Abstract
Dietary glycemic load (GL), glycemic index (GI), and carbohydrate could be associated with breast cancer risk by influencing long-term blood glucose and insulin concentrations. We examined associations between GL, GI, and carbohydrate and incident breast cancer in 148,767 Women’s Heath Initiative (WHI) participants. Dietary variables were estimated from food frequency questionnaires administered at baseline. Self-reported breast cancers during follow-up were confirmed by medical records review. Cox proportional hazards regression modeled time to breast cancer within quintiles of GL, GI, and carbohydrate. There were 6,115 total breast cancers after a median follow-up of 8.0 yr. We observed no associations between GL, GI, or carbohydrate and total incident breast cancer, with hazard ratios and 95% confidence intervals for the highest vs. lowest quintiles of 1.08, 0.92–1.29 (P for trend = 0.27); 1.01, 0.91–1.12 (P = 0.74); and 0.95, 0.80–1.14 (P = 0.98), respectively. There was a trend toward significance for the positive association between GL and in situ cancers (1.40, 0.94–2.13; P = 0.07). Although there was no evidence of associations between GL, GI, or carbohydrate and total breast cancer risk in WHI participants, the suggestion of an association between GL and risk of in situ cancers requires further investigation.
INTRODUCTION
Although most studies of diet and breast cancer have focused on dietary fat, high carbohydrate intake also may play a role in breast cancer etiology, likely through an increase in blood glucose and insulin concentrations. The quality and quantity of carbohydrates elicit a wide spectrum of postprandial blood glucose and insulin responses, commonly estimated by glycemic index (GI) and glycemic load (GL). Glycemic index (GI) is a ranking of foods based on their postprandial blood glucose responses and is a measure of carbohydrate quality (1). Glycemic load (GL) is a measure that incorporates both the quantity and quality of dietary carbohydrates and is considered by many investigators to be the biologically relevant exposure in epidemiologic studies of carbohydrate intake and disease risk (2,3).
High dietary GI and GL have been associated with an increased risk of breast cancer in some case-control studies (4,5), but not in others (6). The results of cohort studies of GI/GL and breast cancer also have been mixed. High dietary GI was associated with increased risk of breast cancer in postmenopausal women in the Canadian National Breast Screening Study (7), and a positive association between GI and estrogen receptor-negative breast cancer was seen in postmenopausal women in the Diet, Cancer, and Health study (8). GI and GL were positively associated with breast cancer risk in a cohort of Italian women, with the effect particularly evident in premenopausal women and those with BMI <25 kg/m2 (9). However, there were no associations between GI or GL and breast cancer risk in several other cohort studies (10–14).
To further explore this issue, we examined associations between dietary GL, GI, and available carbohydrate and breast cancer risk in women participating in the Women’s Health Initiative (WHI) Clinical Trials (CT) and Observational Study (OS).
METHODS
Study Population
The design and baseline description of the WHI studies and recruitment methods have been published (15–17). Briefly, 68,132 and 93,676 generally healthy postmenopausal women aged 50–79 were randomized into the CT or enrolled into the OS, respectively, at 40 clinical centers across the United States between 1993 and 1998. Inclusion criteria included planning to reside in the area for at least 3 yr, and exclusion criteria included having a medical condition with a predicted survival of less than 3 yr or having conditions such as alcoholism, drug dependency, or dementia. Additional eligibility criteria were applied to the specific clinical trials. The WHI protocol and consent forms were approved by the institutional review board for each participating institution and the Clinical Coordinating Center (CCC; Fred Hutchinson Cancer Research Center, Seattle, WA). All participants provided informed consent.
Data Collection
At baseline, demographic, health history, lifestyle, and diet information was collected by self-report. Height and weight were measured at the clinical centers by certified staff using standardized procedures (18), from which body mass index (BMI) was calculated (kg/m2). Medications were documented during face-to-face interviews and recorded in a pharmacy database (Master Drug Database: Medi-Span, Indianapolis, IN).
Participants in the OS were followed through annual mailings and one in-person clinic visit at Year 3. Participants in the CT were contacted at 6-mo intervals for outcome determination and made yearly clinical visits. Mailings included detailed diet, lifestyle, and health questionnaires. Standardized written protocols, centralized training of local clinic staff, local quality assurance activities, and periodic quality assurance visits by the CCC were used to maintain uniform data collection procedures at all study sites.
Dietary Intake
Usual dietary intake was assessed using a food frequency questionnaire (FFQ) designed for the WHI (19). The FFQ was administered during screening (considered the baseline measure) to all WHI participants. Additional dietary assessments were made during the study, but only baseline FFQ dietary data were used in the present analyses. The methods for assigning GI and GL values used in the WHI FFQ have been reported elsewhere (20). Briefly, GI values were taken from published reports or imputed from GI values of foods with similar composition and prepared in a like manner. GI values with glucose as the standard were used. A composite GI was computed by a weighted average for FFQ line items with multiple foods. The GL was computed by multiplying the GI by grams of carbohydrate by the number and frequency of servings consumed and portion size. Because the intended use of GL is as an indicator of the overall glycemic effect of food, and glycemic effect is inherently a function of carbohydrate which is actually digested and absorbed, we used available carbohydrate—defined as the USDA-based value for grams of carbohydrate per serving minus the USDA value for grams of dietary fiber per serving—in our calculations of GL.
