Abstract
Background and objectives
Abnormal swallowing (dysphagia) among neonates is commonly evaluated using the videofluoroscopic swallow study (VSS). Radiological findings considered high risk for administration of oral feeding include nasopharyngeal reflux, laryngeal penetration, aspiration, or pooling. Our aims were to determine pharyngoesophageal motility correlates in neonates with dysphagia and the impact of multidisciplinary feeding strategy.
Methods
Twenty dysphagic neonates (mean gestation ± standard deviation [SD] = 30.9 ± 4.9 weeks; median 31.1 weeks; range = 23.7–38.6 weeks) with abnormal VSS results were evaluated at 49.9 ± 16.5 weeks (median 41.36 weeks) postmenstrual age. The subjects underwent a swallow-integrated pharyngoesophageal motility assessment of basal and adaptive swallowing reflexes using a micromanometry catheter and pneumohydraulic water perfusion system. Based on observations during the motility study, multidisciplinary feeding strategies were applied and included postural adaptation, sensory modification, hunger manipulation, and operant conditioning methods. To discriminate pharyngoesophageal manometry correlates between oral feeders and tube feeders, data were stratified based on the primary feeding method at discharge, oral feeding versus tube feeding.
Results
At discharge, 15 of 20 dysphagic neonates achieved oral feeding success, and the rest required chronic tube feeding. Pharyngoesophageal manometry correlates were significantly different (P <0.05) between the primary oral feeders versus the chronic tube feeders for swallow frequency, swallow propagation, presence of adaptive peristaltic reflexes, oral feeding challenge test results, and upper esophageal sphincter tone. VSS results or disease characteristics had little effect on the feeding outcomes (P = NS).
Conclusions
Swallow-integrated esophageal motility studies permit prolonged evaluation of swallowing reflexes and responses to stimuli under controlled conditions at cribside. The dysfunctional neuromotor mechanisms may be responsible for neonatal dysphagia or its consequences. Manometry may be a better predictor than VSS in identifying patients who are likely to succeed in vigorous intervention programs.
Keywords: Dysphagia, Motility, Neonates, Videofluoroscopic swallow study
Technological advances in perinatal care have resulted in declining neonatal mortality, but with an accelerating infant morbidity (1,2). One important concern that prolongs hospitalization in neonates is dysphagia (dys = abnormal, phagia = swallowing). Dysphagia is a disruption in the ability to move food or liquid from the mouth through the pharynx and esophagus into the stomach safely and efficiently. The incidence of dysphagia is significant in preterm infants (26%) and is double that of the general population (13%) (3). Dysphagia is widely prevalent (up to 90%) in patients with neurological disorders (4,5). Clinicians and parents are faced with uncertainties regarding the ideal long-term feeding strategies in patients who are unsuccessful with oral feeds. Often, these decisions include exclusive chronic gavage feeding and more invasive and lifestyle-changing feeding methods such as gastrostomy placement.
Unfortunately, the current diagnostic methods to evaluate dysphagia in infants have limitations, with videofluoroscopy swallow study (VSS) being the most widely available technology used to determine feeding safety (6). Pharyngoesophageal manometry is emerging as a complementary technique to VSS to provide information on swallowing dynamics in adults and children (6–13). VSS is designed to evaluate the oropharyngeal and esophageal anatomy pertinent to swallowing during brief exposure to fluoroscopy. Oral feeding is commonly recommended based on the safe passage of barium during fluoroscopic observation (6,14,15). Radiological findings considered unsafe for oral feeding include nasopharyngeal reflux, laryngeal penetration, aspiration, pooling, or delayed clearance (15). The significance of these radiological findings or the relevance of current management strategies to manage dysphagia in infants has not been thoroughly investigated.
Using manometry methods in premature and full-term infants, we have evaluated gastrointestinal motility (16,17) and pharyngoesophageal motility and aerodigestive reflexes (18–23). Specifically, we characterized the basal and adaptive peristaltic and upper esophageal sphincter (UES) functions that are critical to the integration and coordination of swallowing reflexes in neonates (18–23). Recently, others have applied manometry methods to evaluate pharyngoesophageal motility in syndromic infants with dysphagia (24). Motility studies offer a means to evaluate abnormal neurophysiology related to swallowing.
The objectives of the present study were to determine pharyngoesophageal motility correlates in neonates with abnormal VSS and the impact of a multidisciplinary feeding strategy on the ability to achieve oral feeding. The multidisciplinary approach was created based on the evidence accrued during clinical and manometric evaluation of swallowing reflexes. To discriminate pharyngoesophageal manometry correlates, data were stratified based on the primary feeding method at discharge, oral feeding versus tube feeding.
