Abstract
Systemic amyloid A (AA) amyloidosis is a serious complication of chronic inflammation. Serum AA protein (SAA), an acute phase plasma protein, is deposited extracellularly as insoluble amyloid fibrils that damage tissue structure and function. Clinical AA amyloidosis is typically preceded by many years of active inflammation before presenting, most commonly with renal involvement. Using dose-dependent, doxycycline-inducible transgenic expression of SAA in mice, we show that AA amyloid deposition can occur independently of inflammation and that the time before amyloid deposition is determined by the circulating SAA concentration. High level SAA expression induced amyloidosis in all mice after a short, slightly variable delay. SAA was rapidly incorporated into amyloid, acutely reducing circulating SAA concentrations by up to 90%. Prolonged modest SAA overexpression occasionally produced amyloidosis after long delays and primed most mice for explosive amyloidosis when SAA production subsequently increased. Endogenous priming and bulk amyloid deposition are thus separable events, each sensitive to plasma SAA concentration. Amyloid deposits slowly regressed with restoration of normal SAA production after doxycycline withdrawal. Reinduction of SAA overproduction revealed that, following amyloid regression, all mice were primed, especially for rapid glomerular amyloid deposition leading to renal failure, closely resembling the rapid onset of renal failure in clinical AA amyloidosis following acute exacerbation of inflammation. Clinical AA amyloidosis rarely involves the heart, but amyloidotic SAA transgenic mice consistently had minor cardiac amyloid deposits, enabling us to extend to the heart the demonstrable efficacy of our unique antibody therapy for elimination of visceral amyloid.
Keywords: 3Rs, disease model
Amyloid A (AA) amyloidosis is a rare but important complication of otherwise treatable chronic inflammatory conditions (1) in which the amyloid deposits are derived from serum amyloid A protein (SAA) (2), an apolipoprotein of HDL. SAA is a nonspecific acute phase protein, with a dynamic range of 1 to >3,000 mg/L in humans, synthesized by hepatocytes under the control of proinflammatory cytokines (3). The deposition of AA-type amyloid in the viscera, connective tissue, and blood vessel walls damages tissue structure and function, with the kidney as the major target organ (4). Control of inflammation and hence of SAA production in patients with AA amyloidosis arrests amyloid accumulation and enables its clinically beneficial regression (4, 5). Although clinical outcomes have improved markedly in recent years through better control of inflammation, AA amyloidosis is still fatal, and there are important gaps in our understanding of the pathogenesis of this disease. For example, the precise nature of the relationship between inflammation and amyloid deposition and the factors governing the onset, progression, and anatomical distribution of amyloid deposition remain unclear.
AA amyloidosis never occurs in the absence of inflammation and the associated acute phase response with increased circulating concentrations of SAA, but only a minority of individuals at risk ever develop AA amyloidosis. Persistently increased plasma SAA concentrations within the range typically seen in chronic inflammatory diseases are thus not generally sufficient for amyloid deposition. Furthermore, because all previous animal models of AA amyloidosis depend on induction of chronic inflammation (6–9) to stimulate persistently increased SAA production, it has not hitherto been possible to identify the respective roles of inflammation and of increased abundance of SAA.
Human AA amyloidosis is typically preceded by many years of active inflammatory disease, and the clinical presentation is then relatively acute, suggesting a slow or long delayed priming process and/or a rare stochastic event that eventually triggers amyloid deposition in some individuals. In mice, greatly accelerated AA deposition can be produced by parenteral administration of an extract of amyloidotic tissue, so called “amyloid-enhancing factor,” the active component of which is amyloid fibrils themselves, followed by a single very aggressive acute phase stimulus. Deposition of AA amyloid in humans may thus also be triggered by such an amyloid fibril seed, but it has not hitherto been possible to investigate this mechanism clinically. Another weakness of previous models is that, although almost all AA amyloidosis patients present with or develop renal dysfunction, kidney involvement in murine AA amyloidosis is late and rarely causes renal failure. The unique tractable and tunable transgenic model reported here, with hepatic SAA production induced independently of inflammation by oral administration of doxycycline (10), allows the underlying mechanisms to be dissected.
Results
Regulated Overexpression of SAA in Mice.