Breast Cancer Ascertainment
Initial self-reports of breast cancer were confirmed by physician adjudicators at the local clinical centers by medical records review. Central adjudication and coding followed at the Clinical Coordinating Center using standard Surveillance, Epidemiology, and End Results (SEER) guidelines. Estrogen receptor (ER) and progesterone receptor (PR) status was based on review of reports following locally determined analytic procedures.
Statistical Analysis
The initial dataset contained 161,808 women—68,132 CT participants and 93,676 OS participants. Participants who had missing baseline information regarding breast cancer status (n = 735) were removed from the dataset. Participants with previously reported breast cancer (n = 5,381), a history of removal of one or both breasts (n = 269), or missing previous breast cancer history (n = 1,569) also were removed. Women with implausible energy intakes (defined as mean intakes <600 kcal/d or >5,000 kcal/d) were excluded (n = 3,906 and 407, respectively), as were those with extreme BMI values (defined as <15 kg/m2 or >50 kg/m2) (n = 774). The final analysis dataset contained 148,767 participants. Of these, 6,115 (4.1%) developed breast cancer (invasive or in situ) after a median follow-up of 8.0 yr. The median time from entry to diagnosis of breast cancer for the 6,115 cases was 4.1 yr. ER and PR status was available for 4,377 cases (71.6%).
Analyses began with descriptive statistics (mean, SD) calculated for continuous covariates (age, alcohol intake, physical activity, BMI, parity, age at menopause, and energy), primary explanatory variables (GL, GI, and available carbohydrate), and outcome measure (time until breast cancer). Although the primary interest was in GL, GI, and carbohydrate as risk factors for breast cancer, we also investigated carbohydrate influencers of GL and GI: total dietary fiber, soluble fiber, insoluble fiber, fructose, added sugars, and total sugars. For categorical covariates, proportions of individuals reporting non-Hispanic White ethnicity, some post-college education, current smoking, age at menarche ≤12 yr, age at first birth ≥30 yr, ever use of oral contraceptive use, ever use of hormone therapy, and breast cancer in first-degree relatives were calculated.
The primary statistical model used to evaluate the association of the explanatory variables with risk of breast cancer was Cox proportional hazards regression, modeling time to event. Graphical examination of the log cumulative hazards plot determined that the proportionality assumption was not violated. To test for linear trends in the risk of breast cancer, quintile ranks of GL, GI, available carbohydrate, and the other explanatory variables were included as continuous predictors in separate hazard regression models. The primary associations assessed in this article were tested in two manners. First, the association was examined in a crude analysis not controlling for covariates. The second approach utilized a standard variable added last test in regression. Specifically, the linear effect of the quintiles of GL was tested for significant association with the outcome after adjustment for the following baseline factors: age, ethnicity, education, individual CT randomization (Hormone Therapy, Dietary Modification, Calcium, and Vitamin D), smoking, alcohol, physical activity, BMI, age at menarche, age at first birth, parity, age at menopause, oral contraceptive use, hormone therapy use, breast cancer in first-degree relative, mammogram within 2 yr prior to enrollment, and energy intake. The decision to include randomization in the individual trials as covariates was based upon a crude analysis that indicated time to event varied by these covariates. Similar models were developed for GI, available carbohydrate, and the other explanatory variables. All analyses were performed using SAS statistical software, v. 9.1 (SAS Institute, Inc., Cary, NC). All P values reported in the text are tests for linear trend.
RESULTS
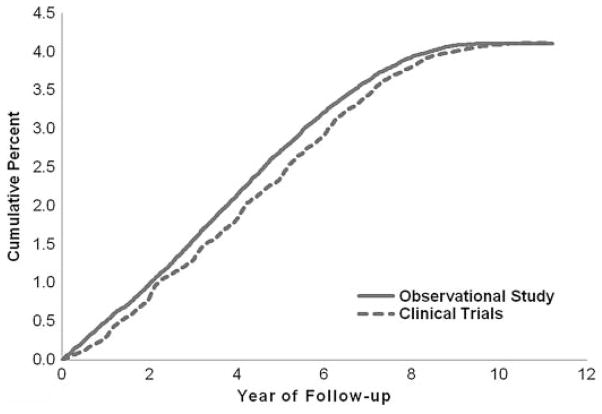
Compared to participants without incident breast cancer, participants who developed incident breast cancer were more likely at baseline to be non-Hispanic White, have some postcollege education, be current smokers, have experienced menarche at age ≤12 yr, given birth for the first time at age ≥30 yr, have reported previous use of postmenopausal hormones, and have reported breast cancer in a first-degree relative (Table 1). Participants with breast cancer also consumed more alcohol and had a slightly higher mean age at menopause than participants without breast cancer. Baseline dietary intakes between the 2 groups, including GL, GI, and carbohydrate, were similar. There was no statistically significant difference in the cumulative percent of CT and OS participants with incident breast cancer by year of follow-up (Fig. 1).
TABLE 1.