PATIENTS AND METHODS
Participants were 20 neonates (9 male, 11 female) of gestational age 31 ± 5 weeks, evaluated at 49 ± 16 weeks postmenstrual age. Subjects had clinical and videofluoroscopic evidence of dysphagia. Feeding problems were heterogeneous and included feeding-related bradycardia and desaturation, coughing, gagging, arching, refusal to feed, and/or poor nippling ability. These infants were considered unsafe for oral feeding and were referred for the establishment of alternative tube-feeding plans. All of the subjects were referred to receive further management at Nationwide Children’s Hospital neonatal intensive care unit. Approval for this study was obtained from the institutional research review board. Informed consent was obtained from parent(s), and the study complied with the Health Insurance Portability and Accountability Act.
Clinical Evaluation
Clinical characteristics of perinatal and neonatal course, airway and neurological status, feeding methods, and oromotor skills were recorded. Next, we evaluated the results of chest radiography, cranial ultrasound, VSS, and upper gastrointestinal fluoroscopy studies undergone by the study infants as part of their routine care. Finally, a swallow-integrated esophageal motility assessment was performed at cribside.
Manometry Protocol
Esophageal motility assessment was performed as previously reported by us (18–22). In brief, pharyngoesophageal motility was evaluated using a micromanometry system concurrent with respiratory inductance plethysmography and monitoring of vital signs and submental electromyography. The pharyngoesophageal motor responses were quantified using a micromanometric water perfusion system and a specially designed catheter with a UES sleeve and 5 side-hole recording sites, positioned as previously reported (19,20). Continuous data acquisition and analysis were completed during the manometric study based on waveform characteristics (18–22).
We first recorded basal pharyngoesophageal motility characteristics of swallows such as the swallow frequency, propagation and distribution of primary peristaltic waveforms, peristaltic velocity, and resting UES and lower esophageal sphincter pressures. All of the measurements were taken at end expiration (18–23). Next, peristaltic responses to wet swallows were evaluated as previously reported (9–11,18,19,23). Infants were allowed a trial of bottle feeding (oral feeding challenge test) during the manometry session while monitoring respiration, electrocardiogram, and pulse oximetry.
Multidisciplinary Feeding Management Strategy
Standard published oral feeding practices were applied, although none can be uniformly applicable to all dysphagic neonates (14,15,25,26). Therefore, a multidisciplinary working plan for feeding was established. The team included a neonatologist with gastrointestinal motility expertise, occupational therapists, clinical psychologists and radiologists, and a dedicated neonatal nursing team. The feeding management strategy complied with the following principles:
Standard occupational therapy methods were used in all of the subjects and included nonnutritive sucking, application of sucrose on a pacifier, and maintenance of optimal position and posture during therapy sessions. Generally, there was 1 feeding session with an occupational therapist per day. Oral feeding attempts were begun cautiously in a stepwise manner, and were advanced based upon feeding performance and absence of symptoms. When a subject was noted to be tachypneic, no oral feedings were offered. A feeding session was attempted using 5 to 10 mL of milk, and the volume was increased at the next session in asymptomatic subjects. In symptomatic subjects, approaches to relieve symptoms included pacing techniques and modification of milk flow rates using slow-flow nipples. Frequent pauses in feeding were made to allow adaptation, particularly when subjects began to feed higher volumes. When symptoms were noted during nipple feeding, the nipple was withdrawn to allow swallowing and clearance.
Hunger manipulation methods (26,27) were the preferred methods, and were used by using a bolus feeding regimen with 3-hour feeding cycles. However, in infants symptomatic during bolus feeds, alternative tube feeding methods were used (transpyloric or continuous).
Manipulation of gut motility responses methods were used. Fasting and fed phases of the feeding cycle have distinct gastrointestinal motility patterns (17,28–30). To develop cyclical fasting–feeding pattern, we used an approach of progressing gradually from continuous intragastric feeds to slow bolus feeds (infusing feed for 2 hours and pausing for 2 hours) to bolus feeds given for 30 minutes, in that order. This approach was based on our previous experience related to manometric responses during fasting, feeding, and swallowing reflexes (28–31).
