Abstract
Purpose
To better understand the human papillomavirus (HPV) vaccine series initiation among 9–17-year-old female Medicaid beneficiaries in Florida programs between June 2006 and December 2008 (n = 237,015).
Methods
Among the Florida Medicaid enrollees with itemized claims collected (non-managed care organization enrollees), we assessed the association between HPV vaccine series initiation (≥1 vaccine claim) and important demographic characteristics (age, race/ethnicity, program enrollment, area of residence, and length of enrollment).
Results
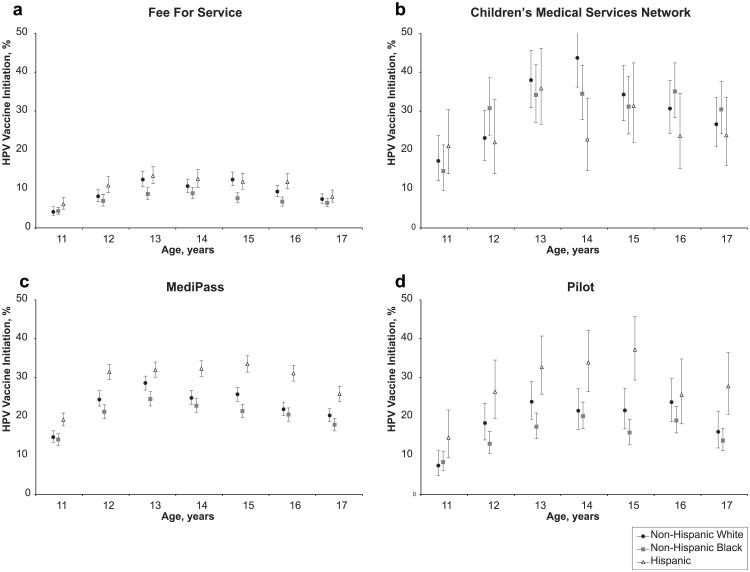
Among 11-17-year-olds, vaccine initiation increased over time from <1% by December 2006 to nearly 19% by December 2008. By December 2008, HPV vaccine initiation increased with respect to age from 9 (1.6%) to 13 years (22.9%), remained relatively stable from ages 13 to 15 years (between 21% and 22%), and decreased among 16- (18.6%) and 17-year-olds (15.7%). Compared with girls in Pilot or Fee for Service programs, the girls in MediPass or Children's Medical Service Network programs were more likely to have initiated the vaccine series. Within three of the four programs, Hispanics were more likely than non-Hispanic white and black girls to have initiated the vaccine series.
Conclusions
This study expands the understanding of HPV vaccine initiation to low-income adolescents eligible for free vaccine through the Federal Vaccine for Children program. Increased understanding of reasons for the observed differences, especially by program and race/ethnicity, will aid in developing interventions to improve HPV vaccine initiation.
Keywords: Human papillomavirus, Vaccines, Racial disparity, Ethnic disparity, Adolescents, Low income, Health insurance, Medicaid
Each year in the United States, 12,000 women are diagnosed with, and 4,000 die from invasive cervical cancer [1]. Prevention of cervical cancer is particularly important within the Florida Medicaid population for two reasons. First, Florida has the fifth highest cervical cancer incidence rate among the United States [1]. Second, cervical cancer rates are disproportionately high among Medicaid enrollees and populations eligible for Medicaid, particularly low-income and minority women [1–3].
The following two vaccines are available to reduce the risk of cervical cancer: a quadrivalent human papillomavirus (HPV) vaccine (Gardasil) and a bivalent HPV vaccine (Cervarix). The Advisory Committee on Immunization Practices recommends either HPV vaccine for females aged 11–26 years, and permits vaccination among girls as young as 9 years (Gardasil since June 2006 and Cevarix since October 2009) [4,5]. The high cost of the vaccine, a frequently cited barrier to HPV vaccination [6,7], is alleviated within Medicaid because girls who are enrolled in all programs receive the vaccine free of cost through the Vaccines for Children Program [8].
HPV vaccine series initiation (the percentage of girls receiving at least one of the three recommended vaccine doses) provides some evidence as to whether the vaccine is acceptable, available, and offered to parents and adolescents. Vaccine series initiation is the first step to reducing the risk of cervical cancer, and is likely associated with different factors than those associated with the completion of the three-dose series required to achieve maximum available protection [6,9]. Among U.S. adolescent girls, estimates of HPV vaccine series initiation range from 5%–47% [6,10,11]. Within Florida, by 2008, 37% of the general population of 13–17-year-old girls had initiated the vaccine series [6].
