Abstract
Twist1 promotes epithelial–mesenchymal transition, invasion, metastasis, stemness, and chemotherapy resistance in cancer cells and thus is a potential target for cancer therapy. However, Twist1-null mice are embryonic lethal, and people with one Twist1 germline mutant allele develop Saethre–Chotzen syndrome; it is questionable whether Twist1 can be targeted in patients without severe adverse effects. We found that Twist1 is expressed in several tissues, including fibroblasts of the mammary glands and dermal papilla cells of the hair follicles. We developed a tamoxifen-inducible Twist1 knockout mouse model; Twist1 knockout in 6-week-old female mice did not affect mammary gland morphogenesis and function during pregnancy and lactation. In both males and females, the knockout did not influence body weight gain, heart rate, or total lean and fat components. The knockout also did not alter blood pressure in males, although it slightly reduced blood pressure in females. Although Twist1 is not cyclically expressed in dermal papilla cells, knockout of Twist1 at postnatal day 13 (when hair follicles have developed) drastically extended the anagen phase and accelerated hair growth. These results indicate that Twist1 is not essential for maintaining an overall healthy condition in young and adult mice and that loss of function facilitates hair growth in adulthood, supporting Twist1 as a preferential target for cancer therapy.
Twist1 is a class B member of the basic helix-loop-helix (bHLH) transcription factor superfamily. Twist1 forms heterodimers with class A members of the same superfamily, such as E12 and E47, to bind NdeI E-box DNA elements to regulate the expression of genes essential for mesodermal induction and organogenesis.1–3 Human and mouse Twist1 proteins are highly homologous and share 96% amino acid sequence identity,2,4 suggesting conserved functions across these species. During mouse embryogenesis, Twist1 mRNA is expressed primarily in mesoderm-derived tissues, including the somites, the neural crest-derived head mesenchyme, the first aortic arches, the lateral mesoderm, the second, third, and fourth branchial arches, and both the anterior and posterior limb buds.4–7 After birth, Twist1 mRNA is expressed in the adult stem cells of the mesenchyme.8,9 Twist1 mRNA has also been detected in primary osteoblastic cells derived from newborn mouse calvariae10 and in both brown and white adipocytes.11,12 However, the expression pattern of Twist1 protein in adult mice has not been well defined.
The critical roles of Twist1 in mesodermal development have been well illustrated by genetic studies. In humans, heterozygous TWIST1 gene mutations cause Saethre–Chotzen syndrome (SCS), which is an autosomal dominant inheritance disease characterized by a broad spectrum of malformations, including short stature, craniosynostoses, high forehead, ptosis, small ears with prominent crus, and maxillary hypoplasia with a narrow and high palate.13–18 Mice with genetic ablation of one of the two Twist1 alleles manifest craniofacial and limb abnormalities resembling those in SCS patients; genetic ablation of both Twist1 alleles results in embryonic lethality.19–22 Although Twist1 is essential for embryonic survival and development, it is not known whether all postnatal SCS symptoms are consequences of developmental defects, nor whether Twist1 is required for maintaining normal physiological function in adulthood after accomplishment of all developmental processes.
Importantly, expression of Twist1 expression is induced in and associated with many types of aggressive cancers, including breast,23 prostate,24,25 gastric,26,27 liver,28,29 bladder,30,31 esophageal,32,33 and pancreatic cancers.34 Twist1 plays multiple roles in cancer initiation, progression, and metastasis. Specifically, Twist1 can override the failsafe cell senescence and apoptotic responses triggered by oncogenes,35–37 increase cancer cell resistance to endocrine therapy and chemotherapy,38,39 enhance cancer stem cell populations,40–42 and facilitate cancer cells to invade and metastasize.23,43–48 Expression of hypoxia-inducible factor 1α up-regulates Twist1 to promote metastasis.49 Twist1 promotes the epithelial–mesenchymal transition (EMT) process in part by directly repressing E-cadherin and estrogen receptor-α expression through recruiting the nucleosome remodeling and deacetylase (NuRD) complex for gene repression and by up-regulating proteins such as Bmi1, AKT2, and YB-1.39,46,50–52 Twist1 also promotes Bmi1 expression to enhance self-renewal of cancer stem cells and promotes PDGFR-α expression to induce invadopodia formation and promote tumor cell local invasion, intravasation, and extravasation.51,53,54 These studies suggest that Twist1 may be an important and useful target for controlling cancer metastasis and enhancing the efficacy of cancer therapy. However, given the lethal phenotype of Twist1-null mice, it remains to be evaluated whether inhibition of Twist1 in cancer patients causes any severe health problems.
The process of carcinogenesis from normal epithelial cells can essentially be considered as a dedifferentiation process that is commonly associated with the expression of many genes that are only or predominantly expressed during early embryonic development. We hypothesize that, although Twist1 is required for embryonic development and for cancer cell EMT and metastasis, Twist1 is not essential for maintaining a generally healthy physiological condition in the adult organism. To test this hypothesis, we first examined Twist1 protein distribution in adult mice, then developed an inducible knockout model to delete Twist1 in adult mice at different stages as needed, and finally defined the effect of Twist1 knockout on physiological functions after embryonic morphogenesis was fully accomplished.
Materials and Methods
Histological Examination, IHC, IHF, and TUNEL Assay
Mouse tissues were dissected and fixed in a 4% paraformaldehyde solution at 4°C overnight. After a PBS wash, the fixed tissues were dehydrated and embedded in paraffin blocks as described previously.55 Tissue sections (5 μm thick) were prepared, deparaffinized in xylene, and hydrated using an ethanol gradient. H&E staining, antigen retrieval, IHC, and immunohistofluorescence (IHF) were performed as described previously.55 For immunostaining, sections were blocked with either 10% normal serum or a M.O.M mouse-on-mouse immunodetection kit (Vector Laboratories, Burlingame, CA) for 1 hour at room temperature and incubated with primary antibodies overnight at 4°C. The primary antibodies were against Twist1 (ab50887; Abcam, Cambridge, MA), α-smooth muscle actin (α-SMA) (Dako M0851; Agilent Technologies, Santa Clara, CA), lymphoid enhancer-binding factor 1 (Lef-1) (C12A5; Cell Signaling Technology, Danvers, MA), vimentin (ab8978; Abcam), and Ki-67 (550609; BD Biosciences, San Jose, CA). Secondary antibodies (Vector Laboratories) were diluted 1:400. The IHC signal was enhanced using a Vectastain ABC system and was visualized with a peroxidase substrate kit containing 3,3′-diaminobenzidine (Vector Laboratories). Slides were counterstained with Harris modified hematoxylin and mounted with Permount mounting medium (Thermo Fisher Scientific, Waltham, MA) for microscopy. For IHF, a tyramide signal amplification kit (Life Technologies, Carlsbad, CA) was used according to the manufacturer’s instructions. TUNEL assay was performed on hydrated mouse skin sections using an apoptosis detection kit (Upstate; EMD Millipore, Billerica, MA) according to the manufacturer’s instructions.
Mouse Lines, Mouse Breeding, and Genotype Analysis
The mouse line with floxed Twist1 alleles (Twist1f/f) for conditional knockout has been described previously56 and was obtained from Mutant Mouse Regional Resource Centers (016842-UNC). In this line, two loxP sites were placed flanking the entire coding region of the Twist1 gene.56 The Rosa26-CreERT2 mouse line has been described previously57 and was provided by T.L. In this mouse line, the Rosa26 locus drives the expression of the Cre-ERT2 fusion protein consisting of the Cre recombinase and the mutant ligand-binding domain of the estrogen receptor α (ER-α). This mutant ligand-binding domain binds only 4-hydroxytamoxifen (the active metabolite of tamoxifen), but not endogenous estrogen. On tamoxifen binding, the Cre-ERT2 fusion protein translocates into the nucleus from the cytoplasm, allowing its Cre recombinase to excise any floxed DNA fragment in the genome.57 Rosa-CreERT2 and Twist1f/f mice were crossbred for two generations to generate Rosa-CreERT2+/−;Twist1f/f (hereafter referred to as CreERT2;Twist1f/f or, after tamoxifen treatment, as Twist1Δ/Δ) and Rosa-CreERT2−/−;Twist1f/f (hereafter referred to as Twist1f/f or control) mice with the same genetic background for experiments. For analyzing the genotypes of these mice, genomic DNA samples were extracted from a small piece of mouse ear tissue to serve as PCR template as described previously.58 Allele-specific PCR primers were synthesized and allele-specific PCR reactions were performed as described previously.57
Animal protocols were approved by the Animal Care and Use Committee of Baylor College of Medicine.