Baseline characteristics of WHI CT and OS participants with and without incident breast cancer
| Participant Characteristic | Breast Cancer (N = 6,115) | No Breast Cancer (N = 142,652) |
|---|---|---|
| Mean (±SD) age (yr) | 63.6 (7.1) | 63.1 (7.2) |
| Non-Hispanic white ethnicity (%) | 5,375 (88) | 118,972 (83) |
| Some post-college education (%) | 2,305 (38) | 40,656 (29) |
| Current smoking (%) | 3,168 (52) | 70,185 (49) |
| Mean (±SD) alcohol intake (servings/wk) | 2.8 (5.3) | 2.4 (4.9) |
| Mean (±SD) physical activity (MET) | 12.2 (13.1) | 12.5 (13.7) |
| Mean (±SD) BMI (kg/m2) | 28.0 (5.5) | 27.8 (5.6) |
| Age at menarche ≤12 yr (%) | 3,027 (50) | 68,616 (48) |
| Age at first birth ≥30 yr (%) | 685 (11) | 12,696 (9) |
| Mean (±SD) parity | 2.5 (1.5) | 2.7 (1.5) |
| Mean (±SD) age at menopause (yr) | 48.3 (6.6) | 47.3 (6.6) |
| Ever use of oral contraceptive use (%) | 2,495 (41) | 59,771 (42) |
| Ever use of postmenopausal hormones (%) | 4,403 (72) | 96,005 (67) |
| Breast cancer in first-degree relative (%) | 2,813 (46) | 53,922 (38) |
| Mean (±SD) energy intake (kcal/d) | 1,659 (622) | 1,643 (644) |
| Mean (±SD) GL (g/d) | 98.4 (39.4) | 98.3 (40.8) |
| Mean (±SD) GI | 52.4 (3.6) | 52.4 (3.7) |
| Mean (±SD) carbohydrate intake (g/d) | 203.6 (77.5) | 203.0 (79.6) |
| Mean (±SD) total dietary fiber intake (g/d) | 16.1 (6.6) | 16.1 (6.8) |
| Mean (±SD) soluble fiber intake (g/d) | 4.3 (1.8) | 4.3 (1.8) |
| Mean (±SD) insoluble fiber intake (g/d) | 11.7 (5.0) | 11.7 (5.1) |
| Mean (±SD) fructose intake (g/d) | 20.9 (12.3) | 21.1 (13.2) |
| Mean (±SD) added sugars intake (g/d) | 47.8 (31.1) | 48.4 (32.9) |
| Mean (±SD) total sugars intake (g/d) | 98.6 (44.7) | 98.3 (45.7) |
WHI = Women’s Health Initiative; CT = Clinical Trials; OS = Observational Study; MET = metabolic equivalent task; BMI = body mass index.
FIG. 1.
Cumulative percent of WHI Clinical Trials and Observational Study participants with incident breast cancer by year of follow-up.
We observed no associations between dietary GL, GI, or available carbohydrate and total incident breast cancer, with hazard ratios (HR) and 95% confidence interval (CI) for the highest vs. lowest quintiles of 1.08, 0.92–1.29 (P for trend = 0.27); 1.01, 0.91–1.12 (P = 0.74); and 0.95, 0.80–1.14 (P = 0.98), respectively (Table 2). While there were no statistically significant associations of these variables with risk of total breast cancer, when in situ and invasive breast cancers were analyzed separately, there was a trend toward significance for the association between GL and risk of in situ breast cancer (HR, 95% CI for highest vs. lowest quintile = 1.40, 0.94–2.13; P for trend = 0.07).
TABLE 2.
Cox proportional hazard modeling of the association between glycemic load, glycemic index, available carbohydrate, and total breast cancer risk in all WHI CT and OS participants and by degree of invasiveness
| Quintile | Median Intake | Total Breast Cancer
|
In Situ Breast Cancer
|
Invasive Breast Cancer
|
||||
|---|---|---|---|---|---|---|---|---|
| n | Univariate HR (95% CI) | Multivariablea HR (95% CI) | n | Multivariablea HR (95% CI) | n | Multivariablea HR (95% CI) | ||
| Glycemic load (g/d) | ||||||||
| 1 | 52.9 | 1,142 | 1.00 (ref) | 1.00 (ref) | 197 | 1.00 (ref) | 952 | 1.00 (ref) |
| 2 | 74.4 | 1,269 | 1.10 (1.02–1.19) | 1.05 (0.94–1.16) | 242 | 1.23 (0.95–1.59) | 1,035 | 1.01 (0.90–1.13) |
| 3 | 92.2 | 1,173 | 1.01 (0.93–1.10) | 0.97 (0.87–1.09) | 250 | 1.37 (1.04–1.80) | 934 | 0.90 (0.79–1.02) |
| 4 | 112.8 | 1,296 | 1.12 (1.03–1.21) | 1.10 (0.97–1.25) | 233 | 1.36 (1.00–1.86) | 1,068 | 1.05 (0.91–1.21) |
| 5 | 150.4 | 1,218 | 1.05 (0.97–1.13) | 1.08 (0.92–1.29) | 240 | 1.40 (0.94–2.13) | 984 | 1.02 (0.84–1.23) |
| P for trend | 0.27 | 0.27 | 0.07 | 0.77 | ||||
