Abstract
The clearance of mucus from the airways protects the lungs from inhaled noxious and infectious materials. Proper hydration of the mucus layer enables efficient mucus clearance through beating of cilia on airway epithelial cells, and reduced clearance of excessively concentrated mucus occurs in patients with chronic obstructive pulmonary disease and cystic fibrosis. Key steps in the mucus transport process are airway epithelia sensing and responding to changes in mucus hydration. We reported that extracellular adenosine triphosphate (ATP) and adenosine were important luminal auto-crine and paracrine signals that regulated the hydration of the surface of human airway epithelial cultures through their action on apical membrane purinoceptors. Mucus hydration in human airway epithelial cultures was sensed by an interaction between cilia and the overlying mucus layer: Changes in mechanical strain, proportional to mucus hydration, regulated ATP release rates, adjusting fluid secretion to optimize mucus layer hydration. This system provided a feedback mechanism by which airways maintained mucus hydration in an optimum range for cilia propulsion. Understanding how airway epithelia can sense and respond to changes in mucus properties helps us to understand how the mucus clearance system protects the airways in health and how it fails in lung diseases such as cystic fibrosis.
INTRODUCTION
The airway epithelium provides a broad innate host barrier designed to maintain the sterility of airway surfaces during normal breathing. The mucus clearance system lies at the center of this innate defense activity by trapping and clearing inhaled noxious and infectious particulates from the lung. Mucus clearance in health depends on the ability of the airway epithelium to hydrate secreted mucins so that mucus concentrations are optimal for cilial-dependent mucus transport (1, 2). Studies of patients with muco-obstructive airway diseases, such as cystic fibrosis, suggest that dys-regulation of airway surface hydration, possibly coupled with alterations in mucin biosynthesis and maturation (3–5), results in failure of mucus transport and airway disease (6).
Epithelia in general must sense and properly regulate the volume and composition of the fluids lining their surfaces. The juxtaglomerular apparatus of the kidney, an epithelia-dominated organ, senses luminal ion concentrations through a cell volume–dependent mechanotransduction mechanism resulting in the release of adenosine triphosphate (ATP) into the extracellular space (7). This process results in the homeostatic regulation of glomerular filtration rates and tubular fluid flow (7). In the distal nephron, luminal fluid flow produces mechanically stimulated luminal ATP release, which activates purinergic receptors, inhibits Na+ reabsorption, and governs rates of diuresis and natriuresis (8).
Airway epithelial cells respond to direct mechanical stresses by modulating the rate of ATP release (9, 10), which stimulates purinoceptors to activate Cl− transport and inhibit Na+ transport. Thus, a change in luminal ATP concentration is predicted to reciprocally alter liquid absorption and liquid secretion rates, thereby optimizing airway surface layer (ASL) hydration and mucociliary clearance (10).
A single, nonmotile primary cilium present on the luminal surface of most mammalian cells is involved in sensing fluid flow in many organ systems in the body (11), such as in the flow-dependent regulation of natriuresis and diuresis in the cortical collecting duct of the kidney (12). Although primary cilia are present on undifferentiated human airway epithelia during lung development, they disappear just before the appearance of motile (beating) cilia (13). Therefore, we hypothesized that airway epithelia must use a mechanism that does not involve a primary cilium to sense and control the hydration state of the airway surface and to maintain effective mucus clearance and lung health.
Here, we tested the hypothesis that airway epithelia control airway surface hydration through a mechanotransduction mechanism that depends on motile cilia. We first investigated whether the airway surface hydration state is governed by the concentrations of ATP and its metabolite, adenosine, on the airway surface. We next tested the hypothesis that mucus hydration properties are sensed by the motile cilia that propel the overlying viscoelastic mucus layer. Specifically, we hypothesized that the mechanical stress on the motile cilium during the beat cycle varies with mucus concentration, producing variable cilial strain–dependent increases in luminal ATP concentration. The rates of nucleotide release and ectonucleotidase metabolism control ASL nucleotide and nucleoside concentrations and, hence, set the balance of ion transport–mediated fluid secretion and absorption required for “normal” mucus hydration and clearance. Finally, we tested the hypothesis that desensitization of purinoceptors receptors protects the airway surfaces from overhydration, such as “flooding,” in response to abnormally high rates of nucleotide release.
RESULTS
Regulation of airway hydration by endogenous or exogenous ATP and adenosine
We first tested the hypothesis that under static conditions, the ATP and adenosine concentrations within the airway surface layer (ASL) regulate steady-state airway surface hydration. Nebulization of a small volume (0.5 μl) of vehicle to the airway surface resulted in a ~3-μm increase in ASL height (Fig. 1A). Immediately after delivery, this extra fluid was rapidly reabsorbed, and ASL height was maintained at the original steady-state ASL height (~7 μm), as previously reported (9). In contrast, nebulization of vehicle containing apyrase (to degrade extracellular ATP) and adenosine deaminase (to degrade extracellular adenine) onto the airway surface resulted in ASL fluid absorption, which persisted until essentially all available ASL was absorbed, leaving only a compressed periciliary layer and flattened cilia (~3 μm) (14). These results suggest that the endogenous release of ATP and the extracellularly formed adenosine regulate basal airway hydration. In the absence of ATP- and adenosine-dependent signaling within the ASL, the default mode of airway epithelia is to absorb salt and water, reflecting a high rate of Na+ absorption and an absence of purinergic-dependent Cl− secretion (15). We speculate that the transient stimulation in ASL fluid absorption that occurred after nebulization of phosphate-buffered saline (PBS) reflected the dilution of purinoceptor agonists, which relieved the block of sodium absorption mediated by the epithelial sodium channel (ENaC) (9) and stimulated chloride secretion through the cystic fibrosis transmembrane conductance regulator (CFTR) and the calcium-activated chloride channel (CaCC) (10) required to maintain steady-state ASL.
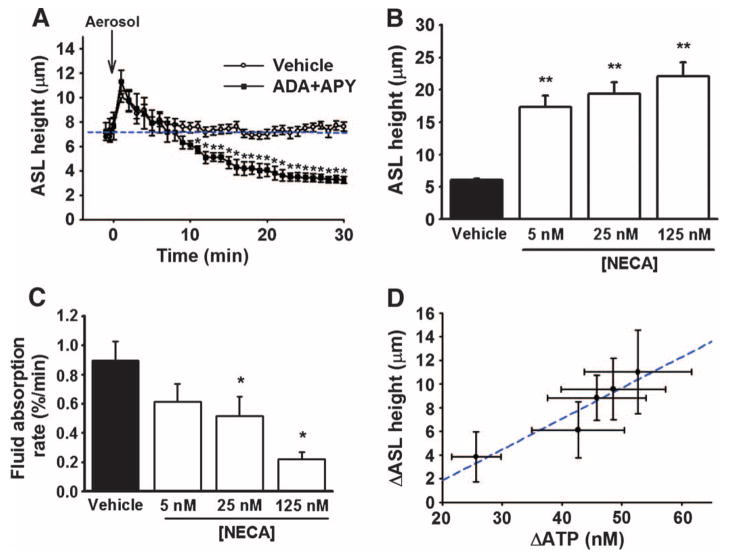
Fig. 1. ATP and adenosine as regulators of airway surface hydration.