Tamoxifen Treatment
Tamoxifen (Sigma-Aldrich, St. Louis, MO) was dissolved in corn oil (Sigma-Aldrich) at a concentration of 20 mg/mL. For inducible knockout of the floxed Twist1 alleles in adult mice, 6-week-old CreERT2;Twist1f/f and Twist1f/f mice were injected with 1 mg tamoxifen/day intraperitoneally for five consecutive days. At 7 days after the last injection, various mouse organs were collected for examining histology, for assessing Twist1 knockout efficiency by PCR-based genotype analysis after organ-specific genomic DNA samples were prepared, and for analyzing Twist1 protein by IHC. The maintenance of tamoxifen-induced Twist1 knockout efficiency was also examined by IHC at different time points after tamoxifen treatment. The body weights of the tamoxifen-treated mice were measured once a week at different time points. For analysis of the hair follicle cycle, 13-day-old CreERT2;Twist1f/f and Twist1f/f mice were injected with 0.4 mg tamoxifen/day intraperitoneally for three consecutive days. Dorsal skin specimens were collected at various time points after tamoxifen treatment for histological examination and immunostaining.
Morphological Analysis of Mouse Mammary Glands
The staining of whole-mounted mammary glands was performed as described previously.59 In brief, whole mammary glands were dissected out from mice and fixed on glass slides in Carnoy’s fixative (1:3:7 glacial acetic acid–CHCl3–ethanol). They were then hydrated in 70% ethanol, rinsed in water, and stained overnight in carmine alum, followed by dehydration in increasing series of alcohol and clearing in xylene. The glands were mounted under coverslips with Permount.
Body Composition Measurement
Body composition was measured using a PIXImus body composition and densitometry system (Piximus, Fitchburg, WI). Mice were weighed on a compact scale (cs200; Ohaus, Pine Brook, NJ), and body lengths were measured from the nose to the base of the tail. Mice were anesthetized with 2% isoflurane during the procedure. Data analysis was performed at the Mouse Phenotyping Core Facility at Baylor College of Medicine using PIXImus software (version 1.43).
Electrocardiography
A MouseMonitor made by Indus Instruments (Webster, TX) was used to obtain the ECG data. Its unique integrated pad incorporates ultra-low noise, high-resolution ECG electronics and a homeothermic heating pad with a steel platform that supports magnetic accessories. During the procedure, mice were anesthetized with isoflurane (0.5% to 2%) and placed on the heated pad (37.5°C). Screenshots with clear wavelengths of ECG and heart rates were recorded by professional staff at the Mouse Phenotyping Core Facility at Baylor College of Medicine.
Blood Pressure Measurement
The mouse blood pressure was recorded using the noninvasive Mouse and Rat Tail Cuff Blood Pressure System (IITC Life Science, Woodland Hills, CA). Mice were pretrained to adapt to the experimental conditions for blood pressure measurement. Specifically, mice were placed in a restrainer for 12 minutes on day 1. On day 2, mice in the restrainer were placed in a warm chamber (∼37°C) for 12 minutes. On day 3, a trial of blood pressure measurement was performed by mounting the blood pressure cuff on the mice in the restrainer located in the warm chamber. On day 4, blood pressure data were collected from three rounds of measurement, each round comprising five tests, for a total of 15 data points. Only those tests providing good signals were counted for data analysis. The systolic pressure was calculated by using the manufacturer’s BPMONWIN software version 1.35 (IITC Life Sciences).
Statistical Analysis
Data are expressed as means ± standard deviation (SD). The unpaired Student's t-test was used for analysis of statistical significance. P < 0.05 was considered significant.
Results
Twist1 Protein Is Detected in Only Several Specific Tissues in Adult Mice
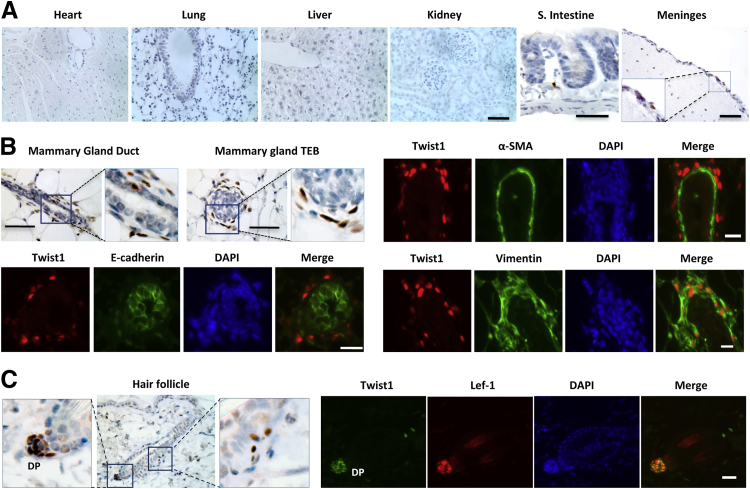
To map the functional sites of Twist1 in adult mice, we performed IHC on tissue sections prepared from various organs of 7-week-old mice using a Twist1-specific antibody. Twist1 protein was not detected in heart, lung, liver, or kidney (Figure 1A), nor in skeletal muscle, adrenal gland, or testis (data not shown). Twist1 protein was also not detected in the epithelial cells of the small intestine, including the intestinal crypts where epithelial stem cells reside (Figure 1A).60 Furthermore, Twist1 protein was not detected in any neuronal or glial cells in the neuronal tissues (data not shown). It was, however, detected in some cells of the meninges membrane that envelops the brain (Figure 1A).
Figure 1.
Detection of Twist1 protein by immunostaining in different tissues of adult mice. Tissues were dissected out from 7-week-old wild-type mice and processed for IHC (brown) or IHF (red or green) staining using Twist1 antibody. The sections stained by IHC were counterstained with hematoxylin. DAPI was used as a nuclear counterstain. Boxed regions are shown enlarged in adjacent panels. A: Twist1 is seen in the meninges of the brain, but not in heart, lung, liver, kidney, or small intestine. B: Twist1 is seen in some fibroblast-like cells surrounding the mammary gland duct and terminal end bud (TEB). These Twist1-positive cells do not express α-SMA or E-cadherin, but do express vimentin. C: Twist1 is detected by IHC in DP cells of the hair follicle and in some cells outside of the outer root sheath above the bulge region of the hair. Both Twist1 and Lef-1 are seen in DP cells with IHF. Scale bars: 100 μm (A and IHC in B); 20 μm (IHC in B and C; IHF in C).
Although Twist1 protein was not detected in the mammary gland epithelial cells, it was detected in the stromal cells surrounding the mammary gland ducts and terminal end buds (Figure 1B). These Twist1-positive cells were negative for the luminal epithelial marker E-cadherin and for the myoepithelial marker α-SMA, but were positive for the fibroblast marker vimentin (Figure 1B). These results indicate that Twist1 is expressed in the stromal fibroblast cells of the mammary gland.