Important demographic characteristics, such as age, race/ ethnicity, and health care access, may influence HPV vaccine initiation [9,12]. Studies on HPV vaccine initiation present conflicting results regarding the importance of demographic characteristics, and do not focus on low-income families, or families with children enrolled in Medicaid [6,10,11,13]. It is particularly important that racial/ethnic differences in HPV vaccination are monitored and addressed among low-income women because racial/ethnic disparities in vaccination will likely increase the disproportional burden of cervical cancer incidence and mortality among underserved women [14,15].
After demographic subgroups that are less likely to initiate the vaccine series are identified, policy makers and public health professionals can explore reasons behind the observed differences, and develop targeted interventions to improve vaccine series initiation. To better understand HPV vaccine initiation among 9–17-year-old Medicaid beneficiaries in Florida programs between June 2006 and December 2008, we examined the association between HPV vaccine series initiation and important individual characteristics (i.e., age, race/ethnicity, program enrollment, area of residence, and number of months enrolled).
Methods
Design and sample
We assessed the HPV vaccine claims among 9–17-year-old girls enrolled in Florida Medicaid for at least 1 month between June 2006 (when the Advisory Committee on Immunization Practices recommended the vaccine) [4] and December 2008. Age and enrollment status were available on a monthly basis for all Florida Medicaid programs from Medicaid enrollment files. HPV vaccine claims were available from the Medicaid claims and encounter data. But, until 2009, the State did not collect itemized claims from the Florida Medicaid Managed Care Organizations (MCO). Because we could not assess HPV vaccine claims among MCO participants (n = 196,517), we restricted our analysis to non-MCO program participants (n = 237,015). No exclusions were made on the basis of pregnancy (a contraindication for the vaccine) because data were not available. The study protocol was approved by the institutional review board at the University of Florida.
Because HPV vaccine initiation changed over the 2.5-year period considered, we assessed 6-month cross-sectional periods (i.e., June and December of each year). Each 6-month cross-sectional period included 9–17-year-old girls who were enrolled in a non-MCO Florida Medicaid program during that month. For example, the June 2006 analysis included all 9–17-year-old girls enrolled in a non-MCO Medicaid plan in June 2006.
The U.S. Medicaid program is funded by the federal and state governments to provide medical care to low-income, disabled, and special interest groups of citizens under Title XIX [16]. Children under 19 years, whose family incomes are at or below 100% of the Federal poverty line are eligible for Medicaid and are excluded from cost-sharing [16,17]. Although girls up to 19 years of age participate in Medicaid, we focused our analysis on 9–17-year-old girls because we were primarily interested in vaccines received by girls whose parents were involved in the decision to receive the vaccine; Parental consent is required for girls ≤17 years of age. Furthermore, evidence suggests that rates of and factors associated with HPV vaccine initiation are different among 9–17-year-old girls as compared with those ≥18 years [9,18].
HPV vaccine series initiation
We assessed claims for the HPV vaccine (current procedural terminology codes for Gardasil: 90649 or 90649-SL) between June 2006 and December 2008 reported by September 30, 2009. We did not include Cevarix because it was not approved by the Advisory Committee on Immunization Practices until after the end of the study, that is, October 2009 [5]. Girls were considered as having initiated the series on and following the date of their first claim for the HPV vaccine. Because we were unable to account for vaccine doses received outside of the Medicaid system, we could not verify whether the first claim was the girl's first dose of the vaccine.
Vaccine series initiation was measured as the percentage of 9–17-year-old girls enrolled in a non-MCO Medicaid program who had at least one HPV vaccine claim. We counted girls who had a claim for the vaccine in or before the month assessed. For example, for the June 2008 analysis, we counted girls who had a claim for the HPV vaccine at any time between June 2006 and June 2008.
Claims and encounter data validation was conducted in Florida on a periodic basis using a protocol recommended by the Center for Medicare and Medicaid Services for sampling medical records, establishing a sample size, and conducting the review [19]. Internal validation suggests the match rates are 88% or better between medical records and key elements in the claims and encounter data (e.g., name of patient, primary through tertiary diagnoses, service date and type).