| Glycemic index | ||||||||
| 1 | 47.8 | 1,212 | 1.00 (ref) | 1.00 (ref) | 205 | 1.00 (ref) | 1,012 | 1.00 (ref) |
| 2 | 50.6 | 1,264 | 1.04 (0.96–1.12) | 1.02 (0.93–1.13) | 250 | 1.12 (0.89–1.41) | 1,029 | 1.02 (0.91–1.13) |
| 3 | 52.5 | 1,242 | 1.02 (0.94–1.10) | 1.01 (0.92–1.12) | 232 | 1.00 (0.79–1.28) | 1,016 | 1.02 (0.91–1.13) |
| 4 | 54.3 | 1,220 | 1.00 (0.92–1.08) | 0.97 (0.88–1.07) | 249 | 1.17 (0.93–1.48) | 978 | 0.93 (0.83–1.04) |
| 5 | 57.0 | 1,160 | 0.95 (0.88–1.03) | 1.01 (0.91–1.12) | 226 | 1.15 (0.90–1.47) | 938 | 0.98 (0.88–1.10) |
| P for trend | 0.12 | 0.74 | 0.25 | 0.33 | ||||
| Available carbohydrate (g/d) | ||||||||
| 1 | 112.3 | 1,166 | 1.00 (ref) | 1.00 (ref) | 203 | 1.00 (ref) | 969 | 1.00 (ref) |
| 2 | 156.1 | 1,227 | 1.04 (0.96–1.13) | 0.94 (0.84–1.05) | 234 | 1.10 (0.85–1.42) | 1,002 | 0.91 (0.81–1.02) |
| 3 | 191.9 | 1,212 | 1.02 (0.95–1.11) | 0.94 (0.84–1.05) | 231 | 1.13 (0.85–1.49) | 989 | 0.90 (0.79–1.02) |
| 4 | 232.9 | 1,274 | 1.08 (0.99–1.16) | 1.00 (0.88–1.14) | 258 | 1.38 (1.01–1.87) | 1,025 | 0.93 (0.81–1.07) |
| 5 | 305.7 | 1,219 | 1.02 (0.95–1.11) | 0.95 (0.80–1.14) | 236 | 1.24 (0.81–1.88) | 988 | 0.89 (0.74–1.08) |
| P for trend | 0.38 | 0.98 | 0.09 | 0.36 | ||||
WHI = Women’s Health Initiative; CT = Clinical Trials; OS = Observational Study; HR = hazard ratio; CI = confidence interval.
Results adjusted for age, ethnicity, education, Hormone Therapy trial randomization, Dietary Modification trial randomization, Calcium and Vitamin D trial randomization, smoking, alcohol, physical activity, body mass index, age at menarche, age at first birth, parity, age at menopause, oral contraceptive use, postmenopausal hormone use, breast cancer in first-degree relative, mammogram within 2 yr prior to enrollment, and energy intake.
There was no apparent effect modification of the association between total breast cancer risk and dietary GL, GI, and available carbohydrate by BMI (<25 kg/m2 and ≥25 kg/m2) (data not shown). When analyses were conducted separately in CT and OS participants, or CT participants receiving active treatment were excluded from the analysis, the results were similar (data not shown). In analyses conducted separately according to ER and PR status, while there were trends toward an inverse association between GL and the risk of ER+/PR+ cancers, an inverse association between GL and the risk of ER+/PR− cancers, and a positive association between GL and the risk of ER−/PR− cancers, none of these trends reached statistical significance (Table 3). Further analyses in all cases revealed no associations between total breast cancer risk and other carbohydrate variables (Table 4).
TABLE 3.
Cox proportional hazard modeling of the association between glycemic load, glycemic index, available carbohydrate, and breast cancer risk in all WHI CT and OS participants by hormone receptor statusa
| Quintile | ER+/PR+
|
ER+/PR−
|
ER−/PR−
|
|||
|---|---|---|---|---|---|---|
| n | Multivariableb HR (95% CI) | n | Multivariableb HR HR (95% CI) | n | Multivariableb HR (95% CI) | |
| Glycemic load | ||||||
| 1 | 543 | 1.00 (ref) | 133 | 1.00 (ref) | 125 | 1.00 (ref) |
| 2 | 618 | 0.92 (0.79–1.08) | 155 | 0.88 (0.62–1.23) | 110 | 1.59 (1.06–2.23) |
| 3 | 601 | 0.88 (0.74–1.04) | 116 | 0.97 (0.64–1.44) | 105 | 1.53 (1.01–2.31) |
| 4 | 671 | 0.85 (0.71–1.01) | 139 | 0.75 (0.48–1.16) | 130 | 1.73 (1.10–2.72) |
| 5 | 583 | 0.81 (0.63–1.04) | 121 | 0.60 (0.33–1.09) | 146 | 1.68 (0.93–3.02) |
| P for trend | 0.07 | 0.14 | 0.07 | |||
| Glycemic index | ||||||
| 1 | 607 | 1.00 (ref) | 136 | 1.00 (ref) | 131 | 1.00 (ref) |
| 2 | 624 | 1.08 (0.94–1.24) | 154 | 0.92 (0.67–1.25) | 105 | 0.90 (0.63–1.29) |
| 3 | 644 | 1.03 (0.89–1.18) | 122 | 0.96 (0.69–1.34) | 121 | 1.16 (0.81–1.62) |
