Abstract
The challenge of osmotic stress is something all living organisms must face as a result of environmental dynamics. Over the past three decades, innovative research and cooperation across disciplines has irrefutably established that cells utilize mechanically gated ion channels to release osmolytes and prevent cell lysis during hypoosmotic stress. Early electrophysiological analysis of the inner membrane of Escherichia coli identified the presence of three distinct mechanosensitive activities. The subsequent discoveries of the genes responsible for two of these activities, the mechanosensitive channels of large (MscL) and small (MscS) conductance, led to the identification of two diverse families of mechanosensitive channels. The latter of these two families, the MscS family, is made up of members from bacteria, archaea, fungi, and plants. Genetic and electrophysiological analysis of these family members has provided insight into how organisms use mechanosensitive channels for osmotic regulation in response to changing environmental and developmental circumstances. Furthermore, solving the crystal structure of E. coli MscS and several homologs in several conformational states has contributed to the understanding of the gating mechanisms of these channels. Here we summarize our current knowledge of MscS homologs from all three domains of life, and address their structure, proposed physiological functions, electrophysiological behaviors, and topological diversity.
Keywords: Mechanosensitive, MscS, MSL, ion channel
INTRODUCTION
I. Ion Channels
Ion channels are membrane-spanning protein complexes that form a gated macromolecular pore. An open channel can facilitate the diffusion of tens of millions of ions per second from one side of the membrane to the other, down their electrochemical gradient 1, 2. The role played by ions in the excitable membranes of muscle and nerve cells has been studied for over a hundred years 3 and the importance of ion channels as mediators of the nervous system and their role in human disease is now well established (several recent reviews include 4–6). However, plant and microbial ion channels have also been important subjects of study 7, 8. It is often forgotten that single-cell action potentials were first described in the giant cells of characean algae and that during the 1930s, the excitation of squid axons and algal membranes was studied side-by-side (reviewed in 9–11). The first measurements of a membrane potential in living cells were performed in the ciliate Paramecium12. The bacterial potassium channel KcsA was the first ion-selective channel to be characterized by X-ray crystallography 13, and it is now understood that bacteria have a wide array of ion-specific, mechanosensitive, and water channels 14. Investigations into plant and microbial ion channels not only inform our understanding of basic cellular physiology, but may also be instrumental in engineering defenses against microbial pathogens and in crop improvement 15–17
Ion channels can be classified according to homology-based family groupings or functional characteristics such as ion selectivity or gating stimulus (in addition to other more subtle behaviors such as conductance, adaptation and opening or closing kinetics). Many channels are specific to the ion or small molecule that they allow to pass (KcsA has a 1000-fold preference for K+ over Na+ ions 18), while others are not (the bacterial mechanosensitive ion channel of large conductance (MscL) has no ionic preference at all 19). Channel conductance, the ease with which current passes from once face of the channel pore to the other, can range over several orders of magnitude in different channel types and organisms. For example, the aforementioned MscL has one of the largest conductances measured, up to 3 nS 19, while the small potassium (SK) channels associated with Parkinson’s disease have a conductance of only 10 pS 20. The burst of ion flux that results from the rapid opening of an ion channel (occurring on the order of milliseconds) can have several downstream effects: a change in membrane potential, which can serve as a signal itself by exciting other channels; a burst of intracellular Ca2+; or the normalization of ion concentrations across a membrane to control cell volume. Many ion channels open (or “gate”) only under certain conditions, such as altered transmembrane voltage, binding of a small ligand, or mechanical force. Mechanosensitive (MS) channels, whose principal gating stimulus is mechanical force, are considered in this review.
II. Mechanosensitive Ion Channels
A. Gating Models
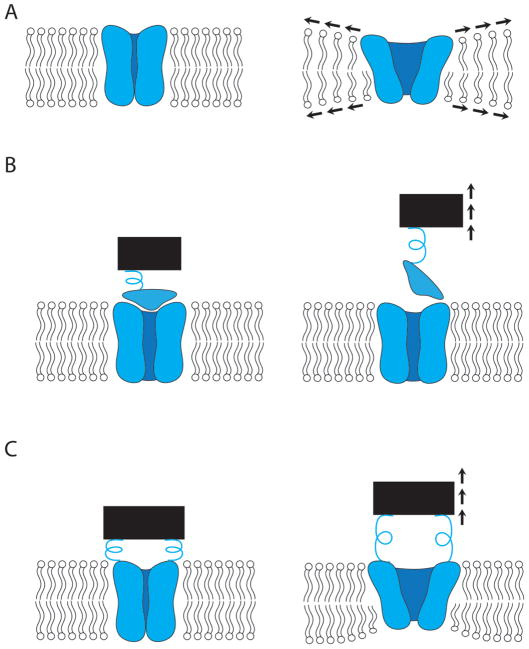
How force administered to a cell is delivered to a mechanosensitive channel, and how the channel subsequently converts that force into ion flux are important questions requiring the purposeful integration of genetic, biochemical, structural, and biophysical approaches. Three simplified models have been proposed for the gating of channels that act directly as mechanoreceptors (that is, there is not an intermediary between the force perception and the channel) 21–23. These models are described below and illustrated in Figure 1.
Figure 1. Schematic representation of models for mechanosensitive channel gating.
(A) The intrinsic bilayer model, wherein lateral membrane tension favors the open state of the channel. (B) The tethered trapdoor model, wherein a tether to an extracellular (in this case) component exerts force on the channel, leading to its gating. (C) The unified model, wherein a tether to an extracellular component leads to reorientation of the channel within the membrane bilayer, thereby gating it.