(A) Effect of removing endogenous ATP and adenosine by nebulizing vehicle alone (PBS; open circle) or PBS containing apyrase (APY) and adenosine deaminase (ADA) (closed square). Steady-state fluid absorption from the luminal surface was reestablished a few minutes after nebulization in vehicle-treated cultures (blue line), but not in cultures treated with APY and ADA. Data are means ± SE from six cultures per group. *P < 0.05 compared to vehicle alone. (B) Comparison of ASL heights after addition of PBS alone (vehicle) and presence of various concentrations of NECA. Data are means ± SE from three cultures per group. **P < 0.001 compared to vehicle alone. (C) Comparison of the fluid absorption rate in the absence (vehicle; PBS) and presence of various concentrations of a nonhydrolyzable adenosine analog (NECA). Data represent the rate of change in ASL height calculated over the first 60 min after volume addition. Data are presented as a percentage of the peak ASL height change after the addition of 25-μl volume. Data are means ± SE from three cultures per group. *P < 0.005 compared to vehicle alone. (D) Relationship between the magnitude of changes in luminal ATP concentrations and ASL height. Human airway cultures were subjected to varying degrees of oscillatory stress before measuring luminal [ATP] and steady-state ASL height. These data revealed a direct relationship between ATP and changes in ASL height (correlation coefficient = 0.95 and slope = 0.3 μm height per steady-state change in ATP concentration).
If the concentrations of nucleotides and nucleosides in the ASL are responsible for controlling the ASL hydration status in airway epithelia, then addition of exogenously added nucleotides or nucleosides in isotonic saline should establish new steady-state ASL concentrations of nucleotides or nucleosides and ASL heights. To test this hypothesis, we added bolus volumes of fluid to static airway epithelial surfaces in the absence and presence of the adenosine analog [NECA (5′-N-ethylcarboxamidoadenosine)], which, unlike adenosine, is not rapidly degraded by endogenous nucleosidases to inactive metabolites (16). Whereas the ASL height in the control group had returned to baseline 60 min after fluid addition, cultures treated with NECA exhibited a significant increase in ASL height over baseline at the same time point (Fig. 1B). Although steady-state ASL height was not altered during exposure to NECA, the rate of fluid reabsorption was significantly slowed in a concentration-dependent fashion (Fig. 1C). Together, these data support the notion that the concentrations of nucleotides and nucleotide metabolites within the ASL represent major regulators of airway surface hydration.
Regulation of ASL height (volume) by mechanical stress stimulation of ATP release
We next tested the hypothesis that variations in the rate of endogenous ATP release would result in different ATP and adenosine concentrations in the ASL and different ASL heights. Using a luciferineluciferase–based approach (17), we measured luminal ATP concentrations in human airway cultures subjected to a range of oscillatory compressive stresses that stimulate the rate of ATP release from airway epithelia (10). In parallel, confocal microscopy was used to measure post-stress ASL heights as an index of ATP-mediated fluid secretion. Graded increases in mechanical stress stimulated proportionate increases in steady-state ATP concentrations and ASL heights (Fig. 1D). On the basis of our previous studies (9, 10), the relationship between increases in ATP concentrations and ASL height is consistent with purinoceptor activation producing net fluid secretion by stimulation of CFTR and CaCC and inhibition of ENaC. Overall, these results support the hypothesis that the hydration status of the airway surface is regulated by the concentrations of luminal nucleotides and nucleosides interacting with purinoceptor-mediated regulation of airway epithelial ion transport.
Sensing of the airway surface hydration status by interactions of cilia with the mucus layer
We hypothesized that, in addition to responding to macroscopic mechanical forces exerted at the epithelial cell surface that occur during tidal breathing, such as shear, stretch, and compression (18), airway epithelia can also sense and respond to changes in the local airway surface microenvironment, providing fine control of local airway surface hydration. We further speculated that because mucus layer hydration enables efficient mucus clearance, hydration of the mucus layer could be locally sensed by the mucus layer–cilia interactions that occur during propulsion of the mucus layer. In this scenario, cilia would experience resistance to beating as a function of mucus hydration, producing cilial strain–dependent regulation of ATP release rates and luminal ATP concentration. Such a system would provide an “autoregulatory” feedback mechanism to regulate ion transport and fluid secretion through local ATP and adenosine concentrations and maintain optimal hydration of the mucus layer and mucociliary clearance.
To test this hypothesis, we assessed the effects of mucus concentration on regulation of luminal ATP concentration in the absence of external mechanical stresses, such as that characteristic of static conditions. Because endogenous mucus is a heterogeneous milieu containing DNA and ~200 different proteins, which can potentially act as signaling molecules on airway epithelia (19), we used a defined viscoelastic “mucus simulant” that exhibits “mucus-like” concentration–dependent changes in both viscosity and elasticity under conditions that did not produce a large partial osmotic pressure, which could result in collapse of the periciliary layer (20). For these studies, we characterized a low–melting point agarose (LMA), a flowable gel at low concentrations, with a cone-and-plate rheometer to obtain values of the elastic storage modulus (G′; Fig. 2A) and the viscous (or loss) modulus (G″; Fig. 2B) over a range of concentrations of LMA at 37°C. For comparison, the average values of G′ and G″ are shown for normal human (2% solids) and dehydrated, cystic fibrosis–like mucus (8% solids), based on rheological studies with mucus harvested from airway epithelia (21). Thus, the concentration of LMA could be varied to alter the biophysical properties to mimic normal airway mucus or the concentrated mucus associated with cystic fibrosis. In the following studies, we refer to 0.15% LMA as a mimic of normal, healthy mucus and 0.3% LMA as an approximation of cystic fibrosis mucus.
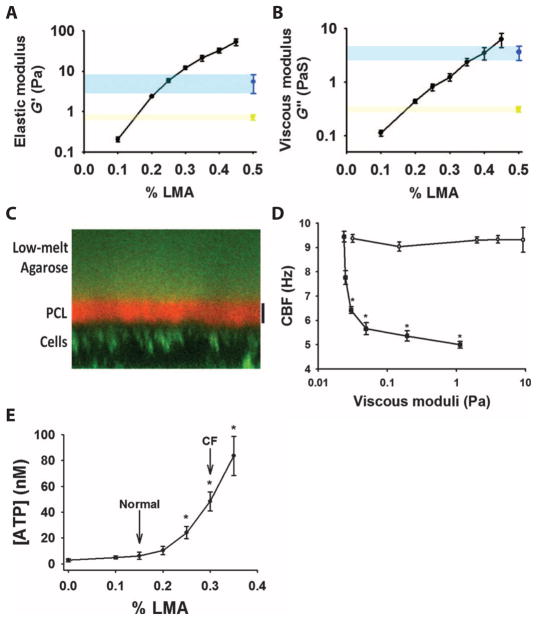
Fig. 2. Airways sense and respond to changes in surface viscoelasticity.