Data and Statistical Analysis
Success was assessed based on the clinical relevance: safe nipple feeding ability at discharge. Subjects with primary oral feeding abilities were grouped under the oral feeding success group, and those that did not achieve safe oral feeding were grouped under the feeding failure group. All variables of interest (subject and disease characteristics, videofluoroscopic characteristics, manometric characteristics) were stratified under these 2 groups and data were compared. Because manometric data have more than 1 dependent variable, multivariate analysis of variance also was used to determine the main effect of categorical variables on multiple dependent variables. Bonferroni adjustments were made to pairwise comparisons and to keep an overall α level of 0.05. Chi-square tests were used to compare proportions. Data are stated as mean ± standard deviation (SD), median, and range, or as percentages, and adjusted P values are reported. Data were assessed using SPSS version 14.0 (SPSS, Chicago, IL).
RESULTS
Demographic, Disease, Videofluoroscopic, and Feeding Outcome Characteristics
Dysphagic infants (N = 20; gestational age = 30.9 ± 4.9 weeks; median 30.14 weeks; range 23.7–38.6 weeks) with abnormalities on VSS were evaluated at 48.9 ± 16.5 weeks postmenstrual age (median 41.36 -weeks). At discharge (52.0 ± 17.8 weeks postmenstrual age), 15 patients fell into the feeding success group (vs feeding failure groups, P <0.001, χ2 = 16), among whom 60% were on exclusive oral feeds and the rest (40%) were supplemented with some gavage feeds (Table 1). Individual subject, disease, VSS, and outcome characteristics are summarized in Table 1, and radiological markers during VSS are compared in Figure 1.
TABLE 1.
Subject and videofluoroscopic swallow study (VSS) characteristics and feeding methods before motility study and at discharge
| ID | Subject characteristics | VSS characteristics
|
Feeding methods (primary/secondary)
|
|||||
|---|---|---|---|---|---|---|---|---|
| NPR | LP | ASP | Pool | DS | Premotility | At discharge | ||
| 1 | PT, CLD | + | Gavage | PO | ||||
| 2 | PT, | + | + | Gavage/POs | PO | |||
| 3 | PT, CLD | + | + | Gavage/POs | PO | |||
| 4 | PT, CLD, GERD | + | + | + | Gavage/POs | PO | ||
| 5 | PT, CLD, GERD | + | + | + | Gavage | PO | ||
| 6 | PT | + | + | + | Gavage/POs | PO | ||
| 7 | PT | + | + | + | Gavage/POs | PO | ||
| 8 | PT | + | + | + | Gavage | PO | ||
| 9 | PT, GERD, temporal lobe hemorrhage | + | + | + | Gavage | PO | ||
| 10 | PT, CLD, IVH, GERD | + | Gavage/POs | PO/gavage | ||||
| 11 | PT, CLD, HIE, GERD | + | Gavage | PO/GT | ||||
| 12 | PT, GERD, PVL | + | + | Gavage/POs | PO/gavage | |||
| 13 | PT, ALTE, GERD | + | + | + | Gavage/POs | PO/gavage | ||
| 14 | PT, CLD | + | + | + | + | Gavage | PO/gavage | |
| 15 | PT | + | + | + | + | Gavage | PO/gavage | |
| 16 | PT, CLD, GERD, occipital lobe hemorrhage | + | + | Gavage | Gavage | |||
| 17 | PT, CLD, GERD, PVL | + | + | + | GJ | GJ | ||
| 18 | CLD, GERD | + | + | GJ | GJ | |||
| 19 | PT, CLD | + | + | GJ | GJ | |||
| 20 | Fetal alcohol syndrome | + | + | Gavage | GT | |||
ALTE = apparent life-threatening event; ASP = aspiration; CLD = chronic lung disease; DS = delayed swallow; GERD = gastroesophageal reflux disease; GJ = gastrojejunal feeds; GT = gastrostomy feeds; HIE = hypoxic-ischemic encephalopathy; IVH = intraventricular hemorrhage; LP = laryngeal penetration; NPR = nasopharyngeal reflux; PO = asymptomatic with oral feeds; Pool = pooling; POs = symptomatic with oral feeds; PT = preterm born; PVL = periventricular leukomalacia.