Intrinsic
In the intrinsic bilayer model (Fig. 1A), force is conveyed to the channel directly through the planar membrane in which it is embedded. Biophysical modeling approaches have identified a number of factors that may favor the closed state of the channel under low membrane tensions, including the energetic cost associated with hydration of the channel pore24, and the cost of membrane deformation at the perimeter of the channel 25. A channel can deform the surrounding membrane due to mismatch between the thickness of the membrane and the thickness of the hydrophobic domain of the channel. In addition, the membrane (which has a lower compressibility modulus than the channel 26) can be locally distorted or bent as it conforms to the shape, or profile, of the embedded channel 22, 27, 28. The energy cost associated with these membrane deformations increases upon channel opening, as the cross-sectional area—and therefore the perimeter—of the channel expands. However, loading the membrane with tension through a patch pipette or osmotic pressure can offset this energy cost; under these conditions the open state is favored. Importantly, membranes are active participants in the gating of MS channels and the pressure exerted by the lipid on the channel is a critical component of the intrinsic bilayer model 29. This model is supported by experimental evidence showing that the fluidity, thickness and curvature of the membrane influence the gating characteristics of MS channels 30–32.
Tethered
It has long been speculated that mechanotransduction by hair cells of the vertebrate inner ear is mediated by the action of tethers comprised of cadherin and protocadherin (called “tip links”) on transducer channels located in the hair cell plasma membrane (reviewed in 33). In the tethered trapdoor model (Fig. 1B), force is conveyed to the channel through tension applied to other cellular components, such as the actin or microtubule cytoskeleton and/or the extracellular matrix. Displacement of the cellular component pulls on the channel through the tether, thereby triggering its opening. The unified model proposes that rather than opening a trapdoor, pulling on the tether leads to reorientation of the channel within the lipid bilayer, which results in channel gating in response to the membrane deformation and tension forces described above for the intrinsic model (Fig. 1C)23, 34, 35. Finally, the hybrid model suggests that force-gated channels could be embedded in a cholesterol-rich platform that is in turn tethered to the cytoskeleton 36. In the latter two models, as with the intrinsic bilayer model, the biophysical properties of the membrane are an important contributor to the lowest energy conformation of a MS channel, and can either restrict or facilitate changes in state.
B. Electrophysiology and Model Systems
The first observations of ion flux in response to mechanical stimuli quickly followed the development of the patch-clamp technique in the early 1980s. This technique allows one to record the current passing across a small patch of membrane tightly sealed to the tip of a thin glass capillary pipette (reviewed in 37). A key aspect of this technique is the formation of a high resistance “gigaseal” between the membrane and the glass (on the order of 1GOhm or higher). When positive or negative pressure is applied to the membrane patch through this glass recording pipette, the membrane (and any associated cytoskeletal components) is deformed. The opening and closing of individual mechanically gated ion channels can then be observed over time 38, 39. Early patch-clamping experiments resulted in the identification of stretch-activated ion channels in animal cells known to be specialized for mechanical perception 40–43. Similar activities were soon identified in non-specialized cells 41, 44, leading to the proposal that sensitivity to mechanical stimuli might be a basic cellular feature 26, 45. In the decades since these first studies, many families of MS channels have been identified and characterized in bacteria, plants, animals, and archaea (reviewed in 46–48). MS channels can be activated by membrane tension introduced through the patch pipette as described above, by the swelling associated with hypoosmotic shock, or by treatment of cells with membrane-bending amphipaths. Their function has been investigated in endogenous membranes, in a variety of heterologous systems, and even reconstituted into artificial membranes. Leading the way in many of these studies is a suite of bacterial channels that are arguably the best studied and best understood mechanoperceptive proteins at the functional, structural, and biophysical levels.
III. Escherichia coli MscL, MscS, and MscM
A. Identification
Identifying MS channels in bacteria by electrophysiological analysis at first presented several challenges as an E. coli cell is smaller than the diameter of a typical patch pipette tip, and has a peptidoglycan layer between the inner and outer membranes 49, 50. This problem was solved by treating cultures with an inhibitor of cell division and then enzymatically digesting the peptidoglycan layer. These treatments result in the production of “giant E. coli protoplasts” amenable to patch clamp electrophysiology 51. Using this approach, the Kung group measured current induced in response to membrane stretch in E. coli and observed a robust tension-sensitive channel activity 49. Subsequent studies established that at least three distinct channel activities are detectable in the inner membrane of E. coli—the mechanosensitive channels of large, small, and mini conductances. MscL, MscS, MscM activities each have different conductances (3 nS, 1 nS and 0.3 nS, respectively) and are activated at decreasing thresholds of pressure 19, 52–54.
B. Cloning
It is now established that the three classic activities of the E. coli membrane, MscL, MscS and MscM, represent a complex combination of activities provided by two distinct families of MS channels. The E. coli mscL gene was cloned through a fractionation/reconstitution and microsequencing strategy 55 and found to be essential and sufficient for MscL activity. The mscS/yggB gene was identified through a combination of forward and reverse genetic approaches, and along with mscL it underlies the primary response of an E. coli cell to rapid increases in membrane tension 56. While the MscS and MscL proteins are structurally and evolutionarily unrelated, at least part of the originally observed MscS activity can now be attributed to the action of another channel with homology to MscS, now referred to as kefA/MscK 57 (for more on MscK, see below). When MscL 55 or MscS 58 channels are reconstituted into artificial liposomes, both show characteristics indistinguishable from that in native E. coli membranes, indicating that neither requires additional cellular structures for mechanosensitivity. Thus, both MscS and MscL are gated in direct response to lipid bilayer deformation, as in the intrinsic bilayer model (Fig. 1A). Relatively less is known about MscM, though recent reports have demonstrated that YjeP and YbdG, two more homologs of MscS, are likely to underlie this elusive activity 59, 60.
