Abstract
Background. In patients with hepatocellular carcinoma, selection criteria for transarterial hepatic selective internal radiotherapy are imprecise. Additionally, radiographic parameters to predict outcome of transarterial hepatic selective internal radiotherapy have not been fully characterized. Patients and methods. Computed tomography (CT) scans of 23 patients with unresectable primary hepatocellular carcinoma before and after transarterial hepatic selective internal radiotherapy with yttrium-90 microspheres were retrospectively reviewed. Selected radiographic parameters were evaluated and correlated with progression-free survival and overall survival. Response to treatment was assessed with Response RECIST 1.1 and Morphology, Attenuation, Size, and Structure (MASS) criteria. Results. On the post-SIRT CT, 68% of tumors demonstrated decreased size (median decrease of 0.8 cm, P = 0.3); 64% had decreased attenuation (median decrease 5.7 HU, P = 0.06), and 48% demonstrated increased tumor necrosis (P < 0.001). RECIST-defined partial response was seen in 10% patients, stable disease in 80%, and 10% had disease progression. Median progression-free survival was 3.9 months (range, 3.3 to 7.3), and median overall survival was 11.2 months (7.1 to 31.1). Pretreatment lower hepatopulmonary shunt fraction, central hypervascularity, and well-defined tumor margins were associated with improved progression-free survival. Conclusion. In patients with unresectable hepatocellular carcinoma, pretreatment CT parameters may predict favorable response to SIRT and improve patient selection.
1. Introduction
Hepatocellular carcinoma is the third leading cause of cancer mortality worldwide [1]. It frequently occurs in patients who have chronic liver disease and cirrhosis [2, 3]. Curative treatment options include liver transplant, resection, and ablation [4]. However, approximately two thirds of patients are not candidates for curative therapy when diagnosed [5]. In selected patients, transarterial chemoembolization or radioembolization may be options for palliative treatment [6–9]. For patients with advanced hepatocellular carcinoma who are not candidates for curative or liver-directed therapies, targeted molecular therapy with sorafenib may improve survival [10, 11].
In the past, hepatocellular carcinoma had been considered a radioresistant tumor because of the limited effect of external beam radiation doses. This was caused, in part, by technical limitations imposed by the overlying anatomy [12, 13]. In selective internal radiotherapy (SIRT) with yttrium-90 microspheres, radioactive particles are injected into the hepatic artery, become trapped at the precapillary level, and emit lethal radiation to the tumor [6, 14, 15]. In SIRT with radioactive yttrium-90 microspheres, radiation exposure to normal liver parenchyma and extrahepatic structures may be limited, and higher radiation doses may be delivered to the tumor than feasible with external beam radiation. Nonetheless, the current patient selection criteria for SIRT are imprecise, and there is limited information available about the effect of SIRT on computed tomography (CT) characteristics of hepatocellular carcinoma. Furthermore, there is limited information about the relation between changes in CT characteristics and progression-free and overall survival after SIRT. Furthermore, it is not well studied whether CT scans can provide clinically useful objective parameters before or after SIRT in patients with hepatocellular carcinoma and whether the availability of these parameters may improve patient selection and treatment outcome from SIRT.
The purpose of this study was to characterize the radiographic parameters of hepatocellular carcinoma before SIRT with yttrium-90 glass microspheres; to evaluate whether these parameters may predict the outcome of SIRT; to characterize the objective changes caused by SIRT; and to evaluate the radiographic response of patients with hepatocellular carcinoma who were treated with SIRT with yttrium-90 glass microspheres. We characterized the features of tumors on CT scans before and after SIRT, assessed the radiographic tumor response by Morphology, Attenuation, Size, and Structure (MASS) criteria, and determined CT features that were associated with better progression-free and overall survival.
2. Materials and Methods
2.1. Patients
This study was a retrospective review of 23 patients who had hepatocellular carcinoma and who underwent SIRT with yttrium-90 glass microspheres between 2008 and 2011 at Karmanos Cancer Center, a tertiary care center. Inclusion criteria included (1) a diagnosis of hepatocellular carcinoma from biopsy or imaging according to criteria of the American Association for the Study of Liver Diseases [16]; (2) treatment with SIRT using yttrium-90 glass microspheres; and (3) survival >6 weeks after SIRT. Patients without proper follow-up imaging were excluded from the study. The study was approved by the Institutional Review Board.
2.2. Clinical Evaluation
The electronic medical records were reviewed for demographic, clinical, radiographic, and pathologic information before and after SIRT. Data included age, race, sex, hepatic function, renal function tests, liver Child Pugh status, imaging studies, and survival outcomes. Patients were staged by Child-Pugh score, United Network for Organ Sharing (UNOS) score, and Barcelona Clinic Liver Cancer (BCLC) score (A, early; B, intermediate; C, advanced; D, end-stage) [17, 18]. Patients survival was determined using the Metropolitan Detroit Cancer Surveillance System (MDCSS) or Detroit Surveillance Epidemiology and End Results (SEER) cancer registry.