(A and B) Results from a cone-and-plate rheometer show the effect of changes in the concentration of LMA on the (A) elastic modulus (G′) and (B) viscous modulus (G″). Comparison values for the G′ and G″ of native mucus are shown for both normal mucus (yellow; ~2% solids) and cystic fibrosis–like mucus (blue; ~8% solids). Data are means ± SE from three to five samples per condition. (C) Sample ZX confocal micrograph showing the distinct layering of fluorescently labeled LMA (0.45%) (green layer at top) from the periciliary layer (PCL) (labeled with Texas Red dextran 6 kD). Airway epithelial cells were labeled green using calcein AM. Scale bar, 7 μm. (D) Comparison of the effects of LMA (open circles, concentration range: 0.1 to 0.45%) and low–molecular weight dextran (closed squares, ~6 kD, concentration range: 1.0 to 30%) on CBF over a wide range of viscous moduli. Unlike dextrans, LMA does not penetrate the periciliary layer, which results in a slowing of the CBF. Data are means ± SE from three cultures per group. *P < 0.05 compared to vehicle alone. (E) Relationship between the concentration of luminal ATP and the concentration of LMA added to the airway surface. Concentrations approximating the viscoelasticity of normal (2% solids) and cystic fibrosis (CF; 8% solids) mucus are shown for comparison (21). Data are means ± SE from six to eight cultures per point. *P < 0.05 compared to vehicle (0% LMA).
With respect to the osmotic properties of LMA, it has a molecular radius of gyration of >50 nm. Therefore, it should not penetrate the periciliary layer and is predicted to be osmotically active with reference to the periciliary layer (20). Confocal microscopy demonstrated that fluorescently labeled LMA, like endogenous mucus (20), did not penetrate the periciliary layer when applied to the luminal surface (Fig. 2C). However, at the concentration of LMA used (0.45%), the cilia were not osmotically collapsed, suggesting that LMA at this concentration exerts low partial osmotic pressure relative to the periciliary layer (20). To verify that the osmotic pressure of LMA did not perturb the periciliary layer properties and cilial function, we exposed ciliated airway cultures to LMA at various concentrations. LMA (0.1 to 0.45%) had negligible effect on cilia beat frequency (CBF) (Fig. 2D). In contrast, exposure of cultures to small dextrans [6 kD average molecular weight, with a radius of gyration of <5 nm (20)], over a range of osmotic pressures (representing 1.0 to 30% dextran), produced significant decreases in CBF (Fig. 2D). We concluded that, in contrast to LMA, the low–molecular weight dextran penetrated the periciliary layer where it presented a large viscous load on the cilia and slowed CBF. Together, these data suggest that LMA represents a reasonable simulant for endogenous mucus.
To test whether ciliated human airway epithelia sense and respond to hydration-dependent variations in mucus viscoelasticity, we measured the effects of LMA concentration on ATP concentrations in airway cultures (Fig. 2E). Under basal (static) conditions, human airway epithelia responded to both physiologically and disease-relevant changes in LMA concentrations and, hence, viscoelasticity, with LMA concentration–dependent changes in luminal ATP concentrations. These findings suggest that airways are capable of sensing subtle changes in the hydration state of mucus.
Role of motile cilia and cilial beating in mucus viscoelasticity sensing
To assess the requirement for motile cilia in the stress sensing of the viscoelastic properties of the mucus layer (or LMA), we measured the magnitude of luminal ATP concentration in well-ciliated or poorly ciliated airway cultures. “Well-ciliated” cultures were defined as exhibiting >80% of the cell surface area covered by ciliated cells, whereas “poorly ciliated” was defined as <15% ciliation (Fig. 3A). To control for possible differences in ATP concentration due to variations in cell differentiation, airway cultures were subjected to two different types of external macroscopic mechanical forces, shear stress (9), or compressive stress (10), after which, ATP concentrations in ASL were measured by luminometry. We chose a magnitude of mechanical stress that approximated that of the shear and compressive stresses associated with tidal breathing (9, 22). Neither basal nor stimulated (shear or compressive stress) ATP concentrations were dependent on the presence of cilia (Fig. 3B). These findings suggest that cell differentiation did not affect ATP release capacity, and cilia were not required for stimulation in luminal ATP concentration in response to macroscopic external stresses.
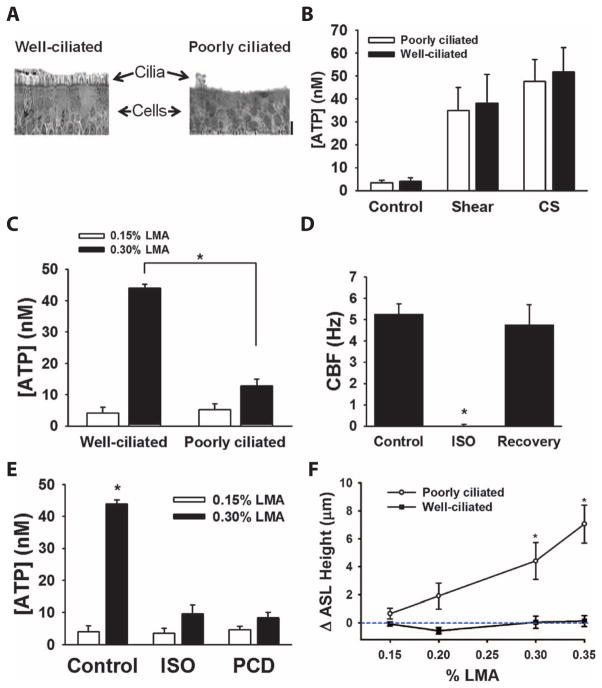
Fig. 3. Role of motile cilia and cilia beating in viscoelastic sensing.
(A) Representative sections of (left) well-ciliated and (right) poorly ciliated airway cultures stained with hematoxylin and eosin. Scale bar, 20 μm. (B) Comparison of the steady-state ASL concentrations of ATP measured in poorly ciliated (open bars) and well-ciliated (closed bars) cultures under control (static) conditions, apical shear stress (0.5 dynes/cm2) (9), or compressive stress (CS; 20 cmH2O) (10). Data are means ± SE from five to six cultures per group. (C) Role of cilia in the ability of airway cells to sense viscoelas-ticity of the overlying surface. ATP was measured after exposure of either 0.15% LMA (open bars) or 0.3% LMA (closed bars) in well-ciliated and poorly ciliated cultures. Data are means ± SE from six to eight cultures per group. *P < 0.05 between groups. (D) Magnitude of cilia beating in normal airway cultures before, immediately after pretreatment with isoflurane (ISO), and 15 min after wash-off (recovery). Data are means ± SE from 6 to 12 cultures per group. *P < 0.001 between groups. (E) Data summarizing the role of cilia beating in control, isoflurance-treated (ISO), and primary ciliary dyskinesia (PCD) airway cultures exposed to 0.15% LMA (open bars) and 0.3% LMA (closed bars). Data are means ± SE from three cultures per group. *P < 0.05 between groups. (F) Relationship between the change in ASL height and concentration of LMA for both poorly ciliated and well-ciliated cultures. Data are plotted as the difference in ASL height between the LMA relative and a vehicle control (PBS) at 30 min after exposure. In the absence of agarose (PBS control), the average ASL height at 30 min was 8.2 ± 0.3 μm for the well-ciliated and 6.1 ± 0.4 μm for the poorly ciliated group. Data are means ± SE from three cultures per point. *P < 0.05 compared to the initial measurement.
