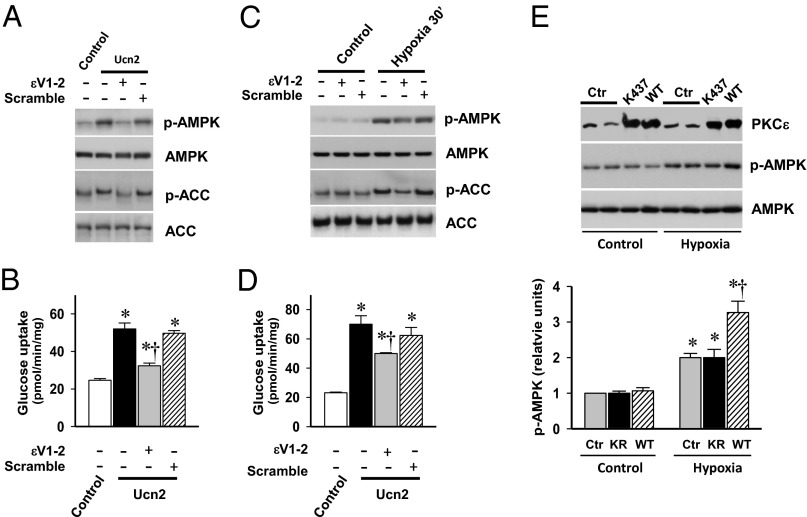
We next examined whether PKCε has a role in the activation of AMPK by Ucn2. When heart muscles were preincubated with the PKCε translocation inhibitor peptide, εV1-2 (19), Ucn2-stimulated AMPK phosphorylation and downstream glucose uptake were diminished (Fig. 3 A and B). In addition, preincubation with εV1-2 led to partial reduction in hypoxia-induced AMPK activation and glucose uptake (Fig. 3 C and D). In contrast, preincubation with the scramble peptide had no significant effect on AMPK activation or glucose uptake (Fig. 3 A–D).
Fig. 3.
Effect of PKCε translocation inhibitor peptide on Ucn2- and hypoxia-stimulated AMPK activation and glucose uptake. Heart muscles were preincubated with the PKCε translocation inhibitor peptide εV1-2 (200 nmol/L) or scramble peptide (200 nmol/L) for 15 min, then (A and B) incubated with or without Ucn2 (100 nmol/L) for 10 min, or (C and D) incubated in oxygenated (control) or hypoxic (hypoxia) buffer for 30 min. (A and C) Representative immunoblots show α subunit p-AMPK, AMPKα, p-ACC, and total ACC. (B) and (D) 2-deoxy-[1-3H] glucose uptake in additional muscles under the same conditions. Values are means ± SE for four experiments. *P < 0.01 vs. control, †P < 0.01 vs. Ucn or hypoxia alone. (E) H9c2 myoblast cells were transfected with either empty vector (Ctr) or expression construct of WT or KD (K437R) forms of PKCε. Cells were starved in 1% FBS medium for 12 h before 6 h of hypoxia treatment. PKCε protein content, p-AMPK, and total AMPKα expression were examined by immunoblotting. The ratio of phosphorylated AMPK to total AMPK was expressed relative to the ratio of control incubation with empty vector. Values are means ± SE for three independent experiments. *P < 0.01 vs. control, †P < 0.01 vs. hypoxic. KR, K437R.
To further investigate the involvement of PKCε in the regulation of AMPK activation during hypoxia, we examined whether overexpression of WT or kinase-inactivated PKCε K437R affected hypoxic AMPK activation. These experiments were done in H9c2 myoblast cells to achieve adequate expression of the PKCε WT and K437R proteins. WT PKCε overexpression enhanced hypoxic activation of AMPK, whereas inactive PKCε K437R did not affect hypoxic activation of AMPK in the H9c2 cells (Fig. 3E). Thus, PKCε appears to play a role in hypoxic activation of AMPK signaling.
Ucn2 Activates AMPK and Reduces Infarct Size After Ischemia/Reperfusion.
