Significance
The pre–T-cell receptor (pre-TCR) marks a functional checkpoint in thymocyte development that controls functions such as survival, proliferation, and differentiation. Despite knowledge accumulated on signaling pathways activated by the pre-TCR, less is known about the specialization of transcription factors downstream from this receptor and the genes they control. The Relish (Rel)-like transcription factors NF-κB and NFATc were previously known to control specific points of thymocyte maturation. Our work now positions the Rel-like protein NFAT5 as a target of the IKKβ pathway in thymocytes and as a downstream effector of the prosurvival role of the pre-TCR through its ability to regulate the expression of the survival-promoting proteins A1 and Bcl2 and attenuate the proapoptotic axis comprised by p53 and Noxa proteins.
Keywords: T-cell development, gene expression
Abstract
The Rel-like transcription factors nuclear factor kappa B (NF-κB) and the calcineurin-dependent nuclear factor of activated T cells (NFATc) control specific points of thymocyte maturation. Thymocytes also express a distinct member of the Rel family, the calcineurin-independent, osmostress response regulator NFAT5. Here we show that IKKβ regulates the expression of NFAT5 in thymocytes, which in turn contributes to the survival of T-cell receptor αβ thymocytes and the transition from the β-selection checkpoint to the double-positive stage in an osmostress-independent manner. NFAT5-deficient thymocytes had normal expression and proximal signaling of the pre–T-cell receptor but exhibited a partial defect in β-chain allelic exclusion and increased apoptosis. Further analysis showed that NFAT5 regulated the expression of the prosurvival factors A1 and Bcl2 and attenuated the proapoptotic p53/Noxa axis. These findings position NFAT5 as a target of the IKKβ/NF-κB pathway in thymocytes and as a downstream effector of the prosurvival role of the pre–T-cell receptor.
Early thymocyte differentiation proceeds through double-negative (DN; CD4− and CD8−) steps (1, 2) from the DN1 to the early DN3 (E-DN3) stages until cells express a recombined β allele of the T-cell receptor (TCR), which, together with the nonvariant pre-Tα and the CD3 complex, constitute a functional pre-TCR (3). The pre-TCR marks the commitment to the αβ T-cell lineage and controls the β-selection checkpoint in a ligand-independent manner by regulating allelic exclusion of the TCRβ locus, proliferation, survival, and differentiation of thymocytes, all of which are required for progression of DN3 to double-positive (DP; CD4+ and CD8+) cells (4–6). At the DP stage, thymocytes recombine the TCRα and express the mature TCRαβ complex, which interacts with self-peptide–MHC complexes of thymic antigen-presenting cells (7). This interaction determines the positive and negative selection of DP thymocytes from which CD4+ or CD8+ single-positive (SP) cells emerge. SP cells then migrate to peripheral organs to populate them with mature T lymphocytes.
Despite knowledge accumulated on signaling pathways activated by the pre-TCR, less is known about the specialization of transcription factors downstream from this receptor and the genes they control (8). The Rel-like transcription factors NF-κB and the calcineurin-dependent NFATc proteins regulate thymocyte development downstream of the pre-TCR (9–16). Thymocytes also express the calcineurin-independent NFAT protein NFAT5, which has hybrid features of both NF-κB and NFATc proteins (17, 18). NFAT5 protects cells from osmotic stress (19), and NFAT5-deficient mice present severe atrophy of the renal medulla, systemic hypernatremia, and a reduced thymocyte compartment and mature T-cell lymphopenia (20, 21). Whereas the thymocyte and T-lymphocyte deficiency of NFAT5-null mice can be explained by their systemic hypernatremia (21, 22), mice expressing a T-lymphocyte–restricted dominant negative NFAT5 transgene were shown to have reduced thymic cellularity and peripheral T lymphopenia (23). Because the tonicity of the thymus is not high enough to activate NFAT5 (21, 24, 25), this suggests an osmostress-independent role of NFAT5 in thymocyte development. Here we describe that NFAT5 expression is regulated by the IκB kinase β (IKKβ) pathway in thymocytes and acts as a survival factor downstream from the pre-TCR, independent of its osmoprotective function.
Results
Reduced Number of Thymocytes and Peripheral T Cells in Nfat5fl/fl, Lck-Cre Mice.