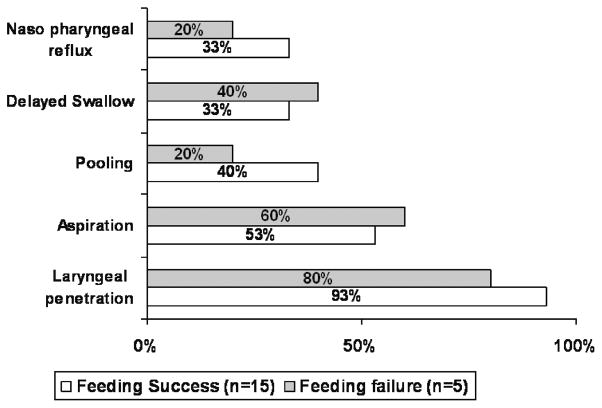
FIG. 1.
Video fluoroscopic swallow study parameters are compared between successful oral feeders and failures (P= NS, chi square).
Between the success versus failure groups, differences with each of the disease characteristics are as follows: gastroesophageal reflux disease (GERD) (40% vs 80%), cranial ultrasound abnormalities (67% vs 80%), chronic lung disease (53% vs 80%), and prematurity (100% vs 80%). Acid suppressive strategy was adopted in GERD cases (n = 6 in success group and n = 4 in failure group). Given the sample size and heterogeneity of the cohort, the study was underpowered to detect the effects of GERD, cranial ultrasound abnormalities, and chronic lung disease.
Characterization of Pharyngoesophageal Motility Mechanisms
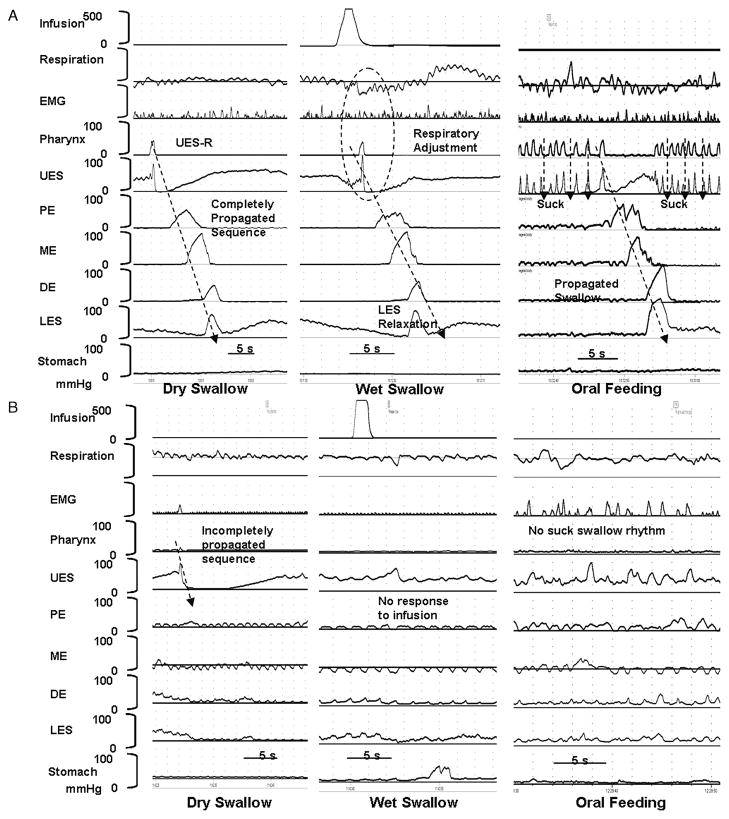
Characteristics of primary peristalsis occurring in response to spontaneous swallows, wet swallows, and milk feed in representative patients with feeding success and failure are shown in Figure 2A and B, respectively. The frequency of occurrence and propagation of swallows during primary peristalsis are reported in Table 2, and the characteristics of UES and proximal and distal esophageal body are reported in Table 3.
FIG. 2.
(A) Charting swallow-integrated pharyngoesophageal motility study in an infant with feeding success describing propagation of spontaneous dry swallow, wet swallow–induced primary peristalsis, and oral feeding challenge test. (B) Charting similar data in an infant with feeding failure describing failed propagation of dry swallow, response failure to wet swallow, and poor response to oral feeds. EMG = electromyography; UES = upper esophageal sphincter; PE = proximal esophagus; ME = mid esophagus; DE = distal esophagus; LES = lower esophageal sphincter.
TABLE 2.