To examine whether pharmacologic treatment with Ucn2 also activates AMPK in the intact heart, we administered Ucn2 (15 μg/kg i.p.) in anesthetized mice (20). Ucn2 stimulated cardiac AMPK and downstream ACC phosphorylation 20 min after injection (Fig. 4A). Urocortin treatment did not increase the circulating serum concentrations of adiponectin, leptin, or macrophage migration inhibitory factor, which are known activators of AMPK (21) (Fig. S1). Urocortin also did not alter the content of myocardial adenine nucleotides or the AMP to ATP ratio (Fig. S2), suggesting that AMPK activation by urocortin does not result from perturbations in the balance of energy production or utilization in the heart.
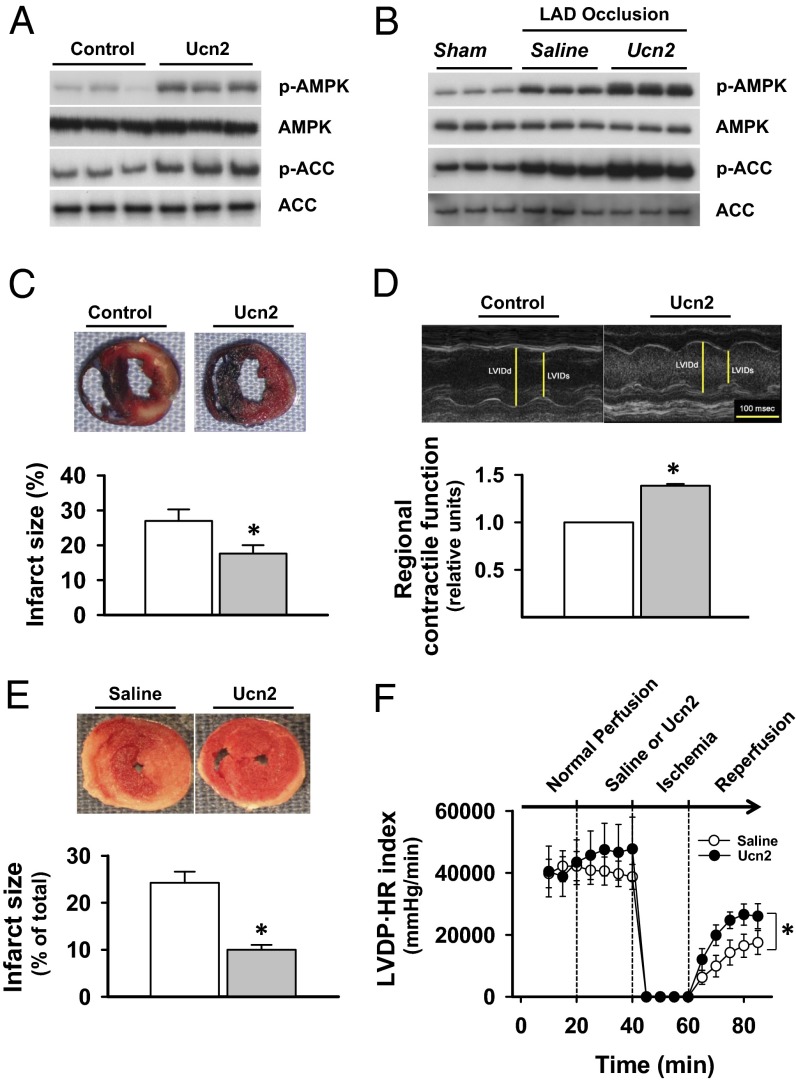
Fig. 4.
Effect of Ucn treatment on ischemic AMPK activation, myocardial necrosis, and contractile function after ischemia/reperfusion. (A) Anesthetized mice were treated with injection with Ucn2 (15 μg/kg i.p.) or saline, and hearts were excised after 20 min. Homogenates were immunoblotted for p-AMPK, total AMPKα, p-ACC, and total ACC. (B) Additional mice were treated with Ucn2 or saline; after 20 min, they were subjected to left anterior descending (LAD) coronary occlusion and hearts were excised after 20 min. Heart homogenates were immunoblotted for ischemic AMPK and ACC activation. (C) Separate mice were treated with saline or Ucn and then subjected to LAD coronary occlusion (20 min) and reperfusion for 3 h. Hearts were excised and dual stained to assess the extent of myocardial necrosis (Materials and Methods). Viable myocardium stains red with TTC, necrotic regions remain white, and blue dye staining defines nonischemic myocardium that was not subjected to coronary occlusion. Representative sections are shown and the % infarct size was calculated from the ratio of the area of necrosis to the ischemic area at risk. (D) Additional mice were treated with saline or Ucn 20 min before LAD coronary occlusion (20 min) and 24 h of reperfusion, and then underwent echocardiography (Upper) to assess regional contractile function in the area of infarction, expressed relative to saline-treated mice. (E) The direct cardiac effects of Ucn2 treatment on ischemic injury in ex vivo-isolated mouse hearts perfused with saline or Ucn2 (100 ng/mL) for 20 min before 20 min of no flow ischemia and 30 min of reperfusion. Infarct size was assessed by TTC staining and expressed relative to ventricular volume. (F) In these same hearts, the recovery of LV contractile function after ischemia was assessed by the product of LVDP and HR. Values are means ± SE for four to six independent experiments. *P < 0.05 vs. saline control.