The hair follicle consists of multiple cell types, including dermal papilla (DP) cells with a mesenchymal origin, epithelial dividing and differentiating cells located in the hair matrix, epithelial inner and outer root sheath cells, and epithelial stem cells located in the bulge region of the outer root sheath.61 Interestingly, Twist1 protein was specifically detected in DP cells, an identification that was further supported by the colocalization of Twist1 and the DP cell protein Lef-1 in the nuclei of all DP cells (Figure 1C). In addition, a few Twist1-positive cells were identified in the connective tissue outside of the outer root sheath above the bulge region (Figure 1C). Double-IHF staining analysis revealed that some of these Twist1-positive cells were positive for the glial cell marker S100B (data not shown), indicating that some of these cells may be glial cells wrapping the nerves that regulate the hair follicle.
Inducible Knockout of Twist1 in Adult Mice
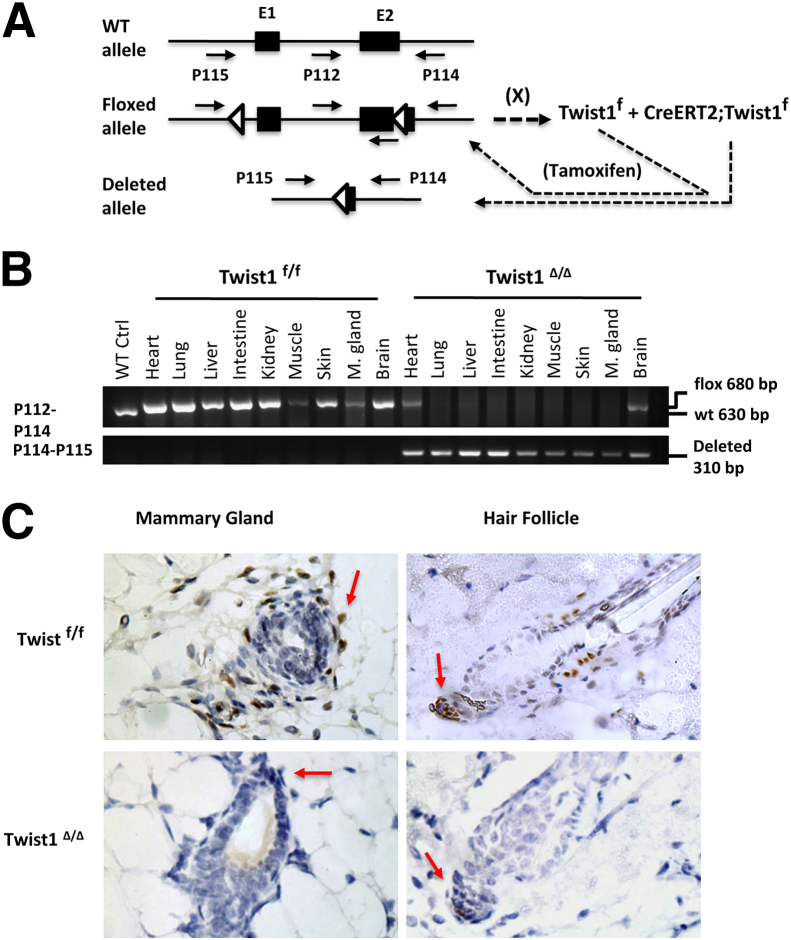
To define the physiological function of Twist1 in adulthood and to test whether Twist1 can be a tolerable in vivo molecular target for cancer therapy, we generated both the CreERT2;Twist1f/f experimental mice and the control Twist1f/f mice with a similar genetic strain background and fully functional floxed Twist1 alleles but no CreERT2 (Figure 2A). In this system, the ROSA26 locus directs universal expression of CreERT2 in all cells of CreERT2;Twist1f/f mice.57 The 6-week-old mice in both genotype groups were treated with tamoxifen for 5 days. We then examined the deletion efficiency of the floxed Twist1 alleles on day 7 after the final tamoxifen injection in CreERT2;Twist1f/f mice versus Twist1f/f control mice by Twist1f allele-specific and Twist1Δ allele-specific PCR reactions using genomic DNA templates prepared from different organs. Only the Twist1f/f alleles were detected in tamoxifen-treated Twist1f/f control mice (indicative of the absence of CreERT2 expression). In contrast, only the Twist1Δ/Δ alleles were detected in the lung, liver, small intestine, kidney, skeletal muscle, skin, and mammary gland of the tamoxifen-treated CreERT2;Twist1f/f mice. These data indicate that the floxed Twist1 alleles were effectively and efficiently excised by the tamoxifen-activated CreERT2 in the tissues examined (Figure 2B). Intriguingly, the Twist1f/f alleles in the heart and brain of these tamoxifen-treated CreERT2;Twist1f/f mice were not completely converted into Twist1Δ/Δ alleles, suggesting that the floxed Twist1 alleles in these organs are partially excised (Figure 2B). However, because Twist1 protein was not detected in the heart, nor in the neuronal or glial cells of the brain, this incomplete genetic deletion of the Twist1 gene in these organs may not significantly affect the assessment of Twist1 physiological function in adult mice.
Figure 2.
Generation of the inducible Twist1-knockout mouse model. A: Schematic of the Twist1 wild-type (WT), floxed, and conditionally deleted alleles. Tamoxifen treatment does not induce the deletion in mice with only the Twist1f allele, but does induce deletion in mice with both the Twist1f allele and the CreERT2 transgene. Exons 1 and 2 are indicated as E1 and E2; loxP sites are indicated by triangles; cross-breeding is indicated by X. B: Genotype analysis by PCR using allele-specific primer pairs as shown in panel A. Genomic DNA samples were prepared from different tissues isolated from WT mice (control), tamoxifen-treated Twist1f/f mice, and Twist1Δ/Δ mice. C: With IHC staining, Twist1 is detected in the mammary gland and hair follicle cells (arrows) in tamoxifen-treated 7-week-old Twist1f/f mice, but is absent in tamoxifen-treated 7-week-old Twist1Δ/Δ mice (arrows point to the expected location). Original magnification, ×400.
Next, we used IHC to compare Twist1 protein expression in the hair follicles and mammary glands of the tamoxifen-treated Twist1f/f control and Twist1Δ/Δ tester mice on day 7 after the final tamoxifen injection. Consistent with the Twist1 expression pattern in untreated wild-type mice (Figure 1, B and C), Twist1 protein was detected in some fibroblast cells of the mammary gland and in hair follicle DP cells, as well as in some neighboring cells in the tamoxifen-treated Twist1f/f mice. In contrast, Twist1 protein was absent in these cells in Twist1Δ/Δ mice (Figure 2C). These results demonstrate that Twist1 protein was reliably knocked out in Twist1Δ/Δ mice after tamoxifen treatment.