Frequency and propagation of swallow-induced primary peristalsis
| Characteristics | Feeding success (n = 15) | Feeding failure (n = 5) | P |
|---|---|---|---|
| No. completely propagated swallows/min | 2.4 ± 0.3 | 0.4 ± 0.2 | 0.001 |
| Completely propagated swallows, % | 69.4 ± 6.9 | 32.5 ± 21.4 | 0.043 |
| Peristaltic response to wet swallows, % | 86.7 ± 5.7 | 28.7 ± 16.5 | 0.001 |
| Complete propagation of esophageal peristaltic waveforms during oral feeding challenge, % | 100 | 33 | 0.002 |
| Presence of suck–swallow rhythm during oral feeding challenge, % | 100 | 0 | <0.0001 |
Values as mean ± standard error of measurement (SEM) or percentages.
TABLE 3.
Characteristics of upper esophageal sphincter (UES) tone and esophageal waveforms
| Characteristics | Feeding success (n = 15) | Feeding failure (n = 5) |
|---|---|---|
| Resting UES pressure, mmHg | 21.8 ± 1.8 | 34.0 ± 7.5 |
| UES relaxation time, s | 0.4 ± 0.1 | 1.0 ± 0.3 |
| Rate of UES relaxation, mmHg/s | 87.2 ± 13.0 | 50.7 ± 17.1 |
| PE peristaltic waveform duration, s | 2.6 ± 0.2 | 2.4 ± 0.2 |
| DE peristaltic waveform duration, s | 3.2 ± 0.4 | 4.9 ± 0.8 |
Values as mean ± standard error of means (SEM). Individual comparisons after Bonferroni correction were similar. Including all 5 variables in multivariate analysis of variance, P = 0.06. PE = proximal esophagus; DE = distal esophagus.
DISCUSSION
The mechanisms of dysphagia or the management of neonatal dysphagia are rather complex and are not well understood. As a result, there is a lack of structured algorithms associated with successful feeding patterns. This is largely because of the heterogeneity of the problem that so far has defied attempts at developing uniformly recognized classifications. The dysphagic neonates in this study can be considered to be problematic cases, and had been referred for a decision regarding need for chronic tube feedings. The pharyngoesophageal motility mechanisms and multidisciplinary clinical observations guided us toward the development of structured feeding plans. In 75% of the cohort, the diagnostic and management strategies were beneficial in preventing lifestyle-changing chronic tube feeding practices. The rest of the cohort failed to achieve safe oral feeds and required chronic tube feeds exclusively. The findings of this study potentially have important clinical implications in the evaluation and management of neonatal dysphagia, in that manometry may be a better predictor than VSS in identifying those patients that are more likely to succeed with oral feeding abilities using a vigorous intervention program. This is the first study that has integrated diagnostic evaluation using pharyngoesophageal motility studies and management strategies in neonatal dysphagia and studied their impact on oral feeding.
The application of VSS methods in the evaluation of neonatal dysphagia is well known (6), and dysphagic mechanisms are recognized by the presence of aspiration, laryngeal penetration, nasopharyngeal reflux, delayed swallow, or pooling. These phenomena may be the result of inappropriate adaptive responses to protect the airway. However, there are limitations in the interpretation of the VSS, possibly owing to application of restraint and stress, use of contrast media as a sensory stimulus to provoke swallows, impaired adaptive responses under restraint, and need for short-term exposure to radiation to define complex swallowing phases. VSS offers an advantage in characterizing structural pathology, although in this study no anatomical problems were identified.
Manometry methods to evaluate motility related to swallowing and esophageal functions have been used across this age range by others and us (12,13,18,19, 21,24). We previously characterized the sequences of normal propagating swallows and adaptive reflex responses to pharyngeal or esophageal stimulation in healthy neonates (18,19,21). However, mechanisms of neonatal dysphagia have not been characterized before. In this study, clinical and manometric observations were made during physiological states using physiological stimuli for prolonged periods under cardiorespiratory monitoring. Hence, multiple continuous observations were recorded with respect to initiation and propagation of swallowing, adaptive response to spontaneous and induced swallows, and the oral feeding challenge. Furthermore, integration of cardiorespiratory measures permitted us to monitor safety.
In comparison with the infants with feeding success, infants in the feeding failure group had a different set of pharyngoesophageal dysmotility sequences, characterized by the infrequency of swallows, failure of complete peristalsis propagation, less frequent responses to water swallows, poor response to oral feeding challenge, prolonged distal esophageal waveforms, higher UES tone, and prolonged UES relaxation. These dysfunctional mechanisms may be responsible for neonatal dysphagia or its consequences. The study population was heterogeneous, although all of the subjects were neonates admitted to the neonatal intensive care for various reasons. Early life events, experiences, or noxious aerodigestive stimulations encountered by the study population may have potentially contributed to the food refusal on the basis of visceral hyperalgesia, impaired gastric accommodation, or central nervous system neuropathology (32,33). This may in part explain the outcomes in the feeding failure subgroup in the present study.