2.3. Selective Internal Radiotherapy with Yttrium-90 Glass Microspheres
The SIRT procedure was performed by percutaneous transarterial injection of radioactive glass microspheres (β-radiation; activity per microsphere, 2500 Bq; diameter, 20 to 30 μm) into the hepatic artery. The microspheres were supplied in a V-bottom, shielded glass vial (range, 1.2 million to 8 million microspheres per vial); the number of microspheres determined the radioactivity of the vial (range, 3 GBq (81 mCi) to 20 GBq (540 mCi)). Radiation doses were customized for each patient by administering the beads at a defined time on the radiation decay curve. The SIRT dose was determined from CT-based liver volumetric analysis. Desired calculated doses per tumor-containing lobe ranged from 80 to 150 Gy [19–21].
The patients were evaluated with a CT scan and liver function tests before the SIRT procedure. Digital subtraction angiography was done with fluoroscopy to provide a map of the vascular anatomy, and vessels supplying extrahepatic structures such as the stomach or intestine were coil-embolized. A technetium-labeled protein was injected to determine the lung shunt fraction. Patients were excluded from SIRT when the hepatopulmonary shunt fraction resulted in a lung dose for a single session >30 Gy or cumulative lifetime >50 Gy. Patients had a second angiogram, and the radioactive beads were injected in a lobar fashion [19–21].
2.4. Computed Tomography Evaluation
The abdominal CT scans were performed before and after SIRT (Toshiba Aquilion 64 slice scanner, Toshiba Medical, Otawara-shi, Tochigi-ken, Japan or Siemens Somatom 64, Siemens, Munich, Germany). An abdominal radiologist who was blinded to the clinical outcome reviewed all CT scans.
Primary tumor parameters were determined from the CT scans including (1) bidimensional tumor size; (2) tumor attenuation (density in Hounsfield units (HU)); (3) tumor margins (well or poorly defined); (4) tumor enhancement (homogenous or heterogeneous); (5) extent of tumor necrosis (<50%; ≥50% to <95%; or ≥95%); (6) hepatopulmonary shunt fraction; and (7) hypervascularity pattern (central or peripheral). The CT scans were also evaluated for the presence of portal venous thrombosis and lymphadenopathy.
The radiographic features of the primary tumor were determined on CT scans obtained at 3 times: before SIRT (baseline), after SIRT, and at the time of documented disease progression. The response of the primary tumor to SIRT was evaluated from the CT images using RECIST 1.1 [22] and MASS criteria [23]. The radiographic tumor responses by MASS criteria were categorized as favorable response, indeterminate response, or unfavorable response (Table 1) [23].
Table 1.
Response to treatment graded by Morphology, Attenuation, Size, and Structure (MASS) criteria*.
| Response category | MASS criteria description |
|---|---|
| Favorable response | No new lesions and either of the following: (1) decrease in tumor size ≥20%; (2) ≥1 predominantly solid enhancing lesion with marked central necrosis or marked decreased attenuation |
|
| |
| Indeterminate response | Does not fit criteria for favorable or unfavorable response |
|
| |
| Unfavorable response | Either of the following: (1) increase in tumor size ≥20% without marked central necrosis or marked decreased attenuation; (2) new metastases, marked central fill-in, or new enhancement of a previously homogeneously hypoattenuating, nonenhancing mass |
*Described by Smith et al. [23].
2.5. Data Analysis
Categorical data were reported as number (%). Measured data were reported as median (range, minimum to maximum). Categorical variables (tumor margins, enhancement, and percent necrosis) before and after SIRT were compared. The differences between continuous variables (tumor size and attenuation) before and after SIRT were dichotomized at the median for survival analysis. Changes between CT parameters before and after treatment were analyzed with Wilcoxon Signed-Rank Test (continuous variables) or Fisher's Exact Test (categorical variables).
The primary endpoint of this study was progression-free survival (PFS), defined as the time from SIRT to disease progression (defined by RECIST 1.1 or death). The one patient who did not have a documented progression event was censored at the last evaluation for progression. The secondary endpoint was overall survival (OS), defined as time from SIRT until death. Patients who did not die were censored at the last time when they were known to be alive. Each of the categorical covariates was tested for association with progression-free and overall survival using the log-rank test. Continuous covariates were tested using Cox proportional hazards regression model. Median survival and 95% confidence intervals were estimated using Kaplan-Meier method. Bayesian analysis was applied to a multivariate Cox proportional hazards regression model to identify a parsimonious set of statistically significant covariates. Statistical significance was defined by P ≤ 0.05.
3. Results
Most patients were African American men who did not have ascites or extrahepatic metastases (Table 2). Baseline CT scans showed that most tumors had <50% necrosis, poorly defined margins, and heterogeneous enhancement (Table 2).
Table 2.