Inducible Knockout of Twist1 Does Not Impair Mammary Gland Development and Function
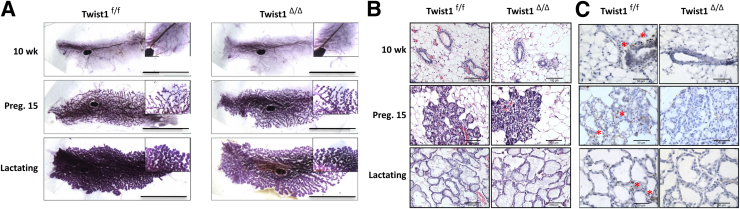
To determine whether Twist1 expression in the fibroblast cells of the mammary gland plays a role in mammary gland development, we compared mammary gland morphologies at different developmental phases after the 6-week-old female Twist1f/f and Twist1Δ/Δ mice had been treated with tamoxifen for 5 days. Because of the inhibitory effects of tamoxifen treatment at the age of 6 weeks on estrogen-stimulated mammary gland growth, mammary ductal growth in both groups of 10-week-old (4 weeks after the final tamoxifen injection) mice was blunted and had failed to fill the fat pads by this age. To generate a model for examining the responses of both types of mammary glands to pregnancy and lactation hormones, we crossbred tamoxifen-treated female Twist1f/f and Twist1Δ/Δ mice with normal male mice and generated pregnant and lactating Twist1f/f and Twist1Δ/Δ female mice. Upon the stimulation of pregnancy hormones, the mammary glands in both groups of mice exhibited extensive ductal growth, branch morphogenesis, and alveolar structures, as examined on day 15 of pregnancy (Figure 3A). Lactating glands of both groups also demonstrated normal alveolar differentiation, as examined by whole-mount staining (Figure 3A). Furthermore, both groups of mammary glands also exhibited the same normal tissue architectures at the virgin, pregnant, and lactation stages and plentiful milk production at the lactation stage, as examined by H&E staining of tissue sections (Figure 3B). Moreover, both Twist1f/f and Twist1Δ/Δ mice were able to sustain their pups throughout lactation. No weight differences were observed between pups from Twist1f/f and Twist1Δ/Δ dams (data not shown). Finally, IHC analysis demonstrated that the Twist1 protein was expressed in stromal cells in the mammary glands of Twist1f/f but not Twist1Δ/Δ mice at the virgin, pregnant, or lactating stages, indicating that Twist1 knockout was maintained in the mammary glands throughout the entire experimental process (Figure 3C). These results indicate that Twist1 is not essential for mammary gland development and function in adult mice. Furthermore, these findings suggest that female Twist1Δ/Δ mice maintain reproductive function, because they are able to get pregnant and foster their pups.
Figure 3.
Inducible knockout of Twist1 does not alter mammary gland morphogenesis. A: Whole-mount analysis of the fourth-pair mammary glands of tamoxifen-treated Twist1f/f and Twist1Δ/Δ littermate mice at 10 weeks of age, at pregnancy day 15, and lactation day 1. Insets show enlarged regions from the same image. B: H&E-stained mammary gland sections prepared from the mice shown in panel A. C: IHC detected Twist1 in some fibroblast cells (asterisks) in the mammary glands of Twist1f/f mice but not Twist1Δ/Δ mice. Scale bars: 1 cm (A), 100 μm (B); 50 μm (C).
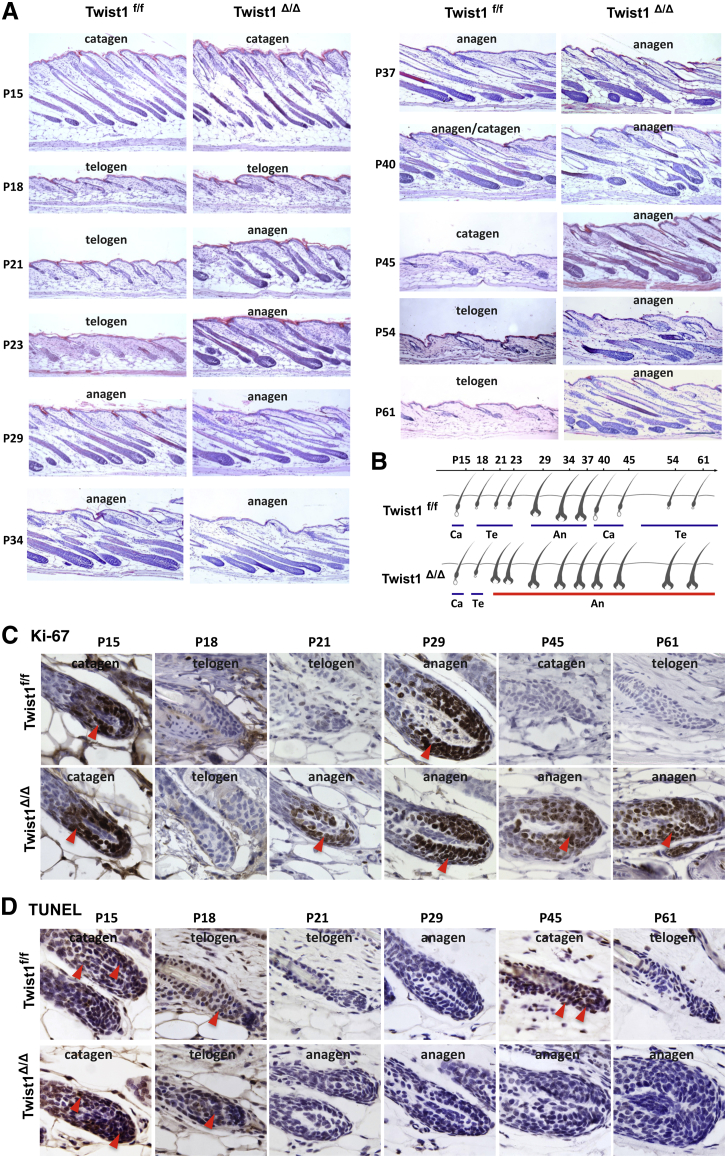
Inducible Knockout of Twist1 Significantly Extends the Anagen Phase of the Hair Follicle Cycle
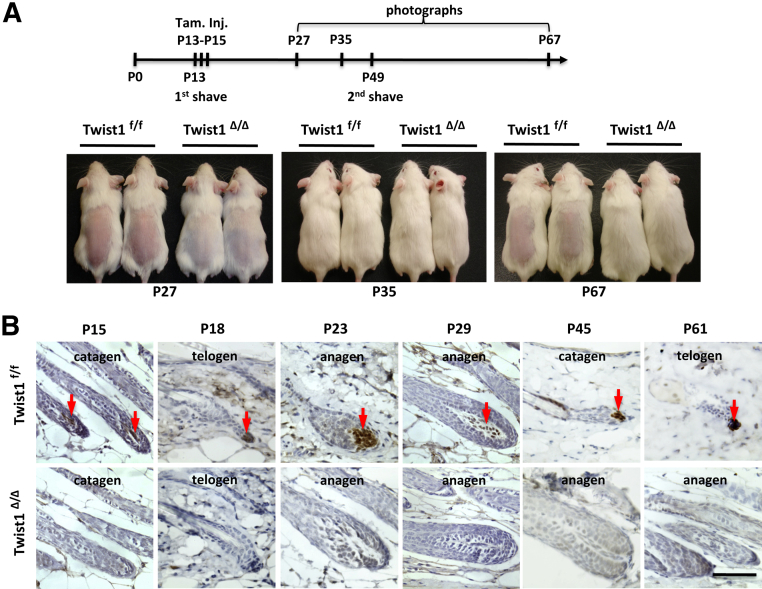
In mice, the morphogenesis of hair follicle starts at a late embryogenic stage and is completed on postnatal day 14 (P14); the first two hair follicle cycles are synchronized.62 The DP cells of the hair follicle play a pivotal role in regulation of hair formation, growth, and cycling.63 Because our data indicated a specific expression of Twist1 protein in DP cells, we hypothesized that Twist1 may play an important role in DP cells to regulate hair follicles. To define the role of Twist1 in hair follicle cycling, we treated 13-day-old Twist1f/f and CreERT2;Twist1f/f (referred to as Twist1Δ/Δ after tamoxifen treatment) mice with tamoxifen for 3 days, shaved the hair on the back twice (on P13 and P49), and examined hair growth from P15 to P67. After the first shave on P13, the hair grew significantly faster in Twist1Δ/Δ mice versus Twist1f/f mice (photographed on P27). On P35, the hair in Twist1f/f mice had grown back, and showed similar thickness as the hair of Twist1Δ/Δ mice (Figure 4A). After the second shave on P49, when the hair follicles of normal mice are in the telogen phase of the hair growth cycle, the hair in Twist1f/f control mice showed very little growth until P67, whereas the hair in Twist1Δ/Δ mice still maintained rapid growth as examined on P67 (Figure 4A). Importantly, Twist1 protein was detected in DP cells in Twist1f/f mice but not in Twist1Δ/Δ mice, as examined by IHC on P15, P18, P23, P29, P45, and P61 (Figure 4B), validating the constant status of Twist1 knockout in the hair follicles of Twist1Δ/Δ mice (data not shown). These results indicate that Twist1 plays an essential role in regulating the hair growth cycle, and that inducible knockout of Twist1 significantly extends the hair-growth phase in adult mice.