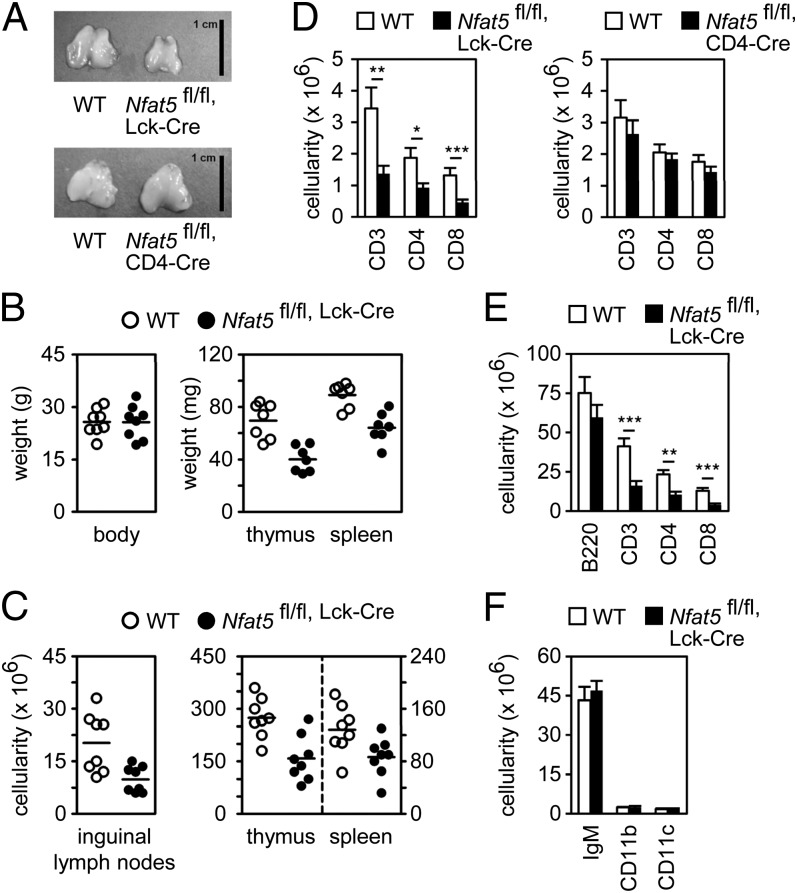
To gain insight into the role of NFAT5 in thymocyte development, conditional deletion of Nfat5 at early (DN2) or later (DP) thymocyte stages was induced by crossing mice with LoxP sites flanking both Nfat5 alleles (Nfat5fl/fl) with mice expressing Cre at DN2 (Lck-Cre) or DP (CD4-Cre). Nfat5fl/fl, Lck-Cre mice, but not Nfat5fl/fl, CD4-Cre mice, had smaller thymi than their wild-type (WT) littermates (Nfat5 wt/wt, Lck-Cre or Nfat5fl/fl mice) (Fig. 1 A and B) and decreased cellularity in lymphoid organs (Fig. 1C), with fewer mature CD4 and CD8 cells in lymph nodes and spleen (Fig. 1 D and E and ref. 21), but normal numbers of B cells (B220+, IgM+), macrophages (CD11b), and dendritic cells (CD11c) (Fig. 1 E and F). These results indicated that loss of NFAT5 in DN cells affected thymocyte development.
Fig. 1.
Reduced number of thymocytes and peripheral T cells in Nfat5fl/fl, Lck-Cre mice. (A) Thymus size in control (WT), Nfat5fl/fl, Lck-Cre (Upper), or Nfat5fl/fl, CD4-Cre (Lower) mice. (Scale bar, 1 cm.) (B) Body, thymus, and spleen weight in WT and Nfat5fl/fl, Lck-Cre mice. (C) Cell numbers in inguinal lymph nodes, thymus, and spleen in WT and Nfat5fl/fl, Lck-Cre mice. Each circle in B and C represents a single mouse, and the horizontal lines show the mean. (D) Numbers of CD3, CD4, and CD8 cells in lymph nodes of WT and Nfat5fl/fl, Lck-Cre mice (Left) and WT and Nfat5fl/fl, CD4-Cre mice (Right) (mean ± SEM); n ≥ 6. Numbers of B220-positive, CD3-positive, CD4-positive, and CD8-positive (E) and IgM-positive, CD11b-positive, and CD11c-positive (F) splenocytes in WT and Nfat5fl/fl, Lck-Cre mice (mean ± SEM); n = 8. *P < 0.05; **P < 0.01; ***P < 0.001.
NFAT5 Deficiency Causes a Thymocyte-Intrinsic Defect in Development.
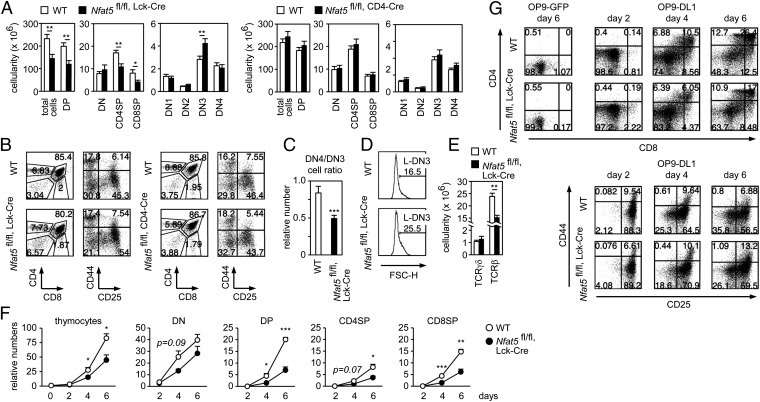
Nfat5fl/fl, Lck-Cre mice had significantly fewer DP and SP thymocytes than WT littermates and Nfat5fl/fl, CD4-Cre mice (Fig. 2 A and B). They also had an altered ratio of DN3 (CD44− and CD25+) to DN4 thymocytes (CD44− and CD25−) (Fig. 2 A–C), with a greater proportion of large, late DN3 cells (L-DN3) (Fig. 2D) and fewer αβ thymocytes, but normal numbers of γδ precursors (Fig. 2E). We did not observe obvious defects in markers of the DN to DP transition, such as expression of surface CD3, intracellular TCRβ in DN cells, and mRNA levels of Ptcra, Tcra, Lef1, and Tcf7 (SI Appendix, Fig. S1). DN cells of Nfat5fl/fl, Lck-Cre mice also displayed normal levels of activated PDK1 and Erk1/2 (SI Appendix, Fig. S2), indicating a normal function of proximal pre-TCR signaling.
Fig. 2.