C. Physiological Function
Bacterial cells are found in a variety of dynamic environments, frequently requiring them to adapt to changing osmotic conditions. In order to maintain turgor pressure during exposure to hyperosmotic stress, bacterial cells accumulate osmolytes that are compatible with cellular metabolism 61. On the other hand, a sudden shift to hypoosmotic conditions will cause a rapid influx of water across the lipid bilayer, leading to increased membrane tension (reviewed in 39, 62). It has been estimated that a mere 20 mM drop in external osmolarity can result in membrane tensions that approach lytic levels if unrelieved 39. A hypoosmotic shock of this type might occur when soil bacteria are caught in the rain, when marine bacteria migrate to freshwater or during the transmission of enteric bacteria through excrement. Without a rapid response, these shocks would lead to a compromised cell wall, leaving the cell vulnerable to lysis 63.
It had long been proposed that bacterial cells were capable of relieving this type of environmental hypoosmotic stress by facilitating the exit of osmolytes from the cell, thus ensuring the physical integrity of the cell under increased turgor 50, 61, 64. We now know that the primary mechanism for hypoosmotic shock survival is the activation of MS channels, which allows the diffusion of nonspecific osmolytes out of the cell, relieving membrane tension and preventing cellular lysis. E. coli strains with lesions in both mscL and mscS show reduced survival of hypoosmotic shock though single mutations have no discernable effect 55, 56. Mutants lacking YbdG also show a small defect in osmotic shock survival 59 and the overexpression of YjeP promotes survival in the absence of all other MS channels 60. Thus, these bacterial MS channels are often referred to as osmotic “safety valves” 65 and have been proposed to provide a graded series of responses allowing the bacteria to tune its response to different osmotic challenges under different environmental or developmental conditions 14, 50, 53, 57, 66.
MSCS and MSCS-LIKE CHANNELS: CONSERVATION AND DIVERSITY
These classic mechanosensitive channels from E. coli described above not only serve important biological functions, but MscL and MscS have also become leading model systems for the study of MS channel structure and function. Here we focus on the structure and function of the bacterial mechanosensitive channel MscS and its homologs in E. coli, other microbes, and in eukaryotes. Several excellent reviews on MscL have recently been published 62, 67, 68.
I. Structure
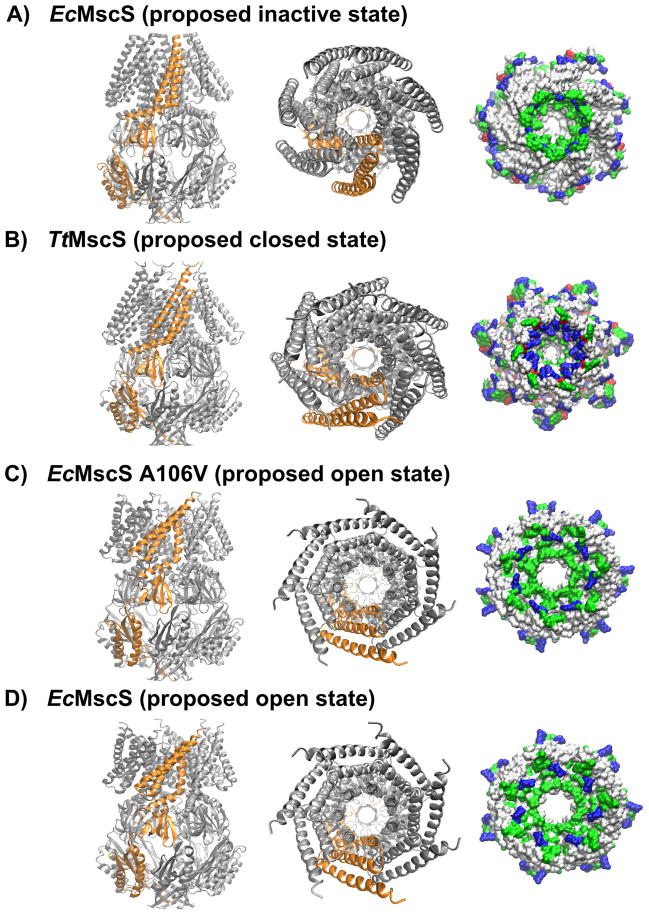
Crystallographic studies of MscS structure are beginning to answer the fundamental question of how mechanosensitivity is accomplished in MscS-type channels (recently reviewed in 69). At present, five structures of prokaryotic MscS homologs have been solved: wild type E. coli MscS (EcMscS) in both open and nonconducting (not necessarily closed, see below) conformations 70–72, a point mutation of EcMscS that likely represents the open conformation, and MscS homologs from Thermoanaerobacter tengcongensis (TtMscS)73 and Helicobacter pylori (HpMscS)70 in nonconducting conformations. Four of these structures are shown in Figure 2. A cartoon representation of each is shown from the side (left panel), and both cartoon and space-filling models are shown from the periplasmic surface (middle and right panels). A fragment containing the three TM domains and the upper vestibule from a single monomer of each of these structures (including amino acids 27–175 for MscS) is shown in Figure 3. Despite the inevitable possibility of artifacts associated with packing contacts and protein-detergent interactions 23, 74, 75, these structures provide an invaluable source of information about the molecular mechanism of gating and the relationship between channel structure and electrophysiological behavior.