Figure 4.
Inducible knockout of Twist1 results in faster hair growth. A: Twist1f/f and Twist1Δ/Δ mice (sex-matched littermates) were treated with tamoxifen from P13 to P15. The hair on the back area of these mice was shaved on P13 and P49. Mice were photographed to document hair growth on P27, P35, and P67. Faster hair growth in Twist1Δ/Δ mice, compared with control, was observed at P27 and P67. B: Twist1 is detected in DP cells at all phases of the Twist1f/f mouse hair follicles (arrows), but is absent in the hair follicles of Twist1Δ/Δ mice, as examined by IHC on P18, P23, P29, P45, and P61. Scale bar = 50 μm.
Twist1 IHC also demonstrated that Twist1 protein is expressed at similar levels in DP cells of the control Twist1f/f mice across different phases of the hair follicle cycle, including catagen, telogen, and anagen phases, suggesting that Twist1 expression is not cyclically regulated during the hair follicle growth cycle (Figure 4B). Upon brief examination of the morphology of Twist1Δ/Δ hair follicles on the IHC-stained sections, we noticed changes of the hair follicle phases from catagen on P45 and telogen on P61 to anagen on both P45 and P61 (Figure 4B). We further examined the histological changes of the mid-dorsal skin in Twist1Δ/Δ mice at multiple time points during the first two hair growth cycles. After Twist1f/f and Twist1Δ/Δ mice were treated with tamoxifen from P13 to P15, no histological differences in hair follicles were observed between the two groups of mice at the catagen phase on P15 and at the telogen phase on P18 of the first hair follicle cycle (Figure 5A). However, although all hair follicles in the dorsal skin of Twist1f/f mice were still maintained in the telogen phase of the first hair cycle on P21, the hair follicles in Twist1Δ/Δ mice had already progressed into the anagen phase of the second hair growth cycle. These observations suggest that the telogen phase of the first hair cycle is drastically shortened in Twist1Δ/Δ mice. On P23, the Twist1f/f hair follicles had just started the anagen phase of the second hair cycle. This anagen phase was sustained to approximately P40, and then was followed by the catagen phase observed at P45 and the telogen phase observed at P54 and P61 (Figure 5A). In contrast, the Twist1Δ/Δ hair follicles always remained in the anagen phase, as histologically examined on P21, P23, P29, P34, P37, P40, P45, P54, and P61 (Figure 5A). A schematic comparison of these differences in the hair follicle growth cycle is presented in Figure 5B. These results clearly indicate that Twist1 plays an essential role in maintaining the hair growth cycle by converting the anagen phase into the catagen phase, which is then followed by the telogen phase. The loss of Twist1 function keeps the hair growth cycle at its anagen phase in adult mice.
Figure 5.
Histological analysis of hair follicle cycles in Twist1f/f and Twist1Δ/Δ mice. A: Twist1f/f and Twist1Δ/Δ mice (sex-matched littermates) were treated with tamoxifen from P13 to P15. Dorsal skin specimens were isolated from these mice at different time points, from P15 to P61, and the skin sections were stained with H&E. Specific phases of the hair follicle growth cycle were determined by previously established criteria. Scale bar = 200 μm. B: Observed differences in hair follicle cycles between Twist1f/f and Twist1Δ/Δ mice. C: Proliferating cells detected by Ki-67 IHC (brown) at different hair follicle phases of Twist1f/f and Twist1Δ/Δ mice. Areas with many Ki-67–positive proliferating cells are indicated by arrowheads. D: Apoptotic cells detected by TUNEL assay (brown) at different hair follicle phases of Twist1f/f and Twist1Δ/Δ mice. Areas with many TUNEL-positive apoptotic cells are indicated by arrowheads. An, anagen; Ca, catagen; Te, telogen. Original magnification, ×200 (C and D).
Consistent with the hair follicle cycle, IHC for Ki-67 detected numerous proliferating cells in the hair follicles of Twist1f/f mice at the transition phase between anagen and catagen on P15 and the anagen phase on P29, and no proliferating cells were detected in these follicles at the telogen phase on P18, P21, and P61 or at the catagen phase on P45. As in control mice, proliferating cells were observed in Twist1Δ/Δ hair follicles on P15, but not on P18. However, proliferating cells were detected in Twist1Δ/Δ hair follicles on P21, P29, P45, and P61, which is consistent with the sustained anagen phase from P21 to P61 (Figure 5C). On the other hand, TUNEL assay detected extensive cell apoptosis in the control Twist1f/f hair follicles at the early and late catagen phase on P15 and P45, as well as the early telogen phase on P18, but not at the late telogen phase on P21 and P61 or the anagen phase on P29. However, apoptotic cells were detected in the Twist1Δ/Δ hair follicles only at the catagen phase on P15 and the telogen phase on P18, but not in the anagen phase from P21 to P61 (Figure 5D). These results further support the notion that inducible knockout of Twist1 in young mice leads to arrest of the hair follicle cycle at the anagen phase in adulthood.
Inducible Knockout of Twist1 Has Subtle Negative Effects on General Health in Adult Mice
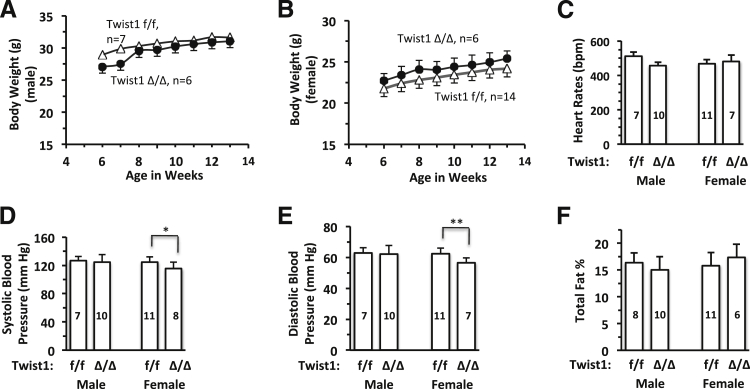
To evaluate the overall effects of inducible Twist1 ablation on the general health of adult mice, we treated 6-week-old Twist1f/f and CreERT2;Twist1f/f (referred as Twist1Δ/Δ after tamoxifen treatment) mice with tamoxifen for 5 days and then monitored body weight change over time and measured a series of general health parameters at 4 weeks after the final tamoxifen injection. There were no significant differences in body mass between Twist1f/f and Twist1Δ/Δ mice of either sex, as measured from 6 to 13 weeks (Figure 6, A and B). These results indicate that Twist1 is not required for mouse somatic growth and body weight maintenance after 6 weeks of age. The heart rates and ECGs of Twist1f/f and Twist1Δ/Δ mice, measured at 4 weeks after the final tamoxifen treatment, were normal and very similar (Figure 6C and ECG data not shown). There were no significant differences in systolic and diastolic blood pressures between male Twist1f/f and Twist1Δ/Δ mice (Figure 6, D and E). Although the systolic and diastolic blood pressure values were significantly decreased in the female Twist1Δ/Δ mice, compared with female Twist1f/f mice, the changes expressed as ratios were subtle, and both blood pressures were still within the normal range (Figure 6, D and E). There were also no significant differences in fat percentage between the two groups of mice (Figure 6F), nor in lean mass or fat mass (data not shown). These results demonstrate that Twist1 is not essential for maintaining generally normal health in adult mice.
Figure 6.