When Ucn2 treatment was administered before coronary occlusion, ischemic activation of AMPK and downstream ACC were both augmented (Fig. 4B). We then investigated whether Ucn2 limited myocardial injury during ischemia/reperfusion. Mice were injected with Ucn2 before a 20-min occlusion of the left coronary artery that was followed by 3 h of reperfusion (Fig. 4C). The extent of necrosis was measured by vital staining of myocardial slices (22). Ucn2 treatment significantly reduced the area of necrosis compared with controls (Fig. 4C). To determine whether Ucn2 treatment also prevents cardiac contractile dysfunction, we assessed left ventricular (LV) function by echocardiography 24 h after coronary occlusion/reperfusion. Mice treated with Ucn2 had better regional contractile function in the previously ischemic area compared with controls (Fig. 4D). Thus, these results indicate that Ucn2 activates AMPK and has a cardioprotective effect to prevent ischemic injury.
To exclude the possibility that the protective effects of Ucn2 treatment were indirectly mediated by indirect, noncardiac systemic effects, we also treated isolated perfused mouse hearts ex vivo with Ucn2 before ischemia/reperfusion. Ucn2 reduced the extent of myocardial necrosis (Fig. 4E) and improved recovery of cardiac contractile function (Fig. 4E), demonstrating the direct cardiac protective effects of Ucn2.
To better understand the contribution of AMPK in mediating the cardioprotective effects of Ucn2 in limiting ischemia/reperfusion injury, Ucn2 was administered to transgenic mice expressing an AMPK kinase dead (KD) α2 subunit, which have partially inhibited cardiac AMPK activity (12). AMPK KD mice are more sensitive to ischemic injury (12). AMPK KD mice were injected with saline or Ucn2 before coronary occlusion and reperfusion, as in prior WT mouse experiments. We observed more necrosis in the AMPK KD mice, but Ucn2 treatment had the residual effect of diminishing injury in their hearts (Fig. S3), consistent with the additional known cardioprotective effects of Ucn2 (8, 23–26).
Discussion
These results implicate Ucn2 in the regulation of AMPK activation in the heart and further elucidate the mechanisms responsible for the protective effects of Ucn2 treatment during myocardial ischemia. First, Ucn2 treatment activated the AMPK pathway in isolated heart muscles via stimulation of CRFR2 and in the intact heart in vivo. Ucn2 treatment also had prototypical downstream AMPK metabolic actions to phosphorylate ACC and stimulate heart glucose uptake. Second, endogenous cardiac Ucn2 had an autocrine/paracrine effect to augment AMPK activation and glucose transport in heart muscle, enhancing the effect of energetic stress to activate the AMPK pathway during hypoxia. Ucn2 treatment similarly activated AMPK in the ischemic heart and had a cardioprotective effect against ischemic injury. Third, we found that AMPK activation by Ucn2 required PKCε translocation during hypoxia. Taken together, these results define a Ucn2-CRFR2-PKCε-AMPK pathway that is highly relevant to both the actions of the endogenous Ucn2 pathway and to pharmacologic Ucn2 therapy during ischemia.