| 4 | 592 | 1.13 (0.98–1.31) | 135 | 1.15 (0.80–1.67) | 125 | 1.15 (0.83–1.68) |
| 5 | 549 | 1.05 (0.90–1.22) | 117 | 1.01 (0.71–1.43) | 134 | 1.07 (0.74–1.52) |
| P for trend | 0.41 | 0.61 | 0.32 | |||
| Available carbohydrate | ||||||
| 1 | 546 | 1.00 (ref) | 140 | 1.00 (ref) | 130 | 1.00 (ref) |
| 2 | 614 | 1.01 (0.87–1.18) | 148 | 1.02 (0.72–1.44) | 97 | 1.30 (0.88–1.92) |
| 3 | 624 | 0.97 (0.82–1.15) | 114 | 0.93 (0.62–1.41) | 124 | 1.23 (0.83–1.83) |
| 4 | 645 | 0.85 (0.71–1.03) | 137 | 1.03 (0.67–1.60) | 122 | 1.35 (0.86–2.12) |
| 5 | 587 | 0.99 (0.77–1.27) | 125 | 0.75 (0.42–1.34) | 143 | 1.33 (0.75–2.38) |
| P for trend | 0.20 | 0.56 | 0.29 | |||
WHI = Women’s Health Initiative; CT = Clinical Trials; OS = Observational Study; ER = estrogen receptor; PR = progesterone receptor; HR = hazard ratio; CI = confidence interval.
Analysis included cases with hormone receptor status. ER−/PR+ not included because of the small number of cases.
Results adjusted for age, ethnicity, education, Hormone Therapy trial randomization, Dietary Modification trial randomization, Calcium and Vitamin D trial randomization, smoking, alcohol, physical activity, body mass index, age at menarche, age at first birth, parity, age at menopause, oral contraceptive use, postmenopausal hormone use, breast cancer in first-degree relative, mammogram within 2 yr prior to enrollment, and energy intake.
TABLE 4.
Cox proportional hazard modeling of the association between other carbohydrate variables and total breast cancer risk in all WHI CT and OS participants
| Quintile | Median Intake (g/d) | n | Univariate HR (95% CI) | Multivariablea HR (95% CI) |
|---|---|---|---|---|
| Total fiber | ||||
| 1 | 8.2 | 1,152 | 1.00 (ref) | 1.00 (ref) |
| 2 | 11.9 | 1,237 | 1.06 (0.98–1.15) | 1.04 (0.93–1.15) |
| 3 | 15.1 | 1,237 | 1.06 (0.98–1.15) | 0.95 (0.85–1.06) |
| 4 | 18.8 | 1,239 | 1.06 (0.98–1.15) | 0.99 (0.88–1.10) |
| 5 | 25.1 | 1,233 | 1.05 (0.97–1.14) | 0.93 (0.82–1.07) |
| P for trend | 0.29 | 0.22 | ||
| Soluble fiber | ||||
| 1 | 2.2 | 1,176 | 1.00 (ref) | 1.00 (ref) |
| 2 | 3.2 | 1,225 | 1.03 (0.95–1.12) | 0.97 (0.88–1.08) |
| 3 | 4.1 | 1,259 | 1.06 (0.98–1.15) | 0.94 (0.84–1.05) |
| 4 | 5.1 | 1,216 | 1.02 (0.94–1.11) | 0.94 (0.84–1.06) |
| 5 | 6.7 | 1,222 | 1.03 (0.95–1.11) | 0.90 (0.79–1.03) |
| P for trend | 0.66 | 0.13 | ||
| Insoluble fiber | ||||
| 1 | 5.8 | 1,142 | 1.00 (ref) | 1.00 (ref) |
| 2 | 8.5 | 1,237 | 1.07 (0.99–1.16) | 1.02 (0.93–1.14) |
| 3 | 10.9 | 1,261 | 1.09 (1.00–1.18) | 0.99 (0.89–1.10) |
| 4 | 13.7 | 1,235 | 1.06 (0.98–1.15) | 1.02 (0.91–1.14) |
| 5 | 18.4 | 1,223 | 1.05 (0.97–1.14) | 0.93 (0.82–1.06) |
| P for trend | 0.32 | 0.32 | ||
| Fructose | ||||
| 1 | 8.5 | 1,135 | 1.00 (ref) | 1.00 (ref) |
| 2 | 13.9 | 1,285 | 1.12 (1.03–1.21) | 1.11 (1.00–1.23) |
| 3 | 18.7 | 1,241 | 1.08 (1.00–1.18) | 1.08 (0.97–1.20) |
| 4 | 24.4 | 1,246 | 1.09 (1.00–1.19) | 1.10 (0.99–1.23) |
| 5 | 35.0 | 1,191 | 1.05 (0.97–1.14) | 1.07 (0.95–1.21) |
| P for trend | 0.47 | 0.36 | ||
| Added sugars | ||||
| 1 | 18.1 | 1,198 | 1.00 (ref) | 1.00 (ref) |
| 2 | 29.7 | 1,222 | 1.01 (0.93–1.10) | 0.97 (0.88–1.08) |
| 3 | 41.0 | 1,259 | 1.04 (0.96–1.12) | 0.99 (0.89–1.10) |
| 4 | 55.5 | 1,209 | 0.99 (0.92–1.08) | 1.00 (0.90–1.12) |
| 5 | 85.2 | 1,210 | 0.99 (0.92–1.08) | 1.01 (0.89–1.16) |
| P for trend | 0.71 | 0.71 | ||
| Total sugars | ||||
| 1 | 48.5 | 1,188 | 1.00 (ref) | 1.00 (ref) |
| 2 | 71.5 | 1,197 | 1.00 (0.92–1.08) | 1.01 (0.91–1.12) |
| 3 | 91.2 | 1,256 | 1.05 (0.97–1.14) | 1.06 (0.95–1.18) |
| 4 | 114.2 | 1,213 | 1.01 (0.93–1.09) | 1.00 (0.89–1.12) |
| 5 | 155.4 | 1,244 | 1.04 (0.96–1.12) | 1.06 (0.92–1.21) |
| P for trend | 0.37 | 0.60 | ||
WHI = Women’s Health Initiative; CT = Clinical Trials; OS = Observational Study; HR = hazard ratio; CI = confidence interval.
