Abstract
Background
Little is known about factors influencing time to severe Alzheimer’s disease (AD).
Methods
Incident cases of AD in the Cache County Memory Study were identified. Severe AD was defined as Mini-Mental State Examination score of ≤10 or Clinical Dementia Rating Scale score of 3; cases with either Mini-Mental State Examination score of ≥16 or Clinical Dementia Rating <2 were not categorized as severe AD. Kaplan–Meier, log-rank tests, and Cox analyses were used to identify demographic, clinical, and genetic correlates of time to progression to severe AD.
Results
Sixty-eight of 335 cases of incident AD developed severe dementia. In bivariate analyses, female gender, less than high school education, at least one clinically significant Neuropsychiatric Inventory domain at baseline, and the youngest and oldest ages exhibited shorter time to severe AD. In competing risk analysis, subjects with mild or at least one clinically significant Neuropsychiatric Inventory domain score, and subjects with worse health were more likely to progress to severe dementia or death.
Conclusions
Demographic and clinical variables predict progression to severe AD. Further study should examine whether these relationships are causal or correlational.
Keywords: Incident dementia, Severe dementia, Severe AD, Rate of decline
1. Introduction
The identification of factors that influence the progression of Alzheimer’s disease (AD) might offer targets for the development of interventions that slow disease progression and diminish the morbidity, an approach advocated for chronic conditions for which primary prevention has failed [1]. Previous studies have identified younger age of onset, higher education, greater severity of cognitive impairment, presence of psychosis or neuropsychiatric symptoms, and parkinsonism as correlates of rate of progression [2,3].
One challenge to determining rate of progression of AD is the difficulty in identifying when the disease begins. The use of longitudinally followed samples in which cognition is reassessed on a regular basis offers one solution to this issue [4]. One benefit of staging AD is that it avoids the assumption that progression is linear. Also, as expenses such as long-term care make up a substantial proportion of the cost of the disease in countries where it is widely available [5], preventing or delaying progression to late-stage dementia is an outcome that deserves attention.
The Cache County Memory Study [6,7] and its successor, that is, the Dementia Progression Study, offer an opportunity to address the question of factors affecting rate of progression of dementia. These studies have followed individuals with dementia and cognitively normal comparison subjects since 1995 and assessed them at regular intervals. Here, we report on factors affecting the rate of progression to severe AD in individuals who were initially cognitively normal and who developed AD during the follow-up period.
2. Methods
The Cache County Memory Study has been described elsewhere in detail [6,7]. In brief, 5657 community-residing individuals of age >65 years were contacted, and 5092 agreed to participate, an 89% participation rate. Rate of dementia was 9.6% in the prevalence wave, similar to many epidemiological samples. Individuals were subsequently rescreened and assessed at 3- to 5-year intervals (mean = 3.53, standard deviation [SD] = 0.6) in three incidence waves. Diagnoses of dementia and type of dementia were made by a consensus panel. Analyses were limited to individuals who converted from no dementia to AD and were subsequently followed in the Dementia Progression Study [8]. Follow-up rates, excluding mortality, exceeded 90%.
Severe AD was defined as Mini-Mental State Examination [9] score of ≤10 or Clinical Dementia Rating Scale score equal to 3.0 [10]. If only one of these criteria was met, inclusion required a Clinical Dementia Rating Scale score of at least 2 or a Mini-Mental State Examination score of <16. To identify individual factors associated with time to develop severe AD, we constructed Kaplan–Meier (KM) plots and ran log-rank test. Variables examined were as follows:
Demographics: gender, education (less than high school vs high school graduate or above);
Age of dementia onset determined as the age at which the individual met Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised criteria for dementia [6];
Clinical variables: Neuropsychiatric Inventory (NPI) [11] score across all domains trichotomized as 0 symptoms, at least one domain score 1 to 3 (mild) or ≥4 (clinically significant);
-
General medical health (General Medical Health Rating Scale [12] of excellent/good or fair/poor);
Apolipoprotein E epsilon 4 (APOE ε4) status: ε4/× versus no ε4).
NPI and General Medical Health Rating Scale scores were obtained at the time of diagnosis of AD. Examination of the distribution of age at dementia onset revealed a nonlinear association with time to severe dementia. Therefore, we centered onset age at the mean. However, for ease of interpretation, we depict results in KM plots as a trichotomized variable.