Treatment with Ucn2 stimulated AMPK and downstream ACC phosphorylation and glucose uptake. ACC is a classic AMPK target and phosphorylation inhibits the action of ACC to synthesize malonyl-CoA, a potent inhibitor of CPT-1, the rate-limiting step for mitochondrial fatty acid oxidation (27). AMPK is also known to activate glucose uptake and glycolysis (28) as well as fatty acid uptake (29) in the heart. These effects of Ucn2 to promote cardiac metabolism further elucidate its pharmacologic actions in the heart (30, 31). Interestingly, both Ucn2 and AMPK also modulate whole-body energy homeostasis, although they have opposing actions in the hypothalamus, with Ucn2 suppressing appetite (2) and AMPK stimulating appetite (32).
AMPK has a critical role in the heart during ischemia/reperfusion. We found that Ucn2 treatment activates AMPK and protects the heart against ischemic injury. These results expand upon prior studies that showed that Ucn2 treatment protects isolated cardiac myocytes against hypoxia/reoxygenation and reduces injury in the intact heart during ischemia/reperfusion (4, 7), activating ERK (7) and PI3K/Akt signaling pathways (6). We observed that Ucn2 has a residual cardioprotective effect in mice with inactivated AMPK in the heart, consistent with the multiple mechanisms involved in Ucn2 action. Interestingly, recent data show that urocortin also up-regulates the expression of the α catalytic subunit of AMPK in the heart (23), potentially indicating dual mechanisms through which Ucn2 might activate the AMPK pathway.
Our results also suggest that endogenous Ucn2 has a specific autocrine/paracrine function to activate the AMPK pathway and protect the heart against ischemic injury. They expand on earlier findings that cardiac myocytes release Ucn2 from intracellular stores and Ucn2 mRNA abundance increases during simulated ischemia/reperfusion (33). We found that neutralizing Ucn2 antibody, which selectively binds extracellular Ucn2, blunts the activation of AMPK during hypoxia. In addition, treatment with a-SVG-30 partially inhibited the activation of AMPK during hypoxia. Thus, these results support the concept that endogenously secreted Ucn2 modulates the cardioprotective AMPK pathway in an autocrine/paracrine fashion in the heart.
Our findings also implicate PKCε as having a role in Ucn2 activation of AMPK. Ucn2 is known to stimulate PKCε translocation (34), but only limited prior data exist linking PKCε with AMPK (35). Pharmacologic PKC inhibitors abolished AMPK activation in the ischemic heart, but an unexpected high degree of AMPK inhibition was observed that likely reflected the known lack of specificity of these inhibitors (36). We found that the PKCε translocation inhibitor εV1-2 partially inhibited hypoxic activation of AMPK, more clearly implicating PKCε in both hypoxia and urocortin-mediated activation of AMPK. The detailed mechanisms through which PKCε activation activates AMPK are unknown, but appear to involve phosphorylation of the activating Thr172 residue in the catalytic domain of the α subunit. The phosphorylation state of Thr172 reflects the balance of action of AMPK upstream kinases, LKB1 and calcium-calmodulin-dependent protein kinase kinase β (22, 31), and less well-defined protein phosphatases. In noncardiac cells, different PKC isoforms also appear to modulate AMPK activation: specifically, PKCα activates AMPK in cancer cells (37) and PKCζ activates AMPK in endothelial cells (38). Although PKCζ has been reported to modulate LKB1 action in endothelial cells, this PKC isoform is not activated in the ischemic heart (39).
Ucn2 treatment had a preconditioning action to prevent injury during ischemia in our mouse model, consistent with prior studies (14). Interestingly, AMPK is also activated during ischemic preconditioning (40, 41), the physiologic phenomenon by which preceding short durations of ischemia decrease the susceptibility to necrosis during more prolonged and potentially injurious ischemia. AMPK is also activated by other treatment regimens that prevent ischemic injury, such as heat shock (10), adrenergic stimulation (42), and nitric oxide (43). AMPK activation is sufficient to precondition the heart, based the results of recent pharmacologic studies with a direct AMPK activator (41). PKCε activation is also involved in ischemic preconditioning, and previous studies have linked PKCε to the activation of mitochondrial ATP-sensitive potassium channels (44, 45). Thus, there is a complex interplay between Ucn2, PKCε, and both AMPK and non-AMPK pathways that appears to work in concert to favorably impact on the cardiac response to ischemia.