Figure 2. Crystal structures of E. coli MscS and homologs.
(A) EcMscS in inactive/non-conductive state (2OAU, Steinbacher, 2007); (B) TtMscS from T. tengcongensis in closed state (3UDC, Zhang, 2012); (C) A106V EcMscS mutant in open state (2VV5, Wang, 2008); (D) EcMscS in open state (4HWA, Lai, 2013). Left panel, side view of the heptameric channel. Middle panel, view from the periplasmic side. Right panel, space-filling representation of the pore from the periplasmic side; the channels are truncated at I175 (for EcMscS) for an unobstructed view. Basic residues are blue, acidic residues are red, polar residues are green, non-polar residues are white. The images were generated with VMD software (University of Illinois).
Figure 3. The conserved region of EcMscS and TtMscS monomers in different conformations.
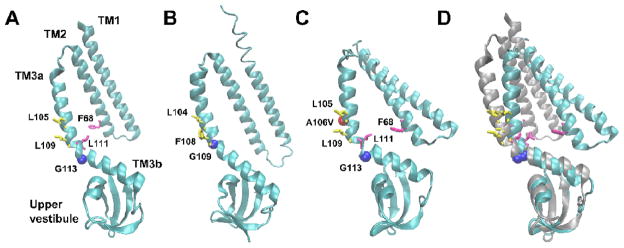
A single monomer of (A) EcMscS (aa 27-175) in a nonconducting state (2OAU, Steinbacher, 2007); (B) TtMscS (aa 13-175) in a nonconducting state (3UDC, Zhang, 2012); (C) EcMscS A106V (aa 25-175) in an open state (2VV5, Wang, 2008). (D) Superposition of panel A with a single monomer of EcMscS (27-175) in an open state (4HWA, Lai, 2013). The kink-forming residues G113 (EcMscS) and G109 (TtMscS) are represented as blue spheres and the A106V mutation as a red sphere. The vapor-lock residues L105 and L109 are labeled in yellow. F68 and L111, residues proposed to mediate the TM2-TM3 interaction (Belyy, 2010) are labeled magenta. Images were generated with VMD software (University of Illinois).
A. Nonconducting and Open Conformations of EcMscS and Homologs
Nonconducting Conformations
The first crystal structure of EcMscS was solved by the Rees group at 3.7 Å resolution 71, 72 (Fig 2A) and revealed a homoheptameric channel with three transmembrane alpha helices per monomer and a large, soluble C-terminal domain. This oligomeric state and topology were subsequently verified experimentally 76–78. As shown in Figure 3, each monomer contributes three N-terminal transmembrane (TM) alpha helices to the transmembrane region. TM1 (residues 28 - 60) and TM2 (residues 63 - 90) face the membrane, while TM3 (residues 93 - 128) lines the channel pore. (The residues assigned to each helix are as in 69). One striking feature of the structure is a sharp kink at Q112/G113, which divides TM3 into TM3a, which is roughly perpendicular to the membrane, and TM3b, which is almost parallel to the membrane (Fig. 3A). The narrowest constriction of the pore has a diameter of 4.8 Å, and is created by two rings of Leucine residues (L105 and L109) with inward facing side chains. These hydrophobic rings are proposed to prevent the wetting of the pore and thereby serve as a “vapor lock” to the movement of ions through the channel 79, 80. Mutational analysis of L105 confirmed its importance in maintaining the closed state 76. The C-terminal region of each monomer contributes to a large hollow structure referred to here as the “vestibule”. The vestibule comprises seven side portals and one axial portal located at the base of the vestibule, formed by a seven-stranded β-barrel.
Originally thought to be the open conformation, this structure it is now generally agreed to represent a nonconducting state. It is unlikely to represent the normal closed conformation, because TM1 and TM2 are not in contact with TM3, an expected requirement for tension-sensitive gating (see the section on “force-sensing” below)39, 81. A number of molecular dynamics (MD) simulations further support this conclusion 79, 82, 83. The recently reported structures of TtMscS (Fig 2B) and HpMscS (not shown) exhibit similar transmembrane helix organization and pore size as the original EcMscS structure, and therefore are also considered to represent nonconducting states 70, 73. The C-terminal vestibule of TtMscS has several differences in structure from that of EcMscS, which are shown to modulate the conducting properties of the channel and are discussed below.