Nfat5fl/fl, Lck-Cre thymocytes have a cell-intrinsic defect in development. (A) Cell numbers for thymocyte subsets in WT, Nfat5fl/fl, Lck-Cre, and Nfat5fl/fl, CD4-Cre mice (mean ± SEM); n ≥ 6. (B) Representative flow cytometry analysis of CD4 versus CD8 in total thymocytes, and CD44 versus CD25 gated in DN cells of WT and Nfat5fl/fl, Lck-Cre mice (Left) and WT and Nfat5fl/fl, CD4-Cre mice (Right). Numbers indicate percentages. (C) Ratio of DN4 to DN3 thymocytes in WT and Nfat5fl/fl, Lck-Cre mice (mean ± SEM); n = 8. (D) Percentage of L-DN3 cells in WT and Nfat5fl/fl, Lck-Cre mice, determined by cell size (forward light scatter height parameter, FSC-H). One representative experiment is shown out of six independently performed. (E) Number of TCRγδ+ or TCRβ+ thymocytes in WT and Nfat5fl/fl, Lck-Cre mice (mean ± SEM); n ≥ 8. (F) Relative numbers of thymocyte subsets after in vitro differentiation with OP9-DL1 cells for 2, 4, and 6 d (mean ± SEM); n = 3. (G) Representative flow cytometry analysis of CD4 versus CD8 (Upper) in total thymocytes, and CD44 versus CD25 (Lower) in gated DN cells, from thymocytes cocultured with OP9 control or OP9-DL1 cells. Percentages are indicated in the quadrants. *P < 0.05; **P < 0.01; ***P < 0.001.
We tested whether NFAT5 had a thymocyte-intrinsic role by analyzing the differentiation of isolated precursors cocultured with stromal cells expressing the Notch ligand Delta-like 1 (OP9-DL1) (26). Thymic precursors from Nfat5fl/fl, Lck-Cre mice exhibited poorer differentiation capacity than WT ones, producing fewer DP and SP thymocytes and showing defective progression from DN3 to DN4 (Fig. 2 F and G). These results reproduce the defects observed in vivo and support a cell-autonomous role for NFAT5 in thymocyte development.
NFAT5 Has Different Effects on Thymocyte Proliferation and Survival.
It has been suggested that the slightly hypertonic milieu of the thymus could be harmful for NFAT5-deficient thymocytes (24), but it is unclear whether thymic tonicity can activate NFAT5 (25). Nfat5fl/fl, Lck-Cre mice had normally isotonic plasma, and their DN3, DN4, and DP thymocytes expressed normal levels of the osmostress-inducible, NFAT5-dependent genes Akr1b3 and Slc5a3 (SI Appendix, Fig. S3 A and B). This result indicated that thymocytes are not undergoing an osmotic stress response and that NFAT5 regulates their development independent of its osmoresponsive function. This view is consistent with the in vitro differentiation experiments in Fig. 2 F and G, done in controlled isotonic medium.
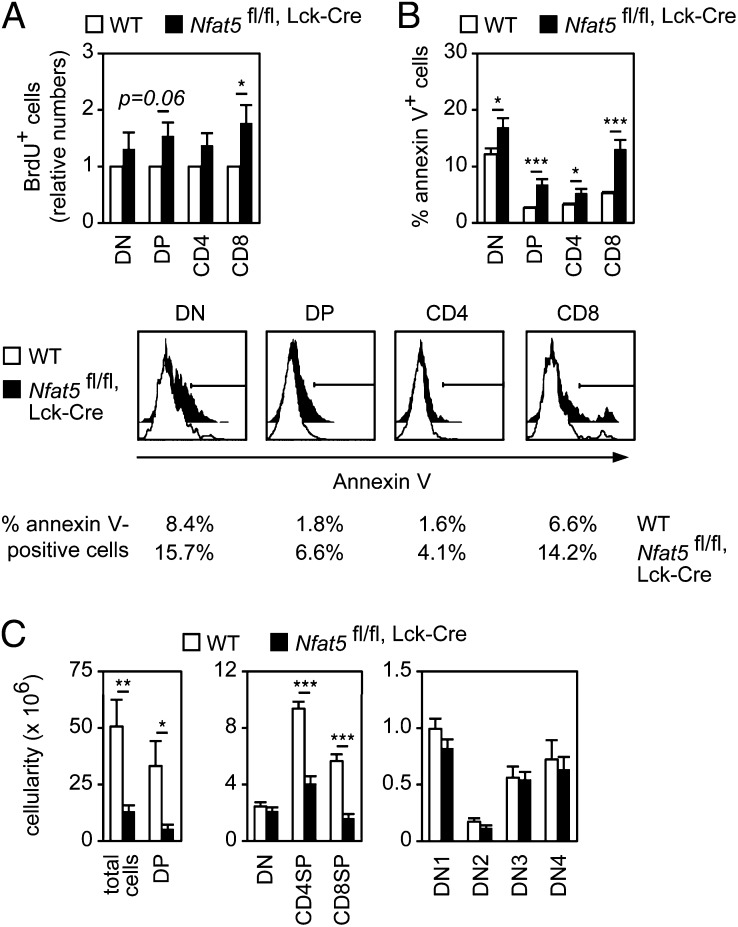
Because our results were consistent with a role for NFAT5 in thymocyte survival or proliferation, we examined both functions. NFAT5-deficient DP and CD8 thymocytes showed a mild increase in proliferation rate in vivo and a slightly elevated proportion of cells in S and G2/M cell cycle phases (Fig. 3A and SI Appendix, Fig. S4 A and B). Nonetheless, Nfat5fl/fl, Lck-Cre thymocytes expressed normal levels of the pre-TCR– or NFAT-regulated cyclins D3 or A2 (22, 27, 28) during their DN3 to DP transition (SI Appendix, Fig. S4C). In contrast with the moderate change in proliferation, freshly isolated thymocytes from Nfat5fl/fl, Lck-Cre mice displayed a higher proportion of apoptotic cells in the DN, DP, and SP compartments (Fig. 3B), suggesting that the overall effect of lacking NFAT5 in thymocytes was a reduced survival capacity. Consistent with this, enforced induction of apoptosis in vivo through CD3/TCR stimulation (29) caused a greater depletion of DP thymocytes in Nfat5fl/fl, Lck-Cre mice than in WT ones (Fig. 3C vs. Fig. 2A). Altogether, these data indicate that pre-TCR–driven thymocyte survival required NFAT5.
Fig. 3.