Inducible knockout of Twist1 in adult mice has subtle effect on general health. A and B: Male and female Twist1f/f (white symbols) and Twist1Δ/Δ (black symbols) mice were treated with tamoxifen for 5 days at 6 weeks of age, and body weights were measured once a week for 7 weeks. C–F: Heart rate (C), blood pressure (D and E), and total fat percentage (F) were measured at 4 weeks after the final tamoxifen treatment. Data are expressed as means ± SD, and sample size is indicated on each data bar (C–F). ∗P < 0.05, ∗∗P < 0.01, unpaired Student’s t-test.
Discussion
Twist1 has been identified in many types of metastatic cancers and is considered one of the master transcription factors that drive cancer cell EMT, metastasis, stemness, and drug resistance (reviewed in2,64). However, given that Twist1-null mice die in utero and that heterozygous mutant mouse and human develop SCS,13–22 it is unknown whether Twist1 can be targeted in cancer patients with tolerable side effects. Developing Twist1 as a clinical target for cancer therapy would require understanding of the physiological role of Twist1 and the consequence of Twist1 inactivation in young and adult cancer patients. In the present study, through mapping the expression pattern of Twist1 protein and characterizing the inducible Twist1 knockout mouse model, we have shown that inducible knockout of Twist1 in young and adult mice has no severe effects on general health condition. Specifically, Twist1 protein is detected in only a few tissues and cell types, including some meninges cells, mammary gland fibroblasts, and hair follicle DP cells. Inducible knockout of Twist1 in 6-week-old mice had no effect on mammary gland morphogenesis or lactating function in response to pregnancy and lactation hormones. Knockout of Twist1 at this age also had no effect on somatic growth, heart rate, or total fat components. Furthermore, inducible knockout of Twist1 at 6 weeks did not affect blood pressures in male mice, and only slightly reduced the blood pressures of female mice. Taken together, these results indicate that Twist1 is not essential for adult mice to maintain overall health and suggest that Twist1 may be a safe cancer-specific molecular target in adult patients.
EMT induced by expression of Twist1 or other EMT-inducing factors is reported to enhance the stemness features of epithelial and cancer cells.40,42 Thus, the question is whether adult tissue stem cells express and require Twist1 to maintain stemness. In the present study, we did not detect Twist1 protein in the stem cells of hair follicles and skin, suggesting that Twist1 is not required for the stem cells in these tissues.
Hair growth occurs in a cyclic manner, through growth (anagen), cessation (catagen), and rest (telogen) phases. In mice, hair follicle morphogenesis (the initial anagen phase) is completed on P14, which is followed by a short catagen regression phase and a resting telogen phase for the first cycle. After the first cycle, a new anagen phase is initiated with the start of the second cycle. The first and second cycles are synchronized, but the third and later cycles become unsynchronized.65,66 In the present study, high levels of Twist1 protein were detected in DP cells of the hair follicle, which is consistent with previously reported expression data.67 Interestingly, inducible knockout of Twist1 in these DP cells by tamoxifen treatment from P13 to P15 (the transition time between anagen and catagen phases of the first cycle) resulted in faster hair growth by P27 (the time point with early anagen phase in control mice) and P61 (the time point with telogen phase in control mice). These observations indicate that Twist1 plays a role in arresting hair growth, so that loss of function accelerates hair growth and changes the normal hair growth cycle.
Our histological examination over a long time course after the knockout of Twist1 provided explanations for this phenotype. Inducible knockout of Twist1 by tamoxifen treatment from P13 to P15 significantly shortens the telogen phase of the first cycle and drastically extends the anagen phase of the second cycle. The hair follicles of control mice have transitioned into the catagen phase on P45, which is shortly followed by the telogen phase. The hair follicles of knockout mice still remain in anagen phase even on P61. Importantly, the hair follicle phases characterized by histological features in control and inducible Twist1 knockout mice are perfectly associated with the patterns of follicular cell proliferation and apoptosis, respectively. This suggests that Twist1 may play a role in suppressing follicular cell proliferation and promoting follicular cell apoptosis at the catagen and telogen phases. In addition, it is not clear whether the hair follicles of the knockout mice will remain at anagen phase for the remaining lifetime. These findings clearly indicate an essential role of Twist1 in regulation of the hair follicle cycle.
Our data also show that the Twist1 protein level in DP cells does not change significantly at different phases of the hair follicle, suggesting that the function but not the expression level of Twist1 is regulated during the hair growth cycle. Because it is still unknown how the hair follicle growth cycle is regulated, the identification of Twist1 as a key regulator of hair cycle in the present study should largely facilitate studies designed to understand the molecular mechanisms responsible for controlling the hair follicle cycle. Our findings may also have some implication in preventing hair loss through inhibiting Twist1 function.
The rapid growth of the early-stage embryo somewhat simulates the rapid growth of a tumor. Some genes expressed during early embryonic development and required for early embryonic development are also expressed in cancer cells. For example, Twist1 is expressed at specific locations of the embryo at the gastrulation stage to induce mesodermal formation, and Twist1-null mice die in utero. Twist1 is also expressed in many types of metastatic cancers to drive EMT, metastasis, stemness, and drug resistance (reviewed by Qin et al2). COUP transcription factor 2 (COUP-TFII, encoded by Nr2f2), a member of the nuclear receptor superfamily, is highly expressed during embryonic development to enhance angiogenesis and Nr2f2-null mice also die in utero.68 On the other hand, COUP-TFII is also highly expressed in prostate cancer cells to promote carcinogenesis.69 Interestingly, both the present study and a previous study70 demonstrate that inducible knockout of either Twist1 or Nr2f2 in young or adult mice causes neither death nor severe phenotype. These findings support the notion that some genes or proteins required for embryonic development and cancer cell growth and/or metastasis are not essential for maintaining a generally healthy condition in adulthood. Thus, such genes or proteins could be used as specific targets for cancer therapy without severe side effects.
Acknowledgments
We thank the Mouse Phenotyping Core Facility at Baylor College of Medicine for phenotypic analysis of the inducible Twist1 knockout mice.
Footnotes
Supported in part by NIH grants R01-CA112403 and R01-DK058242 (J.X.) and center core grant P30-CA125123 (Dan L. Duncan Cancer Center Genetically Engineered Mouse Core), by Susan G. Komen for the Cure promise grant PG12221410, by Cancer Prevention and Research Institute of Texas (CPRIT) grant RP120732-P5, and by a Chinese State Scholarship (Y.X.).