Open Conformations
Though invaluable for establishing the basic structure of MscS, nonconducting structures give limited insight into the channel’s gating mechanism. In a directed attempt to solve the structure of MscS in an alternate conformation, the Booth and Naismith groups crystallized the A106V point mutation of EcMscS at 3.45 Å resolution 84 (Fig. 2C). The resulting structure has a substantially increased pore size (approximately 13 Å in diameter) due to a rearrangement of transmembrane helices. TM1 and TM2 are angled away from TM3b and the channel core, while TM3a is tilted away from the plane of the membrane and rotated slightly away from the pore (compare Fig 3A and C). TM3b and the upper vestibule are mostly unchanged compared to the nonconducting structures. These rearrangements place the vapor lock residues out of the pore, as previously predicted based on experimental and modeling data 85–87. A pulsed electron-electron double resonance (PELDOR) approach 88 revealed that two EcMscS mutants, spin-labeled at D67C (PDB 4AGE) or L124C (4AGF), took a similar conformation in solution, indicating that it is not an artifact of crystal packing nor of the particular A10V mutation 89. Further confirmation that the A106V structure properly resembles the open state comes from a recent report describing wild type EcMscS solubilized in a different detergent (β-dodecylmaltoside instead of fos-choline-14), at a resolution of 4.4 Å 70 (Fig. 3D). This structure closely resembles the A106V EcMscS structure, establishing a solid consensus regarding the open state structure of EcMscS.
B. Gating Mechanism
Despite having multiple crystal structures attributed to different states of MscS, as well as an array of mutational and functional data that have determined functionally important residues, the actual mechanism of transition between closed and open states is still not completely clear. While several models have been proposed based on MD simulations 86, 90 and electron paramagnetic resonance (EPR) spin labeling 87, the model which is currently favored is one wherein membrane tension induces the rotation and tilting of TM1 and TM2 as a whole, immersing them more deeply into the surrounding lipid bilayer. This movement pulls TM3a away from the pore until it’s oriented almost normal to the membrane plane, effectively removing the L105 and L109 vapor lock side chains and opening the channel to ion flux 69, 84. In all of the crystal structures described above, the positioning of TM1 and TM2 with respect to each other is the same, as if they act like a rigid lever (compare Fig 3A, B to Fig. 3C and D). Assuming that the newly obtained crystal structures described above indeed represent nonconducting and open states, the “rigid-body” movement model of transition into the open state may be considered the most probable.
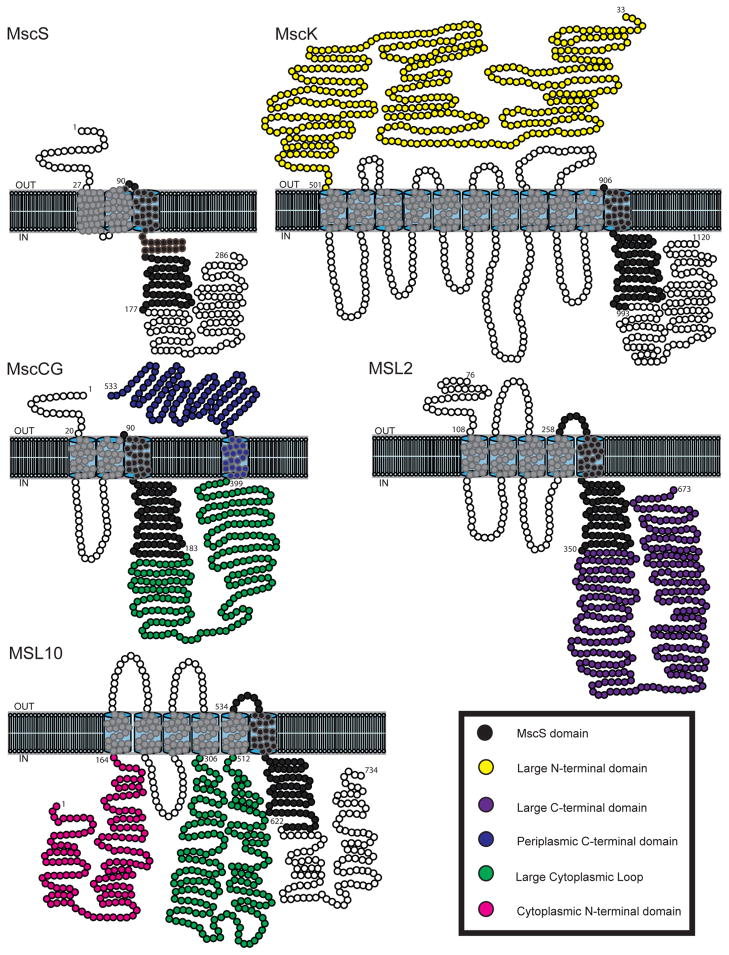
Lipid-protein interactions must occur at the periphery of the channel, which in MscS is likely to be comprised of TM1 and TM2. Hydrophobic residues in the protein-lipid interface of TM1 and TM2 were shown in several site-directed mutagenesis studies to affect tension sensitivity and osmotic shock protection 91, 92. In addition, an interaction between F68 in TM2 and L111 in TM3 was shown by electrophysiology and mutational analysis to be of critical importance for force transmission from lipid-facing helices to the pore region; disruption of this inter-helical contact results in channel inactivation 81. These data are consistent with a model wherein TM1 and TM2 serve as a tension sensor, transmitting force from the membrane to TM3; subsequent rearrangement of TM3 helices results in channel gating. It is intriguing to consider MscS homologs that possess additional N-terminal transmembrane helices (for several examples, see Figure 4). Additional helices may shield TM2 and TM3 from lipid environment of membrane or serve as tension sensors themselves, transmitting force to the pore-lining helix through a different (yet unknown) mechanism 93.
Figure 4. Monomer topologies of representative MscS family members.
MscS monomer topology was rendered based on Naismith and Booth, 2012. The domain conserved among all MscS homologs is indicated in black; other domains are colored as indicated in the legend. For the purpose of clarity TM3b of MscS is represented outside the lipid bilayer. MscK and MscCG topologies were predicted with TOPCONS (http://topcons.net/) and ARAMEMNON (http://aramemnon.botanik.uni-koeln.de/) for MSL2 and MSL10. Processed versions of MscK and MSL2 are presented.