NFAT5 has different effects on thymocyte proliferation and survival. (A) Relative numbers of BrdU-positive thymocyte populations 5 h after BrdU injection in WT and Nfat5fl/fl, Lck-Cre mice (mean ± SEM); n = 4. (B) Percentage of annexin V-positive cells in thymocytes freshly isolated from WT and fl/fl, Lck-Cre mice (mean ± SEM); n > 8. Histograms in bottom panels show one representative experiment. (C) Number of thymocytes in each subset in WT and Nfat5fl/fl, Lck-Cre mice 48 h after anti-CD3 injection (mean ± SEM); n ≥ 6. *P < 0.05; **P < 0.01; ***P < 0.001.
Inefficient TCRβ Allelic Exclusion in Nfat5fl/fl, Lck-Cre Thymocytes.
Although pre-TCR signaling appeared to be normal in Nfat5fl/fl, Lck-Cre mice, defects in other pre-TCR functions were suggested by their having more L-DN3 thymocytes, which is the earliest stage at which the pre-TCR is expressed, and increased apoptosis since DN cells. In addition to promoting thymocyte survival and proliferation, the pre-TCR controls TCRβ allelic exclusion by preventing further rearrangements at the TCRβ locus (30). We observed that Nfat5fl/fl, Lck-Cre mice had normal levels of intracellular TCRβ in DN thymocytes (SI Appendix, Fig. S1) and a largely normal representation of TCR Vβ chain species in mature T cells (SI Appendix, Fig. S5A) but showed a higher percentage of thymocytes coexpressing Vβ8 together with Vβ5, Vβ7, or Vβ9 (SI Appendix, Fig. S5B), suggestive of anomalous TCRβ allelic exclusion. Although some degree of faulty allelic exclusion occurs in normal mice (30, 31), this defect was more pronounced in NFAT5-deficient cells. The increased allelic inclusion of Nfat5fl/fl, Lck-Cre thymocytes was not linked to defects in the expression of the transcription factors Ets-1 or E47, two known regulators of this process (31, 32) (SI Appendix, Fig. S5 C and D). In addition, the Ets-1 target gene Runx3 (33) was normally expressed in DN3 to DP thymocytes of Nfat5fl/fl, Lck-Cre mice (SI Appendix, Fig. S5E).
Thymocyte markers expressed beyond the β-selection checkpoint (CD62L, CD44, IL-7Rα, CD24, and TCRβ), as well as positive selection markers, were normal in NFAT5-deficient cells (SI Appendix, Fig. S6 A–C). However, analysis of their progression through the DP steps of large preselection, small preselection, and small postselection cells (34) showed that they accumulated as large CD69− preselection cells and had a mild reduction in CD69 expression in cells with high intensity of TCRβ (SI Appendix, Fig. S6 D and E). To exclude the possibility that impaired survival of DP thymocytes could be a result of defective positive selection, we crossed Nfat5fl/fl, Lck-Cre mice with Marilyn mice and assessed positive and negative selection of CD4 cells, respectively, in their female and male progeny (35). We observed that positive and negative selection proceeded similarly in Marilyn mice regardless of NFAT5 (SI Appendix, Fig. S7).
Expression of NFAT5 in Thymocytes Is Enhanced by the Pre-TCR and Regulated by IKKβ.
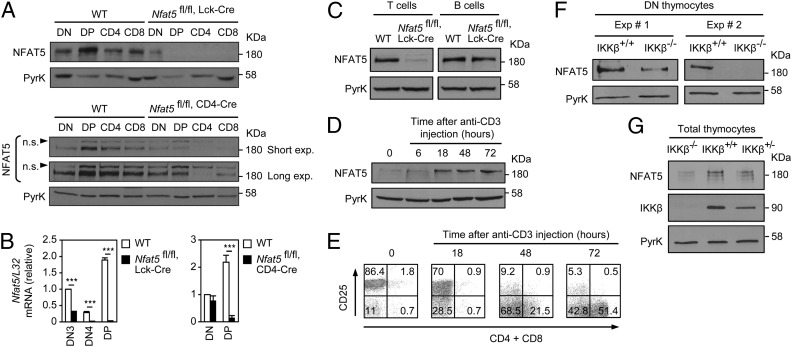
NFAT5 expression was higher in DP thymocytes than in DN and SP CD4 or CD8 cells (WT; Fig. 4A). Likewise, its mRNA increased in the DN to DP transition (WT, Fig. 4B). Nfat5fl/fl, Lck-Cre mice had reduced NFAT5 expression in DN cells and lost it completely in DP and SP thymocytes (Fig. 4 A and B). The deficiency in NFAT5 was maintained in peripheral mature T cells (Fig. 4C). In contrast, Nfat5fl/fl, CD4-Cre mice had NFAT5 in DN cells and still expressed moderate levels of it in DP thymocytes (Fig. 4 A and B). Residual expression of NFAT5 in CD8+ thymocytes of Nfat5fl/fl, CD4-Cre mice (Fig. 4A) likely is a result of immature CD8+ CD4− SP cells that do not express Cre. These findings indicated that the thymic hypocellularity and peripheral T-cell lymphopenia of Nfat5fl/fl, Lck-Cre mice were caused by the loss of NFAT5 between late DN and early DP thymocytes.
Fig. 4.