Results adjusted for age, ethnicity, education, Hormone Therapy trial randomization, Dietary Modification trial randomization, Calcium and Vitamin D trial randomization, smoking, alcohol, physical activity, body mass index, age at menarche, age at first birth, parity, age at menopause, oral contraceptive use, postmenopausal hormone use, breast cancer in first-degree relative, mammogram within 2 yr prior to enrollment, and energy intake.
DISCUSSION
In this study of WHI participants, we found no evidence of associations between dietary GL, GI, or available carbohydrate and total breast cancer risk. There was a trend toward statistical significance for the positive associations between GL and risk of in situ cancers.
Two case-control studies have demonstrated positive associations of GI (4) and GL (4,5) with overall breast cancer risk. In both studies, these associations were limited to post-menopausal women. A third case-control study found no associations between GI or GL and breast cancer risk in pre-or postmenopausal women (6). Previous cohort studies have produced mixed results on the association between GI and GL and breast cancer risk. While one cohort study observed positive associations of GI and GL with overall breast cancer risk in premenopausal and postmenopausal women combined (9), most other cohort studies have failed to demonstrate such associations in all women combined (7,10,11). However, two of these studies observed positive associations between GI and breast cancer risk in postmenopausal women (7,11). Other cohort studies conducted exclusively in postmenopausal women showed no associations of GI or GL with risk (8,13,14), as did a study conducted only in pre-menopausal women (12).
A recent meta-analysis of observational studies showed a modest positive association between GI and breast cancer risk (rate ratio = 1.09, P = 0.015) (21). In another meta-analysis of GI, GL, and cancer risk which included 39 case-control and cohort studies, only GL was associated with increased risk of breast cancer (rate ratio = 1.14, 95% CI = 1.02–1.28), although this association disappeared when publication bias was taken into account (22). However, in a recent analysis of GI, GL, and risk of cancer in the large prospective National Institutes of Health–American Association of Retired Persons Diet and Health Study, neither GI nor GL were associated with breast cancer risk (23).
In contrast to our results, several studies have reported an interaction of BMI with GI/GL and breast cancer risk. Navarro Silvera et al. (7) and Holmes et al. (11) observed positive associations of GI with breast cancer risk in postmenopausal women with BMI <25 kg/m2. GL was inversely associated with breast cancer risk in postmenopausal women with BMI ≥25 kg/m2 (6) and in premenopausal women with BMI ≥25 kg/m2 (11). In an analysis taking tumor grade into account, only Grade I cancers showed a positive association with GL in one study of postmenopausal women (14).
There are plausible mechanisms through which chronically high-GI/GL diets may be expected to influence breast cancer risk. High-GI/GL diets, compared with isoenergetic and nutrient-controlled low-GI diets, resulted in higher 12-h incremental blood glucose accumulations and evidence of higher insulin secretion (24). Hyperinsulinemia may act in several ways to increase breast cancer risk, including stimulation of estrogen, which binds to estrogen receptors, increasing cell proliferation (25) and stimulation of insulin-like growth factor-I (IGF-I), which has proliferative, cell differentiation, and antiapoptotic actions (26,27). Manipulating dietary GI has been shown to influence the IGF axis by altering concentrations of IGF-binding proteins (28). Therefore, high-GI carbohydrates may contribute to a metabolic environment that is conducive to breast tumor growth. One also may expect a reduced risk of breast cancer with higher intakes of dietary fiber, particularly soluble fiber with its effect of reducing postprandial glucose. However, we did not see an effect of total dietary fiber or soluble fiber on the risk of breast cancer, which is similar to findings of earlier epidemiologic studies with premenopausal women (12) and postmenopausal women (29).