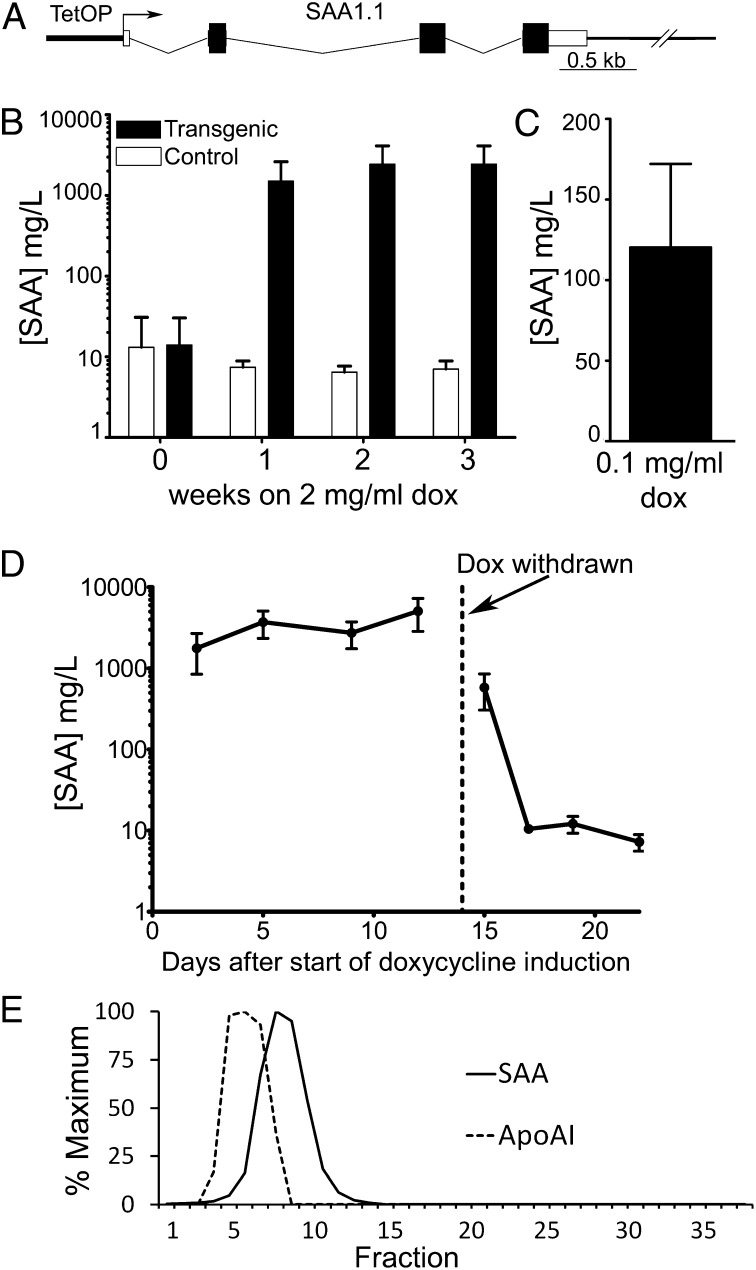
Without exposure to doxycycline, double transgenic mice carrying both a doxycycline-inducible SAA transgene (Fig. 1A) and a liver-specific reverse tet transactivator transgene (11), expressed SAA at the same baseline levels, ∼10 mg/L, as WT C57BL/6 mice. When exposed to drinking water containing doxycycline at 2 mg/mL, SAA production dramatically increased to values otherwise only seen in severe inflammation (Fig. 1B). Doxycycline had no effect on SAA values in nontransgenic mice, or in SAA or rTALap-1 single transgenic mice, but there was a clear dose–response effect in double-transgenic mice. Drinking water containing 0.1 mg/mL doxycycline induced SAA production to yield plasma values of 50–250 mg/L (Fig. 1C), whereas exposure to 2 mg/mL doxycycline induced values of >1,000 mg/L (Fig. 1B). Peak SAA concentrations of 2,000–5,000 mg/L are much greater than seen in any other experimental or naturally occurring model of AA amyloidosis, although among ∼700 patients with clinical AA amyloidosis, we have seen values of ∼2,500 mg/L in a few individuals. Induction of SAA expression by doxycycline was reversible, with the serum SAA concentration falling to baseline within 72 h of doxycycline withdrawal (Fig. 1D). As with acute phase SAA in WT mice, the SAA produced in response to doxycycline was associated with HDL, eluting in size exclusion chromatography of whole serum in the trailing edge of the apoAI peak (Fig. 1E), consistent with the known displacement by SAA of apoAI from HDL particles (12).
Fig. 1.
Inducible expression of SAA. (A) Schematic of the doxycycline-responsive SAA transgene. (B) Sustained high-level expression of SAA specifically in transgenic mice on drinking water containing 2 mg/mL doxycycline (mean ± SD; n = 5 transgenic, n = 6 controls). (C) Modest SAA expression in transgenic mice on drinking water containing 0.1 mg/mL doxycycline [mean ± SD; n = 10 (5 ♂; 5 ♀)]. (D) SAA concentrations rapidly return to normal baseline values following doxycycline removal [mean ± SD; n = 8 (4 ♂; 4 ♀)]. (E) Elution profile of SAA and apoAI in size exclusion chromatography of whole serum from mouse with high-level transgenic SAA expression.
Deposition of AA Amyloid Without Inflammation.
Neither doxycycline alone, nor massively induced production of mouse SAA, caused increased production of murine serum amyloid P component (SAP) (Fig. S1). Mouse SAP is a major murine acute phase reactant that responds sensitively to almost all inflammatory or tissue-damaging processes (13). Doxycycline-induced overexpression of SAA is thus totally independent of inflammation. Double-transgenic mice exposed to drinking water containing 2 mg/mL doxycycline developed major systemic amyloid deposits after a median of 5 wk (range, 4–16 wk) as shown by massive whole body retention of i.v.-administered 125I-human SAP (125I-hSAP), a validated, specific, quantitative in vivo tracer for systemic amyloid deposits in mice (14, 15) and humans (16–18). Heavy AA amyloid deposits were confirmed histologically in the spleen and liver, correlating closely with the major organ localization of 125I-hSAP. Significant minor amyloid deposits were also present in the kidneys, heart, and gut (Fig. S2). The total amyloid load was much greater than we have ever seen, or has previously been reported, but organ function was preserved (Table S1). A substantial proportion, 13 of 60, of these amyloidotic mice had normal SAP concentrations (≤10 mg/L), showing unequivocally that amyloid deposition can occur in the absence of inflammation. The remaining amyloidotic animals had only modestly elevated SAP concentrations, probably as a result of tissue damage caused by the massive amyloid deposits. For example, in one experiment, the SAP values were 17.0 ± 5.9 mg/L (mean ± SD) in mice after deposition of amyloid compared with 7.0 ± 1.0 mg/L in the same animals after induction of SAA expression but before amyloid deposition (n = 18).
Latency of Amyloid Deposition.