Ucn2 treatment has additional important physiologic effects in vivo, including dose-dependent increases in cardiac output and coronary blood flow as well as a decrease in systemic vascular resistance (46). These Ucn2 actions also appear to be mediated by CRFR2 receptors (47). The combined CRFR2-mediated myocardial and cardiovascular effects have potential relevance to the treatment of heart failure (48). In addition, the CRFR2 receptor inhibits tumor neovascularization and growth (49). Thus, CRFR2 activation has important biological actions that might prove to be the basis for treating human disease. Therapeutic strategies may also be influenced by alterations of CRFR2 expression under pathologic conditions, recognizing that inflammatory cytokines and stress-induced increases in corticosterone reduce cardiac CRFR2 expression (50). Nonetheless, our studies provide evidence to explore the therapeutic potential of the Ucn2-CRFR2-PKCε-AMPK mechanism in the ischemic heart and potentially in other solid organs.
Materials and Methods
Animals.
Animals were housed in accordance with guidelines from the American Association for Laboratory Animal Care. All procedures were approved by the Yale University Institutional Animal Care and Use Committee.
Heart Muscle Incubations.
Male Sprague-Dawley rats weighing 250–300 g were allowed access to standard chow and water ad libitum. They were anesthetized with sodium pentobarbital (60 mg/kg i.p.), and left ventricular papillary muscles were excised (51). Muscles were incubated in oxygenated buffer containing 5 mmol/L glucose, or under hypoxic conditions in buffer equilibrated with nitrogen at 37 °C. Muscles were treated with Ucn2 (100 nmol/L, Sigma) for variable times. Muscles were also preincubated with various antagonists or their vehicles for 30 min before Ucn2 or hypoxic treatments. a-SVG-30 (100 nmol/L) was used as a CRFR2 antagonist (14); εV1-2 [EAVSLKPT; PKCε (14–21)] was used as a PKCε translocation inhibitor peptide (19); scramble peptide (LSETKPAV, 200 nmol/L) as negative control for εV1-2; and compound C (10 μmol/L) as an AMPK inhibitor (16). Glucose transport was assessed by measuring 2-deoxy-d-[1-3H]glucose accumulation in the muscles (51).
Peptide Delivery into Cells.
The PKCε translocation inhibitor peptides (100–200 nmol/L) were introduced into heart muscles after transient permeabilization using saponin (52) with sham permeabilization as control. The heart muscles were incubated with freshly prepared permeabilization buffer (20 mmol/L Hepes, pH 7.4, 10 mmol/L EGTA, 140 mmol/L KCl, 50 µg/mL saponin, 5 mmol/L NaN3, and 5 mmol/L oxalic acid dipotassium salt) containing the desired peptides for 10 min at 4 °C. The muscles were then gently washed four times with PBS at 4 °C. After a 20-min recovery, the heart muscles were incubated for 2 min at 25 °C and then for 2 min at 37 °C. The muscles were then incubated in standard buffer with glucose 5 mmol/L for 15 min at 37 °C before use in other studies.
Cell Culture and Transfection.
H9c2 myoblast cells (ATCC) were maintained in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin (53). Cells were made quiescent in DMEM supplemented with 1% FBS for 12 h before Ucn2 or hypoxia treatments. H9c2 cells were transfected with WT and KD (K437R) forms of PKCε (54), using Lipofectamine 2000 (Invitrogen) and examined for the expression of transfected proteins by immunoblotting.
Isolated Heart Perfusions.
Mouse hearts were perfused in the Langendorff mode with oxygenated Krebs–Henseleit buffer containing 7 mmol/L glucose, 0.4 mmol/L oleate, 1% BSA, and a low fasting concentration of insulin (10 µU/mL), as previously described (22, 55). For ischemia/reperfusion studies, hearts were stabilized at a flow rate of 4 mL/min for 30 min before 15–20 min of no-flow global ischemia, with or without 30 min of reperfusion. The extent of myocardial necrosis was evaluated by 2, 3, 5-triphenyltetrazolium chloride (TTC) staining of viable tissue.
Coronary Occlusion Model.
C57BL/6 male mice (4–6 mo of age) were anesthetized with sodium pentobarbital (60 mg/kg i.p.), intubated, and mechanically ventilated. Mice underwent thoracotomy and the proximal left anterior descending coronary artery was occluded with a suture for 20 min and then reperfused, as previously described (22). Control mice underwent a sham thoracotomy with suture placement without occlusion of the artery. Ucn2 (15 μg/kg) (7) or saline were administered by i.p. injection 20 min before coronary occlusion. Transgenic C57BL/6 male mice, expressing a KD rat α2 isoform (K45R mutation) in heart and skeletal muscle (12), were also studied at 4–6 mo of age.