References
- 1.Thisse B., el Messal M., Perrin-Schmitt F. The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987;15:3439–3453. doi: 10.1093/nar/15.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin Q., Xu Y., He T., Qin C., Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 2012;22:90–106. doi: 10.1038/cr.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jan Y.N., Jan L.Y. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell. 1993;75:827–830. doi: 10.1016/0092-8674(93)90525-u. [DOI] [PubMed] [Google Scholar]
- 4.Wolf C., Thisse C., Stoetzel C., Thisse B., Gerlinger P., Perrin-Schmitt F. The M-twist gene of Mus is expressed in subsets of mesodermal cells and is closely related to the Xenopus X-twi and the Drosophila twist genes. Dev Biol. 1991;143:363–373. doi: 10.1016/0012-1606(91)90086-i. [DOI] [PubMed] [Google Scholar]
- 5.Füchtbauer E.M. Expression of M-twist during postimplantation development of the mouse. Dev Dyn. 1995;204:316–322. doi: 10.1002/aja.1002040309. [DOI] [PubMed] [Google Scholar]
- 6.Stoetzel C., Weber B., Bourgeois P., Bolcato-Bellemin A.L., Perrin-Schmitt F. Dorso-ventral and rostro-caudal sequential expression of M-twist in the postimplantation murine embryo. Mech Dev. 1995;51:251–263. doi: 10.1016/0925-4773(95)00369-x. [DOI] [PubMed] [Google Scholar]
- 7.Gitelman I. Twist protein in mouse embryogenesis. Dev Biol. 1997;189:205–214. doi: 10.1006/dbio.1997.8614. [DOI] [PubMed] [Google Scholar]
- 8.Figeac N., Daczewska M., Marcelle C., Jagla K. Muscle stem cells and model systems for their investigation. Dev Dyn. 2007;236:3332–3342. doi: 10.1002/dvdy.21345. [DOI] [PubMed] [Google Scholar]
- 9.Zhao P., Hoffman E.P. Embryonic myogenesis pathways in muscle regeneration. Dev Dyn. 2004;229:380–392. doi: 10.1002/dvdy.10457. [DOI] [PubMed] [Google Scholar]
- 10.Murray S.S., Glackin C.A., Winters K.A., Gazit D., Kahn A.J., Murray E.J. Expression of helix-loop-helix regulatory genes during differentiation of mouse osteoblastic cells. J Bone Miner Res. 1992;7:1131–1138. doi: 10.1002/jbmr.5650071004. [DOI] [PubMed] [Google Scholar]
- 11.Pan D., Fujimoto M., Lopes A., Wang Y.X. Twist-1 is a PPARdelta-inducible, negative-feedback regulator of PGC-1alpha in brown fat metabolism. Cell. 2009;137:73–86. doi: 10.1016/j.cell.2009.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettersson A.T., Laurencikiene J., Mejhert N., Näslund E., Bouloumié A., Dahlman I., Arner P., Rydén M. A possible inflammatory role of twist1 in human white adipocytes. Diabetes. 2010;59:564–571. doi: 10.2337/db09-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reardon W., Winter R.M. Saethre-Chotzen syndrome. J Med Genet. 1994;31:393–396. doi: 10.1136/jmg.31.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.el Ghouzzi V., Le Merrer M., Perrin-Schmitt F., Lajeunie E., Benit P., Renier D., Bourgeois P., Bolcato-Bellemin A.L., Munnich A., Bonaventure J. Mutations of the TWIST gene in the Saethre-Chotzen syndrome. Nat Genet. 1997;15:42–46. doi: 10.1038/ng0197-42. [DOI] [PubMed] [Google Scholar]
- 15.Krebs I., Weis I., Hudler M., Rommens J.M., Roth H., Scherer S.W., Tsui L.C., Füchtbauer E.M., Grzeschik K.H., Tsuji K., Kunz J. Translocation breakpoint maps 5 kb 3′ from TWIST in a patient affected with Saethre-Chotzen syndrome. Hum Mol Genet. 1997;6:1079–1086. doi: 10.1093/hmg/6.7.1079. [DOI] [PubMed] [Google Scholar]
- 16.Gripp K.W., Zackai E.H., Stolle C.A. Mutations in the human TWIST gene. Hum Mutat. 2000;15:150–155. doi: 10.1002/(SICI)1098-1004(200002)15:2<150::AID-HUMU3>3.0.CO;2-D. [Erratum appeared in Hum Mutat 2000, 15:479] [DOI] [PubMed] [Google Scholar]
- 17.Rose C.S., Patel P., Reardon W., Malcolm S., Winter R.M. The TWIST gene, although not disrupted in Saethre-Chotzen patients with apparently balanced translocations of 7p21, is mutated in familial and sporadic cases. Hum Mol Genet. 1997;6:1369–1373. doi: 10.1093/hmg/6.8.1369. [DOI] [PubMed] [Google Scholar]
- 18.Howard T.D., Paznekas W.A., Green E.D., Chiang L.C., Ma N., Ortiz de Luna R.I., Garcia Delgado C., Gonzalez-Ramos M., Kline A.D., Jabs E.W. Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre-Chotzen syndrome. Nat Genet. 1997;15:36–41. doi: 10.1038/ng0197-36. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z.F., Behringer R.R. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 20.Bourgeois P., Bolcato-Bellemin A.L., Danse J.M., Bloch-Zupan A., Yoshiba K., Stoetzel C., Perrin-Schmitt F. The variable expressivity and incomplete penetrance of the twist-null heterozygous mouse phenotype resemble those of human Saethre-Chotzen syndrome. Hum Mol Genet. 1998;7:945–957. doi: 10.1093/hmg/7.6.945. [DOI] [PubMed] [Google Scholar]
- 21.Bialek P., Kern B., Yang X., Schrock M., Sosic D., Hong N., Wu H., Yu K., Ornitz D.M., Olson E.N., Justice M.J., Karsenty G. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6:423–435. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- 22.Soo K., O’Rourke M.P., Khoo P.L., Steiner K.A., Wong N., Behringer R.R., Tam P.P. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Dev Biol. 2002;247:251–270. doi: 10.1006/dbio.2002.0699. [DOI] [PubMed] [Google Scholar]
- 23.Yang J., Mani S.A., Donaher J.L., Ramaswamy S., Itzykson R.A., Come C., Savagner P., Gitelman I., Richardson A., Weinberg R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Kwok W.K., Ling M.T., Lee T.W., Lau T.C., Zhou C., Zhang X., Chua C.W., Chan K.W., Chan F.L., Glackin C., Wong Y.C., Wang X. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65:5153–5162. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 25.Yuen H.F., Chua C.W., Chan Y.P., Wong Y.C., Wang X., Chan K.W. Significance of TWIST and E-cadherin expression in the metastatic progression of prostatic cancer. Histopathology. 2007;50:648–658. doi: 10.1111/j.1365-2559.2007.02665.x. [DOI] [PubMed] [Google Scholar]
- 26.Luo G.Q., Li J.H., Wen J.F., Zhou Y.H., Hu Y.B., Zhou J.H. Effect and mechanism of the Twist gene on invasion and metastasis of gastric carcinoma cells. World J Gastroenterol. 2008;14:2487–2493. doi: 10.3748/wjg.14.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng M.Y., Wang K., Song H.T., Yu H.W., Qin Y., Shi Q.T., Geng J.S. Metastasis-induction and apoptosis-protection by TWIST in gastric cancer cells. Clin Exp Metastasis. 2009;26:1013–1023. doi: 10.1007/s10585-009-9291-6. [DOI] [PubMed] [Google Scholar]
- 28.Lee T.K., Poon R.T., Yuen A.P., Ling M.T., Kwok W.K., Wang X.H., Wong Y.C., Guan X.Y., Man K., Chau K.L., Fan S.T. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–5376. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 29.Yang M.H., Chen C.L., Chau G.Y., Chiou S.H., Su C.W., Chou T.Y., Peng W.L., Wu J.C. Comprehensive analysis of the independent effect of Twist and Snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z., Xie D., Li X., Wong Y.C., Xin D., Guan X.Y., Chua C.W., Leung S.C., Na Y., Wang X. Significance of TWIST expression and its association with E-cadherin in bladder cancer. Hum Pathol. 2007;38:598–606. doi: 10.1016/j.humpath.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Wallerand H., Robert G., Pasticier G., Ravaud A., Ballanger P., Reiter R.E., Ferrière J.M. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol Oncol. 2010;28:473–479. doi: 10.1016/j.urolonc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki K., Natsugoe S., Ishigami S., Matsumoto M., Okumura H., Setoyama T., Uchikado Y., Kita Y., Tamotsu K., Sakamoto A., Owaki T., Aikou T. Significance of Twist expression and its association with E-cadherin in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2009;28:158. doi: 10.1186/1756-9966-28-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuen H.F., Chan Y.P., Wong M.L., Kwok W.K., Chan K.K., Lee P.Y., Srivastava G., Law S.Y., Wong Y.C., Wang X., Chan K.W. Upregulation of Twist in oesophageal squamous cell carcinoma is associated with neoplastic transformation and distant metastasis. J Clin Pathol. 