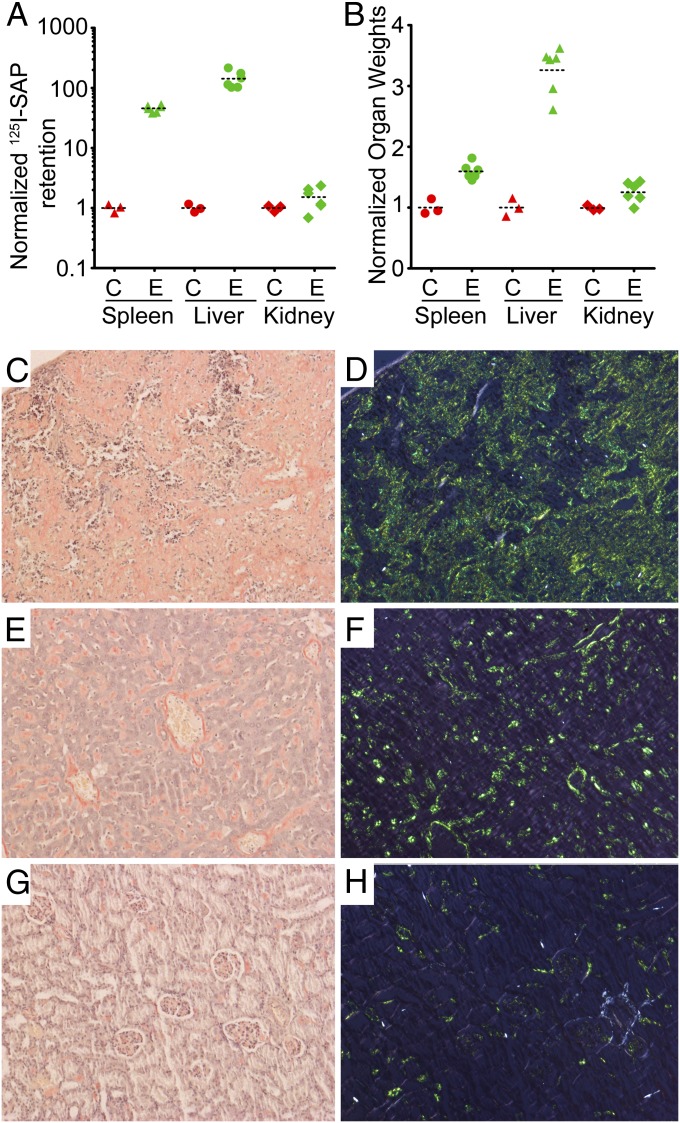
Induction of murine AA amyloidosis by sustained chronic inflammation typically takes several weeks (7). However, mice in which AA amyloid deposits have regressed following remission of chronic inflammation are primed for rapid reaccumulation when they receive a new inflammatory stimulus (19), and previously untreated mice are readily primed by i.v. injection of amyloidotic tissue extracts or isolated amyloid fibrils, so-called amyloid enhancing factor (AEF) (20, 21). In double-transgenic mice, a single AEF injection 4 d after starting on drinking water containing high-dose doxycycline at 2 mg/mL produced heavy amyloid deposits just 2 d later. By 21 d, there was massive amyloidosis in all animals tested, with markedly increased liver and spleen sizes (Fig. 2). In contrast, the median time before amyloid deposition was detected in mice receiving doxycycline treatment alone was 5 wk, demonstrating the requirement, even in the face of abundant SAA, for a delayed and/or rare seeding or triggering event.
Fig. 2.
Amyloidosis in SAA overexpressing transgenic mice. (A) Retention of 125I-human SAP in organs of control (C) and experimental transgenic (E) mice treated with doxycycline for 3 wk after AEF injection. The 125I retained in spleens, livers, and kidneys was counted 24 h after injection of 106 cpm of 125I-labeled human SAP. The counts retained are shown normalized to the means of the control values (spleen 8.1 × 102 cpm; liver 4.5 × 103 cpm; kidney 2.8 × 103 cpm). (B) Weights of amyloidotic and control mouse organs normalized to the means of the control values (spleen 0.072 g; liver 0.846 g; kidney 0.265 g). (C–H) Representative histology of amyloidotic spleen (C and D), liver (E and F), and kidneys (G and H) of transgenic mice stained with Congo red. C, E, and G, bright field; D, F, and H, crossed polarized light.
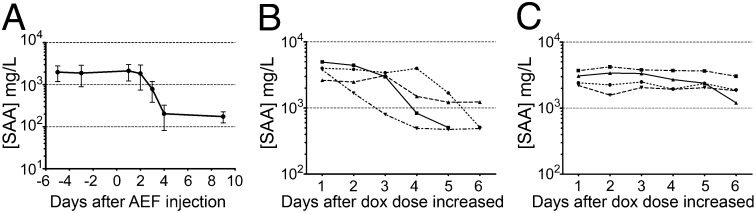
When only 0.1 mg/mL doxycycline was present in the drinking water, inducing more modestly increased mouse SAA expression and a mean ± SD concentration of 120 ± 52 mg/L (n = 10), no amyloid was detected by 125I-hSAP retention in more than 1 y. One mouse then became amyloidotic between 58 and 66 wk, resembling the long latency and low incidence of clinical AA amyloidosis. In principle, these findings could reflect delayed seeding of amyloid deposition, insufficient SAA expression to permit net accumulation of amyloid, or both. When mice received AEF and low-dose doxycycline at 0.1 mg/mL in their drinking water, only 1 of 10 animals developed amyloidosis between 9 and 13 wk, 2 became amyloidotic after 34–50 wk, and 7 had no detectable amyloid at 58 wk. The drinking water doxycycline concentration was then increased to 2 mg/mL, and all seven mice became amyloidotic within 4 d, demonstrating both that the primed state persisted and that these animals had not previously become amyloidotic because SAA abundance was limiting. In contrast, when eight mice that had received water containing 0.1 mg/mL of doxycycline for 21 mo were switched to 2 mg/mL, four of them promptly developed amyloidosis within 4 d, and a further two did so at 7 d, whereas two others remained amyloid free at 7 d. Amyloid was deposited significantly later in a control cohort of previously unexposed, double-transgenic, age-matched animals started on water containing 2 mg/mL doxycycline at exactly the same time as the dose was increased in the long-term low-dose group (Fig. S3; P = 0.022). These results clearly differentiate between endogenous priming for amyloid deposition and bulk amyloid accumulation and show that priming can occur when levels of SAA are elevated modestly and insufficiently for net amyloid deposition. Sustained modest elevation of circulating SAA concentration does not potently initiate or promote amyloid deposition but can cause amyloidosis occasionally and also primes for rapid deposition if SAA production sharply rises.