Demographic characteristics
Demographic characteristics of the members were obtained from the Medicaid enrollment files. Age was calculated at each month based on the girl's birthday, and categorized for analysis by 1 year age groups. Race/ethnicity was defined from self-reports as non-Hispanic white, non-Hispanic black, or Hispanic. Members who did not identify with one of these racial/ethnic groups (9%) were excluded from analyses that assessed race/ethnicity.
Program enrollment was available by month, and was assessed for the month considered in the analysis. Within Florida Medicaid, there are six programs for members: Children's Medical Services Network (CMSN), Pilot, MediPass, Provider Service Network (PSN), Fee for Service (FFS), and MCO. Children with a chronic medical or developmental problem that requires additional medical visits can enroll in CMSN, Florida's Title V program, and receive managed care along with care coordination and access to specialty providers. Parents whose children do not have chronic health conditions severe enough to qualify for CMSN can choose one of the remaining programs available in their area. Five Florida counties participated in Pilot where it is the only option available. Two counties allow choices of PSN, Med-iPass, FFS, and MCO. Of the remaining counties, some counties allow choices of MediPass, FFS, and MCO. Other counties only have MediPass or FFS options. Members who do not select a program are assigned to FFS.
The structure of service delivery and provider reimbursement differs between programs. MediPass is a primary care case management system wherein participants have a primary care provider who provides access to care, referrals to specialists, and monitors health care. MediPass providers receive a monthly fee for each member and reimbursement for the services he or she provides. PSN is a network of affiliated health care providers through which members receive most of their services. PSN providers are reimbursed on a fee-for-service or per member basis. Pilot is a managed care program with customizable benefit packages, and providers are reimbursed per member. FFS providers are reimbursed for services delivered.
We categorized the girls on the basis of their residential zip codes into four categories of rural and urban status using Rural-Urban Communing Area Codes version 2.0 [20]. We excluded participants whose zip codes were not in the Medicaid enrollment file or could not be mapped to categories of urban status (1.3%) from analyses that considered area of residence. We also calculated the total number of months between June 2006 and December 2008 that each girl was enrolled in any non-MCO Florida Medicaid program, and for analysis, categorized the total months enrolled into approximate 6-month intervals.
Statistical analysis
We used logistic regression to determine whether key individual characteristics (i.e., age, race/ethnicity, program enrollment, area of residence, and number of months enrolled) were predictors of HPV vaccine initiation when adjusting for demographic risk factors and interaction terms (combinations of race/ethnicity, age, and program enrollment). Analyses were performed for each 6-month cross-sectional period.
Early in the analysis, we detected effect measure modification of HPV vaccine initiation by an interaction between age, race/ethnicity, and program enrollment. To control for this interaction and preserve model stability, multivariable models were stratified by age and included an interaction of race/ethnicity and program enrollment. Multivariable models also included length of enrollment and area of residence as potential confounders. PSN participants (<1%) were omitted from analyses that included the interaction of race/ethnicity and program enrollment because of sparse cells (<50 girls). Because of differences in the length of enrollment by program, we performed a sensitivity analysis restricting to girls who had enrolled for 8 months or more.
We used SUDAAN version 10.0.1 (Research Triangle Institute, Research Triangle Park, NC) assuming simple random sampling and no clustering for all analyses [21]. HPV vaccine initiation was calculated with predicted marginals [22,23]. Statistical significance was determined by non-overlapping 95% confidence intervals.
Results
Between June 2006 and December 2008, a total of 433,532 girls were enrolled in Florida Medicaid for at least 1 month. Itemized claims were not collected by the State until 2009 for girls enrolled in MCO programs, and therefore, these 196,517 girls were excluded from the analysis. Among the 237,015 girls enrolled in non-MCO Florida Medicaid plans, most girls lived in urban areas (89%) and were participants of MediPass (52%) or FFS (38%). As compared with non-MCO participants, MCO participants were more likely to be non-Hispanic black (41% vs. 33%), more likely to live in urban areas (97% vs. 89%), and less likely to be enrolled for only 1–7 months (26% vs. 33%).
Results of unadjusted and multivariable models showed similar associations between the girls' demographic characteristics and HPV vaccine initiation for each period studied; thus, we present data to best fit the needs of interpretation. For simplicity, unadjusted results by 6-month cross-sectional period are shown. For relevance to the most recent state of vaccination, multivariable results are presented for December 2008.