To determine whether beating cilia were required for sensing the local loads imposed by the LMA (or mucus) layer, we measured changes in luminal ATP concentration in the absence and presence of LMA in well-ciliated and poorly ciliated airway cultures exposed to LMA (Fig. 3C). Changes in ATP concentration in response to changes in the LMA concentration were significantly reduced in poorly ciliated cultures compared to well-ciliated cultures, suggesting that unlike macroscopic external stress (shear or compressive stress), viscoelastic-mediated changes in luminal ATP concentration were dependent on beating of motile cilia.
It is possible, however, that the sensing of viscoelasticity depends on the presence of cilia but does not require the beating of cilia. To address this possibility, we compared well-ciliated airway cultures with beating cilia to those with nonbeating cilia. To produce ciliostasis, we either exposed normal human cultures to the anesthetic isoflurane, which reversibly inhibits cilia beating (Fig. 3D) (20), or used cultures derived from patients with primary ciliary dyskinesia, which exhibit nonbeating, immotile cilia (23). Luminal ATP concentrations increased in normal human airway cell cultures after exposure to LMA, but not in those pretreated with isoflurane or those derived from primary ciliary dyskinesia patients (Fig. 3E).
Response of airway surface hydration to mucus layer–cilia interactions
To test the prediction that increases in mucus viscoelasticity and cilial-mediated increases in luminal ATP concentrations stimulate increased fluid secretion, we directly measured the effect of exposure to LMA on ASL height in ciliated or nonciliated airway cultures. ASL height increased in response to increasing LMA concentrations in ciliated, but not poorly ciliated, airway cultures (Fig. 3F). From these experiments, we conclude that beating cilia are essential for sensing the viscoelastic properties of the mucus layer and transmitting this information into increases in luminal ATP concentration and, consequently, ASL hydration.
Protection of the airway from flooding
How do airways protect themselves from prolonged or unregulated nucleotide release and flooding of airway surfaces due to persistent fluid secretion? We hypothesized that desensitization of purinoceptors, including P2Y2, could be a protective mechanism against persistent fluid secretion by unregulated increases in luminal ATP concentrations (24). To test this hypothesis, we treated static airway cultures with high concentrations of a long- acting, poorly hydrolysable P2Y2 receptor agonist, denufosol (INS37217), which exhibits an EC50 (median effective concentration) of ~10 μM for P2Y2 receptor activation (25) and could be maintained above the EC50 point for 6 to 8 hours on the airway surface of airway cultures (Fig. 4A). Denufosol produced a significant increase in ASL height, reflecting P2Y2 receptor–mediated fluid secretion, which was terminated rapidly, likely reflecting P2Y2 receptor desensitization (Fig. 4B). The secreted ASL was reabsorbed over time, despite the continued presence of denufosol at values exceeding its EC50, albeit at a rate slower than without denufosol exposure (compared to vehicle group in Fig. 1A). This latter finding is consistent with the observation that P2Y2 receptor–mediated inhibition of Na+ absorption may persist for larger intervals than P2Y2 receptor activation of Cl− secretion (26).
Fig. 4. Desensitization protects the airways from excessive puriner-gic stimulation.
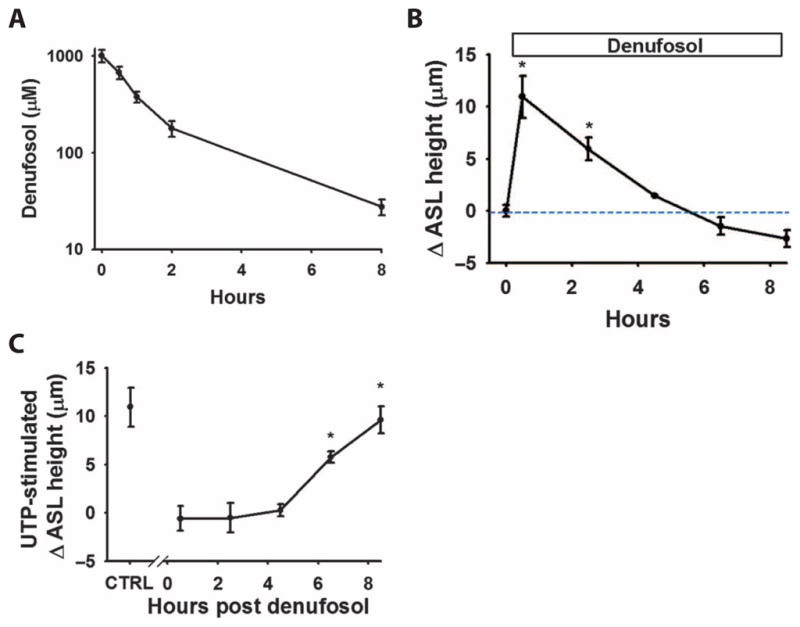
(A) Pharmaco-kinetic data showing the lifetime of denufosol concentration on the surface of the airways. Data are means ± SE from three cultures per point. (B) Summary of the effect of purinoceptor desensitization on nucleotide-stimulated changes in ASL fluid secretion. Changes in ASL height (from baseline) were measured at various times after the addition of denufosol. Data are means ± SE from three to six cultures per group. *P < 0.005 compared to the initial (t = 0) measurement. (C) In parallel cultures, also treated with denufosol, the effect of UTP (100 μM) on ASL height was determined. At earlier time points (<4 hours), UTP was ineffective at altering ASL height as compared to the control (minus denufosol) point. Data are means ± SE from three to six cultures per group. *P < 0.005 compared to the initial (t = 0) measurement.
To further test the notion that P2Y2 receptor desensitization inhibits secretion, small boluses of the natural P2Y2 receptor agonist UTP (uridine 5′-triphosphate) were added to parallel cultures at designated time points after denufosol administration to produce maximal purinoceptor activation (Fig. 4C). UTP did not stimulate further fluid secretion for at least 6 hours after denufosol administration. These results suggest that P2Y2 receptor desensitization protects airways against the deleterious effects of persistent fluid secretion and, ultimately, airway luminal flooding in the presence of high nucleotide or nucleoside concentrations.
DISCUSSION
Effective mucus clearance requires the movement of the mucus layer, composed in health of mucins (~0.2 to 0.3%), globular proteins (~0.5%), salt (~0.9%), and water (~98%), atop a periciliary layer surrounding and including the cilia (6, 27). Mucus transport failure can result in pathological conditions in which mucus water content approaches 94% (27).