Sequestration of Circulating SAA During Active Amyloid Deposition.
While amyloid was being deposited, between 2 and 4 d following AEF injection in mice on 2 mg/mL doxycycline, we observed a precipitous ∼90% fall in circulating SAA concentrations (Fig. 3A). The fall was not an effect of exogenous AEF, as SAA values fell similarly in mice that, after long-term treatment with 0.1 mg/mL doxycycline, were then given 2 mg/mL doxycycline and developed amyloid within 4 d (Fig. 3B). SAA values did not fall in mice that did not develop amyloid despite receiving identical treatment (Fig. 3C). SAA mRNA abundance in the livers of doxycycline-treated mice did not differ between those receiving AEF and controls (Fig. S4A) despite the sharp fall in circulating SAA concentration during amyloid deposition in the AEF-treated group (Fig. S4B). The evident rapid sequestration of SAA into nascent amyloid deposits was quantitatively consistent with the increase in amyloidotic organ weights. Assuming a peak steady-state SAA concentration of 2 mg/mL, blood volume of 1.5 mL, and SAA t1/2 of 90 min (22), maximal SAA production is 24 mg daily. Assuming constant SAA secretion rate and otherwise normal turnover, the 90% fall in circulating SAA concentration during amyloid deposition corresponds to loss of 21.6 mg daily from the plasma. The total of ∼0.45 g over 3 wk, based on conservative assumptions, is consistent with the observed ∼2-g increase in liver weight of mice after AEF and 3 wk on doxycycline, considering that amyloid deposits contain abundant proteoglycans and SAP, as well as amyloid fibrils (1).
Fig. 3.
Circulating SAA concentration falls during amyloid deposition. (A) SAA concentrations fell sharply following AEF injection as amyloid was deposited (note the logarithmic scale for SAA values; mean ± SD; n = 8; P < 0.0001, repeated-measures ANOVA). (B and C) Circulating SAA concentrations following increase of drinking water doxycycline content to 2 mg/mL in mice treated long term with water containing 0.1 mg/mL doxycycline. (B) Mice amyloidotic by day 4: SAA concentrations fell (n = 4; P = 0.0062, repeated-measures ANOVA). (C) Mice not detectably amyloidotic on day 4: SAA concentrations were stable (n = 4; P = 0.3378, repeated-measures ANOVA).
Amyloid Regression, Recurrence, and Onset of Renal Failure.
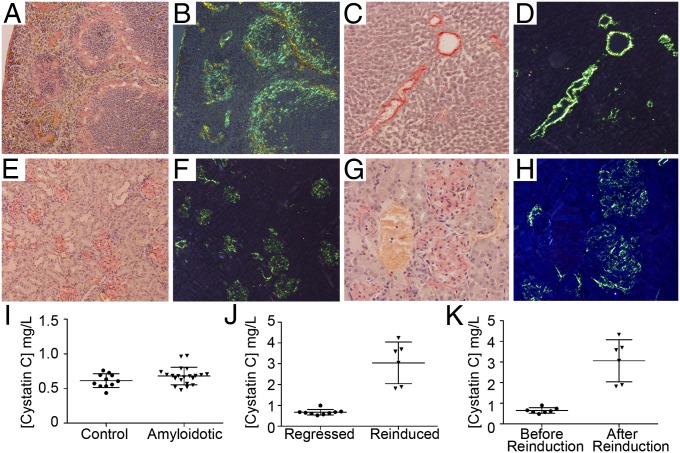
Consistent with clinical experience, amyloid load diminished very slowly after withdrawal of doxycycline, despite prompt extinction of SAA transgene expression. Retention of 125I-hSAP took at least 4 mo to return to baseline, remained abnormal after >1 y in some animals, and specks of typical Congophilic amyloid (23), undetected by the whole body method, were sometimes present histologically. After 6 and 17 mo off doxycycline, reexposure of previously amyloidotic mice to drinking water containing 2 mg/mL of doxycycline triggered extremely rapid new amyloid deposition with interestingly different pathological and clinical features. There were massive typical splenic marginal zone and interfollicular deposits (Fig. 4 A and B), but hepatic amyloid was predominantly in and around portal tracts and central veins rather than scattered in the parenchyma (Fig. 4 C and D). Most strikingly, major renal glomerular amyloidosis developed (Fig. 4 E–H). The glomerular amyloid deposits were far more abundant than we have previously observed in mice, and remarkably, all glomeruli were affected. Renal function monitored by serum cystatin C assay (24) was normal before amyloid regression (Fig. 4I) or immediately before doxycycline reexposure but was markedly impaired within 3 d of reinduction of SAA expression and the ensuing renewed amyloid deposition (Fig. 4 J and K).
Fig. 4.
Remission and relapse lead to glomerular amyloidosis and renal failure. Congo red staining of reaccumulated amyloid in spleen (A and B), liver (C and D), and renal cortex (E–H) showing widespread, heavy glomerular amyloid deposits; bright field illumination (A, C, E, and G) and crossed polarized light (B, D, F, and H). (I) Normal serum cystatin C in mice amyloidotic for the first time (mean ± SD; n = 11 controls; n = 21 amyloidotic; P = 0.135; t test). (J and K) Increased serum cystatin C concentration caused by renal failure in mice with massive glomerular amyloid deposition after reinduction. (J) Comparison of reinduced (n = 6) and control (n = 5) groups (mean ± SD; P = 0.0025; Mann–Whitney test). (K) Before and after reinduction values in individual mice (n = 6; mean ± SD; P = 0.0156; Wilcoxon matched-pairs signed rank test).