As anticipated, small percentages of 9- (1.6%) and 10-year-old (3.8%) girls initiated the vaccine series by December 2008. Because of the small percentage of vaccinated 9–10-year-old girls, we could not obtain reliable estimates for multivariable models among 9-, 10-, or 9–10-year-old girls. Furthermore, to facilitate comparisons to other studies, we focused the presentation of the results on 11–17-year-old girls.
Among the 11–17-year-old girls, HPV vaccine initiation increased over time from <1% by December 2006 to nearly 19% by December 2008 (Table 1). We found evidence that the rapid increase in HPV vaccine initiation seen during 2007 was leveling off during 2008, especially during the last half of 2008. Specifically, there were smaller increases in HPV vaccine initiation in 2008 (total increase 5.4%) than in 2007 (total increase 12.8%), and the increase in the last half of 2008 (1.0%) was less than the first half of 2008 (4.4%).
Table 1. Unadjusted associations between HPV vaccine uptake and girls' characteristics from December 2006 to December 2008.
| Characteristic | Unadjusted percent of girls who received the HPV vaccine | ||||
|---|---|---|---|---|---|
|
| |||||
| December 2006 (N = 87,735) | June 2007 (N = 83,428) | December 2007 (N = 82,908) | June 2008 (N = 84,435) | December 2008 (N = 98,880) | |
| Total | .4% | 5.1% | 13.2% | 17.6% | 18.6% |
| Age (years) | |||||
| 11 | .4% | 5.7% | 10.6% | 12.1% | 11.7% |
| 12 | .4% | 6.7% | 15.8% | 19.9% | 19.7% |
| 13 | .5% | 5.4% | 15.3% | 20.9% | 22.9% |
| 14 | .4% | 5.2% | 14.7% | 19.7% | 21.6% |
| 15 | .5% | 5.1% | 13.8% | 19.4% | 21.0% |
| 16 | .5% | 4.4% | 12.3% | 17.2% | 18.6% |
| 17 | .2% | 3.5% | 10.1% | 14.3% | 15.7% |
| Race/ethnicity | |||||
| Non-Hispanic white | .6% | 5.7% | 12.4% | 16.4% | 17.3% |
| Non-Hispanic black | .3% | 4.1% | 10.9% | 14.9% | 16.7% |
| Hispanic | .3% | 5.7% | 16.6% | 22.3% | 22.5% |
| Programa | |||||
| FFS | .2% | 1.7% | 3.9% | 5.7% | 6.6% |
| CMSN | .6% | 9.7% | 21.8% | 28.4% | 32.6% |
| MediPass | .5% | 6.4% | 16.7% | 22.0% | 27.4% |
| Pilot | .1% | 3.3% | 13.6% | 19.2% | 19.9% |
| PSN | .2% | 3.5% | 8.1% | 11.5% | 17.2% |
| Area of residenceb | |||||
| Urban | .4% | 5.1% | 13.4% | 17.8% | 18.7% |
| Large rural | .7% | 5.8% | 14.8% | 19.1% | 21.8% |
| Small rural | 1.1% | 5.1% | 10.6% | 14.8% | 16.1% |
| Isolated | .6% | 3.8% | 7.9% | 10.9% | 12.1% |
| Length of enrollment | |||||
| 1–7 months | .4% | 2.8% | 4.7% | 4.9% | 3.5% |
| 8–13 months | – | 5.6% | 11.5% | 13.9% | 12.8% |
| 14–19 months | – | – | 16.4% | 17.3% | 19.0% |
| 20–25 months | – | – | – | 22.8% | 22.2% |
| 26–31 months | – | – | – | – | 28.4% |
FFS = fee for service; CMSN = children's medical services network; PSN = provider service network.
Girls were assigned to programs based on their enrollment during the month accessed. For example, for analysis of December 2006, girls were assigned to the program they were enrolled in during December 2006.
Residence is defined with zip code approximation to Rural-Urban Commuting Area codes [20].
HPV vaccine initiation differed considerably by age with relatively consistent patterns across programs and racial/ ethnic groups. Vaccine initiation increased among 11–13-year-old girls, remained relatively stable among 13–15-year-olds, and decreased among those aged 16 and 17 (Figure 1, Table 1). For comparison with national statistics [6], 19.8% (95% CI: 19.5%–20.1%) of 13–17-year-old girls had initiated the HPV vaccine series by December 2008.
Figure 1.