Assessment of Myocardial Infarction in Vivo.
Myocardial infarction was assessed in hearts excised after 20 min ischemia and 3 h of reperfusion. The heart was stained to delineate the extent of necrosis as a percent of the nonperfused area at risk distal to the coronary occlusion (22, 55).
Assessment of Contractile Dysfunction in Vivo.
In a separate group of mice, cardiac contractile function was assessed 24 h after infarction (20 min coronary occlusion and reperfusion) using echocardiography (VisualSonics 2100). Mice were lightly anesthetized with 1–2% (vol/vol) isoflurane and body temperature was maintained constant. Anatomic M-mode tracings were acquired from parasternal short-axis images and fractional shortening through the center of infarct region was calculated.
Immunoblotting and Immunohistochemistry.
Heart tissue homogenates underwent immunoblotting as previously described (51). Rabbit polyclonal antibodies were used: phospho-AMPK (Thr172), total AMPKα, and total ACC (Cell Signaling), PKCε (Santa Cruz), and phospho-ACC (Ser79) (Millipore).
Mouse heart sections were incubated with Ucn2 antibody (diluted 1:50) followed by incubation with anti-rabbit peroxidase-conjugated secondary antibody. The sections were counterstained with hematoxylin.
Ucn2 Measurement.
Plasma Ucn2 concentrations were measured in duplicate by ELISA (Phoenix Pharmaceuticals Inc.).
Nucleotide Measurement.
Myocardial adenine nucleotides were measured in methanol-extracted heart homogenates by liquid chromatography–tandem MS analysis on an Applied Biosystems API4000 QTrap interfaced to a Shimadzu HPLC, as previously described (56).
Statistical Analysis.
Data were expressed as means ± SEM. Significance was assessed by Student's two-tail t tests with Bonferroni correction or two-way repeated measures ANOVA with post hoc analysis. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Gary Cline for measuring adenine nucleotide concentratrions.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312775110/-/DCSupplemental.
References
- 1.Vaughan J, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378(6554):287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 2.Spina M, et al. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273(5281):1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- 3.Kishimoto T, Pearse RV, 2nd, Lin CR, Rosenfeld MG. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci USA. 1995;92(4):1108–1112. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentim L, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40(6):846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence KM, et al. Cardioprotection mediated by urocortin is dependent on PKCepsilon activation. FASEB J. 2005;19(7):831–833. doi: 10.1096/fj.04-2506fje. [DOI] [PubMed] [Google Scholar]
- 6.Brar BK, Stephanou A, Knight R, Latchman DS. Activation of protein kinase B/Akt by urocortin is essential for its ability to protect cardiac cells against hypoxia/reoxygenation-induced cell death. J Mol Cell Cardiol. 2002;34(4):483–492. doi: 10.1006/jmcc.2002.1529. [DOI] [PubMed] [Google Scholar]
- 7.Schulman D, Latchman DS, Yellon DM. Urocortin protects the heart from reperfusion injury via upregulation of p42/p44 MAPK signaling pathway. Am J Physiol Heart Circ Physiol. 2002;283(4):H1481–H1488. doi: 10.1152/ajpheart.01089.2001. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence KM, et al. The cardioprotective effect of urocortin during ischaemia/reperfusion involves the prevention of mitochondrial damage. Biochem Biophys Res Commun. 2004;321(2):479–486. doi: 10.1016/j.bbrc.2004.06.170. [DOI] [PubMed] [Google Scholar]
- 9.Cross HR, Murphy E, Bolli R, Ping P, Steenbergen C. Expression of activated PKC epsilon (PKC epsilon) protects the ischemic heart, without attenuating ischemic H(+) production. J Mol Cell Cardiol. 2002;34(3):361–367. doi: 10.1006/jmcc.2001.1518. [DOI] [PubMed] [Google Scholar]
- 10.Khaliulin I, et al. Temperature preconditioning of isolated rat hearts—a potent cardioprotective mechanism involving a reduction in oxidative stress and inhibition of the mitochondrial permeability transition pore. J Physiol. 2007;581(Pt 3):1147–1161. doi: 10.1113/jphysiol.2007.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison A, Li J. PPAR-γ and AMPK—advantageous targets for myocardial ischemia/reperfusion therapy. Biochem Pharmacol. 2011;82(3):195–200. doi: 10.1016/j.bcp.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Russell RR, 3rd, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114(4):495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bale TL, et al. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology. 2003;144(6):2580–2587. doi: 10.1210/en.2002-0091. [DOI] [PubMed] [Google Scholar]