While suggestive, associations between GL and breast cancer risk stratified by hormone receptor status did not reach statistical significance in this study. Some previous cohort studies have shown associations between GL and breast cancer risk which varied according to hormone receptor status (30,31), while others did not (8,14). The biologic mechanisms for possible differential associations between GL and breast cancer defined by hormone receptor status are unclear. Because it has been hypothesized that estrogen and IGF-1 may be able to stimulate the ER on breast cancer cells in a synergetic way (32) and high-GL diets may result in stimulation of estrogen and IGF-1, an association between GL and breast cancer risk may be expected to be stronger or limited to ER+ tumors (31).
The strengths of this study include the large and race/ethnicity-diverse sample, the comprehensive data collection, the central adjudication of breast cancer, and the use of a comprehensive GI/GL database, which was added to the existing WHI FFQ nutrient database. A limitation was that only relatively healthy, postmenopausal women were studied. Other limitations relate to the dietary assessment method utilized. First, most FFQs in common use were not designed to assess GI and GL and thus may be unable to capture the true range of GI and GL (22). Though the WHI FFQ was not designed initially to assess GI and GL, the addition of GI/GL values to the FFQ nutrient database was done in a systematic and well-documented manner (20), which we believe minimizes this limitation. Second, for ease of application, analysis, and economics in epidemiologic studies, FFQs are composed of fixed food lists, which may compromise assessment of dietary intake. Open-ended dietary assessment methods such as dietary recalls or food records may be more reflective of food intake, and thus may yield different results when investigating diet–disease associations. However, dietary GI and GL values are available only in the FFQ nutrient database within the WHI. Furthermore, the use of FFQs to assess dietary GI and GL in large epidemiologic studies is a well-accepted method, having been utilized in virtually all previous studies of GI, GL, and breast cancer risk. A third limitation was the fairly narrow range of GI values observed in the study population. The fixed food lists on most FFQs, and consumption of a relatively narrow range of GI foods in the United States, can limit the ability to test GI–disease associations. For example, in the current analysis, the median of the first quintile of GI intake was 47.8, whereas the median of the fifth quintile was 57.0 (a spread of only 9.2 GI units). A similarly narrow range of 12 GI units between the medians of the first and fifth quintiles has been reported elsewhere (11). Future studies in populations with a wider range of overall dietary GI and GL values are needed. Fourth, it is possible that changing dietary intakes since the baseline assessment could have resulted in misclassification bias, which could have attenuated the results. Finally, dietary assessment methods limitation that may have prevented us from comprehensively investigating dietary sugars intake with breast cancer risk was that the USDA sugars database is still not completely populated and there are many missing values.
In summary, there was no evidence prospectively of associations of total breast cancer and dietary GL, GI, or available carbohydrate in the WHI postmenopausal participants. Refined assessment of dietary GI and GL with related biomarkers would be advantageous in future research. However, considering our findings collectively with others, the likelihood that GI and GL influence breast cancer appears to be low.
Acknowledgments
This research was supported by Grant 1 R01 HL 73114-01 from the National Institutes of Health.
Contributor Information
James M. Shikany, Division of Preventive Medicine, Department of Medicine, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA
David T. Redden, Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama, USA
Marian L. Neuhouser, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA
Rowan T. Chlebowski, David Geffen School of Medicine, University of California, Los Angeles; and Division of Medical Oncology and Hematology, Harbor-UCLA Medical Center, Los Angeles, California, USA
Thomas E. Rohan, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA
Michael S. Simon, Population Studies Program, Karmanos Cancer Institute at Wayne State University, Detroit, Michigan, USA
Simin Liu, Department of Epidemiology, School of Public Health, University of California–Los Angeles, Los Angeles, California, USA.
Dorothy S. Lane, Department of Preventive Medicine, School of Medicine, Stony Brook University, Stony Brook, New York, USA
Lesley Tinker, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA.
References
- 1.Jenkins DJ, Wolever TM, Taylor RH, Barker HM, Fielden H, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 2.Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 3.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, et al. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 4.Augustin LS, Dal Maso L, La Vecchia C, Parpinel M, Negri E, et al. Dietary glycemic index and glycemic load, and breast cancer risk: a case-control study. Ann Oncol. 2001;12:1533–1538. doi: 10.1023/a:1013176129380. [DOI] [PubMed] [Google Scholar]
- 5.Lajous M, Willett W, Lazcano-Ponce E, Sanchez-Zamorano LM, Hernandez-Avila M, et al. Glycemic index, glycemic load, and the risk of breast cancer among Mexican women. Cancer Causes Control. 2005;16:1165–1169. doi: 10.1007/s10552-005-0355-x. [DOI] [PubMed] [Google Scholar]