NFAT5 expression is up-regulated by the pre-TCR and IKKβ. (A) Western blot for NFAT5 in thymocyte subpopulations (DN, DP, CD4, CD8) from WT and Nfat5fl/fl, Lck-Cre mice (Upper), and WT and Nfat5fl/fl, CD4-Cre mice (Lower). n.s., nonspecific band. (B) Nfat5 mRNA levels in thymocyte subpopulations from WT and Nfat5fl/fl, Lck-Cre mice (Left) or Nfat5fl/fl, CD4-Cre mice (Right). Graphs show the mean ± SEM after normalization to L32 mRNA; n ≥ 4, ***P < 0.001. (C) NFAT5 expression in cultured T- and B-cell blasts from WT and Nfat5fl/fl, Lck-Cre mice. (D) Western blot of NFAT5 in thymocytes from Rag2−/− mice in basal conditions (0) and 6, 18, 48, or 72 h after anti-CD3 injection. (E) Surface expression of CD25 versus CD4 plus CD8 in thymocytes from Rag2−/− mice treated as in D. Western blot of NFAT5 in isolated DN cells (F) or total thymocytes (G) from IKKβ-deficient (Ikbkbfl/fl,Mx-Cre, IKKβ−/−) or control (Ikbkbwt/wt, IKKβ+/+; Ikbkbwt/fl,Mx-Cre, IKKβ+/−) mice. Pyruvate kinase was used as loading control, and one (A, D, E, and G) or two (F) representative experiments are shown of three independently performed.
Because the pre-TCR is a major player in the transition from DN to DP thymocytes, and NFAT5 expression was higher in DP than in DN cells, we tested whether this increase could be induced by stimulating the pre-TCR. Rag2−/− mice were injected with the anti-CD3 antibody 2C11 to enforce pre-TCR–induced DN to DP progression in developmentally arrested thymocytes in a manner independent of a functional mature αβ TCR (36). Anti-CD3 treatment increased the expression of NFAT5 protein in Rag2−/− thymocytes before they presented the surface markers CD4 and CD8 (Fig. 4 D and E). Given that the IKKβ/NF-κB pathway regulates NFAT5 expression in macrophages (37) and that this pathway promotes thymocyte survival downstream from the pre-TCR (12), we tested its involvement in the induction of NFAT5 in thymocytes. IKKβ-deficient thymocytes had substantially fewer DP and SP cells than control mice, but normal DN cell numbers (SI Appendix, Fig. S8). Nonetheless, both total thymocytes and isolated DN cells from these mice showed reduced expression of NFAT5 (Fig. 4 F and G). Altogether, these results indicated that NFAT5 is present in different thymocyte populations and that pre-TCR–activated IKKβ could further up-regulate its expression.
Altered Expression of Genes That Control the Balance Between Cell Death and Survival in Thymocytes of Nfat5fl/fl, Lck-Cre Mice.
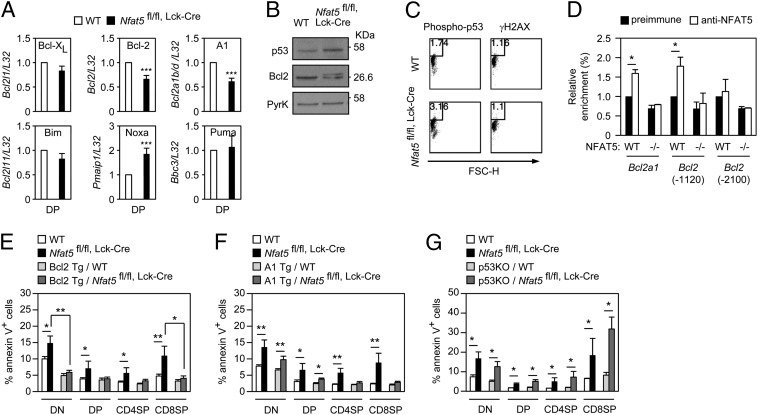
Analysis of the expression of genes that regulate cell death and survival (38) showed that NFAT5-deficient thymocytes had reduced mRNA levels of the antiapoptotic genes Bcl2a1 (Bcl2-related protein A1, or A1) and Bcl2 (B-cell leukemia/lymphoma 2, Bcl2), increased expression of the proapoptotic gene Pmaip1 (Noxa), and normal expression of other cell survival [Bcl2l1 (Bcl-xL)] or apoptosis [Bcl2l11 (Bim), Bbc3 (Puma)] regulators (Fig. 5A). Moreover, although enforced CD3 signaling in vivo reduced the number of NFAT5-deficient thymocytes, it did not reveal additional NFAT5 targets in control of cell survival (including Mcl1, Bax, and Bad; SI Appendix, Fig. S9). The ratio of A1 or Bcl2 to Noxa was around three times lower in NFAT5-deficient thymocytes, in line with their higher proportion of apoptotic cells. In addition to reduced levels of Bcl2 mRNA and protein, NFAT5-deficient DP thymocytes also expressed a lower-mobility form of Bcl2 associated with increased cell death (39) (Fig. 5B). We could not analyze A1 protein because of the lack of available antibodies. The elevated level of Noxa mRNA suggested that p53, its upstream regulator, could be more active in NFAT5-deficient DP cells. We detected a moderate increase in p53 expression and phosphorylation in NFAT5-deficient thymocytes (Fig. 5 B and C) without increased γH2AX (Fig. 5C), a marker of persistent double-strand DNA breaks.
Fig. 5.
Altered expression of anti- and proapoptotic genes in Nfat5fl/fl, Lck-Cre thymocytes. (A) mRNA level of antiapoptotic (Bcl2l1, Bcl2, Bcl2a1b/d) and proapoptotic (Bcl2l11, Pmaip1, Bbc3) Bcl2 family genes in WT and Nfat5fl/fl, Lck-Cre DP thymocytes. Values normalized to each L32 mRNA are represented relative to WT cells (value of 1) (mean ± SEM); n ≥ 3. ***P < 0.001. (B) Western blot of p53 and Bcl2 in DP thymocytes of WT and Nfat5fl/fl, Lck-Cre mice. Pyruvate kinase was used as loading control. One representative experiment is shown of three independently performed. (C) Percentage of phospho–p53-positive (Ser15; Left) and γH2AX-positive (Right) thymocytes in WT and Nfat5fl/fl, Lck-Cre mice. Data are representative of three independent experiments. (D) Chromatin immunoprecipitation analysis of NFAT5 binding to Bcl2 and Bcl2a1 regulatory regions in total thymocytes. Values show the enrichment in chromatin immunoprecipitated by the NFAT5-specific antibodies in the indicated genomic regions relative to the preimmune serum (value of 1) after correction for the input signal and are shown as mean ± SEM (n = 3, *P < 0.05). (E–G) Percentage of annexin V-positive thymocyte subsets in control and Nfat5fl/fl, Lck-Cre mice overexpressing Bcl2 (E) or A1 (F), or lacking p53 (G). Data are the mean ± SEM from three or more independent experiments including four or more individual mice of each genotype (*P < 0.05; **P < 0.01).