Adjusted (for area of residence and length of enrollment) HPV vaccine series initiation by program enrollment, race/ethnicity, and age. Point estimates for series initiation are represented by symbols and the 95% confidence intervals for these estimates are represented by lines. Figure subparts separate girls into the programs they were enrolled in during December 2008. (a) 40,600 girls enrolled in Fee for Service; (b) 3,981 girls enrolled in Children's Medical Services Network; (c) 47,559 girls enrolled in MediPass; (d) 6,659 girls enrolled in Pilot.
Consistently across time, the area of residence and length of enrollment were associated with HPV vaccine initiation (Table 1). From December 2007 to December 2008, HPV vaccine series initiation was similar among girls living in urban and large rural areas, and nearly doubled that of girls living in isolated areas (Table 1). As expected, HPV vaccine initiation increased with length of time enrolled in Medicaid (Table 1). By December 2008, HPV vaccine initiation estimates were over eight times greater among the girls enrolled in Medicaid for nearly the entire time the HPV vaccine was available (at least 26 of the 31 months) compared with girls enrolled for 7 or fewer months (Table 1).
In general, in December 2008, demographic characteristics of 11–17-year-old female enrollees were similar by program, but there were differences by race/ethnicity and length of enrollment in some programs. Hispanics were underrepresented in Pilot (15%) and CMSN (21%). As compared with members of other programs, FFS participants were least likely to be enrolled for more than 26 months (16% compared with 75% for CMSN, 63% for MediPass, and 52% for Pilot).
HPV vaccine initiation varied dramatically on the basis of Medicaid program enrollment (Figure 1, Table 1). Vaccine initiation was lowest among girls in FFS (6.6%; Table 1). CMSN participants were most likely to have initiated the vaccine series within each time point and nearly all age groups assessed (Figure 1, Table 1). By December 2008, we found the highest estimates of HPV vaccine initiation among CMSN participants, that is, 33% overall and 41% among 14-year-old non-Hispanic white girls.
We found considerable racial/ethnic disparities in HPV vaccine initiation within the Medicaid programs (Figure 1, Table 1). Within all programs except CMSN, HPV vaccine initiation was higher among Hispanics than among non-Hispanic blacks (Figure 1). We found the greatest racial/ethnic disparity within MediPass enrollees where HPV initiation was 5%–12% higher among Hispanic girls compared with non-Hispanic white and compared with non-Hispanic black girls (Figure 1c). When restricting to girls enrolled for ≥8 months, vaccine initiation was between 2% and 5% higher for each group considered, but overall patterns, including differences between racial/ethnic groups and Medicaid programs, were found to be similar.
Discussion
By December 2008, 19% of 11–17 year-old and approximately 20% of 13–17-year-old girls enrolled in Florida Medicaid non-MCO programs had initiated the HPV vaccine series. Despite free vaccination for Medicaid-enrolled girls, HPV vaccine series initiation among 13–17-year-old girls was considerably lower among Florida non-MCO Medicaid enrollees (19.8%) than among the general population of the United States (37.2%) and Florida (36.7%) [6]. Among 11–17 year olds, our estimate of HPV vaccine series initiation was also lower than other studies (range: 26% –36%) [24,25]. Similar to other populations, HPV vaccine initiation within Florida Medicaid is well below the 70% that is needed for realization of the vaccine's potential to reduce cervical cancer rates [26,27]. The low HPV vaccine initiation within Florida Medicaid is particularly concerning because of the increased rates of late stage cervical cancer among Medicaid enrollees [3].
Comparing our findings with private insurance claims suggests that privately insured girls are more likely to be vaccinated than Medicaid insured girls. To the best of our knowledge, the only published HPV vaccine initiation data from private insurance claims is from California Kaiser Permanente [9]. For every age group considered, girls with California Kaiser Permanente private insurance were two to seven times more likely than girls enrolled in non-MCO Florida Medicaid programs to have claims for the HPV vaccine. Vaccination among private vs. Medicaid enrollees is given as follows: for girls aged 9 (7% vs. 2%), 10 (28% vs. 4%), 11 (38% vs. 12%), 12 (40% vs. 20%), 13 (46% vs. 23%), 14 (47% vs. 22%), 15 (46% vs. 21%), 16 (43% vs. 19%), and 17 years (35% vs. 16%) [9]. The National Immunization Survey found the opposite relationship when considering income (a proxy for medical insurance) [6]; HPV series initiation among girls with family income below poverty (46.4%), and therefore, eligible for Medicaid, was higher than among girls with family income at or above poverty (35.8%) [6]. Because insurance status itself was not considered, the percentage of uninsured girls both above and below poverty is unknown.