Therapeutic Reduction of Cardiac Amyloid Deposits.
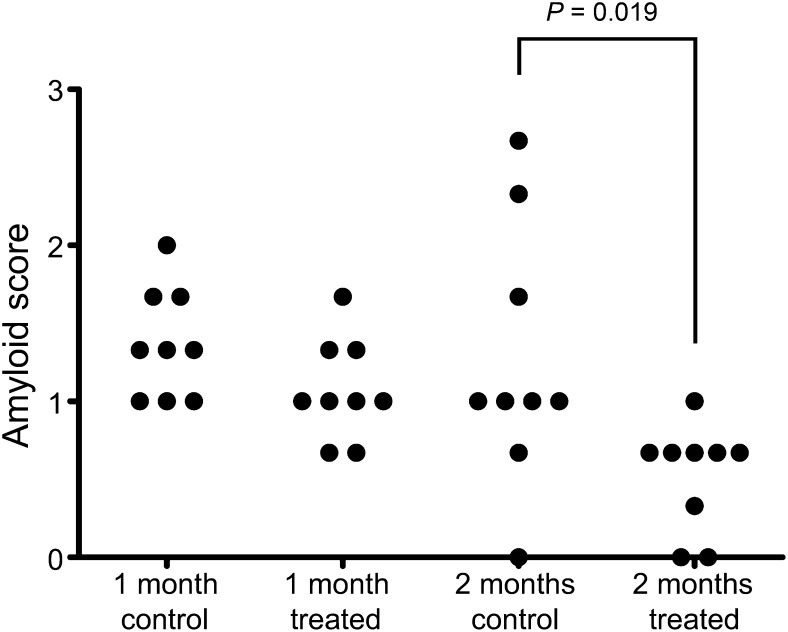
We recently showed that administration of anti-human SAP antibodies to AA amyloidotic mice in which the amyloid deposits had been loaded with human SAP leads to macrophage-mediated elimination of hepatic and splenic amyloid (25). In contrast to the standard mouse AA amyloidosis model, cardiac amyloid is consistently present in the new SAA transgenic model. SAA transgenic mice with abundant amyloid after exposure to doxycycline were therefore given three daily parenteral injections of human SAP to load up the amyloid deposits, and 5 d after the last injection, when all human SAP had left the circulation, they were treated with monoclonal mouse IgG2a anti-human SAP antibodies. After a single dose of anti-SAP, spleen and liver amyloid deposits were, as usual, substantially reduced (both P < 0.001 compared with amyloidotic controls not given the antibody), but there was no significant difference in extent of cardiac amyloid. However, after a second treatment with SAP and anti-SAP 1 mo later, the cardiac amyloid deposits were substantially diminished (P = 0.014; Fig. 5).
Fig. 5.
Reduction of cardiac amyloid deposits following antibody therapy. Amyloidotic mice received an injection of human SAP to load the amyloid deposits, followed by injection of monoclonal anti-human SAP antibody. Control amyloidotic mice received no treatment. Half the treated and control mice were killed 30 d later, and the remaining treated animals received a second identical treatment. All remaining mice were then killed after a further 30 d. Amyloid scores of individual mice are plotted. Kruskal–Wallis test P = 0.002; multiple comparison test: one anti-SAP dose vs. control, P = 0.38; two anti-SAP doses vs. control, P = 0.019.
Discussion
Systemic AA amyloid deposition is always accompanied by increased SAA production, whether triggered by tissue damage, infection, or abnormal proinflammatory cytokine activity (26–30). The transgenic mice reported here allowed us to induce expression of the amyloidogenic mouse SAA isoform, SAA2, by oral administration of doxycycline, completely independently of any inflammatory stimulus. Neither doxycycline exposure nor induction of SAA production itself triggered an acute phase response of other proteins. Thus, contrary to claims in the literature, circulating mouse SAA, like authentic human SAA (31), is clearly not intrinsically proinflammatory. Furthermore, although AA amyloidosis has hitherto been inextricably linked to systemic inflammation, the present results demonstrate that isolated overproduction of SAA, without inflammation, is sufficient.
In common with models in which AA amyloidosis is evoked by potently inducing inflammation, transgenic mice induced to express SAA at high levels deposited amyloid after a variable latent period, and the process was accelerated and made absolutely consistent by injection of AEF containing preformed amyloid fibrils that seed amyloid fibrillogenesis.
In contrast with experimental AA amyloidosis in mice, human AA amyloidosis affects only a small minority of patients with inflammatory diseases, usually presents long after the onset of inflammation [median (range), 17 y (0–68 y)] (4, 5), most commonly with proteinuria and/or renal failure (4, 5), and plasma SAA concentrations [median (range), 28 mg/L (1–1,610 mg/L)] (4, 5) are typically more modest than in the mouse model. The reasons why some patients develop amyloidosis and most others do not are obscure. The ability to dissociate priming and deposition experimentally raises the possibility that either (or both) of these could be limiting in patients. Using low-dose doxycycline to induce modestly increased SAA production, we replicated the long latency of the human disease and the occasional late-onset development of amyloidosis, resembling the low frequency of its incidence in patients. Amyloid deposition occurred only occasionally in mice in which prolonged modest overexpression of SAA was induced after priming with AEF. Thus, SAA levels can be elevated for an extended period and yet be insufficient to drive net accumulation of amyloid, whether or not amyloid deposition has been primed. Although extended modest overproduction of SAA did not inexorably lead to amyloid deposition, some animals that remained free of detectable amyloid were primed for amyloid deposition when SAA expression was increased. If these findings accurately reflect amyloid pathogenesis in patients, a significant proportion of individuals with prolonged chronic inflammatory disease may already be primed, and amyloid deposition may then be precipitated by an episode of severe inflammation. In patients with a long history of chronic inflammation, it would be wise to treat episodes of severe inflammation aggressively and without delay. Prompt evaluation of such patients may lead to early detection of AA amyloidosis, perhaps before the onset of significant renal failure.