Variables for which the univariate KMplot’s log-rank test of equality had P <.10 were then entered into a multivariate Cox proportional hazards model that controlled for the quadratic effect of age of onset, to account for the nonlinear association with severe AD. We also stratified by whether there were ≤2 or >2 years between age at the visit at which the person was ascertained to be without dementia and age at the visit at which the person was identified with dementia to address differences in dementia duration at diagnosis. Because dementia is a risk factor for mortality, we conducted additional analyses jointly considering the competing risks of severe dementia and death. All analyses met the proportional hazards assumption and were conducted with SPSS version 18 (IBM corp, Armonk, NY), with the exception of the competing risk analyses, which were conducted with SAS version 9.2.
3. Results
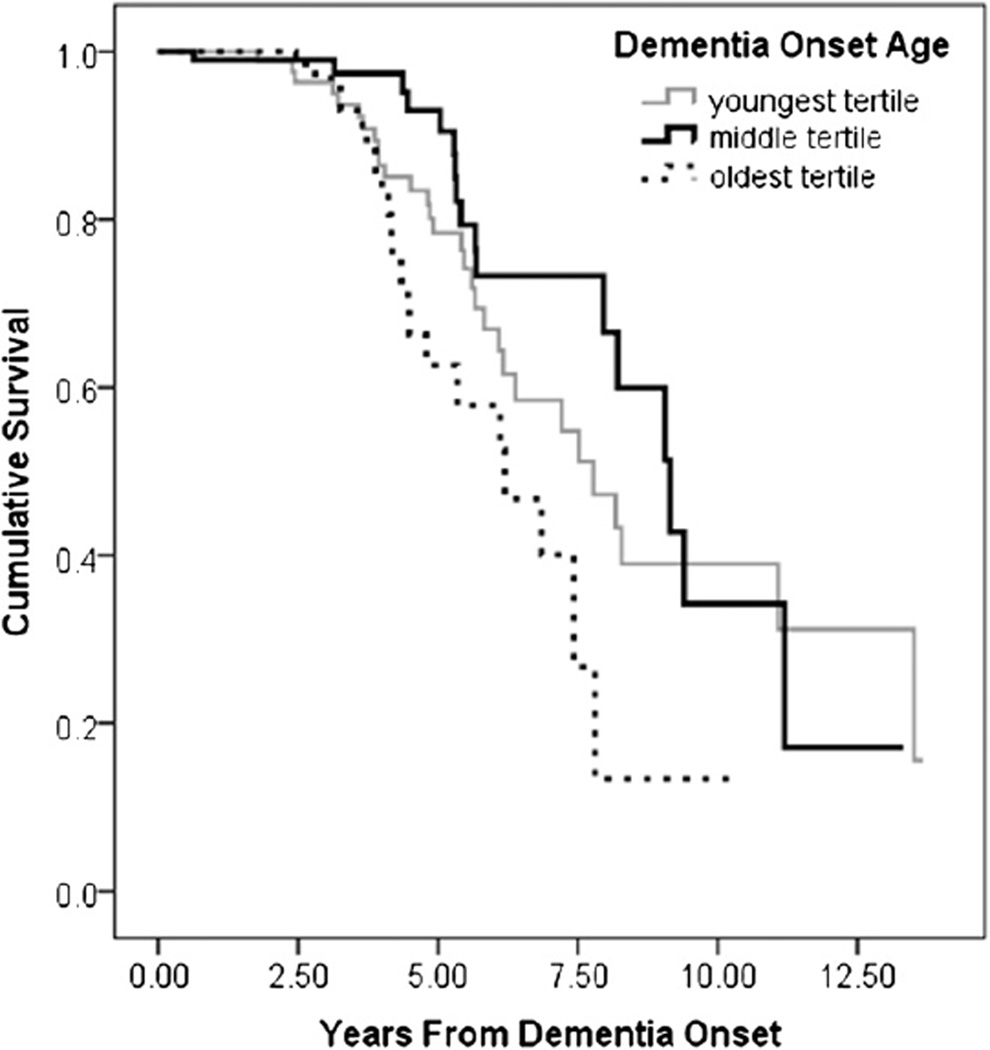
Three hundred thirty-five incident cases of possible or probable AD were identified. Two hundred seventy-three cases were deceased at the last data point analyzed (October 12, 2010). Mean age at onset was 84.3 (SD = 6.4) years, and mean time period between dementia onset and diagnosis was 1.7 (SD = 1.3) years. The KM plot displayed in Figure 1 shows shorter time to severe AD for those with younger and older onset ages.