How does the airway epithelium sense the hydration status of the airway surface and maintain it through regulation of airway epithelial ion transport processes? Airway epithelia are unresponsive to the effects of systemic hormones that regulate ion and water transport rates in other epithelia, such as mineralocorticoids and steroid hormones, in the context of total body volume homeostasis (28). Rather, airway epithelia appear to respond to local signals generated at airway surfaces to maintain a sufficient volume of water (hydration) for normal mucus clearance, such as extracellular ATP, which links mechanical stimulation of epithelial surfaces to regulation of luminal salt and water transport (6).
Our data suggest that the concentrations of nucleotides (ATP) and nucleosides (adenosine) on the airway surface regulate the balance of ion transport and, thus, fluid volume on human airway surfaces. Removal of ATP and adenosine from ASL blocked the ability of the airway epithelia to maintain ASL height at physiological values, such as 7 μm, the length of the outstretched cilia (Fig. 1A). It may be biologically important for the airway that the default, unregulated mode of airway ion transport is absorption, which would be expected to keep airway lumens clear of liquid for proper airflow. Conversely, activation of A2b receptors resulted in ASL heights that were substantially higher than basal values (Fig. 1C). Thus, the rate of ATP release and metabolism may set the concentrations of nucleotides and nucleosides that regulate ASL fluid height under static conditions. Furthermore, these data suggest that there is no absolute “volume sensor” and, therefore, no fixed hydration state of the airway surface.
External mechanical stresses (including shear, compression, stretch, and cell swelling) stimulate ATP release. For example, various cell types, including renal epithelial cells (29), osteoblasts (30) and endothelial cells (31), release ATP into the extracellular compartment through flow-induced mechanisms. The lung is a dynamic organ, exposed to multiple macroscopic physical forces derived from breathing, blood flow, and surface tension, which contribute to the regulation of lung development, structure, metabolism, and function (32–34). ATP is released from human airway epithelia as a result of mechanical forces in the lungs, such as compression and membrane stretching of the airways (10, 35, 36), and the shear stresses associated with air movement across the epithelial surface during tidal breathing and coughing (9, 17, 37). Our data demonstrate that shear stress–dependent variations in the concentration of airway surface ATP were associated with rebalancing steady-state ASL volumes to variable heights (Fig. 1D), which again suggests that there is no fixed volume (height) of airway surface hydration. Rather, ASL hydration is regulated in response to the magnitude of mechanical stress–induced ATP release and proportional to ASL concentrations of ATP and adenosine. We speculate that this capacity allows for normal airway epithelia to respond to macroscopic external airway stresses, such as physical exercise, with an appropriate volume of ASL.
We observed that luminal ATP concentration can also be regulated locally in response to changes in the hydration status of the mucus layer. Luminal ATP concentrations were regulated at mucus simulant concentrations mimicking the viscoelastic properties of normal airway mucus (Fig. 2E), suggesting that this feedback system is “tuned” to maintain rheological properties that ensure efficient mucus clearance under normal conditions. However, airway epithelia also responded with increased ATP concentrations when exposed to concentrations of LMA, at which it has viscoelastic proprieties similar to “dehydrated” mucus, suggesting that airway epithelia can respond to disease-associated airway surface hydration needs. Indeed, addition of LMA to the airway lumen resulted in concentration-dependent increases in ASL height (Fig. 3F). These results demonstrate that airway epithelial cells can “sense” changes in the visco-elastic status (hydration) of the mucus layer and use a feedback system to readjust airway surface hydration by regulating ATP concentration and, hence, fluid transport through the activation state of apical membrane purinoceptors.
Primary cilia sense flow in various types of cells. However, differentiated human airway epithelia lack primary cilia (13) and use the beating of motile cilia to sense changes in mucus concentration. We observed that airway epithelia that lacked cilia because of poor differentiation or that exhibited immotile cilia because of primary ciliary dyskinesia or isoflurane treatment did not detect changes in overlying mucus concentration by stimulating an increase in luminal ATP (Fig. 3, C and E) or ASL height (Fig. 3F). In contrast, we observed (Fig. 3B) that motile cilia were not required for sensing external physical stresses. Similarly, intercalated cells in the distal nephron, which lack both a primary cilium and motile cilia, release ATP in response to flow-induced shear stresses (38).
These findings might suggest that the airway epithelia of individuals with immotile cilia, such as those with primary ciliary dyskinesia, cannot regulate mucus concentration. Although mucus hydration has not been measured in primary ciliary dyskinesia subjects, radiotracer mucus clearance studies have demonstrated a nearly normal rate of mucus clearance over time that is mediated solely by frequent coughing (39). The mechanical stresses associated with coughing are large, producing transpulmonary pressures, reaching pressures as high as 200 cmH2O and shear rates of 1700 dynes/cm2 (40). We suggest that these stresses are associated with sufficient stimulation in ATP release and fluid secretion to at least partially hydrate the mucus layer in individuals with primary ciliary dyskinesia.
Why does this feedback system seemingly fail in cystic fibrosis, so that mucus transport ceases and the airways become plugged with dehydrated mucus? One possibility is that other properties of cystic fibrosis mucus could override the feedback system. These include the presence of DNA (41), a different ratio of two mucin subtypes that make up the airway mucus (Muc5AC and Muc5B) as a result of goblet cell hyperplasia (42), and proteolytic cleavage of mucins by bacterial- and neutrophil-released proteases (43). Furthermore, the mucins themselves on the airway epithelia of cystic fibrosis patients may be abnormal, reflecting possible defects in mucin biosynthesis (3, 44) or defective unfolding during release from intracellular granules as a result of defective CFTR-mediated HCO3− transport (4, 5). How such alterations in cystic fibrosis airway mucus might affect the ability of cilia to sense and respond to changes in viscoelasticity is unknown. Alternatively, cystic fibrosis patients may be susceptible to insults that alter ATP regulation of fluid transport, because cystic fibrosis airways have only one mechanism to initiate Cl− secretion, the ATP-regulated CaCC channel, rather than the dual mechanisms (CFTR and CaCC) in normal or primary ciliary dyskinesia individuals. Indeed, infection of airway epithelia by specific respiratory viruses is associated with an increase in epithelial ecto-ATPase activity, which reduces the luminal concentration of ATP (9). Under such conditions, it is likely that the airway epithelia cannot respond to changes in mucus concentration, producing collapse of the periciliary layer and cessation of cilia beating (20).