Mice have two distinct acute phase SAA proteins, SAA1 and SAA2, encoded by different genes and produced in roughly equal amounts (2), but only SAA2 is amyloidogenic. As WT mice with chronic inflammation become amyloidotic, the SAA1:SAA2 concentration ratio increases sharply, and the total circulating SAA value falls modestly, consistent with selective sequestration of SAA2 into amyloid (32, 33). Our present finding of a dramatic fall of SAA2 values in all of the transgenic mice as amyloid was being deposited was similarly consistent with such sequestration. In humans, there are also two different acute phase SAA proteins (34) encoded by different genes, but unlike in the mouse, both human acute phase SAA proteins are deposited as amyloid fibrils (35). Our transgenic model, overexpressing only amyloidogenic murine SAA2, thus more closely resembles the human situation than studies in WT mice, and the present finding of rapid depletion of circulating SAA may have important implications for clinical management. Plasma SAA values in AA amyloidosis patients correlate closely with progression of amyloid deposition, morbidity, and mortality (4, 5), and the aim of treatment is to maximally reduce the SAA concentration. However, the transgenic mouse results now suggest that SAA values may be misleadingly low during active amyloid deposition, perhaps explaining clinical observations of amyloid accumulation in the face of modest plasma SAA concentrations (4). If such depression of plasma SAA does occur in patients, it is likely to be most significant in those who have the heaviest amyloid loads because the large amounts of amyloid in those individuals will provide an abundant sink for circulating SAA.
The most common important clinical consequence of AA amyloidosis is renal dysfunction, with >10% of patients having end-stage renal failure at first diagnosis (4). Renal failure developed very rapidly on reinduction of SAA expression in transgenic mice in which amyloid had previously been induced and spontaneously regressed. This response closely parallels the rapid onset or relapse of renal failure precipitated by intercurrent flares of inflammation in some AA amyloidosis patients in remission (4, 5) and suggests that glomerular amyloid deposition may be responsible, as in the transgenic mice. The factors that determine the sites of amyloid deposition in general are unknown, but the dramatically altered pattern of amyloid reaccumulation following regression could reflect a different distribution of amyloid template. Amyloid regresses much more rapidly in the spleen and liver than in the kidney, making the latter more susceptible to amyloid deposition when SAA production increases. Thus, the presence of large amounts of template in spleen and/or liver may, through sequestration of SAA, limit the rate of deposition in the kidney. A related phenomenon may explain the discrepancy between clinical AA amyloidosis, which is almost invariably accompanied by renal dysfunction, and experimental murine AA amyloidosis, in which kidney function is rarely affected. Experimental amyloidosis is usually induced rapidly in a single phase, whereas clinical amyloidosis typically occurs after many years of inflammatory disease, providing opportunity for cycles of amyloid deposition and regression.
As well as providing insights into aspects of the pathogenesis of AA amyloidosis, amyloidotic transgenic mice have proved to be a valuable model for applied studies, and they offer some significant advantages over other models. Although cardiac involvement is very rare in clinical AA amyloidosis (4), the transgenic mice reproducibly developed cardiac amyloid deposits. The heart is usually involved in systemic monoclonal Ig light chain (AL) disease, which is by far the most common type of human amyloidosis. Cardiac amyloid of transthyretin type is also common in the elderly (36, 37), although until recently it was rarely diagnosed in life. The development of cardiac amyloidosis in the transgenic model allowed us to show that our recently developed antibody therapy (25) effectively reduces cardiac amyloid load, as it does amyloid in the spleen and liver. The present mouse model is also proving useful for development of unique noninvasive in vivo amyloid imaging methods (38).
Methods
Transgenic Mice.
The SAA transgene comprises the tetracycline-responsive promoter from pTRE-myc (Clontech) linked within their respective 5′ UTRs to a 3.7-kb genomic DNA fragment containing the amyloidogenic mouse SAA isoform SAA2 (2), also known as SAA1.1 (9), with 1.25 kb of 3′ flanking sequence (ENSEMBL ID ENSMUSG00000057465). The transgene fragment was isolated from the plasmid vector and microinjected into pronuclei of one-cell eggs obtained from C57BL/6 × CBA F1 donors. Transgenic mice were identified by PCR with primers GTGTACGGTGGGAGGCCTAT and CCAGGAGGTTCCACTGAGAG from the promoter and the SAA gene, respectively. Ten lines of transgenic mice were established and crossed with rTALap-1 reverse tet-transactivator (rtTA) mice (11), which express the reverse tet transactivator rtTA2S-S2 (39) in hepatocytes, obtained from the European Mouse Mutant Archive (stock no. EM:00404). Presence of the rtTA transgene was assayed by PCR using primers CTGGGAGTTGAGCAGCCTAC and AGAGCACAGCGGAATGACTT. All of the reported work was done with a single line of mice selected because, in conjunction with rTALap-1, they had normal baseline serum SAA values when untreated with doxycycline and highly inducible SAA expression when doxycycline was administered. Double-transgenic mice were backcrossed onto the C57BL/6J background. All experiments reported here used hemizygous transgenic mice.
Induction of SAA Expression and of Amyloid Deposition.