- 6.McCann SE, McCann WE, Hong CC, Marshall JR, Edge SB, et al. Dietary patterns related to glycemic index and load and risk of premenopausal and postmenopausal breast cancer in the Western New York Exposure and Breast Cancer Study. Am J Clin Nutr. 2007;86:465–471. doi: 10.1093/ajcn/86.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro Silvera SA, Jain M, Howe GR, Miller AB, Rohan TE. Dietary carbohydrates and breast cancer risk: a prospective study of the roles of overall glycemic index and glycemic load. Int J Cancer. 2005;114:653–668. doi: 10.1002/ijc.20796. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen TG, Olsen A, Christensen J, Overvad K, Tjonneland A. Dietary carbohydrate intake is not associated with the breast cancer incidence ratio in postmenopausal Danish women. J Nutr. 2005;135:124–128. doi: 10.1093/jn/135.1.124. [DOI] [PubMed] [Google Scholar]
- 9.Sieri S, Pala V, Brighenti F, Pellegrini N, Muti P, et al. Dietary glycemic index, glycemic load, and the risk of breast cancer in an Italian prospective cohort study. Am J Clin Nutr. 2007;86:1160–1166. doi: 10.1093/ajcn/86.4.1160. [DOI] [PubMed] [Google Scholar]
- 10.Higginbotham S, Zhang ZF, Lee IM, Cook NR, Buring JE, et al. Dietary glycemic load and breast cancer risk in the Women’s Health Study. Cancer Epidemiol Biomarkers Prevent. 2004;13:65–70. doi: 10.1158/1055-9965.epi-03-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes MD, Liu S, Hankinson SE, Colditz GA, Hunter DJ, et al. Dietary carbohydrates, fiber, and breast cancer risk. Am J Epidemiol. 2004;159:732–739. doi: 10.1093/aje/kwh112. [DOI] [PubMed] [Google Scholar]
- 12.Cho E, Spiegelman D, Hunter DJ, Chen WY, Colditz GA, et al. Pre-menopausal dietary carbohydrate, glycemic index, glycemic load, and fiber in relation to risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1153–1158. [PubMed] [Google Scholar]
- 13.Jonas CR, McCullough ML, Teras LR, Walker-Thurmond KA, Thun MJ, et al. Dietary glycemic index, glycemic load, and risk of incident breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2003;12:573–577. [PubMed] [Google Scholar]
- 14.Giles GG, Simpson JA, English DR, Hodge AM, Gertig DM, et al. Dietary carbohydrate, fibre, glycemic index, glycemic load and the risk of postmenopausal breast cancer. Int J Cancer. 2006;118:1843–1847. doi: 10.1002/ijc.21548. [DOI] [PubMed] [Google Scholar]
- 15.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative Clinical Trial and Observational Study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 16.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13 (Suppl 1):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 17.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 18.Anderson G, Manson J, Wallace R, Lund B, Hall D, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(Suppl 1):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 19.Patterson RE, Kristal AR, Carter RA, Fels-Tinker L, Bolton MP, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–197. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 20.Neuhouser ML, Tinker LF, Thomson C, Caan B, Van Horn L, et al. Development of a glycemic index database for food frequency questionnaires used in epidemiologic studies. J Nutr. 2006;136:1604–1609. doi: 10.1093/jn/136.6.1604. [DOI] [PubMed] [Google Scholar]
- 21.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, et al. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 22.Gnagnarella P, Gandini S, La Vecchia C, Maisonneuve P. Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am J Clin Nutr. 2008;87:1793–1801. doi: 10.1093/ajcn/87.6.1793. [DOI] [PubMed] [Google Scholar]
- 23.George SM, Mayne ST, Leitzmann MF, Park Y, Schatzkin A, et al. Dietary glycemic index, glycemic load, and risk of cancer: a prospective cohort study. Am J Epidemiol. 2009;169:462–472. doi: 10.1093/aje/kwn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins DJ, Wolever TM, Collier GR, Ocana A, Rao AV, et al. Metabolic effects of a low- glycemic-index diet. Am J Clin Nutr. 1987;46:968–975. doi: 10.1093/ajcn/46.6.968. [DOI] [PubMed] [Google Scholar]
- 25.Castagnetta LA, Miceli MD, Sorci CM, Pfeffer U, Farruggio R, et al. Growth of LNCaP human prostate cancer cells is stimulated by estradiol via its own receptor. Endocrinology. 1995;136:2309–2319. doi: 10.1210/endo.136.5.7536668. [DOI] [PubMed] [Google Scholar]
- 26.Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 28.Brand-Miller JC, Liu V, Petocz P, Baxter RC. The glycemic index of foods influences postprandial insulin-like growth factor-binding protein responses in lean young subjects. Am J Clin Nutr. 2005;82:350–354. doi: 10.1093/ajcn.82.2.350. [DOI] [PubMed] [Google Scholar]
- 29.Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Manson J, et al. Dietary fat and fiber in relation to risk of breast cancer. JAMA. 1992;268:2037–2044. [PubMed] [Google Scholar]
- 30.Lajous M, Boutron-Ruault MC, Fabre A, Clavel-Chapelon F, Romieu I. Carbohydrate intake, glycemic index, glycemic load, and risk of post-menopausal breast cancer in a prospective study of French women. Am J Clin Nutr. 2008;87:1384–1391. doi: 10.1093/ajcn/87.5.1384. [DOI] [PubMed] [Google Scholar]
- 31.Larsson SC, Bergkvist L, Wolk A. Glycemic load, glycemic index and breast cancer risk in a prospective cohort of Swedish women. Int J Cancer. 2009;125:153–157. doi: 10.1002/ijc.24310. [DOI] [PubMed] [Google Scholar]
- 32.Yee D, Lee AV. Crosstalk between the insulin-like growth factors and estrogens in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5:107–115. doi: 10.1023/a:1009575518338. [DOI] [PubMed] [Google Scholar]