Characteristics of patients with hepatocellular carcinoma who were treated with selective internal radiotherapy with Yttrium-90 microspheres*.
| Characteristic | |
|---|---|
| Age (y) | 63 (50 to 87) |
| Sex | |
| Men | 18 (79%) |
| Women | 5 (21%) |
| Race | |
| African American | 14 (61%) |
| White | 6 (26%) |
| Other | 3 (13%) |
| Ascites | 5 (24%) |
| Extrahepatic metastatic disease | 4 (17%) |
| Laboratory values | |
| Albumin (g/dL) | 2.7 (2.7 to 4.9) |
| Total bilirubin (mg/dL) | 0.8 (0.2 to 2.6) |
| α-fetoprotein (μg/L) | 108 (1.4 to 462400) |
| Computed tomography of tumors† | |
| Tumor size (cm) | 8.1 (2.3 to 17) |
| Attenuation (HU) | 35 (20 to 65.2) |
| <50% necrosis | 21 (95%) |
| Well-defined margins | 9 (39%) |
| Enhancement (heterogeneous) | 21 (95%) |
| Central hypervascularity | 12 (52%) |
| Hepatopulmonary shunt fraction | 0.056 (0.014 to 0.165) |
| Peripheral hypervascularity pattern | 6 (43%) |
*N = 23 patients. Data reported as median (range, minimum to maximum) or number (%) patients.
†Before selective internal radiotherapy.
Median tumor size was 8.1 cm (2.3–17 cm), median tumor attenuation was 35 HU (20–65.2 HU), and more than half of the tumors (52%) demonstrated central hypervascularity pattern. On the angiogram performed before treatment the median percentage of hepatopulmonary shunt fraction was 5.6%. The median dose of yttrium-90 glass microspheres administered to the patient was 2.0 GBq (0.6–4.3).
Comparing the radiographic features of the CT scan obtained at median 1.5 months after SIRT to the CT scan obtained at baseline, most tumors margins were unchanged, and almost half of the number of tumors (48%) had increased percentage of necrosis (P < 0.001) (Table 3). Sixty-four percent of tumors demonstrated decreased attenuation (median decrease 5.7 HU, P = 0.06), and 68% of the tumors had reduction in longest diameters (median decrease of 0.8 cm, P = 0.3) (Table 3).
Table 3.
Changes in computed tomography parameters of hepatocellular carcinoma after treatment with selective internal radiotherapy with Yttrium-90 microspheres*.
| Parameter | Number (%) of tumors with change† | Median change | P |
|---|---|---|---|
| Decreased tumor size (n = 22) (Figure 3) | 15 (68%) | −0.8 (−4.8 to +4.1) cm | 0.3 |
| Decreased attenuation (n = 11) (Figures 1 and 2) | 7 (64%) | −5.7 (−14.8 to +6.1) HU | 0.06 |
| Increased percentage of necrosis (Figures 1 and 2) | 10 (48%) | — | ≤0.001 |
| Margin status (n = 20)‡ | |||
| Improved | 3 (15%) | — | 0.2 |
| Remained the same | 14 (70%) | ||
| Worsened | 3 (15%) |
*Computed tomography was done at median 1.5 mo (95% confidence interval, 1.3 to 3.3 mo) after selective internal radiotherapy. Data reported as number (%) or median (range, minimum to maximum).
†Change from before to after treatment.
‡Improved, poorly defined became well defined; worsened, well defined became poorly defined.
Following SIRT, the majority of patients (80%) had stable disease by RECIST 1.1. criteria; 10% of the patients demonstrated partial response, while 10% showed disease progression (Table 4). Only nine patients were response evaluable by MASS criteria; of theses 9 patients, 44% demonstrated favorable response while 22% and 33% of the patients demonstrated indeterminate and unfavorable response, respectively (Table 4). The median progression-free survival was 3.9 months (3.3, 7.3), and the median overall survival was 11.2 months (7.1, 31.1) for all patients.
Table 4.
Response of hepatocellular carcinoma to treatment with selective internal radiotherapy with Yttrium-90 microspheres*.
| Response criteria | No. (%) patients | P † |
|---|---|---|
| RECIST‡ | ||
| Partial response | 2 (10%) | 0.09 |
| Stable disease | 17 (80%) | |
| Progressive disease | 2 (10%) | |
| MASS criteria¶ | ||
| Favorable response | 4 (44%) | 0.027 |
| Indeterminate response | 2 (22%) | |
| Unfavorable response | 3 (33%) |
†log-rank test; association of response criteria with overall survival.
‡RECIST: response evaluation criteria in solid tumors; n = 21 patients.
¶MASS: morphology, attenuation, size, and structure; n = 9 patients.
Multivariate analysis of baseline CT parameters showed that prolonged progression-free survival was associated with lower hepatopulmonary shunt fraction, central hypervascularity pattern, and well-defined tumor margins, while shorter progression-free survival was associated with abutment of portal vein by tumor (Table 5).
Table 5.