Doxycycline hyclate (Sigma D9891) was administered in drinking water containing 5% (wt/vol) sucrose. Amyloid loads were assessed essentially as described (14) by whole-body retention of 125I-hSAP 24 and 48 h after injection of tracer; 0.006% (wt/vol) potassium iodide was included in the drinking water to prevent retention of free 125I iodide. AEF (20, 21) was administered by tail vein injection. Abundant amyloid deposits were reliably and consistently produced by a single AEF injection and administration of doxycycline; e.g., 2 mg/mL doxycycline was administered for 3 d before and 7 d after AEF injection. The inconvenience of using (double) transgenic mice is mitigated because we bred mice homozygous for both transgenes. This refined transgenic model of AA amyloidosis minimizes the severity of experimental animal procedures because it eliminates the need for injections to stimulate severe or chronic inflammation. All animal studies were ethically reviewed and approved by the UCL Royal Free Campus Ethics and Welfare Committee and the UK Home Office, and were carried out in accordance with European Directive 86/609/EEC.
Biochemical Assays.
Commercial ELISAs were used for SAA (Tridelta or Immune Systems Ltd.), apolipoprotein AI (USCN Life Science), and cystatin C (Biovendor) and were performed according to the manufacturers’ instructions. Mouse SAP was assayed by electroimmunoassay with monospecific sheep anti-mouse SAP antiserum (13). All other assays were performed at MRC Harwell using an Olympus AU400 automated clinical chemistry analyzer. Size exclusion chromatography was performed using a Superdex 200 column on the ÄKTA Explorer apparatus (GE Healthcare). The column was equilibrated and eluted at 0.5 mL/min with PBS buffer containing 5 mM EDTA, pH 7.4.
Quantitative RT-PCR.
Assay of SAA mRNA levels was performed by quantitative RT-PCR (qRT-PCR) using transgene-specific primers ATAGAAGACACCGGGACCGAT and TAGCTTCATCCTGCTGGTGTCT, specific for the tet-responsive promoter and the SAA gene, respectively. The results were normalized against reference genes Sdha (primers TCATCACAGAAGGGTGTCGT and GCAACAGGTGCGTATCTCTC) and Hprt (primers TCCTCCTCAGACCGCTTTT and ACCTGGTTCATCATCGCTAATC).
Histology.
Formalin-fixed wax-embedded tissues were sectioned at 7 µm, stained with alkaline alcoholic Congo red (23), and viewed under strong cross-polarized light.
Antibody Therapy.
Amyloidosis was induced in female transgenic mice by treatment with AEF and doxycycline and verified by assaying whole body retention of 125I-hSAP. For therapy, the amyloid deposits were loaded with unlabeled human SAP by injection of 10 mg/d for 3 d. After a delay of 5 d to allow elimination of circulating hSAP, the mice were injected with 5 mg of monoclonal mouse anti-hSAP antibody (clone SAP-5; GlaxoSmithKline) (25). Amyloid load was scored as described previously (15); the values reported are the average of the scores assigned by three expert individuals who were blinded to the treatments given.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs), the UK Medical Research Council, the Royal Free London NHS Foundation Trust, and the Wolfson Foundation. Core support for the Wolfson Drug Discovery Unit is provided by the UK National Institute for Health Research Biomedical Research Centre and Unit Funding Scheme. K.B. was supported by the Erik and Edith Fernströms Foundation for Medical Research the Swedish Research Council. S.M. is supported by Biotechnology and Biological Sciences Research Council Grants BB/G021163/1 and BB/I007806/1.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306621110/-/DCSupplemental.
References
- 1.Pepys MB. Amyloidosis. Annu Rev Med. 2006;57:223–241. doi: 10.1146/annurev.med.57.121304.131243. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JS, Ericsson LH, Eriksen N, Walsh KA, Benditt EP. Murine tissue amyloid protein AA. NH2-terminal sequence identity with only one of two serum amyloid protein (ApoSAA) gene products. J Exp Med. 1984;159(2):641–646. doi: 10.1084/jem.159.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benditt EP, Eriksen N, Hanson RH. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc Natl Acad Sci USA. 1979;76(8):4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lachmann HJ, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356(23):2361–2371. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 5.Gillmore JD, Lovat LB, Persey MR, Pepys MB, Hawkins PN. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet. 2001;358(9275):24–29. doi: 10.1016/S0140-6736(00)05252-1. [DOI] [PubMed] [Google Scholar]
- 6.Jaffé RH. Amyloidosis produced by injections of proteins. 1926. Arch Pathol Lab Med. 2001;125(1):25–37. doi: 10.5858/2001-125-0025-HP. [DOI] [PubMed] [Google Scholar]
- 7.Janigan DT. Experimental amyloidosis: Studies with a modified casein method, casein hydrolysate and gelatin. Am J Pathol. 1965;47(1):159–171. [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Guo JT, Zhu H, Kindy MS. Amyloid formation in the rat: Adenoviral expression of mouse serum amyloid A proteins. Amyloid. 2000;7(1):32–40. doi: 10.3109/13506120009146822. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Zhu H, Guo JT, de Beer FC, Kindy MS. Expression of mouse apolipoprotein SAA1.1 in CE/J mice: Isoform-specific effects on amyloidogenesis. Lab Invest. 2000;80(12):1797–1806. doi: 10.1038/labinvest.3780191. [DOI] [PubMed] [Google Scholar]
- 10.Kistner A, et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA. 1996;93(20):10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schönig K, Schwenk F, Rajewsky K, Bujard H. Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res. 2002;30(23):e134. doi: 10.1093/nar/gnf134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parks JS, Rudel LL. Alteration of high density lipoprotein subfraction distribution with induction of serum amyloid A protein (SAA) in the nonhuman primate. J Lipid Res. 1985;26(1):82–91. [PubMed] [Google Scholar]