- 14.Brar BK, et al. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: An essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145(1):24–35, discussion 21–23. doi: 10.1210/en.2003-0689. [DOI] [PubMed] [Google Scholar]
- 15.Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277(2 Pt 2):H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young LH, Li J, Baron SJ, Russell RR. AMP-activated protein kinase: A key stress signaling pathway in the heart. Trends Cardiovasc Med. 2005;15(3):110–118. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda K, et al. Urocortin, a newly identified corticotropin-releasing factor-related mammalian peptide, stimulates atrial natriuretic peptide and brain natriuretic peptide secretions from neonatal rat cardiomyocytes. Biochem Biophys Res Commun. 1998;250(2):298–304. doi: 10.1006/bbrc.1998.9297. [DOI] [PubMed] [Google Scholar]
- 19.Mochly-Rosen D, et al. Cardiotrophic effects of protein kinase C epsilon: Analysis by in vivo modulation of PKCepsilon translocation. Circ Res. 2000;86(11):1173–1179. doi: 10.1161/01.res.86.11.1173. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K, Totsune K, Murakami O, Shibahara S. Urocortins as cardiovascular peptides. Peptides. 2004;25(10):1723–1731. doi: 10.1016/j.peptides.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Zaha VG, Young LH. AMP-activated protein kinase regulation and biological actions in the heart. Circ Res. 2012;111(6):800–814. doi: 10.1161/CIRCRESAHA.111.255505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller EJ, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451(7178):578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 23.Barry SP, et al. New targets of urocortin-mediated cardioprotection. J Mol Endocrinol. 2010;45(2):69–85. doi: 10.1677/JME-09-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brar BK, et al. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J Biol Chem. 2000;275(12):8508–8514. doi: 10.1074/jbc.275.12.8508. [DOI] [PubMed] [Google Scholar]
- 25.Cserepes B, et al. Cardioprotective action of urocortin in early pre- and postconditioning. Ann N Y Acad Sci. 2007;1095:228–239. doi: 10.1196/annals.1397.027. [DOI] [PubMed] [Google Scholar]
- 26.Latchman DS. Urocortin. Int J Biochem Cell Biol. 2002;34(8):907–910. doi: 10.1016/s1357-2725(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 27.Kudo N, et al. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996;1301(1-2):67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 28.Marsin AS, Bouzin C, Bertrand L, Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem. 2002;277(34):30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- 29.Habets DD, et al. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem Biophys Res Commun. 2007;355(1):204–210. doi: 10.1016/j.bbrc.2007.01.141. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Chen P, Vaughan J, Lee KF, Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci USA. 2007;104(10):4206–4211. doi: 10.1073/pnas.0611641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young LH. AMP-activated protein kinase conducts the ischemic stress response orchestra. Circulation. 2008;117(6):832–840. doi: 10.1161/CIRCULATIONAHA.107.713115. [DOI] [PubMed] [Google Scholar]
- 32.Andersson U, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279(13):12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 33.Brar BK, et al. CRH-like peptides protect cardiac myocytes from lethal ischaemic injury. Mol Cell Endocrinol. 1999;158(1-2):55–63. doi: 10.1016/s0303-7207(99)00183-5. [DOI] [PubMed] [Google Scholar]