2007;60:510–514. doi: 10.1136/jcp.2006.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoh K., Hamada S., Kimura K., Kanno A., Hirota M., Umino J., Fujibuchi W., Masamune A., Tanaka N., Miura K., Egawa S., Motoi F., Unno M., Vonderhaar B.K., Shimosegawa T. Up-regulation of MSX2 enhances the malignant phenotype and is associated with twist 1 expression in human pancreatic cancer cells. Am J Pathol. 2008;172:926–939. doi: 10.2353/ajpath.2008.070346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maestro R., Dei Tos A.P., Hamamori Y., Krasnokutsky S., Sartorelli V., Kedes L., Doglioni C., Beach D.H., Hannon G.J. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 1999;13:2207–2217. doi: 10.1101/gad.13.17.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valsesia-Wittmann S., Magdeleine M., Dupasquier S., Garin E., Jallas A.C., Combaret V., Krause A., Leissner P., Puisieux A. Oncogenic cooperation between H-Twist and N-Myc overrides failsafe programs in cancer cells. Cancer Cell. 2004;6:625–630. doi: 10.1016/j.ccr.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 37.Ansieau S., Bastid J., Doreau A., Morel A.P., Bouchet B.P., Thomas C., Fauvet F., Puisieux I., Doglioni C., Piccinin S., Maestro R., Voeltzel T., Selmi A., Valsesia-Wittmann S., Caron de Fromentel C., Puisieux A. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Li Q.Q., Xu J.D., Wang W.J., Cao X.X., Chen Q., Tang F., Chen Z.Q., Liu X.P., Xu Z.D. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009;15:2657–2665. doi: 10.1158/1078-0432.CCR-08-2372. [DOI] [PubMed] [Google Scholar]
- 39.Cheng G.Z., Chan J., Wang Q., Zhang W., Sun C.D., Wang L.H. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 40.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M., Campbell L.L., Polyak K., Brisken C., Yang J., Weinberg R.A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vesuna F., Lisok A., Kimble B., Raman V. Twist modulates breast cancer stem cells by transcriptional regulation of CD24 expression. Neoplasia. 2009;11:1318–1328. doi: 10.1593/neo.91084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Battula V.L., Evans K.W., Hollier B.G., Shi Y., Marini F.C., Ayyanan A., Wang R.Y., Brisken C., Guerra R., Andreeff M., Mani S.A. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–1445. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puisieux A., Valsesia-Wittmann S., Ansieau S. A twist for survival and cancer progression. Br J Cancer. 2006;94:13–17. doi: 10.1038/sj.bjc.6602876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Firulli A.B., Conway S.J. Phosphoregulation of Twist1 provides a mechanism of cell fate control. Curr Med Chem. 2008;15:2641–2647. doi: 10.2174/092986708785908987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin L., Liu Z., Chen H., Xu J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res. 2009;69:3819–3827. doi: 10.1158/0008-5472.CAN-08-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu J., Qin L., He T., Qin J., Hong J., Wong J., Liao L., Xu J. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Res. 2011;21:275–289. doi: 10.1038/cr.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vernon A.E., LaBonne C. Tumor metastasis: a new twist on epithelial-mesenchymal transitions. Curr Biol. 2004;14:R719–R721. doi: 10.1016/j.cub.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 48.Karreth F., Tuveson D.A. Twist induces an epithelial-mesenchymal transition to facilitate tumor metastasis. Cancer Biol Ther. 2004;3:1058–1059. doi: 10.4161/cbt.3.11.1302. [DOI] [PubMed] [Google Scholar]
- 49.Yang M.H., Wu M.Z., Chiou S.H., Chen P.M., Chang S.Y., Liu C.J., Teng S.C., Wu K.J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 50.Fu J., Zhang L., He T., Xiao X., Liu X., Wang L., Yang L., Yang M., Zhang T., Chen R., Xu J. TWIST represses estrogen receptor-alpha expression by recruiting the NuRD protein complex in breast cancer cells. Int J Biol Sci. 2012;8:522–532. doi: 10.7150/ijbs.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang M.H., Hsu D.S., Wang H.W., Wang H.J., Lan H.Y., Yang W.H., Huang C.H., Kao S.Y., Tzeng C.H., Tai S.K., Chang S.Y., Lee O.K., Wu K.J. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 52.Kalra J., Sutherland B.W., Stratford A.L., Dragowska W., Gelmon K.A., Dedhar S., Dunn S.E., Bally M.B. Suppression of Her2/neu expression through ILK inhibition is regulated by a pathway involving TWIST and YB-1. Oncogene. 2010;29:6343–6356. doi: 10.1038/onc.2010.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckert M.A., Lwin T.M., Chang A.T., Kim J., Danis E., Ohno-Machado L., Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai J.H., Donaher J.L., Murphy D.A., Chau S., Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuang S.Q., Liao L., Zhang H., Lee A.V., O’Malley B.W., Xu J. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 2004;64:1875–1885. doi: 10.1158/0008-5472.can-03-3745. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y.T., Akinwunmi P.O., Deng J.M., Tam O.H., Behringer R.R. Generation of a Twist1 conditional null allele in the mouse. Genesis. 2007;45:588–592. doi: 10.1002/dvg.20332. [DOI] [PubMed] [Google Scholar]
- 57.Guo K., McMinn J.E., Ludwig T., Yu Y.H., Yang G., Chen L., Loh D., Li C., Chua S., Jr., Zhang Y. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148:3987–3997. doi: 10.1210/en.2007-0261. [DOI] [PubMed] [Google Scholar]
- 58.Liu Z., Zhou S., Liao L., Chen X., Meistrich M., Xu J. Jmjd1a demethylase-regulated histone modification is essential for cAMP-response element modulator-regulated gene expression and spermatogenesis. J Biol Chem. 2010;285:2758–2770. doi: 10.1074/jbc.M109.066845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J., Liao L., Ning G., Yoshida-Komiya H., Deng C., O’Malley B.W. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA. 2000;97:6379–6384. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itzkovitz S., Blat I.C., Jacks T., Clevers H., van Oudenaarden A. Optimality in the development of intestinal crypts. Cell. 2012;148:608–619. doi: 10.1016/j.cell.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross M.H., Kaye G.I., Pawlina W. ed 4. Lippincott Williams & Wilkins; Hagerstown, MD: 2003. Histology: a text and atlas: with correlated cell and molecular biology. [Google Scholar]
- 62.Lin K.K., Chudova D., Hatfield G.W., Smyth P., Andersen B. Identification of hair cycle-associated genes from time-course gene expression profile data by using replicate variance. Proc Natl Acad Sci USA. 2004;101:15955–15960. doi: 10.1073/pnas.0407114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuzaki T., Yoshizato K. Role of hair papilla cells on induction and regeneration processes of hair follicles. Wound Repair Regen. 1998;6:524–530. doi: 10.1046/j.1524-475x.1998.60605.x. [DOI] [PubMed] [Google Scholar]
- 64.Yang J., Mani S.A., Weinberg R.A. Exploring a new twist on tumor metastasis. Cancer Res. 2006;66:4549–4552. doi: 10.1158/0008-5472.CAN-05-3850. [DOI] [PubMed] [Google Scholar]
- 65.Fuchs E., Merrill B.J., Jamora C., DasGupta R. At the roots of a never-ending cycle. Dev Cell. 2001;1:13–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 66.Stenn K.S., Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 67.Rendl M., Lewis L., Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pereira F.A., Qiu Y., Zhou G., Tsai M.J., Tsai S.Y. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qin J., Wu S.P., Creighton C.J., Dai F., Xie X., Cheng C.M., Frolov A., Ayala G., Lin X., Feng X.H., Ittmann M.M., Tsai S.J., Tsai M.J., Tsai S.Y. COUP-TFII inhibits TGF-beta-induced growth barrier to promote prostate tumorigenesis. Nature. 2013;493:236–240. doi: 10.1038/nature11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin J., Tsai M.J., Tsai S.Y. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS One. 2008;3:e3285. doi: 10.1371/journal.pone.0003285. [DOI] [PMC free article] [PubMed] [Google Scholar]