Our results complement the mixed results from previous studies regarding whether HPV vaccine initiation differs by race/ethnicity by suggesting context (i.e., program enrollment) influences the association [6,9,12,13]. Consistent with private health insurance claims and nationally representative findings [6,9], within most Medicaid program groups, Hispanic girls were more likely than those of other racial/ ethnic groups to have received the HPV vaccine. These results support and expand previous findings showing Hispanics are equally or more likely than non-Hispanics to receive adolescent vaccines [6]. The relatively high vaccine initiation found among Hispanic girls is encouraging, because historically Hispanic women have been less likely than non-Hispanic white or non-Hispanic black women to complete regular cervical cancer screenings [28,29]. Acceptability of the HPV vaccine as a method to prevent cervical cancer is high among Hispanics, and in some cases higher among non-Hispanics [30,31]. Thus, the less invasive cervical cancer prevention offered by the HPV vaccine may be more acceptable and accessible to Hispanics, and in turn, could reduce rates of cervical cancer among Hispanics.
The differences found in HPV vaccine initiation on the basis of program suggest that Medicaid can influence HPV vaccine initiation. But, the differences by program cannot be explained fully by the distribution of race/ethnicity, age, length of enrollment, or area of residence characteristics of the enrollees. As compared with other Medicaid programs, CMSN and MediPass enrollees may have higher HPV vaccine initiation rates because they are assigned a primary care doctor who likely provides a medical home [32–34]. CMSN enrollees also have frequent contact with the health care system because of their chronic conditions. Frequent patient–provider contact may increase HPV vaccine initiation by giving providers more opportunity to focus on and encourage preventive measures. Alternatively, there may be disparities by program in provider recommendation of the vaccine or referral of vaccine administration to health departments [12,35]. Finally, differences in HPV vaccine initiation by program may also represent differences in the percentage of claims reported for vaccines.
Consistent with previous findings [9,36], the area of residence and length of enrollment were also associated with HPV vaccine initiation. The lower HPV vaccine initiation among rural compared with urban residents is consistent with the nationwide rural/urban disparitiesinpreventive care, and is possibly a result ofhaving fewer doctors per person and longer travel distances to health care facilities [37–39]. Longer enrollment may reflect an increased number of visits with primary care providers, establishment of a medical home, or continuity of care [9,33,40].
There are three important limitations to this study. First, our results are limited to representing the girls who participated in Florida Medicaid non-MCO programs (FFS, CMSN, MediPass, and Pilot), because itemized claims were not collected for MCO enrollees. If Florida Medicaid MCOs had a higher rate of vaccination, the inability to include MCOs may account for the lower rates of HPV vaccine series initiation in this study compared with the general population. Second, HPV vaccine doses received outside of the Medicaid system (e.g., from the state health department) are not recorded in the Medicaid claims and encounter data. Consequently, the percentage of vaccinated girls may be underestimated overall or for specific programs. The percentage of girls who received the HPV vaccine outside of the Medicaid systems is likely low because few (14%) providers report referring Medicaid children to other clinics for vaccinations [35]. Third, our results are limited to vaccine series initiation, and do not indicate whether girls received all three doses required for maximum available protection.
There are two important strengths to this study. First, the large sample of girls enabled assessment of differences between demographic subgroups in multivariable analyses. Second, the results of the study are not reliant on self-report because claims and encounter data were used to assess vaccination and demographic characteristics. The claims likely accurately reflect the medical practice because internal consistency checks suggest that claims and medical records are similar.
Despite eligibility for free vaccination, most (81%) of the 11–17-year-old girls participating in non-MCO Florida Medicaid programs have not initiated the HPV vaccine series. Interventions proven to be effective should be developed to improve HPV vaccine initiation. To develop interventions, a better understanding of the reasons behind the observed racial/ethnic and program disparities in HPV vaccine initiation is needed. Possibilities to explore include understanding provider behaviors (vaccine discussions, recommendations, and referrals), and evaluating differences between Medicaid programs. Providers and public health practitioners should strive toward having equal, if not higher, HPV vaccine initiation among the Medicaid population than the general population because the vaccine is available free of charge to these girls from low-income families who are at an increased risk of cervical cancer.