ATP release rates appear to have a linear, nonsaturating relationship with ASL volume (Fig. 1D). A persistently high ATP concentration, which could be caused by cellular ATP release from large numbers of inflammatory cells (45) or inhalation of exogenous P2Y2 receptor agents for therapeutic effects (46), could conceivably raise ASL volumes to the point where airway lumens could be “flooded” and result in altered or blocked airflow. The ATP signaling system has intrinsic properties to avoid this result, such as P2Y2 receptor desensitization at high concentrations of agonists (24). Here, we demonstrated that purinoceptor desensitization blunted the secretory response to the sustained presence of a high concentration of a poorly metabolized P2Y2 receptor agonist, and that P2Y2 receptor desensitization blocked the response to addition of a physiologic agonist (Fig. 4C). Fluid secretion ceased after P2Y2 receptor desensitization, thus preventing flooding of the airway surface (Fig. 4B). However, other mechanisms could protect airways against flooding during prolonged stimulation by ATP release. For example, in the absence of purinergic inhibition of ENaC, mechanoactivation of ENaC (47) coupled with dilution of inhibitory ENaC factors, such as SPLUNC1 (48), could also protect airways from flooding by accelerating the rate of fluid absorption. Other possibilities include reduced ATP release due to cell adaptation to a continued stress (10) or increased extracellular ATPase activity (9).
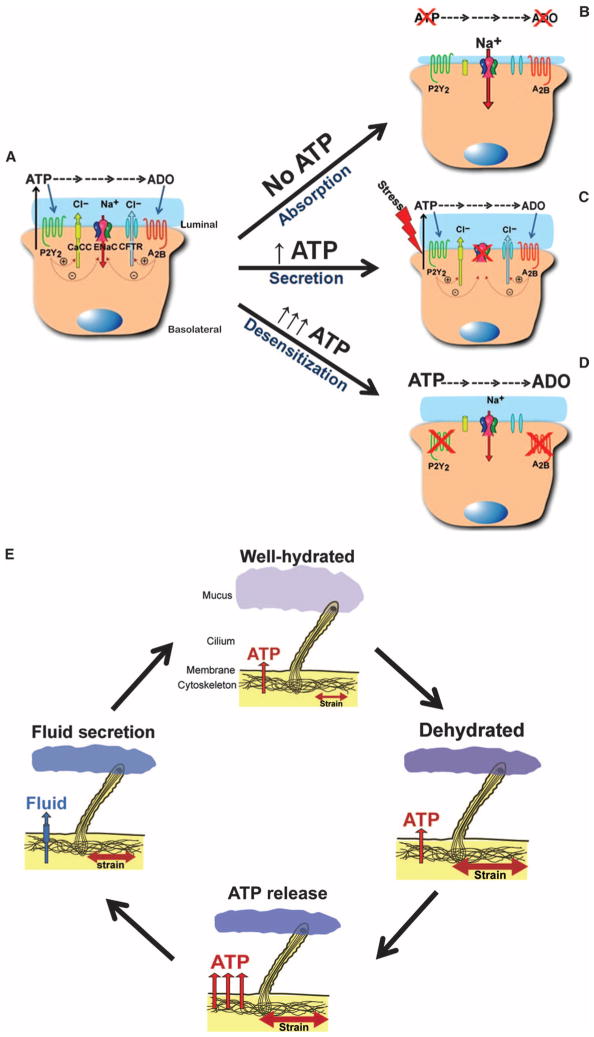
In summary, airway epithelia autoregulate airway surface hydration through regulation of luminal ATP release rates. The mechanisms of ATP release in airway epithelia are still under investigation but appear to include both vesicular (49) and hemichannel release (50). At the macroscopic level, airway epithelia exhibit a “basal” ATP release rate under static conditions that is adequate to maintain ASL hydration in normal airways (Fig. 5A). In the absence of ATP release, or an increase in ectoenzyme activities, luminal ATP and adenosine concentrations decrease and unregulated ion absorption occurs, producing luminal dehydration (Fig. 5B). Superimposed on basal release, there is a variable ATP mechanosensitive release component that modifies the ATP concentration in the ASL (Fig. 5C). At the organ level of regulation, the macroscopic strains and shear stresses associated with airway wall expansion and airflow during tidal breathing regulate ATP concentrations within the ASL that are appropriate for efficient airway surface hydration. In part through purinoceptor desensitization, there are checks to prevent inappropriate secretion and luminal flooding (Fig. 5D). At the microscopic level, hydration of airways surface mucus is sensed by cilia-mucus interactions, which, through feedback on local ATP release rates, ensure that the hydration status of mucus is optimal for transport (Fig. 5E). Thus, these mechanosensitive ATP release systems seem ideally suited to maintain hydration of mucus for effective lung defense.
Fig. 5. Hypothetical models of ASL volume regulation.
Predicted model of ASL regulation by differing conditions of ASL [ATP]. (A) Basal regulation of ASL height under normal conditions of ATP release and subsequent regulation of ion transporters. (B) In the absence of ATP and adenosine, the epithelium absorbs fluid because of the absence of Cl− secretion and the inability to regulate Na+ absorption. (C) During increases in ATP release (for example, shear stress), ASL height is increased as a result of increased Cl− secretion and inhibition of Na+ absorption. (D) Under conditions of excessive ATP release, purinoceptors desensitize, resulting in cessation of fluid secretion and prevention of airway flooding. (E) Proposed feedback model of stimulation of ATP release and subsequent fluid secretion by increased membrane stress during conditions of increased mucus concentration. Increased mucus concentration is sensed by beating cilia and leads to increased fluid secretion through ATP release. Once the mucus is rehydrated and has a lower viscoelasticity, the stress on the cilia is reduced, ATP release is decreased, and fluid homeostasis returns to the normal state.
MATERIALS AND METHODS
Experimental preparations
A combination of confocal measurements of ASL height and direct measurements of ATP was used in this study. Well-differentiated air-liquid interface human bronchial epithelia cultures were used under both (i) static conditions, in which basal ATP release rates were sufficiently low that ASL ATP concentrations were below those required for P2Y2 activation, but adenosine concentrations were within the activation range for A2b receptors (17), and (ii) cyclic mechanical stress conditions to increase ATP release rates and ASL ATP concentrations sufficient to activate P2Y2 purinoceptors receptors.
Human airway cell culture
Human bronchial epithelial cells were obtained from the University of North Carolina (UNC)–Cystic Fibrosis Tissue Culture Core under protocols approved by the UNC Institutional Review Board. Normal and primary ciliary dyskinesia airway epithelial cells were derived from donor lungs. Cells from the trachea, main stem, and lobar bronchi were isolated by protease digestion (51). Isolated cells (106/cm2) were seeded on 12-mm permeable support (0.45-μm pore diameter, Transwell-Clear; Costar) pre-coated with human placental collagen (Sigma), in Ham’s F12–based medium supplemented with insulin (10 μg/ml), transferrin (5 μg/ml), 1 μM hydrocortisone, 30 nM triiodothyronine, epidermal growth factor (25 ng/ml), and endothelial cell growth substance (3.75 μg/ml), as previously described (51). Cells were maintained under air-liquid conditions, washed every 48 to 72 hours to remove accumulated mucus, and studied as fully differentiated cultures (3- to 4-week cultures with transepithelial resistances of ~200 to 400 Ω cm2). Culture incubations were performed in a well-humidified (>95%) tissue culture incubator (5% CO2) at 37°C. Excess mucus on the cultures was removed by washing the cultures three times with serum-free Ham’s F12–based medium 16 hours before experimentation (14).