This study had been designed to identify the adaptive neuromotor mechanisms in neonatal dysphagia. This study was not designed to predict outcomes because outcomes can be the result of appropriate intervention targeted to a specific dysfunctional mechanism. However, swallowing is a complex task to accomplish and requires participation of 6 pairs of cranial nerves and 31 pairs of muscles, requiring close regulation and coordination between aerodigestive reflexes and respiratory status (31,34). There are no methods available that can reliably and simultaneously assess oral phase, pharyngeal phase, UES phase, esophageal phase, or the gastric phase of swallowing in neonates. We attempted to identify the presence or absence of reflexes at different swallowing phases in this study. The presence of such findings provided guidance in offering multidisciplinary feeding rehabilitation strategies, as opposed to making chronic tube feeding decisions, such as suggesting gastrostomy in all cases.
In this study, an organized multidisciplinary approach was used in all infants with varying success. The feeding methods that may have helped with success (75% of cohort) may have their basis in the occupational therapy methods, manipulation of hunger, sensory modification strategy, or oromotor stimulation. The reasons for the feeding failure may lie in the abnormalities in the neurophysiology of the swallowing or failure of the rehabilitation approaches used.
The strengths of this study are the recognition of the mechanisms of neonatal dysphagia and assessment of the impact of structured multidisciplinary feeding practice. Indeed, this approach prevented lifestyle-changing decisions and permitted oral feeding in 15 neonates over time. The study and the nature of the problem, however, have limitations. The study is under-powered to control for potential confounder variables, such as tube feeding methods, severity of illness, respiratory management, or neurological injury, although we investigated these factors in each subject. Furthermore, repeat manometric and videofluoroscopic evaluations among the successful feeders at discharge were not deemed to be ethically justified. Also, inclusion of a control group of healthy orally feeding infants to undergo both testing modalities is not ethically allowed. Further studies are needed to narrow down the heterogeneous cohort into better classified groups, either based on the neonatal diseases or upon the motility indices.
We are not attempting to define 1 method as superior to the other, vis-á-vis manometry versus videofluoroscopy; rather, we are describing the limitations of VSS in its inability to permit prolonged evaluation to recognize dysfunctional mechanisms. Thus, strategies based on VSS alone may result in delayed initiation and presentation of appropriate oromotor sensory stimuli, which may lead to behavioral feeding disorders (25). Also, the consequences of abnormal VSS can heighten fear of aspiration and limits prescription for oral feeding attempts, although microaspiration has been recognized even in normal subjects (26,35,36). However, VSS may help identify those patients requiring further pharyngoesophageal manometry, and the information gathered can be additive and complementary. Pharyngoesophageal motility sequences and the dysfunctional neuromotor mechanisms may provide clues to develop an individualized approach to manage neonatal dysphagia.
Feeding failure can be certain if the caregiver does not recognize opportunities to initiate feeding and use the natural motivation to engage in swallowing, namely hunger. In this study, hunger was manipulated so as to be active at the time of feeding. Byars et al (26) report the successful initiation of oral feeds in gastric feeding tube–dependent infants and toddlers using behavior- and hunger-inducement procedures. There is evidence from the present study that despite VSS abnormalities, it was possible to institute appropriate rational interventions leading to successful feeders. This has major cost savings implications, given that it has been estimated that the health care costs for children on feeding tubes is $46,875 for the first year and up to $180,000 over 5 years (35).
In conclusion, pharyngoesophageal motility correlates combined with clinical observations during evaluation provide guidance for the development of well-structured multidisciplinary feeding paradigms. Prevention of chronic tube feeding decisions is possible when infants have adaptive aerodigestive reflexes and providers are consistent with appropriate evidence-based feeding practices.
Acknowledgments
The authors are thankful to the Nationwide Children’s Hospital occupational therapy team—Diana Hinton, OTR/L; Melissa Hanin, MOTR/L; Shellie Bolyard, OTR/L; and Sommer Wells, OTR/L—for their help with providing consistent occupational therapy to the infants with dysphagia.
Footnotes
The authors report no conflicts of interest.