Acknowledgments
The authors thank Deepa Ranka, MS; Dandan Xu, MS; and Katie Eddleton, MPH, of the University of Florida for data management and technical assistance. They also thank Syzygy Graphics for figure creation. The research was supported by funding from the University of Florida.
References
- 1.U.S. Cancer Statistics Working Group. [Accessed July 13, 2010];United States cancer statistics: 1999–2006 incidence and mortality web-based report. Available at: www.cdc.gov/uscs.
- 2.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socio-economic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101:1051–7. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 3.O'Malley CD, Shema SJ, Clarke LS, et al. Medicaid status and stage at diagnosis of cervical cancer. Am J Public Health. 2006;96:2179–85. doi: 10.2105/AJPH.2005.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. ACIP VFC vaccines to prevent human papillomavirus: Resolution No 010/09-1. Atlanta, GA: Centres for Disease Control and Prevention; 2009. [Google Scholar]
- 6.Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13–17 years: United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:997–1001. [PubMed] [Google Scholar]
- 7.Downs LS, Jr, Scarinci I, Einstein MH, et al. Overcoming the barriers to HPV vaccination in high-risk populations in the US. Gynecol Oncol. 2010;117:486–90. doi: 10.1016/j.ygyno.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. [Accessed July 13, 2010];Vaccines included in the VFC Program: Resolution No 06/06–1. Available at: http://www.cdc.gov/vaccines/programs/vfc/downloads/resolutions/0606vaccines.pdf.
- 9.Chao C, Velicer C, Slezak JM, et al. Correlates for human papillomavirus vaccination of adolescent girls and young women in a managed care organization. Am J Epidemiol. 2010;171:351–67. doi: 10.1093/aje/kwp365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn JA, Rosenthal SL, Jin Y, et al. Rates of human papillomavirus vaccination, attitudes about vaccination, and human papillomavirus prevalence in young women. Obstet Gynecol. 2008;111:1103–10. doi: 10.1097/AOG.0b013e31817051fa. [DOI] [PubMed] [Google Scholar]
- 11.Gerend MA, Weibley E, Bland H. Parental response to human papillomavirus vaccine availability: Uptake and intentions. J Adolesc Health. 2009;45:528–31. doi: 10.1016/j.jadohealth.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: Importance of a physician's recommendation. [Accessed July 22, 2010];Vaccine. (in press). Corrected Proof [Online, January 5, 2010].Available at: http://www.sciencedirect.com/science/article/B6TD4-4Y3BK1J-F/2/27bd806a52b6385eeb9ff607e44d58fc.
- 13.Caskey R, Lindau S, Alexander G. Knowledge and early adoption of the HPV vaccine among girls and young women: Results of a national survey. J Adolesc Health. 2009;45:453–62. doi: 10.1016/j.jadohealth.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Goldhaber-Fiebert JD, Stout NK, Salomon JA, et al. Cost-effectiveness of cervical cancer screening with human papillomavirus DNA testing and HPV-16,18 vaccination. J Natl Cancer Inst. 2008;100:308–20. doi: 10.1093/jnci/djn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith JS. Ethnic disparities in cervical cancer illness burden and subsequent care: A prospective view in managed care. Am J Manag Care. 2008;14:S193–9. [PubMed] [Google Scholar]
- 16.Klees BS, Wolfe CJ, Curtis CA. [Accessed July 13, 2010.];Brief summaries of Medicare and Medicaid: Title XVIII and Title XIX of the Social Security Act, as of November 1, 2009. Available at: http://www.cms.hhs.gov/MedicareProgramRatesStats/Downloads/MedicareMedicaidSummaries2009.pdf.
- 17.Cohen RD, Horn A, Marks C. [Accessed July 13, 2010];Health coverage for children and families in Medicaid and SCHIP: State efforts face new hurdles. Available at: http://www.kff.org/medicaid/7740.cfm.
- 18.Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for completion of 3-dose regimen of HPV vaccine in female members of a managed care organization. Mayo Clin Proc. 2009;84:864–70. doi: 10.4065/84.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Department of Health and Human Services. Validating encounter data: A protocol for use in conducting external quality review of Medicaid Managed Care Organizations and Prepaid Health Plans. Baltimore, MD: Centres for Medicare and Medicaid Services; 2002. [Google Scholar]
- 20.Health Resources and Service Administration, United States Department of Agriculture, University of Washington. [Accessed July 13, 2010];RUCA (Rural-Urban Commuting Area Codes) Version 2.0. Available at: http://depts.washington.edu/uwruca/index.php.