Application of shear and compressive stress to airway cultures
For studies involving the application to external mechanical forces, we used devices and methods previously described to subject cultures to shear stress (9) and compressive stress (10, 18). Briefly, to generate shear stress, cultures with a known volume of liquid on the apical surface were positioned in a custom rotational device that rotated in a stop-go fashion. By controlling the time and rate of acceleration, the shear stress across the surface was established to produce 0.5 dynes/cm2, a value that corresponds to the magnitude of shear stress exerted on the airway surface during tidal breathing (9). Compressive stress was produced by transiently increasing the transepithelial pressure on airway cultures for a 3-s cycle, composed of 2 s at atmospheric pressure (0 cmH2O transepithelial) and 1 s at 20 cmH2O pressure, at a frequency of 20 cycles/min (10). These parameters were designed to mimic the mechanical stimulation observed in the airways at a frequency consistent with normal tidal breathing (18).
Measurement of ASL height
The height of the ASL, an index of ASL hydration state, was measured over time with a cell impermeant marker to visualize the ASL {Texas Red dextran 70 kD (Molecular Probes) in isotonic TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] buffered Ringer} and XZ confocal microscopy. In studies with LMA, LMA combined with Texas Red was cooled to 37°C before being added to the airway surface to produce a change in ASL height of ~3 μm (~0.5-μl volume). These studies were performed at 37°C with an environmental chamber encompassing the confocal microscope to ensure that the LMA remained a flowable gel. Images of the Texas Red–labeled ASL were acquired by laser scanning confocal microscopy (SP5; Leica) at various time points with the appropriate filters for Texas Red (540-nm excitation and 630-nm emission). In all confocal studies, 100 μl of immiscible perfluorocarbon (Fluorinert-77, 3M Corporation) was added to the airway surface after the addition of the labeling dye and test agents to avoid evaporation of the thin ASL layer in low-humidity environments (52). The height of the ASL in a single culture was determined by averaging the heights obtained from between 10 and 30 XZ scans of the culture’s surface.
Nebulization of apyrase and adenosine deaminase
In human airway cultures under static conditions, ASL height was maintained at ~7 μm, approximating the length of the outstretched cilium. Once steady state was achieved, vehicle alone or apyrase (500 U/ml) and adenosine deaminase (250 U/ml) were added to the ASL by a brief nebulization to minimize perturbation of ASL volume. Solutions were nebulized with a specially modified ultrasonic nebulizer (Aeroneb Pro, Aerogen Inc.) at a rate of ~200 nl/min for 2 min. Immediately after nebulization, 100 μl of perfluorocarbon was gently added to the luminal surface to prevent evaporation (52).
Measurement of ATP by luciferin-luciferase
ATP concentrations in microsampled ASL (10) were determined with luciferin-luciferase bioluminescence as previously described (17). Briefly, 1 μl of sample was added to a test tube, and the volume was adjusted to 100 μl with high-performance liquid chromatography–grade water. Fifty microliters of the luciferin-luciferase reaction mix [300 μM luciferin, luciferase (5 μg/ml), 6.25 mM MgCl2, 0.63 mM EDTA, 75 μM dithiothreitol, bovine serum albumin (1 mg/ml), 25 mM Hepes (pH 7.8)] was added to samples with the built-in autoinjector of a luminometer (LB953; Berthold). Luminescence was detected by the photomultiplier and integrated over 10 s. The recorded arbitrary counts from each sample, counted in duplicate, were compared against an ATP standard curve performed in parallel. The resulting luminescence was linear between 0.1 and 1000 nM ATP, based on the standard curve.
Agarose rheology
Measurements of the viscoelastic properties of LMA were carried out with a Bohlin Gemini rheometer (Bohlin Instruments Ltd.) fitted with 42-mm-diameter cone plate, set at a gap width of 1 mm. For each study, about 1 ml of molten LMA (42°C) was placed on the rheometer preheated and maintained at 37°C. The sample was allowed to equilibrate for 15 min before analysis. Oscillatory sweeps were carried out at stress-strain values in the shear-independent plateau at a frequency of 10 Hz to determine the storage modulus (G′) and the loss modulus (G″) of the LMA samples. Data are presented as the average of at least three samples at each LMA concentration.
Analysis of CBF
CBF was measured in ciliated airway cultures with a technique previously described (10). In these studies, cultures were previously washed three times with PBS (10 min each) to remove accumulated mucus. Cultures were placed on a glass coverslip on an inverted phase contrast microscope (TE2000, Nikon) with a 20× objective to record cilial movement. Videos of cilia beating were recorded digitally with a high-speed (125 Hz) camera (GS-310 Turbo, Megaplus). Specialized software, based on Sisson-Ammons Video Analysis (53), was used to analyze the acquired video images and estimate the average CBF of all motile cilia in each frame. Videos of 5 to 10 random locations were used to compute an average CBF. In experiments where cilia beating was arrested, cultures were exposed to an emulsion of isoflurane [5% (v/v) in PBS] for 10 min before analysis.
Statistical analysis
Results are expressed as means ± SE and were evaluated by Student’s t test for single comparisons or one-way analysis of variance (ANOVA) followed by Tukey’s or Holm-Sidak post hoc comparison for multiple comparisons, depending on the experimental design. Results were confirmed by a minimum of three independent experiments. All statistical tests were performed with SigmaPlot (version 11, Systat Software Inc.). P < 0.05 was considered statistically significant.
Acknowledgments
We thank E. Lazarowski, C. W. Davis, and R. Superfine for their discussion on this work; S. Olson and L. Brown for the graphical and editorial assistance; S. Randell for the human airway epithelial cells; L. Ostrowski for the cultures from primary ciliary dyskinesia patients; D. Hill for the mucus rheology assistance; and K. Burns for the histology assistance.
Funding: Funding for this work was provided by the Cystic Fibrosis Foundation (grant nos. BUTTON07XX0 and BUTTON11G0) and NIH (grant nos. K01DK080847, P50HL107168, P01HL34322, P01HL110873-01, and P50HL107168-01).