References
- 1.Jadcherla SR, Breitzman R, Domnitz A, et al. Impact of feeding milestones on resource utilization among premature neonates. Pediatr Res. 2002;51:408A. [Google Scholar]
- 2.Kliegman RM. Neonatal technology, perinatal survival, social consequences, and the perinatal paradox. Am J Public Health. 1995;85:909–13. doi: 10.2105/ajph.85.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercado-Deane MG, Burton EM, Harlow SA, et al. Swallowing dysfunction in infants less than 1 year of age. Pediatr Radiol. 2001;31:423–8. doi: 10.1007/s002470100456. [DOI] [PubMed] [Google Scholar]
- 4.Reilly S, Skuse D, Poblete X. Prevalence of feeding problems and oral motor dysfunction in children with cerebral palsy: a community survey. J Pediatr. 1996;129:877–82. doi: 10.1016/s0022-3476(96)70032-x. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan PB, Lambert B, Rose M, et al. Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study. Dev Med Child Neurol. 2000;42:674–80. doi: 10.1017/s0012162200001249. [DOI] [PubMed] [Google Scholar]
- 6.American College of Radiology. ACR practice guideline for the performance of pediatric contrast examinations of the upper gastrointestinal tract. American College of Radiology Practice Guidelines and Technical Standards; 2001. pp. 207–11. http://www.acr.org/SecondaryMainMenuCategories/quality_Safety/guidelines/dx/gastro/pediatric_contrast_upper_gi.aspx. [Google Scholar]
- 7.Cook IJ, Dodds WJ, Dantas RO, et al. Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989;4:8–15. doi: 10.1007/BF02407397. [DOI] [PubMed] [Google Scholar]
- 8.Pouderoux P, Shi G, Tatum RP, et al. Esophageal solid bolus transit: studies using concurrent videofluoroscopy and manometry. Am J Gastroenterol. 1999;94:1457–63. doi: 10.1111/j.1572-0241.1999.01126.x. [DOI] [PubMed] [Google Scholar]
- 9.Kahrilas PJ, Clouse RE, Hogan WJ. American Gastroenterological Association technical review on the clinical use of esophageal manometry. Gastroenterology. 1994;107:1865–84. doi: 10.1016/0016-5085(94)90835-4. [DOI] [PubMed] [Google Scholar]
- 10.Pandolfino JE, Kahrilas PJ. American Gastroenterological Association American Gastroenterological Association medical position statement: clinical use of esophageal manometry. Gastroenterology. 2005;128:207–8. doi: 10.1053/j.gastro.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Gilger MA, Boyle JT, Sondheimer JM, et al. A medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. Indications for pediatric esophageal manometry. J Pediatr Gastroenterol Nutr. 1997;24:616–8. doi: 10.1097/00005176-199705000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Hewson EG, Ott DJ, Dalton CB, et al. Manometry and radiology: complementary studies in the assessment of esophageal motility disorders. Gastroenterology. 1990;98:626–32. [PubMed] [Google Scholar]
- 13.Massey BT, Dodds WJ, Hogan WJ, et al. Abnormal esophageal motility: an analysis of concurrent radiographic and manometric findings. Gastroenterology. 1991;101:344–54. [PubMed] [Google Scholar]
- 14.Rudolph CD. Rudolph’s Pediatrics. 21. New York: McGraw-Hill; 2003. Developmental–behavioral pediatrics. [Google Scholar]
- 15.Arvedson JC, Lefton-Greif MA. Pediatric Videofluoroscopic Swallow Studies: A Professional Manual With Caregiver Guidelines. San Antonio, TX: Psychological Corp; 1998. [Google Scholar]
- 16.Koenig WJ, Amarnath RP, Hench V, et al. Manometrics for preterm and term infants: a new tool for old questions. Pediatrics. 1995;95:203–6. [PubMed] [Google Scholar]
- 17.Jadcherla SR, Berseth CL. Acute and chronic intestinal motor activity responses to two infant formulas. Pediatrics. 1995;96 (2 Pt 1):331–5. [PubMed] [Google Scholar]
- 18.Jadcherla SR, Duong HQ, Hoffmann RG, et al. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr. 2003;143:31–8. doi: 10.1016/S0022-3476(03)00242-7. [DOI] [PubMed] [Google Scholar]