C. Contributions by the C-terminus
Though the structure of the C-terminal vestibule is virtually unchanged in all the crystal structures assigned to open and nonconducting states of EcMscS, other evidence indicates that this portion of the channel may be subject to conformational changes during opening, closing, inactivation and desensitization transitions. Analyses of multiple deletion and substitution mutants have established that the vestibule is important for channel function and stability 76, 94, 95, and that interactions between the upper surface of the vestibule and the TM domain can affect gating as well as inactivation behavior 96–99. Co-solvents that induce compaction of the C-terminal domain have been shown to facilitate MscS desensitization and inactivation 100, while experiments utilizing FRET to quantify the diameter of the cytoplasmic domain showed that it swells during gating 101. Taken together, these data indicate that gross structural remodeling of the vestibule and its interactions with the transmembrane domain likely accompanies inactivation and gating cycles, and it has been speculated that the C-terminus may serve as a sensor for molecular crowding in the cytoplasm 102.
In addition, the C-terminal vestibule appears to serve as an ion selectivity filter. While MscL forms a large, completely nonselective pore, MscS is slightly anion-selective, preferring Cl− ions to K+ ions by a factor of as much as 3 (PCl− : PK+ = 1.2 – 3) 58, 103–105. Ions likely do not enter the vestibule through the axial β-barrel, as the portal that it forms is too narrow (1.75 Å in its narrowest part); rather, they probably travel through the seven side portals into the vestibule and then cross the pore. MD simulations suggest the vestibule serves to filter and balance charged osmolytes prior to their release from the cell 106 and it was recently demonstrated that an electronegative domain adjacent to the side portals contributes to anion selectivity, likely by hindering the passage of cations 107. Another correlation between the structure of the C-terminus and ion selectivity comes from the functional study of TtMscS 73. Compared to EcMscS, TtMscS has smaller side portals but a much wider axial portal; at the same time it has a much higher selectivity for anions. A version of TtMscS where the axial beta;-barrel sequence (amino acids 271 to 282) was replaced with the corresponding portion of EcMscS lost this preference for anions. Taken together, these data indicate that both the β-barrel and the C-terminal vestibule are important determinants of channel behavior.
Thus, the five independently derived crystal structures of bacterial MscS homologs available to date have revolutionized our understanding of the overall architecture of bacterial MscS homologs, provided context for the interpretation of mutagenic data and MD simulations, and established a sophisticated foundation for furthering our understanding of the gating cycle. We note that no crystal structures have yet been reported for archaeal or eukaryotic MscS homologs; such a structure would be a major step forward for those interested in the evolutionary diversification of this family of proteins.
II. Evolutionary History
The MscS protein superfamily is vast and diverse, with members found in most bacterial, protozoan, archaeal, some fungal, and all plant genomes so far analyzed 108–114. However, MscS family members have not yet been found in animals. It has been suggested that MS channels first evolved in an ancestor common to all cell-walled organisms and have been maintained throughout these lineages as a solution to osmotic stress and regulation of turgor pressure 108, 109, 115. Another explanation is that the membrane reservoirs of animal cells allow hypoosmotic swelling without producing membrane tension, or that mammalian membranes do not stretch due to their close association with the cytoskeleton 116, 117. Alternatively, MscS homologs could simply be unrecognizable in animal genomes by current homology-based searches.
Mapped onto the MscS structure, the conserved domain comprises the pore-lining helix (in MscS, this is TM3) and the upper part of the cytoplasmic vestibule. Outside of this domain MscS family members vary greatly in sequence and topology. The number of predicted TM helices for MscS family members ranges from 3 to 12 and a variety of conserved domains, including those associated with the binding of Ca2+ and cyclic nucleotides, have been identified in some subfamilies 56, 108, 118, 119. Furthermore, multiple MscS homologs are frequently identified within a single organism (including many bacterial and all plant genomes analyzed to date), suggesting that functional specialization of MscS homologs has evolved both between species and within a single organism. Our current understanding of the physiological function of MscS homologs from bacteria, fungi, plant cells and plant organelles is described below and summarized in Table 1.
Table 1.