The 3-kb upstream regions of the genes encoding for A1 (Bcl2a1, isoforms b and d) and Bcl2 (Bcl2) contain consensus NFAT5 binding sites (ref. 17; 5′-TGGAAA-3′). Chromatin immunoprecipitation assays showed specific association of NFAT5 with the regulatory regions of Bcl2a1 and Bcl2 (−1120) in thymocytes, above background levels observed for a non-NFAT5 target region proximal to the Bcl2 gene (−2100), in NFAT5-deficient thymocytes, or with the preimmune serum (Fig. 5D).
We then analyzed whether overexpression of Bcl2 or A1, or deletion of p53, could rescue the survival defect of NFAT5-deficient thymocytes. Bcl2 had an overall protective effect in NFAT5-deficient thymocytes, as it reduced the proportion of annexin V-positive cells throughout different stages of maturation (Fig. 5E) and rescued the numbers of SP CD4 and CD8 cells (SI Appendix, Fig. S10A). Bcl2 overexpression also reduced the number of DP cells in WT thymi, attenuating the cellularity difference between WT and NFAT5-deficient DP thymocytes (SI Appendix, Fig. S10A). Overexpression of A1 had a mild effect on the survival of NFAT5-deficient thymocytes, which was more noticeable as cells progressed through development (Fig. 5F) but did not rescue their hypocellularity (SI Appendix, Fig. S10B). Finally, lack of p53 in NFAT5-deficient thymocytes only caused a subtle decrease in the percentage of annexin V-positive DN cells (Fig. 5G and SI Appendix, Fig. S10C). These findings support the idea that NFAT5-induced up-regulation of Bcl2 could be relevant in the prosurvival function of the pre-TCR. Altogether, our work shows that NFAT5 is induced by the pre-TCR in an IKKβ-regulated manner and controls a survival checkpoint during thymocyte development by balancing the expression of specific pro- and antiapoptotic effectors that act downstream from the pre-TCR.
Discussion
This work indicates that NFAT5 acts as an IKKβ-regulated survival factor downstream of the pre-TCR to facilitate the transition between DN and DP thymocytes. NFAT5-deficient thymocytes accumulated at the late DN3 stage, which marks the activation of the pre-TCR, had increased apoptosis and defective β-chain allelic exclusion. NFAT5 regulated the balance between specific antiapoptotic Bcl2 family members and the p53/Noxa axis in thymocyte differentiation. Our findings show that NFAT5 has specific functions during T-cell development independent of its role as an osmoprotective transcription factor, and place it as a regulator of distinct pre-TCR–induced outcomes relevant for thymocyte survival.
Although the participation of NF-κB and NFATc transcription factors downstream from the pre-TCR is well recognized (8, 40, 41), knowledge of their targets in this process and their specialization in particular pre-TCR functions is still quite limited. In this regard, the prosurvival function of NFAT5 more resembles the role of the conventional NF-κB pathway than that of the NFATc pathway (9–16). Moreover, the finding that NFAT5 expression downstream of the pre-TCR is under the control of IKKβ suggests that part of the prosurvival function of the NF-κB pathway at this point could be channelled via NFAT5.
NFAT5 may promote thymocyte viability, at least in part, by inducing the expression of A1 and Bcl2, as well as by reducing the p53/Noxa axis. Expression of A1 and repression of p53 are well recognized to control cell survival downstream from the pre-TCR (42–45). However, in addition to the role of NF-κB on A1 expression (45), not much was known about other regulators of A1 and p53 downstream from the pre-TCR (8). In this regard, our work identifies NFAT5 as a factor that controls A1, Bcl2, and p53 at this checkpoint of thymocyte development. Although overexpression of Bcl2 reduced the proportion of apoptotic NFAT5-deficient thymocytes, defects of NFAT5-deficient thymocytes were not rescued by overexpressing A1 or deleting p53. This could suggest that A1 and p53 do not play major roles downstream from NFAT5 or that changes in both need to occur in a coordinated manner, or in combination with other factors, to rescue NFAT5-deficient thymocytes. This possibility seems consistent with the notion that defects in NFAT5-deficient thymocytes could result from the cumulative effect of moderate changes in various genes.
Defective pre-TCR signals leading to inefficient allelic exclusion could facilitate persistent TCRβ rearrangements and, therefore, increase p53 activation and cause cell death of NFAT5-deficient thymocytes. However, as no functional connection has been established between A1 expression and β-chain allelic exclusion, it is likely that NFAT5 might modulate more than one pre-TCR–induced function. In contrast, NFAT5-deficient thymocytes did not show signs of impaired proliferation or differentiation, indicating that only certain functions of the pre-TCR take place in an NFAT5-regulated manner. Therefore, the specific participation of NFAT5 downstream from the pre-TCR also lends support to the notion that this receptor controls distinct outcomes that are, for the most part, functionally independent.
Materials and Methods
Mouse Models.