- 19.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation of the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatr. 2006;149:77–82. doi: 10.1016/j.jpeds.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A, Jadcherla SR. The relationship between somatic growth and in vivo esophageal segmental and sphincteric growth in human neonates. J Pediatr Gastroenterol Nutr. 2006;43:35–41. doi: 10.1097/01.mpg.0000226368.24332.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadcherla SR, Duong HQ, Hofmann C, et al. Characteristics of upper oesophageal sphincter and oesophageal body during maturation in healthy human neonates compared with adults. Neurogastroenterol Motil. 2005;17:663–70. doi: 10.1111/j.1365-2982.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 22.Jadcherla SR. Manometric evaluation of esophageal-protective reflexes in infants and children. Am J Med. 2003;115 (Suppl 3A):157S–60S. doi: 10.1016/s0002-9343(03)00215-8. [DOI] [PubMed] [Google Scholar]
- 23.Jadcherla SR, Gupta A, Stoner E, et al. Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. J Pediatr. 2007;151:597–603. doi: 10.1016/j.jpeds.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacques Baudon J, Renault F, Michel Goutet J, et al. Motor dysfunction of the upper digestive tract in Pierre Robin sequence as assessed by sucking-swallowing electromyography and esophageal manometry. J Pediatr. 2002;140:719–23. doi: 10.1067/mpd.2002.124313. [DOI] [PubMed] [Google Scholar]
- 25.Linscheid TR, Budd KS, Rasnake LK. Pediatric feeding disorders. In: Roberts MC, editor. Handbook of Pediatric Psychology. 3. New York: Guilford Press; 2003. pp. 481–98. [Google Scholar]
- 26.Byars KC, Burklow KA, Ferguson K, et al. A multicomponent behavioral program for oral aversion in children dependent on gastrostomy feedings. J Pediatr Gastroenterol Nutr. 2003;37:473–80. doi: 10.1097/00005176-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Dollberg S, Kuint J, Mazkereth R, et al. Feeding tolerance in preterm infants: randomized trial of bolus and continuous feeding. J Am Coll Nutr. 2000;19:797–800. doi: 10.1080/07315724.2000.10718080. [DOI] [PubMed] [Google Scholar]
- 28.Jadcherla SR, Berseth CL. Antroduodenal motility and feeding outcome among neonatal extracorporeal membrane oxygenation survivors. J Pediatr Gastroenterol Nutr. 2005;41:347–50. doi: 10.1097/01.mpg.0000174331.00711.6d. [DOI] [PubMed] [Google Scholar]
- 29.Jadcherla SR, Klee G, Berseth CL. Regulation of migrating motor complexes by motilin and pancreatic polypeptide in human infants. Pediatr Res. 1997;42:365–9. doi: 10.1203/00006450-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Jadcherla SR, Sty JR, Rudolph CD. Mechanical small bowel obstruction in premature infants diagnosed by intestinal manometry. J Pediatr Gastroenterol Nutr. 2005;41:247–50. doi: 10.1097/01.mpg.0000172326.82076.f4. [DOI] [PubMed] [Google Scholar]
- 31.Goyal RK, Padmanabhan R, Sang Q. Neural circuits in swallowing and abdominal vagal afferent-mediated lower esophageal sphincter relaxation. Am J Med. 2001;111 (Suppl 8A):95S–105S. doi: 10.1016/s0002-9343(01)00863-4. [DOI] [PubMed] [Google Scholar]
- 32.Hyman PE. Gastroesophageal reflux: one reason why baby won’t eat. J Pediatr. 1994;125 (6 Pt 2):S103–9. doi: 10.1016/s0022-3476(05)82933-6. [DOI] [PubMed] [Google Scholar]
- 33.Anand P, Aziz Q, Willert R, et al. Peripheral and central mechanisms of visceral sensitization in man. Neurogastroenterol Motil. 2007;19 (Suppl 1):29–46. doi: 10.1111/j.1365-2982.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 34.Sivarao DV, Goyal RK. Functional anatomy and physiology of the upper esophageal sphincter. Am J Med. 2000;108 (Suppl 4a):27S–37S. doi: 10.1016/s0002-9343(99)00337-x. [DOI] [PubMed] [Google Scholar]
- 35.Piazza CC, Carroll-Hernandez TA. Assessment and treatment of pediatric feeding disorders. In: Trembley RE, Barr RG, Peters RD, editors. Encyclopedia on Early Childhood Development. Montreal, Quebec: Centre of Excellence for Early Childhood Development; 2004. [Accessed May 27, 2008]. pp. 1–7. http://www.enfant-encyclopedie.com/Pages/PDF/Piazza-Carroll-HernandezANGxp.pdf. [Google Scholar]
- 36.Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111:1266–72. doi: 10.1378/chest.111.5.1266. [DOI] [PubMed] [Google Scholar]