Physiological functions of MscS family members
| Organism | Gene Name | Amino Acids | Physiological Function | Mutant Phenotype | Subcellular Localization | References | |
|---|---|---|---|---|---|---|---|
| Prokaryotes | Escherichia Coli | YggB (MscS) | 286 | Release of ions during hypoosmotic shock | mscS mscL mutant exhibits loss of viability during osmotic down-shock; mscS mscK mutant has complete loss of MscS channel activity | Plasma membrane | Levina et al., 1999 |
| MscK (KefA) | 1120* | Release of ions in high K+ environments | mscS mscK mutant has complete loss of MscS channel activity | Plasma membrane | Levina et al., 1999; Mclaggan et al., 2002 | ||
| YbiO | 741 | Release of osmolytes in high NaCl environments | ybiO mutant has loss of 20 pA channel activity | Plasma membrane | Edwards et al., 2012 | ||
| YjeP | 1107 | Release of ions during hypoosmotic shock | yjeP mutant has loss of 7.5-13pA channel activity | Plasma membrane | Edwards et al., 2012 | ||
| YnaI | 343 | NR | ynaI mutant has loss of 2 pA channel activity | Plasma membrane | Edwards et al., 2012 | ||
| Campylobacter jejuni | Cjj0263 | 627 | Osmotic protection and host colonization | cjj0263 has decreased viability after osmotic down-shock; cjj0263 cjj1025 mutant exhibits impaired chick ceca colonization | Plasma membrane | Kakuda et al. 2012 | |
| Cjj1025 | 523 | Host colonization | cjj0263 cjj1025 mutant exhibits impaired chick ceca colonization | Plasma membrane | Kakuda et al. 2012 | ||
| Bacillus subtilis | YkuT | 267 | Osmotic protection | mscL ykuT mutant strain has increased sensitivity to osmotic down-shock | Plasma membrane | Hoffmann et al., 2008; Wahome and Setlow, 2008; Wahome et al., 2009 | |
| Corynebacterium glutanicum | MscCG | 533 | Involved in betaine and glutamate efflux | mscCG mutant is impaired in betaine efflux during hyper and hypoosmotic shock and exhibits a 70% decreases in glutamate export | Plasma membrane | Yao et al., 2009; Börngen et al., 2010; Nottebrock et al., 2003; Nakamura et al., 2007; Becker et al., 2013 | |
| Synechocystis sp. PCC 6803 | PamA | 680 | Involved in the transcriptional control of sugar and nitrogen metabolism genes | pamA mutant is glucose sensitive; shows decreased levels of nitrogen-response genes and the stress sigma factor SigE | NR | Osanai et al., 2005 | |
| Eukaryotes | Arabidopsis thaliana | MSL2 | 673* | Plastid osmotic stress response; division ring placement | msl2 null mutants show defective leaf shape; msl2 msl3 mutant has enlarged, round non-green plastids and enlarged chloroplast exhibiting multiple division rings | Plastid envelope | Haswell and Meyerowitz, 2006; Jensen and Haswell, 2011; Wilson et al., 2011; Veley et al., 2012 |
| MSL3 | 678* | Plastid osmotic stress response; division ring placement | msl2 msl3 mutant has enlarged, round non-green plastids and enlarged chloroplast exhibiting multiple division rings | Plastid envelope | Haswell and Meyerowitz, 2006; Wilson et al., 2011; Veley et al., 2012 | ||
| MSL4 | 881 | NR | Loss of predominant MS channel activity in root of the msl4 msl5 msl6 msl9 msl10 quintuple mutant | NR | Haswell, Peyronnet et al., 2008 | ||
| MSL5 | 881 | NR | Refer to MSL4 | NR | Haswell, Peyronnet et al., 2008 | ||
| MSL6 | 856 | NR | Refer to MSL4 | NR | Haswell, Peyronnet et al., 2008 | ||
| MSL9 | 742 | NR | msl9 null mutant is associated with a loss of 45 pS activity in root protoplast | Plasma membrane | Haswell, Peyronnet et al., 2008 | ||
| MSL10 | 734 | NR | msl10 null mutant is associated with a loss of 137 pS activity in root protoplast | Plasma membrane | Haswell, Peyronnet et al., 2008 | ||
| Chamydomonas reinhardtii | MSC1 | 522 | Chloroplast organization | RNAi-mediated knockdown lines show reduced chlorophyll autofluorescence and loss of chloroplast integrity | Chloroplast envelope | Nakayama et al., 2007 | |
| Schizosaccharomyces pombe | Mys1 | 1011 | Involved in regulating intracellular Ca2+ and cell volume during hypoosmotic stress | mys1− mys2− mutants show decreased viability during osmotic down-shock and treatment with CaCl2 | Perinucler ER | Nakayama et al., 2012 | |
| Mys2 | 840 | Involved in regulating intracellular Ca2+ and cell volume during hypoosmotic stress | mys2− and mys1− mys2− mutants show decreased viability during osmotic down-shock and treatment with CaCl2 | Cortical ER | Nakayama et al., 2012 |
NR = Not Reported
= Unprocessed protein
III. Physiological Function
While it has been clearly established that MscL and MscS serve to protect cells from extreme environmental hypoosmotic shock, it is becoming evident that the functions of the members of this family may be more complex. An emerging theme is that MscS homologs have evolved specific functions tailored to the needs of the organism, including the release of specific cellular osmolytes in response to specific environmental or developmental osmotic triggers.
A. Prokaryotes
E. coli
We know by far the most about the six MscS family members encoded in the E. coli genome (MscS, MscK, YjeP, YbdG, YbiO, and YnaI) 56. Research into their physiological roles suggests that they all serve to release osmolytes from the cell under hypoosmotic stress but that their function is only required for cell viability under specific conditions. Even MscS may serve specialized roles, as MscS protein levels fluctuate. MscS levels are elevated during growth at high osmolarity, possibly a preemptive method of dealing with an impending downshock, and during stationary phase, perhaps to deal with the osmotically vulnerable state of cell wall remodeling 120, 121. MscS co-localizes with the phospholipid cardiolipin at the poles of the cell 122 and abnormal division ring placement was observed in an E. coli strain lacking MscL, MscS, and MscK 123, 124, suggesting that MscS may also function in bacterial cell division. It was recently reported that the transient increase in cytosolic Ca2+ observed in hypoosmotically shocked bacterial cells is dramatically reduced in an E. coli strain lacking MscS, MscK and MscL, opening up the possibility that MS channels may also impact Ca2+ homeostasis 107.