Multivariate analysis of pretreatment computed tomography characteristics and progression-free survival in patients with hepatocellular carcinoma treated with selective internal radiotherapy with Yttrium-90 microspheres*.
| Computed tomography characteristic | Hazard ratio (95% confidence interval) |
P |
|---|---|---|
| Hepatopulmonary shunt fraction | 0.28 (0.09 to 0.85) | 0.02 |
| Central hypervascularity pattern | 0.13 (0.02 to 0.69) | 0.006 |
| Well-defined margins | 0.37 (0.13 to 1.0) | 0.04 |
| Abutment of portal vein | 10.1 (1.78 to 57.50) | 0.002 |
*N = 23 patients.
Subgroup analysis of the 9 patients who were response evaluable by MASS criteria showed that favorable response following SIRT was a predictor of overall survival (P = 0.027) (Table 6; Figure 6).
Table 6.
Multivariate analysis of response after treatment and overall survival in patients with hepatocellular carcinoma treated with selective internal radiotherapy with Yttrium-90 microspheres*.
| Favorable response after SIRT† | Hazard ratio (95% confidence interval) |
P-value |
|---|---|---|
| Overall survival | 8.2 (0.93, 73.0) | 0.027 |
*N = 9 patients. Response graded by Morphology, Attenuation, Size, and Structure (MASS) criteria.
†SIRT: selective internal radiotherapy. MASS criteria were not included in this multivariate analysis because of the small number of patients who could be evaluated by MASS criteria.
Figure 6.
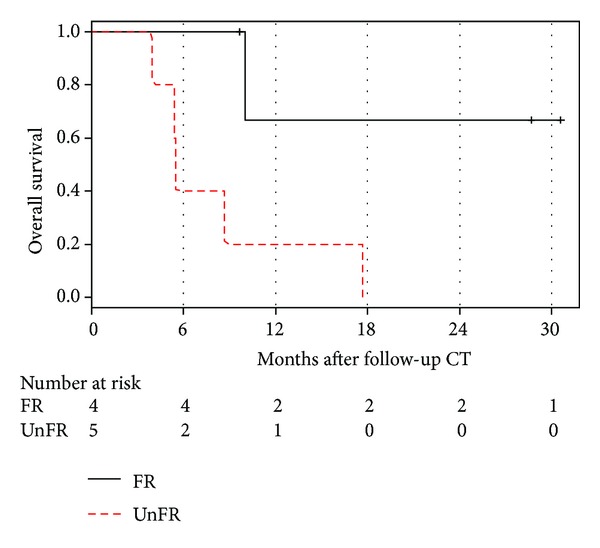
Kaplan-Meier plot showing overall survival stratified by Morphology, Attenuation, Size, and Structure (MASS) criteria (FR, favorable response; UR, unfavorable response).
Additionally, an exploratory analysis of the tumor size at base line (prior to radioembolization) showed that tumors >8.6 cm were associated with worse overall survival (HR: 1.92, 95% CI: (0.73, 5.04)), although the result was not statistically significant (P = 0.18).
4. Discussion
The therapeutic landscape has significantly evolved over the past decade largely because of a better understanding of tumor biology leading to the introduction of targeted therapies as well as technologies that revolutionized local-regional treatments. Yet, such advances have resulted in many challenges and raised several questions such as: which treatment modality is more potent and better tolerated? Can these treatment modalities be combined or applied sequentially? Lastly, how do we accurately assess the clinical efficacy?
The antitumor activity of targeted agents, such as sorafenib or sunitinib, and the local treatment modalities, such as chemoembolization or radioembolization, may result in changes in tumor vascularization, cavitation, and necrosis that do not significantly affect tumor size. Consequently using RECIST criteria to evaluate tumor response after treatment may not predict patient outcomes well. For that reason, although RECIST has been an established tool for assessment of tumor response to conventional cytotoxic chemotherapy, its limitations in assessing the antitumor activity of certain liver-directed therapies and molecularly targeted treatments such as with antiangiogenic agents are increasingly recognized [24–27]. In fact, several studies have shown that response criteria based on size only may be an unreliable indicator of response to treatment [22, 28–31]. This is because of a phenomenon best described as “pseudoprogression” which is an increase in apparent tumor size possibly resulting from central necrosis, peritumoral edema, or intratumoral hemorrhage (Figures 1 and 2). This is perhaps due to the fact that these treatment modalities can reduce tumor vascularization, induce necrosis, and result in cavitations within solid tumors. Such changes may have no major effect on overall tumor size and frequently are read as stable disease or even progression by RECIST criteria. These patterns of tumoral changes have been reported in HCC and also with imatinib, sorafenib, and sunitinib use in other tumor types, such as non-small-cell lung cancer, renal cell carcinomas, and gastrointestinal stromal tumors [32, 33].
Figure 1.