- 34.Townsend PA, et al. Urocortin prevents mitochondrial permeability transition in response to reperfusion injury indirectly by reducing oxidative stress. Am J Physiol Heart Circ Physiol. 2007;293(2):H928–H938. doi: 10.1152/ajpheart.01135.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishino Y, et al. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004;61(3):610–619. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Turrell HE, Rodrigo GC, Norman RI, Dickens M, Standen NB. Phenylephrine preconditioning involves modulation of cardiac sarcolemmal K(ATP) current by PKC delta, AMPK and p38 MAPK. J Mol Cell Cardiol. 2011;51(3):370–380. doi: 10.1016/j.yjmcc.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Mizrachy-Schwartz S, Cohen N, Klein S, Kravchenko-Balasha N, Levitzki A. Up-regulation of AMP-activated protein kinase in cancer cell lines is mediated through c-Src activation. J Biol Chem. 2011;286(17):15268–15277. doi: 10.1074/jbc.M110.211813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117(7):952–962. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ussher JR, et al. Role of the atypical protein kinase Czeta in regulation of 5′-AMP-activated protein kinase in cardiac and skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297(2):E349–E357. doi: 10.1152/ajpendo.00009.2009. [DOI] [PubMed] [Google Scholar]
- 40.Sukhodub A, et al. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K(+) channels. J Cell Physiol. 2007;210(1):224–236. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim AS, et al. A small molecule AMPK activator protects the heart against ischemia-reperfusion injury. J Mol Cell Cardiol. 2011;51(1):24–32. doi: 10.1016/j.yjmcc.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutchinson DS, Bengtsson T. AMP-activated protein kinase activation by adrenoceptors in L6 skeletal muscle cells: Mediation by alpha1-adrenoceptors causing glucose uptake. Diabetes. 2006;55(3):682–690. doi: 10.2337/diabetes.55.03.06.db05-0901. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, et al. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J Biol Chem. 2008;283(41):27452–27461. doi: 10.1074/jbc.M802578200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Ardehali H. Signaling mechanisms in ischemic preconditioning: Interaction of PKCepsilon and MitoK(ATP) in the inner membrane of mitochondria. Circ Res. 2006;99(8):798–800. doi: 10.1161/01.RES.0000247029.31997.a4. [DOI] [PubMed] [Google Scholar]
- 45.Jabůrek M, Costa AD, Burton JR, Costa CL, Garlid KD. Mitochondrial PKC epsilon and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res. 2006;99(8):878–883. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 46.Parkes DG, Weisinger RS, May CN. Cardiovascular actions of CRH and urocortin: An update. Peptides. 2001;22(5):821–827. doi: 10.1016/s0196-9781(01)00396-5. [DOI] [PubMed] [Google Scholar]
- 47.Fisher L, Rivier C, Rivier J, Brown M. Differential antagonist activity of alpha-helical corticotropin-releasing factor9-41 in three bioassay systems. Endocrinology. 1991;129(3):1312–1316. doi: 10.1210/endo-129-3-1312. [DOI] [PubMed] [Google Scholar]
- 48.Davis ME, et al. Urocortin 2 infusion in human heart failure. Eur Heart J. 2007;28(21):2589–2597. doi: 10.1093/eurheartj/ehm340. [DOI] [PubMed] [Google Scholar]
- 49.Hao Z, et al. Urocortin2 inhibits tumor growth via effects on vascularization and cell proliferation. Proc Natl Acad Sci USA. 2008;105(10):3939–3944. doi: 10.1073/pnas.0712366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coste SC, Heldwein KA, Stevens SL, Tobar-Dupres E, Stenzel-Poore MP. IL-1alpha and TNFalpha down-regulate CRH receptor-2 mRNA expression in the mouse heart. Endocrinology. 2001;142(8):3537–3545. doi: 10.1210/endo.142.8.8342. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR, 3rd, Young LH. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res. 2005;97(9):872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- 52.Dorn GW, 2nd, et al. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc Natl Acad Sci USA. 1999;96(22):12798–12803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy AP, Levy NS, Goldberg MA. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996;271(5):2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- 54.Lang W, Wang H, Ding L, Xiao L. Cooperation between PKC-alpha and PKC-epsilon in the regulation of JNK activation in human lung cancer cells. Cell Signal. 2004;16(4):457–467. doi: 10.1016/j.cellsig.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Ma H, et al. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation. 2010;122(3):282–292. doi: 10.1161/CIRCULATIONAHA.110.953208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pongratz RL, et al. Mitochondrial dysfunction contributes to impaired insulin secretion in INS-1 cells with dominant-negative mutations of HNF-1alpha and in HNF-1alpha-deficient islets. J Biol Chem. 2009;284(25):16808–16821. doi: 10.1074/jbc.M807723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.