Nfat5fl/fl, CD4-Cre mice were previously described (22). Nfat5fl/fl, Lck-Cre and littermate Nfat5 wt/wt, Lck-Cre or Nfat5fl/fl control mice (WT) were obtained after consecutive crosses of Nfat5fl/fl mice with mice expressing Cre under the control of the mouse Lck proximal promoter (46) (Jackson Laboratory). p53-deficient mice were from the Jackson Laboratory (Trp53tm1Tyj). Marilyn and Rag2−/− mice were provided by Olivier Lantz (Institut Curie, Paris) (35). Transgenic mice expressing Bcl2 (47) or A1 (48) were provided by Ramón Merino (Universidad de Cantabria, Santander, Spain). IKKβ knockout mice in thymocytes were generated by crossing Ikbkbfl/fl mice with Mx-Cre mice (49, 50) (from Marc Schmidt-Supprian, Max Planck Institute of Biochemistry, Munich). Ikbkb deletion was induced by injecting mice with double-strand poly(I):poly(C) (Amersham Biosciences). NFAT5-deficient mice (21) were used as a source of thymocytes for chromatin immunoprecipitation. Mice were maintained on a pure C57BL/6 background in specific pathogen-free conditions and handled according to guidelines by the institutional ethical committee (Barcelona Biomedical Research Park Animal Care and Use Committee). Injection of mice with anti-CD3 antibody or Bromo-2′-deoxyuridine (BrdU) are described in SI Appendix, SI Materials and Methods.
OP9 and OP9-DL1 Cocultures.
OP9-DL1 cells (26) were used to differentiate thymocyte precursors (CD4− CD8− CD25+) in the presence of 10 ng/mL recombinant murine IL-7 (ImmunoTools) and 5 ng/mL recombinant human Flt3-ligand (ImmunoTools).
Isolation of Thymocyte Subpopulations and Analysis of Cell Death.
Procedures for magnetic or flow cytometry isolation of thymocyte subsets and detection of apoptotic cells are described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Andrea Cerutti, Gabriel Gil, Miguel López-Botet, Andreas Villunger, and José Yélamos for insightful comments. We also thank Drs. Olivier Lantz, Marc Schmidt-Supprian, and Ramón Merino for providing different mouse models; and Drs. Juan Carlos Zúñiga-Pflücker, Thomas Graf, and Meinrad Busslinger for reagents of the OP9-DL1 system. Anna Almor is acknowledged for technical assistance, and Dr. Oscar Fornas for guidance with flow cytometry. This work was supported by Spanish government Grants SAF2009-08066 and SAF2012-36535 (to C.L.-R.) and SAF2011-24268 (to J.A.); Fundació la Marató TV3 Grants 080730 and 122530 (to C.L.-R. and J.A.); Spanish Ministry of Health Grant ISCIII-RETIC RD06/0009-FEDER; and Generalitat de Catalunya Grant 2009SGR601. This work was also supported by a predoctoral fellowship from the Spanish government (to R.B.-B.) and predoctoral fellowships from Generalitat de Catalunya and the Spanish government (to M.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215934110/-/DCSupplemental.
References
- 1.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150(10):4244–4252. [PubMed] [Google Scholar]
- 2.Ciofani M, Zúñiga-Pflücker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol. 2007;23:463–493. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]
- 3.von Boehmer H, Fehling HJ. Structure and function of the pre-T cell receptor. Annu Rev Immunol. 1997;15:433–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]
- 4.Dudley EC, Petrie HT, Shah LM, Owen MJ, Hayday AC. T cell receptor beta chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1994;1(2):83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 5.Kruisbeek AM, et al. Branching out to gain control: how the pre-TCR is linked to multiple functions. Immunol Today. 2000;21(12):637–644. doi: 10.1016/s0167-5699(00)01744-8. [DOI] [PubMed] [Google Scholar]
- 6.Michie AM, Zúñiga-Pflücker JC. Regulation of thymocyte differentiation: pre-TCR signals and beta-selection. Semin Immunol. 2002;14(5):311–323. doi: 10.1016/s1044-5323(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 7.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 8.Aifantis I, Mandal M, Sawai K, Ferrando A, Vilimas T. Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol Rev. 2006;209:159–169. doi: 10.1111/j.0105-2896.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 9.Oukka M, et al. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity. 1998;9(3):295–304. doi: 10.1016/s1074-7613(00)80612-3. [DOI] [PubMed] [Google Scholar]
- 10.Ranger AM, et al. Delayed lymphoid repopulation with defects in IL-4-driven responses produced by inactivation of NF-ATc. Immunity. 1998;8(1):125–134. doi: 10.1016/s1074-7613(00)80465-3. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, et al. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8(1):115–124. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 12.Voll RE, et al. NF-kappa B activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity. 2000;13(5):677–689. doi: 10.1016/s1074-7613(00)00067-4. [DOI] [PubMed] [Google Scholar]
- 13.Aifantis I, Gounari F, Scorrano L, Borowski C, von Boehmer H. Constitutive pre-TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF-kappaB and NFAT. Nat Immunol. 2001;2(5):403–409. doi: 10.1038/87704. [DOI] [PubMed] [Google Scholar]
- 14.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20(3):255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 15.Canté-Barrett K, Winslow MM, Crabtree GR. Selective role of NFATc3 in positive selection of thymocytes. J Immunol. 2007;179(1):103–110. doi: 10.4049/jimmunol.179.1.103. [DOI] [PubMed] [Google Scholar]
- 16.Koltsova EK, et al. Early growth response 1 and NF-ATc1 act in concert to promote thymocyte development beyond the beta-selection checkpoint. J Immunol. 2007;179(7):4694–4703. doi: 10.4049/jimmunol.179.7.4694. [DOI] [PubMed] [Google Scholar]
- 17.López-Rodríguez C, Aramburu J, Rakeman AS, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci USA. 1999;96(13):7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Rodríguez C, et al. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001;15(1):47–58. doi: 10.1016/s1074-7613(01)00165-0. [DOI] [PubMed] [Google Scholar]