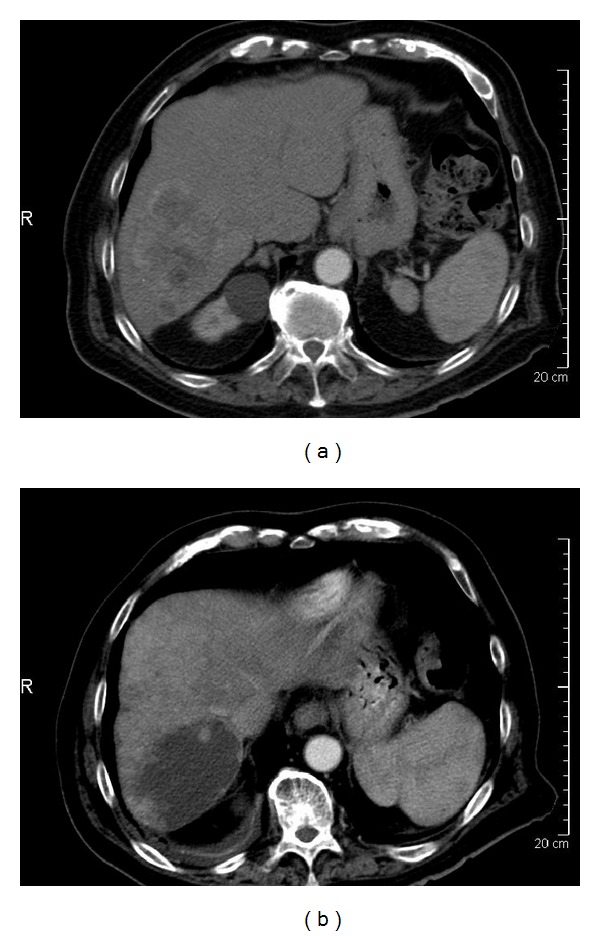
An 89-year-old man with hepatocellular carcinoma involving liver segment VI. (a) Before selective internal radiotherapy (SIRT), the tumor (10.2 cm) had poorly defined margins and peripheral enhancement. (b) After SIRT, the tumor had increased size (12.9 cm), increased necrosis, decreased vascularity, and decreased attenuation. This tumor was rated as progressive disease by RECIST 1.1 but favorable response by MASS criteria.
Figure 2.
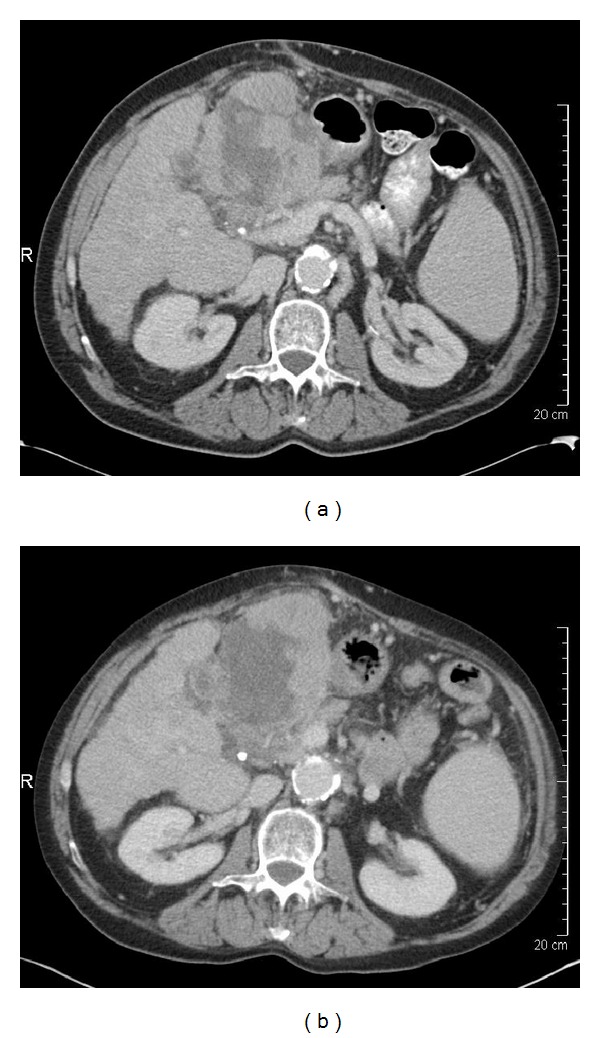
A 61-year-old woman with cirrhosis with multifocal hepatocellular carcinoma in liver segments II and III. (a) Before selective internal radiotherapy (SIRT), the tumor (13.3 cm) had central necrosis. (b) After SIRT, the tumor had minimal increase in size, increased necrosis, and decreased mean attenuation.
In the present study we attempted to objectively quantify the radiographic tumor features on CT scans prior to treatment with SIRT. We hypothesized that CT images provided information on tumor size, tissue density, percent necrosis, hypervascularity pattern, margin irregularity, and portal vein invasion (Figures 4, 5(c) and 5(d)). This in conjunction with other tumor characteristics such as hepatopulmonary shunt fraction may enable the stratification of patients and improve selection of patients who may benefit from SIRT and serve as biomarkers in predicting response to SIRT.