Fig. 1.
Cumulative survival to severe dementia by age of onset tertile.
Sixty-eight persons (20% of the incident sample) developed severe AD over the course of the study. Median survival time to severe AD for the sample was 8.4 years (95% confidence interval [CI]: 7.6–9.2). Univariate analysis with KM plots revealed survival time varied by onset age, education, and NPI score. Median time to severe AD was 7.8 years (95% CI: 5.86–9.7) in the youngest age tertile, 9.2 (95% CI: 7.8–10.5) in the middle tertile, and 6.2 (95% CI: 4.5–7.9) in the oldest tertile (log-rank χ2(2) = 8.792, P = .012). Those with less than high school education (median survival = 6.86, 95% CI: 3.9–9.9) had shorter time to severe AD than those with at least a high school education (median survival = 8.2, 95% CI: 7.4–9.0) (log-rank χ2(1) = 5.144, P = .023). Those with at least one clinically significant NPI domain score (≥4; median survival = 6.9, 95% CI: 5.2–8.5) had shorter time to severe AD compared with those with mild symptoms (median survival = 8.3, 95% CI: 6.5–10.0) and those with no NPI symptoms (median survival = 9.06, 95% CI: 5.8–12.4) (log-rank χ2(2) = 7.706, P = .021). APOE genotype (log-rank χ2(1) = 0.018, P = .893), baseline general health (log-rank χ2(1) = 1.34, P = .246), and sex (log-rank χ2(1) = 2.065, P = .151) failed to predict time to severe dementia.
In bivariate Cox models, stratifying by the time interval from onset age to diagnosis, women (relative risk [RR] = 1.83, 95% CI: 1.01–3.31, P = .047) and those with older onset of AD (RR = 1.78, 95% CI: 0.99–3.2, P = .06), less than high school education (RR = 1.98, 95% CI: 1.07–3.65, P = .029), and at least one clinically significant NPI domain at baseline (RR = 2.25, 95% CI: 1.27–3.99, P = .005) had shorter time to severe AD. In multivariate Cox models, the nonlinear relationship between onset age and severe AD was confirmed. Women (RR = 1.90, 95% CI: 1.02–3.56, P = .044) and having at least one clinically significant NPI domain predicted time to developing severe AD (RR = 2.60, 95% CI: 1.42–4.78, P = .002; see Table 1).
Table 1.
Multivariate Cox regression model
| 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | B | SE | Wald | df | Significance | Exp (B) | Lower | Upper |
| NPI* | 9.94 | 2 | 0.007 | |||||
| All domains 1–3 | 0.10 | 0.35 | 0.08 | 1 | 0.779 | 1.10 | 0.56 | 2.17 |
| At least one domain 4+ | 0.91 | 0.31 | 8.70 | 1 | 0.003 | 2.48 | 1.36 | 4.54 |
| AD onset age | −1.09 | 0.32 | 11.67 | 1 | 0.001 | 0.34 | 0.18 | 0.63 |
| AD onset age2 | 0.01 | 0.002 | 12.38 | 1 | <0.001 | 1.01 | 1.003 | 1.011 |
| Female | 0.64 | 0.32 | 4.05 | 1 | 0.044 | 1.90 | 1.02 | 3.56 |
| Education—less than high school† | 0.55 | 0.34 | 2.63 | 1 | 0.105 | 1.73 | 0.89 | 3.35 |
Reference category is NPI total score = 0.
Reference category is greater than or equal to high school.
To examine the consistency of these risk factors with respect to both severe dementia and death, we additionally carried out a competing risk analysis that considered these outcomes simultaneously. The effect of gender varied significantly between death and severe dementia (χ2 = 36.1, P < .0001, df = 8), with males having a greater hazard of death (RR = 1.30, 95% CI: 0.97–1.76) but lower risk of severe dementia (RR = 0.56, 95% CI: 0.30–1.05). Having at least mild or one clinically significant neuropsychiatric symptom at baseline (compared with no symptoms) predicted both severe dementia and death (P < .0001; mild NPI domain score: RR = 1.41, 95% CI: 1.04–1.89; clinically significant NPI: RR = 2.02, 95% CI: 1.49–2.74). Worse general health also predicted both severe dementia and death (RR = 1.38, 95% CI: 1.05–1.80). Being a carrier of the APOE ε4 allele did not increase the risk for either severe dementia or death (RR = 1.08, 95% CI: 0.80–1.46).