- 13.Pepys MB, Baltz M, Gomer K, Davies AJS, Doenhoff M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979;278(5701):259–261. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins PN, Myers MJ, Epenetos AA, Caspi D, Pepys MB. Specific localization and imaging of amyloid deposits in vivo using 123I-labeled serum amyloid P component. J Exp Med. 1988;167(3):903–913. doi: 10.1084/jem.167.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botto M, et al. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med. 1997;3(8):855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990;323(8):508–513. doi: 10.1056/NEJM199008233230803. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins PN, Wootton R, Pepys MB. Metabolic studies of radioiodinated serum amyloid P component in normal subjects and patients with systemic amyloidosis. J Clin Invest. 1990;86(6):1862–1869. doi: 10.1172/JCI114917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins PN. Serum amyloid P component scintigraphy for diagnosis and monitoring amyloidosis. Curr Opin Nephrol Hypertens. 2002;11(6):649–655. doi: 10.1097/00041552-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins PN, Pepys MB. A primed state exists in vivo following histological regression of amyloidosis. Clin Exp Immunol. 1990;81(2):325–328. doi: 10.1111/j.1365-2249.1990.tb03339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Axelrad MA, Kisilevsky R, Willmer J, Chen SJ, Skinner M. Further characterization of amyloid-enhancing factor. Lab Invest. 1982;47(2):139–146. [PubMed] [Google Scholar]
- 21.Baltz ML, Caspi D, Hind CRK, Feinstein A, Pepys MB. Isolation and characterization of amyloid enhancing factor (AEF) In: Glenner GG, et al., editors. Amyloidosis. New York: Plenum Press; 1986. pp. 115–121. [Google Scholar]
- 22.Hoffman JS, Benditt EP. Plasma clearance kinetics of the amyloid-related high density lipoprotein apoprotein, serum amyloid protein (apoSAA), in the mouse. Evidence for rapid apoSAA clearance. J Clin Invest. 1983;71(4):926–934. doi: 10.1172/JCI110847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puchtler H, Sweat F, Levine M. On the binding of Congo red by amyloid. J Histochem Cytochem. 1962;10(3):355–364. [Google Scholar]
- 24.Song S, et al. Serum cystatin C in mouse models: A reliable and precise marker for renal function and superior to serum creatinine. Nephrol Dial Transplant. 2009;24(4):1157–1161. doi: 10.1093/ndt/gfn626. [DOI] [PubMed] [Google Scholar]
- 25.Bodin K, et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468(7320):93–97. doi: 10.1038/nature09494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon A, et al. Transgenic mouse model of AA amyloidosis. Am J Pathol. 1999;154(4):1267–1272. doi: 10.1016/S0002-9440(10)65378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lachmann HJ, et al. Clinical and subclinical inflammation in patients with familial Mediterranean fever and in heterozygous carriers of MEFV mutations. Rheumatology (Oxford) 2006;45(6):746–750. doi: 10.1093/rheumatology/kei279. [DOI] [PubMed] [Google Scholar]
- 28.Agostini L, et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20(3):319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 29.Lachmann HJ, Gilbertson JA, Gillmore JD, Hawkins PN, Pepys MB. Unicentric Castleman’s disease complicated by systemic AA amyloidosis: A curable disease. QJM. 2002;95(4):211–218. doi: 10.1093/qjmed/95.4.211. [DOI] [PubMed] [Google Scholar]
- 30.Rivas AL, Tintle L, Kimball ES, Scarlett J, Quimby FW. A canine febrile disorder associated with elevated interleukin-6. Clin Immunol Immunopathol. 1992;64(1):36–45. doi: 10.1016/0090-1229(92)90057-u. [DOI] [PubMed] [Google Scholar]
- 31.Björkman L, et al. The proinflammatory activity of recombinant serum amyloid A is not shared by the endogenous protein in the circulation. Arthritis Rheum. 2010;62(6):1660–1665. doi: 10.1002/art.27440. [DOI] [PubMed] [Google Scholar]
- 32.Meek RL, Hoffman JS, Benditt EP. Amyloidogenesis. One serum amyloid A isotype is selectively removed from the circulation. J Exp Med. 1986;163(3):499–510. doi: 10.1084/jem.163.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hébert L, Gervais F. apo-SAA1/apo-SAA2 isotype ratios during casein- and amyloid-enhancing-factor-induced secondary amyloidosis in A/J and C57BL/6J mice mice. Scand J Immunol. 1990;31(2):167–173. doi: 10.1111/j.1365-3083.1990.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 34.Eriksen N, Benditt EP. Isolation and characterization of the amyloid-related apoprotein (SAA) from human high density lipoprotein. Proc Natl Acad Sci USA. 1980;77(11):6860–6864. doi: 10.1073/pnas.77.11.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liepnieks JJ, Kluve-Beckerman B, Benson MD. Characterization of amyloid A protein in human secondary amyloidosis: The predominant deposition of serum amyloid A1. Biochim Biophys Acta. 1995;1270(1):81–86. doi: 10.1016/0925-4439(94)00076-3. [DOI] [PubMed] [Google Scholar]
- 36.Cornwell GG, 3rd, Murdoch WL, Kyle RA, Westermark P, Pitkänen P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med. 1983;75(4):618–623. doi: 10.1016/0002-9343(83)90443-6. [DOI] [PubMed] [Google Scholar]
- 37.Tanskanen M, et al. Senile systemic amyloidosis, cerebral amyloid angiopathy, and dementia in a very old Finnish population. Amyloid. 2006;13(3):164–169. doi: 10.1080/13506120600876757. [DOI] [PubMed] [Google Scholar]
- 38. Campbell-Washburn AE, et al. (2013) Monitoring systemic amyloidosis using MRI measurements of the extracellular volume fraction. Amyloid 20(2):93–98. [DOI] [PubMed]
- 39.Urlinger S, et al. Exploring the sequence space for tetracycline-dependent transcriptional activators: Novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci USA. 2000;97(14):7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.