Figure 4.
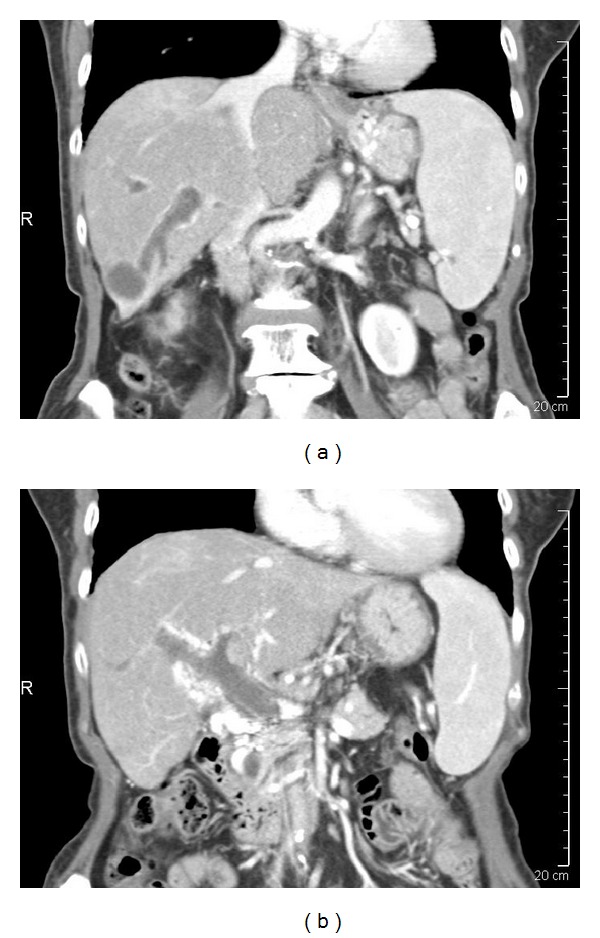
An 81-year-old woman with hepatocellular carcinoma. (a), (b) Before treatment with selective internal radiotherapy, tumor thrombus extended from the hepatocellular carcinoma (liver segment VI) to the right hemiportal vein (a) and main portal vein (b).
Figure 5.
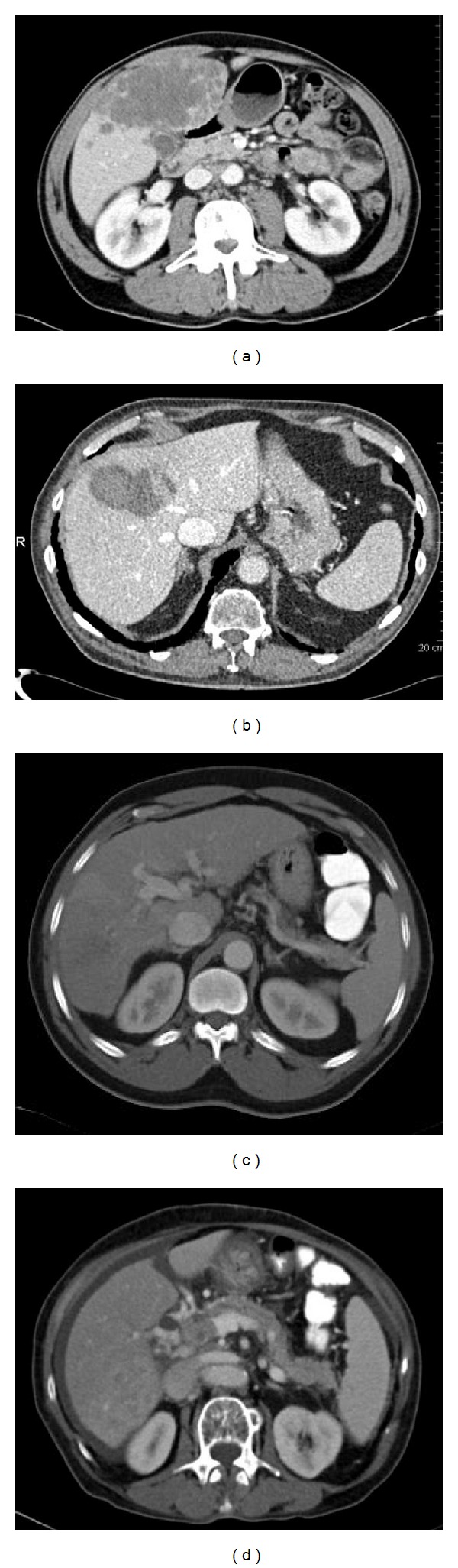
(a) A 63-year-old man with hepatocellular carcinoma. The right lobe of the liver was extensively involved with tumor, which had multiple satellite nodules and poor margins. (b) A 77-year-old man with hepatocellular carcinoma (6.4 cm) in liver segment VII that had well-defined margins. (c) A 59-year-old man with hepatocellular carcinoma that invaded the right hemiportal vein. (d) A 76-year-old woman with hepatocellular carcinoma and tumor thrombus in the main portal vein.