4. Discussion
In this population-based study of individuals with incident AD, women and subjects with at least one clinically significant NPI domain score were more likely to progress to severe dementia. The youngest and oldest age-of-onset cohorts progressed more rapidly to severe AD than the middle tertile (ages: 81–86 years). This raises the possibility that factors such as a more severe form of the disease in younger individuals and/or greater medical comorbidity in the oldest cohort might influence rate of progression. The lack of relationship between APOE status and time to progression to severe dementia suggests that genetic factors do not explain this unexpected U-shaped relationship.
In univariate Cox regression analyses, faster progression to severe dementia occurred in women and in those with less than high school education. The presence of at least one NPI domain score of clinical significance accelerated progression to severe dementia. We have no way of knowing whether this is causally related to the presence of neuropsychiatric symptoms, to treatments used to address these symptoms, or to an interaction between them and disease severity. We have previously found high staff-reported rates of neuropsychiatric symptoms in people with dementia who are hospice eligible [13], suggesting that they remain an important issue throughout the course of AD. Whether non-pharmacological or pharmacological interventions targeting these symptoms influence rate of progression to severe AD is unknown.
Limitations of this study include the lack of incident cases of age <65 years, small number of cases with severe AD, and the homogeneity of the population (low rates of alcohol and illicit substance abuse, low representation of nonwhites, and high representation of members of the Church of Jesus Christ of Latter-day Saints). Strengths of the study include its epidemiologic sampling frame; high participation rate; its prospective, longitudinal data collection; and the use of state-of-the-art clinical diagnostic assessments for AD.
Acknowledgments
This research was supported by the following grants from the National Institute on Aging: R01AG21136, R01AG11380, and R01AG18712.
P.V.R. is supported by NIMH, NIA, Associated Jewish Federation of Baltimore; Legal testimony for Janssen Pharmaceutica. C.L. is supported by (research or CME), NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis.
Consultant/advisor: Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Genentech; Honorarium or travel support: Pfizer, Forest, Glaxo-Smith Kline, Health Monitor.
Funding was obtained from National Institutes of Health grants. All participants provided informed consent, and the study was approved by the Johns Hopkins University, Utah State University, and Duke University Institutional Review Boards.
References
- 1.Glasgow RE, Orleans CT, Wagner EH. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2001;79:579–612. doi: 10.1111/1468-0009.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern Y, Tang M, Albert M, Brandt J, Jacobs D, et al. Predicting time to nursing home care and deathe in individualas with Alzheimer disease. JAMA. 1997;277:806–813. [PubMed] [Google Scholar]
- 3.Lopez OL, Schwam E, Cummings J, Gauthier S, Jones R, Wilkinson D, et al. Predicting cognitive decline in Alzheimer’s disease: an integrated analysis. Alzheimers Dement. 2010;6:431–439. doi: 10.1016/j.jalz.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Storandt M, Grant E, Miller P, Morris J. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Arch Neurol. 2002;59:1034–1041. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- 5.Wim A, Jonsson L, Gustavsson A, McDaid D, Ersek K, Georges J, Gulacsi L, Karpati K, Kenigsberg P, Valtonen H. The economic impact of dementia in Euroipe in 2008–cost estimates from the Eurocode project. Int J Geriatr Psych. doi: 10.1002/gps.2610. (in press). [DOI] [PubMed] [Google Scholar]
- 6.Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 7.Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: the Cache County study. Neurology. 2002;58:209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- 8.Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC, et al. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County Dementia Progression Study. Am J Geriatr Psychiatry. 2011;19:532–542. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 10.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatr. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 11.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 12.Lyketsos CG, Galik E, Steele C, Steinberg M, Rosenblatt A, Warren A, Sheppard JM, Baker A, Brandt J. The General Medical Health Rating: a bedside global rating of medical comorbidity i patients with dementia. J Am Geriatr Soc. 1999;47:487–491. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]
- 13.Kverno KS, Rabins PV, Blass DM, Hicks KL, Black BS. Prevalence and treatment of neuropsychiatric symptoms in advanced dementia. J Gerontol Nurs. 2008;34:8–15. doi: 10.3928/00989134-20081201-03. [DOI] [PMC free article] [PubMed] [Google Scholar]