Footnotes
Author contributions: B.B. and R.C.B. conceived and planned the study; B.B., S.F.O., and W.R.T. performed the experiments; C.B.F. wrote custom Matlab code to analyze confocal microscopy images; and B.B. and R.C.B. wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Randell SH, Boucher RC. University of North Carolina Virtual Lung Group, Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol. 2006;35:20–28. doi: 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paisley D, Gosling M, Danahay H. Regulation of airway mucosal hydration. Expert Rev Clin Pharmacol. 2010;3:361–369. doi: 10.1586/ecp.10.19. [DOI] [PubMed] [Google Scholar]
- 3.Xia B, Royall JA, Damera G, Sachdev GP, Cummings RD. Altered O-glycosylation and sulfation of airway mucins associated with cystic fibrosis. Glycobiology. 2005;15:747–775. doi: 10.1093/glycob/cwi061. [DOI] [PubMed] [Google Scholar]
- 4.Kreda SM, Davis CW, Rose MC. CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring Harb Perspect Med. 2012;2:a009589. doi: 10.1101/cshperspect.a009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinton PM. Role of epithelial HCO3- transport in mucin secretion: Lessons from cystic fibrosis. Am J Physiol Cell Physiol. 2010;299:C1222–C1233. doi: 10.1152/ajpcell.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher RC. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 7.Castrop H. Mediators of tubuloglomerular feedback regulation of glomerular filtration: ATP and adenosine. Acta Physiol. 2007;189:3–14. doi: 10.1111/j.1748-1716.2006.01610.x. [DOI] [PubMed] [Google Scholar]
- 8.Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol. 2006;291:F923–F931. doi: 10.1152/ajprenal.00192.2006. [DOI] [PubMed] [Google Scholar]
- 9.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol. 2007;580:577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salisbury JL. Primary cilia: Putting sensors together. Curr Biol. 2004;14:R765–R767. doi: 10.1016/j.cub.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003;12:517–520. doi: 10.1097/00041552-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Jain R, Pan J, Driscoll JA, Wisner JW, Huang T, Gunsten SP, You Y, Brody SL. Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2010;43:731–739. doi: 10.1165/rcmb.2009-0328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 15.Tarran R. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc Am Thorac Soc. 2004;1:42–46. doi: 10.1513/pats.2306014. [DOI] [PubMed] [Google Scholar]
- 16.Hirsh AJ, Stonebraker JR, van Heusden CA, Lazarowski ER, Boucher RC, Picher M. Adenosine deaminase 1 and concentrative nucleoside transporters 2 and 3 regulate adenosine on the apical surface of human airway epithelia: Implications for inflammatory lung diseases. Biochemistry. 2007;46:10373–10383. doi: 10.1021/bi7009647. [DOI] [PubMed] [Google Scholar]
- 17.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Button B, Boucher RC. University of North Carolina Virtual Lung Group, Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol. 2008;163:189–201. doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, Knight D, Thornton DJ, Sheehan JK. Tracheobronchial air-liquid interface cell culture: A model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol. 2009;296:L92–L100. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui H, Wagner VE, Hill DB, Schwab UE, Rogers TD, Button B, Taylor RM, II, Superfine R, Rubinstein M, Iglewski BH, Boucher RC. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci USA. 2006;103:18131–18136. doi: 10.1073/pnas.0606428103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredberg JJ. A modal perspective of lung response. J Acoust Soc Am. 1978;63:962–966. doi: 10.1121/1.381776. [DOI] [PubMed] [Google Scholar]
- 23.Meeks M, Bush A. Primary ciliary dyskinesia (PCD) Pediatr Pulmonol. 2000;29:307–316. doi: 10.1002/(sici)1099-0496(200004)29:4<307::aid-ppul11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Martin MW, Harden TK. Agonist-induced desensitization of a P2Y-purinergic receptor-regulated phospholipase C. J Biol Chem. 1989;264:19535–19539. [PubMed] [Google Scholar]
- 25.Yerxa BR, Sabater JR, Davis CW, Stutts MJ, Lang-Furr M, Picher M, Jones AC, Cowlen M, Dougherty R, Boyer J, Abraham WM, Boucher RC. Pharmacology of INS37217 [P1-(uridine 5′)-P4- (2′-deoxycytidine 5′)tetraphosphate, tetrasodium salt], a next-generation P2Y2 receptor agonist for the treatment of cystic fibrosis. J Pharmacol Exp Ther. 2002;302:871–880. doi: 10.1124/jpet.102.035485. [DOI] [PubMed] [Google Scholar]
- 26.Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J. 2005;19:142–143. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- 27.Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 28.Grubb BR, Boucher RC. Effect of in vivo corticosteroids on Na+ transport across airway epithelia. Am J Physiol. 1998;275:C303–C308. doi: 10.1152/ajpcell.1998.275.1.C303. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto K, Korenaga R, Kamiya A, Ando J. Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ Res. 2000;87:385–391. doi: 10.1161/01.res.87.5.385. [DOI] [PubMed] [Google Scholar]
- 30.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodin P, Bailey D, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumacker PT. Straining to understand mechanotransduction in the lung. Am J Physiol Lung Cell Mol Physiol. 2002;282:L881–L882. doi: 10.1152/ajplung.00043.2002. [DOI] [PubMed] [Google Scholar]
- 33.Price WA, Stiles AD. New insights into lung growth and development. Curr Opin Pediatr. 1996;8:202–208. doi: 10.1097/00008480-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Pan J, Copland I, Post M, Yeger H, Cutz E. Mechanical stretch-induced serotonin release from pulmonary neuroendocrine cells: Implications for lung development. Am J Physiol Lung Cell Mol Physiol. 2006;290:L185–L193. doi: 10.1152/ajplung.00167.2005. [DOI] [PubMed] [Google Scholar]
- 35.Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- 36.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guyot A, Hanrahan JW. ATP release from human airway epithelial cells studied using a capillary cell culture system. J Physiol. 2002;545:199–206. doi: 10.1113/jphysiol.2002.030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol. 2003;285:F998–F1012. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 39.Möller W, Häussinger K, Ziegler-Heitbrock L, Heyder J. Mucociliary and long-term particle clearance in airways of patients with immotile cilia. Respir Res. 2006;7:10. doi: 10.1186/1465-9921-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basser PJ, McMahon TA, Griffith P. The mechanism of mucus clearance in cough. J Biomech Eng. 1989;111:288–297. doi: 10.1115/1.3168381. [DOI] [PubMed] [Google Scholar]
- 41.Lethem MI, James SL, Marriott C, Burke JF. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur Respir J. 1990;3:19–23. [PubMed] [Google Scholar]
- 42.Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt I, Nicholson AG, Chung KF. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir Med. 2002;96:81–86. doi: 10.1053/rmed.2001.1221. [DOI] [PubMed] [Google Scholar]
- 43.Henke MO, John G, Rheineck C, Chillappagari S, Naehrlich L, Rubin BK. Serine proteases degrade airway mucins in cystic fibrosis. Infect Immun. 2011;79:3438–3444. doi: 10.1128/IAI.01252-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng PW, Boat TF, Cranfill K, Yankaskas JR, Boucher RC. Increased sulfation of glycoconjugates by cultured nasal epithelial cells from patients with cystic fibrosis. J Clin Invest. 1989;84:68–72. doi: 10.1172/JCI114171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piccini A, Carta S, Tassi S, Lasiglié D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1b and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol. 2009;9:262–267. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol. 2001;280:F1010–F1018. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Caballero A, Rasmussen JE, Gaillard E, Watson MJ, Olsen JC, Donaldson SH, Stutts MJ, Tarran R. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci USA. 2009;106:11412–11417. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 50.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 52.Tarran R, Boucher RC. Thin-film measurements of airway surface liquid volume/composition and mucus transport rates in vitro. Methods Mol Med. 2002;70:479–492. doi: 10.1385/1-59259-187-6:479. [DOI] [PubMed] [Google Scholar]
- 53.Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc. 2003;211:103–111. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]