In patients with hepatocellular carcinoma who were treated with SIRT, several CT characteristics of the primary tumors before treatment were associated with improved progression-free survival after SIRT (Table 5). Pretreatment features including well-defined tumor margins (Figures 5(a) and 5(b)), central hypervascularity pattern, and lower hepatopulmonary shunt fraction were associated with improved progression-free survival (Table 5). Therefore, these CT parameters may serve as biomarkers to distinguish patients who will respond to SIRT from those who may benefit from alternative treatment and be spared the potential toxicity and cost of SIRT.
In addition, we evaluated the radiographic response following SIRT using MASS criteria to identify patients who favorably responded to radioembolization therapy versus those who needed additional therapy.
After SIRT, there were no significant changes in tumor size, but a significant increase in the extent of tumor necrosis (as measured by enhancement) was noted (Table 3; Figures 1 and 2), and although it did not reach statistical significance, there was a suggestion of a decrease in tumor attenuation following SIRT (P = 0.06).
Collectively, these findings may explain why RECIST criteria alone are not a reliable indicator for objective tumor response with respect to evaluating response to radioembolization (Table 4). Conversely, the MASS criteria, which include additional radiologic tumor parameters such as morphology, necrosis, attenuation, and structure, were significantly associated with progression-free and overall survival. Thus, MASS criteria after SIRT may be better in predicting progression-free and overall survival compared with RECIST 1.1 (Table 6).
The results of this study are similar to previously described findings in patients with metastatic renal cell carcinoma [23]. A previous study of liver metastases from colorectal cancer showed that RECIST criteria and tumor density were less useful in assessing the response to yttrium-90 radioembolization treatment than 18F-fluorodeoxyglucose positron emission tomography/CT tomography [34].
Limitations of the present study include the retrospective design, post hoc analysis, and small patient population. However, the results may provide justification for a prospective trial to confirm and further evaluate the response to SIRT determined by MASS criteria. In addition, there may have been technical inconsistencies in CT scanning, subjectivity in CT scan interpretation, and variation in the time after SIRT for obtaining the CT scan after treatment. However, this may reflect realistic clinical practice situations. Additionally, the MASS criteria were initially developed in patients with renal cell carcinoma who were treated with tyrosine kinase therapy.
In summary, in hypervascular tumors such as hepatocellular carcinoma, imaging response criteria that account for changes in tumor morphology, percentage of tumor necrosis, attenuation, and size may be more sensitive to the antitumor effects of SIRT than criteria based on size alone. Additionally, certain CT parameters may serve as biomarkers to distinguish patients who will respond to SIRT from those who may benefit from alternative treatment. Future studies may include prospective investigation of the accuracy of MASS criteria as a possible predictor of primary tumor response after SIRT in patients with hepatocellular carcinoma.
Figure 3.
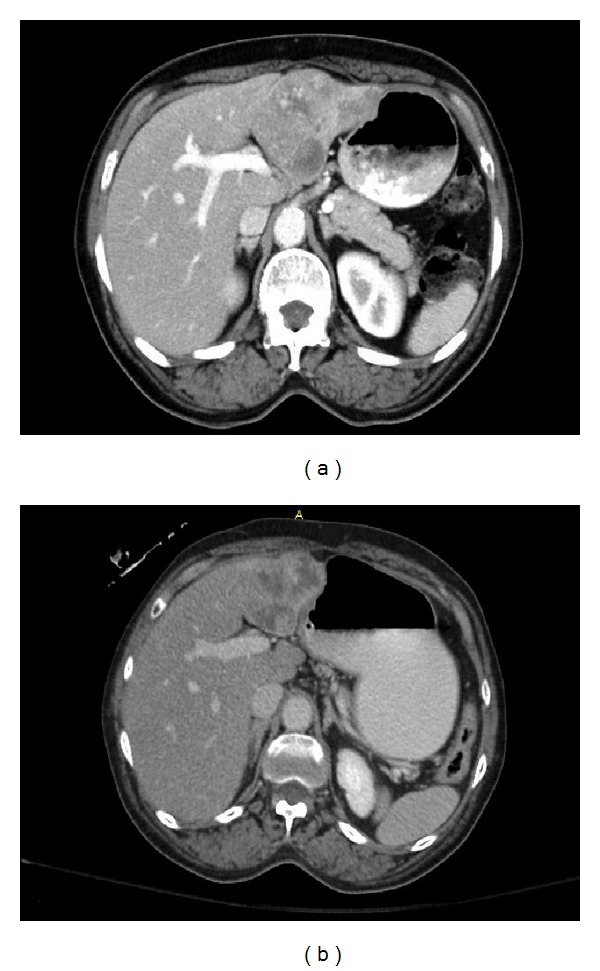
A 64-year-old patient with hepatocellular carcinoma. (a) Before selective internal radiotherapy (SIRT), tumor diameter was 8.4 cm. (b) After SIRT, the tumor had decreased size (6.7 cm; ≥20% decrease).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: globocan 2000. International Journal of Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. Ca: A Cancer Journal for Clinicians. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. The New England Journal of Medicine. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. The Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 6.Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. CardioVascular and Interventional Radiology. 2006;29(4):522–529. doi: 10.1007/s00270-005-0171-4. [DOI] [PubMed] [Google Scholar]
- 7.Lu DSK, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234(3):954–960. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 8.Maddala YK, Stadheim L, Andrews JC, et al. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transplantation. 2004;10(3):449–455. doi: 10.1002/lt.20099. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 10.Cheng A, Kang Y, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The Lancet Oncology. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. The New England Journal of Medicine. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 12.Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. The American Journal of Roentgenology, Radium Therapy, and Nuclear Medicine. 1965;93:200–208. [PubMed] [Google Scholar]