- 19.Aramburu J, et al. Regulation of the hypertonic stress response and other cellular functions by the Rel-like transcription factor NFAT5. Biochem Pharmacol. 2006;72(11):1597–1604. doi: 10.1016/j.bcp.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 20.López-Rodríguez C, et al. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci USA. 2004;101(8):2392–2397. doi: 10.1073/pnas.0308703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berga-Bolaños R, Drews-Elger K, Aramburu J, López-Rodríguez C. NFAT5 regulates T lymphocyte homeostasis and CD24-dependent T cell expansion under pathologic hypernatremia. J Immunol. 2010;185(11):6624–6635. doi: 10.4049/jimmunol.1001232. [DOI] [PubMed] [Google Scholar]
- 22.Drews-Elger K, Ortells MC, Rao A, López-Rodríguez C, Aramburu J. The transcription factor NFAT5 is required for cyclin expression and cell cycle progression in cells exposed to hypertonic stress. PLoS ONE. 2009;4(4):e5245. doi: 10.1371/journal.pone.0005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trama J, Go WY, Ho SN. The osmoprotective function of the NFAT5 transcription factor in T cell development and activation. J Immunol. 2002;169(10):5477–5488. doi: 10.4049/jimmunol.169.10.5477. [DOI] [PubMed] [Google Scholar]
- 24.Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA. 2004;101(29):10673–10678. doi: 10.1073/pnas.0403139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morancho B, Minguillón J, Molkentin JD, López-Rodríguez C, Aramburu J. Analysis of the transcriptional activity of endogenous NFAT5 in primary cells using transgenic NFAT-luciferase reporter mice. BMC Mol Biol. 2008;9:13. doi: 10.1186/1471-2199-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17(6):749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 27.Sicinska E, et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4(6):451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho LD, et al. The NFAT1 transcription factor is a repressor of cyclin A2 gene expression. Cell Cycle. 2007;6(14):1789–1795. doi: 10.4161/cc.6.14.4473. [DOI] [PubMed] [Google Scholar]
- 29.Shi YF, et al. In vivo administration of monoclonal antibodies to the CD3 T cell receptor complex induces cell death (apoptosis) in immature thymocytes. J Immunol. 1991;146(10):3340–3346. [PubMed] [Google Scholar]
- 30.Brady BL, Steinel NC, Bassing CH. Antigen receptor allelic exclusion: an update and reappraisal. J Immunol. 2010;185(7):3801–3808. doi: 10.4049/jimmunol.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eyquem S, Chemin K, Fasseu M, Bories JC. The Ets-1 transcription factor is required for complete pre-T cell receptor function and allelic exclusion at the T cell receptor beta locus. Proc Natl Acad Sci USA. 2004;101(44):15712–15717. doi: 10.1073/pnas.0405546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agata Y, et al. Regulation of T cell receptor beta gene rearrangements and allelic exclusion by the helix-loop-helix protein, E47. Immunity. 2007;27(6):871–884. doi: 10.1016/j.immuni.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Zamisch M, et al. The transcription factor Ets1 is important for CD4 repression and Runx3 up-regulation during CD8 T cell differentiation in the thymus. J Exp Med. 2009;206(12):2685–2699. doi: 10.1084/jem.20092024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Testi R, D’Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15(10):479–483. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 35.Lantz O, Grandjean I, Matzinger P, Di Santo JP. Gamma chain required for naïve CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1(1):54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 36.Shinkai Y, Alt FW. CD3 epsilon-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2-/- mice in the absence of TCR beta chain expression. Int Immunol. 1994;6(7):995–1001. doi: 10.1093/intimm/6.7.995. [DOI] [PubMed] [Google Scholar]
- 37.Buxadé M, et al. Gene expression induced by Toll-like receptors in macrophages requires the transcription factor NFAT5. J Exp Med. 2012;209(2):379–393. doi: 10.1084/jem.20111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez JB, Newton RH, Walsh CM. Life and death in the thymus—cell death signaling during T cell development. Curr Opin Cell Biol. 2010;22(6):865–871. doi: 10.1016/j.ceb.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001;15(4):515–522. doi: 10.1038/sj.leu.2402090. [DOI] [PubMed] [Google Scholar]
- 40.Macián F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 41.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25(51):6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 42.Guidos CJ, et al. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocyte precursors. Genes Dev. 1996;10(16):2038–2054. doi: 10.1101/gad.10.16.2038. [DOI] [PubMed] [Google Scholar]
- 43.Jiang D, Lenardo MJ, Zúñiga-Pflücker JC. p53 prevents maturation to the CD4+CD8+ stage of thymocyte differentiation in the absence of T cell receptor rearrangement. J Exp Med. 1996;183(4):1923–1928. doi: 10.1084/jem.183.4.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haks MC, Krimpenfort P, van den Brakel JHN, Kruisbeek AM. Pre-TCR signaling and inactivation of p53 induces crucial cell survival pathways in pre-T cells. Immunity. 1999;11(1):91–101. doi: 10.1016/s1074-7613(00)80084-9. [DOI] [PubMed] [Google Scholar]
- 45.Mandal M, et al. The BCL2A1 gene as a pre-T cell receptor-induced regulator of thymocyte survival. J Exp Med. 2005;201(4):603–614. doi: 10.1084/jem.20041924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15(5):763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 47.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez J, Orlofsky A, Prystowsky MB. A1 is a growth-permissive antiapoptotic factor mediating postactivation survival in T cells. Blood. 2003;101(7):2679–2685. doi: 10.1182/blood-2002-04-1229. [DOI] [PubMed] [Google Scholar]
- 49.Pasparakis M, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417(6891):861–866. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 50.Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269(5229):1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.