- 21.Research Triangle Institute. SUDAAN language manual, release 10.0. Research Triangle Park, NC: Research Triangle Institute; 2008. [Google Scholar]
- 22.Lane PW, Nelder JA. Analysis of covariance and standardization as instances of prediction. Biometrics. 1982;38:613–21. [PubMed] [Google Scholar]
- 23.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–9. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 24.Yeganeh N, Curtis D, Kuo A. Factors influencing HPV vaccination status in a Latino population; and parental attitudes towards vaccine mandates. Vaccine. 2010;28:4186–91. doi: 10.1016/j.vaccine.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal SL, Rupp R, Zimet GD, et al. Uptake of HPV vaccine: Demographics, sexual history and values, parenting style, and vaccine attitudes. J Adolesc Health. 2008;43:239–45. doi: 10.1016/j.jadohealth.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Kim JJ, Brisson M, Edmunds WJ, et al. Modeling cervical cancer prevention indeveloped countries. Vaccine. 2008;26(Suppl 10):K76–86. doi: 10.1016/j.vaccine.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taira AV, Neukermans CP, Sanders GD. Evaluating human papilloma-virus vaccination programs. Emerg Infect Dis. 2004;10:1915–23. doi: 10.3201/eid1011.040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breen N, Wagener DK, Brown ML, et al. Progress in cancer screening over a decade: Results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93:1704–13. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- 29.Swan J, Breen N, Coates RJ, et al. Progress in cancer screening practices in the United States: Results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–40. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 30.Sanderson M, Coker AL, Eggleston KS, et al. HPV vaccine acceptance among Latina mothers by HPV status. J Womens Health (Larchmt) 2009;18:1793–9. doi: 10.1089/jwh.2008.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watts LA, Joseph N, Wallace M, et al. HPV vaccine: A comparison of attitudes and behavioral perspectives between Latino and non-Latino women. Gynecol Oncol. 2009;112:577–82. doi: 10.1016/j.ygyno.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Ryan S, Riley A, Kang M, Starfield B. The effects of regular source of care and health need on medical care use among rural adolescents. Arch Pediatr Adolesc Med. 2001;155:184–90. doi: 10.1001/archpedi.155.2.184. [DOI] [PubMed] [Google Scholar]
- 33.Starfield B, Shi L. The medical home, access to care, and insurance: A review of evidence. Pediatrics. 2004;113(Suppl 5):1493–8. [PubMed] [Google Scholar]
- 34.Smith PJ, Santoli JM, Chu SY, et al. The association between having a medical home and vaccination coverage among children eligible for the vaccines for children program. Pediatrics. 2005;116:130–9. doi: 10.1542/peds.2004-1058. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman RK, Mieczkowski TA, Mainzer HM, et al. Effect of the vaccines for children program on physician referral of children to public vaccine clinics: A pre-post comparison. Pediatrics. 2001;108:297–304. doi: 10.1542/peds.108.2.297. [DOI] [PubMed] [Google Scholar]
- 36.Centres for Disease Control and Prevention. [Accessed July 13, 2010];Statistics and surveillance: Immunization coverage in the US. Available at: http://www.cdc.gov/vaccines/stats-surv/imz-coverage.htm#nisteen.
- 37.Agency for Healthcare Research and Quality. [Accessed July 13, 2010];National healthcare quality & disparities reports, 2008. Available at: http://www.ahrq.gov/qual/qrdr08.htm.
- 38.Larson SL, Fleishman JA. Rural-urban differences in usual source of care and ambulatory service use: Analyses of national data using Urban Influence Codes. Med Care. 2003;41:III65–74. doi: 10.1097/01.MLR.0000076053.28108.F2. [DOI] [PubMed] [Google Scholar]
- 39.Probst JC, Laditka SB, Wang JY, Johnson AO. Effects of residence and race on burden of travel for care: Cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res. 2007;7:40–52. doi: 10.1186/1472-6963-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halfon N, Wood DL, Valdez RB, et al. Medicaid enrollment and health services access by Latino children in inner-city Los Angeles. JAMA. 1997;277:636–41. [PubMed] [Google Scholar]