- 13.Dawson LA, McGinn CJ, Lawrence TS. Conformal chemoradiation for primary and metastatic liver malignancies. Seminars in Surgical Oncology. 2003;21(4):249–255. doi: 10.1002/ssu.10043. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of 90Y microspheres in man: review of four explanted whole livers. International Journal of Radiation Oncology Biology Physics. 2004;60(5):1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Leoni S, Piscaglia F, Golfieri R, et al. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. American Journal of Gastroenterology. 2010;105(3):599–609. doi: 10.1038/ajg.2009.654. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transplantation. 2004;10:S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. The Lancet. 2003;362(9399):1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 19.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies, part 2: special topics. Journal of Vascular and Interventional Radiology. 2006;17(9):1425–1439. doi: 10.1097/01.RVI.0000235779.88652.53. [DOI] [PubMed] [Google Scholar]
- 20.Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies, part 3: comprehensive literature review and future direction. Journal of Vascular and Interventional Radiology. 2006;17(10):1571–1593. doi: 10.1097/01.RVI.0000236744.34720.73. [DOI] [PubMed] [Google Scholar]
- 21.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies, part 1: technical and methodologic considerations. Journal of Vascular and Interventional Radiology. 2006;17(8):1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European Journal of Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM. Morphology, Attenuation, Size, and Structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. American Journal of Roentgenology. 2010;194(6):1470–1478. doi: 10.2214/AJR.09.3456. [DOI] [PubMed] [Google Scholar]
- 24.Forner A, Ayuso C, Varela M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115(3):616–623. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 25.Kudo M, Kubo S, Takayasu K, et al. Response Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 revised version) Hepatology Research. 2010;40(7):686–692. doi: 10.1111/j.1872-034X.2010.00674.x. [DOI] [PubMed] [Google Scholar]
- 26.Wong C, Salem R, Raman S, Gates VL, Dworkin HJ. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG pet: a comparison with CT or MRI. European Journal of Nuclear Medicine. 2002;29(6):815–820. doi: 10.1007/s00259-002-0787-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhu AX, Holalkere NS, Muzikansky A, Horgan K, Sahani DV. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist. 2008;13(2):120–125. doi: 10.1634/theoncologist.2007-0174. [DOI] [PubMed] [Google Scholar]
- 28.Lencioni R, Llovet JM. Modified recist (mRECIST) assessment for hepatocellular carcinoma. Seminars in Liver Disease. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 29.Kim KW, Lee JM, Choi BI. Assessment of the treatment response of HCC. Abdominal Imaging. 2011;36(3):300–314. doi: 10.1007/s00261-011-9683-3. [DOI] [PubMed] [Google Scholar]
- 30.Cho YK, Chung JW, Kim JK, et al. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer. 2008;112(2):352–361. doi: 10.1002/cncr.23185. [DOI] [PubMed] [Google Scholar]
- 31.Faivre S, Zappa M, Vilgrain V, et al. Changes in tumor density in patients with advanced hepatocellular carcinoma treated with sunitinib. Clinical Cancer Research. 2011;17(13):4504–4512. doi: 10.1158/1078-0432.CCR-10-1708. [DOI] [PubMed] [Google Scholar]
- 32.Horger M, Lauer UM, Schraml C, et al. Early MRI response monitoring of patients with advanced hepatocellular carcinoma under treatment with the multikinase inhibitor sorafenib. BMC Cancer. 2009;9, article 208 doi: 10.1186/1471-2407-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edeline J, Boucher E, Rolland Y, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118(1):147–156. doi: 10.1002/cncr.26255. [DOI] [PubMed] [Google Scholar]
- 34.Zerizer I, Al-Nahhas A, Towey D, et al. The role of early 18F-FDG PET/CT in prediction of progression-free survival after 90Y radioembolization: comparison with RECIST and tumour density criteria. European Journal of Nuclear Medicine and Molecular Imaging. 2012;39:1391–1399. doi: 10.1007/s00259-012-2149-1. [DOI] [PubMed] [Google Scholar]