MscK contributes modestly to cell survival during standard osmotic shock assays 56, 103, 125 and its mechanosensitive channel activity requires the presence of K+ ions in the extracellular solution. It has been proposed that binding of K+ primes the channel for gating. Such an activity may be required for survival in soils with high concentrations of animal urine or within the kidneys during host infection 103. The remaining E. coli MscS family members (YbdG, YjeP, YbiO and YnaI) can provide osmotic shock protection when overexpressed in E coli59, 60, and the latter three activities may simply be expressed at too low levels to contribute under normal laboratory assay conditions. Indeed, the occurrence of the previously uncharacterized mechanosensitive channel activity attributed to YbiO increased dramatically when cells were treated with NaCl prior to patch-clamping 60.
Other species
The 3 MscS homologs (yhdy, yfkC, and yukT) of the gram-positive bacterium Bacillus subtilis are dispensable for osmotic shock survival in the laboratory, though the mscL yukT double mutant strain exhibits enhanced osmotic sensitivity compared to the mscL single deletion strain 126–128. As B. subtilis is found in both the soil and the human gut, there may be specific growth conditions wherein these MscS homologs contribute to osmotic homeostasis that are not replicated in the laboratory environment. Other prokaryotic MscS homologs have been identified that provide tantalizing ideas about the variety of ways in which this family of channels may have evolved to provide osmotic adjustment in response to different environmental and developmental stimuli. The gram-positive bacterium Corynebacterium glutamicum is used in the industrial production of glutamate and other amino acids 129. Its genome encodes homologs of both MscL and MscS (MscCG/NCgl1221), but neither is required for cell survival in laboratory-based osmotic downshock assays 130, 131. Instead, MscCG is involved in regulating the steady state concentration of glycine betaine (the preferred compatible osmolyte of C. glutamicum) in response to both hypo- and hyperosmotic stress 132. MscCG is also essential for glutamate efflux in response to biotin limitation and penicillin treatment, notably in the absence of hypoosmotic stress 131, 133. Several lines of evidence, including the analysis of loss-of-function and gain-of-function lesions in the predicted pore-lining helix, support the model that MscCG directly mediates the efflux of glutamate and that this efflux is dependent on mechanosensitive channel gating 131, 133–135. Thus, MscCG is likely a mechanically gated MscS homolog that is involved in osmotic adjustment of specific compatible solutes in response to multiple stimuli.
Finally, there are indications that MscS family members are important for pathogenesis and metabolism, perhaps indicating the importance of osmotic adjustment in these processes. Two MscS homologs from the food-borne pathogen Campylobacter jejuni, Cjj0263 and Cjj1025, were recently found to be required for colonization of the digestive tract of chicks 136, and a Pseudomonas aeruginosa MscK ortholog has been associated with virulence 137. PamA, a MscS homolog from the photosynthetic cyanobacterium Synechocystis sp.PCC6803 was reported to interact in vitro and in vivo with PII, a highly conserved carbon/nitrogen sensor 138, 139. Furthermore, nitrogen response and sugar metabolic genes show altered expression in the absence of PamA, suggesting that it may serve to integrate carbon and nitrogen metabolism with osmotic conditions. A recent study of MscL-like and MscS-like activities in Vibrio cholera showed that this species has a high density of MS channels in the cell membrane, yet is more sensitive to osmotic shock than wild type E. coli140. Taken together, these preliminary studies illustrate how much more has yet to be revealed regarding MscS homolog function in the prokaryotic world.
B. Eukaryotes
While less studied than their prokaryotic counterparts, recent research offers a few glimpses into the important functions and novel characteristics of the eukaryotic members of the MscS family. Sequence similarities place them into two major classes (described in 110). Class II members are predicted to localize to the plasma membrane or intracellular membranes of both plants and fungi. Class I channels, which show slightly more sequence conservation to MscS than those in class II, are predicted to localize to endosymbiotic organelles (mitochondria and plastids such as chloroplasts), and are found only in plant genomes.
Class I
Considering the origin of endosymbiotic organelles (the engulfment of a primitive bacterium), the MscS homologs found in their envelopes are likely to have a conserved function as osmotic safety valves, but in this case protecting mitochondria and plastids from fluctuations in intracellular rather than extracellular osmotic concentrations 16. The Mechanosensitive Channel (MSC)1, from Chlamydomonas reinhardtii localizes to punctate spots associated with the single plastid found in these cells, and plastid integrity is lost when the MSC1 gene is silenced by RNAi 141. To date, MSC1 is the only Class I MscS homolog to be successfully characterized by electrophysiology (see below). Like MSC1, MscS-Like (MSL)2 and MSL3 of Arabidopsis thaliana localize to distinct foci in the plastid envelope. These two land plant Class I homologs are required for normal plastid shape and size and for proper placement of the plastid division ring 124, 142. The large, round plastid phenotype of the msl2 msl3 mutant can be suppressed by a variety of genetic and environmental treatments that increase cytoplasmic osmolyte levels, indicating that plastids are under hypoosmotic stress from within the cytoplasm and that MSL2 and MSL3 are required to relieve that stress 143. Several Class I MscS homologs from land plants are predicted to localize to the mitochondria 110, 113, but their study has not yet been reported.
Class II
The identification of MscS homologs in plant genomes 108, 109 was exciting for plant biologists because it provided candidate genes for the MS channel activities already known to be widespread in plant membranes 110. However, while the Arabidopsis genome contains seven MSL proteins that are predicated to localize to the plasma membrane, and they exhibit distinct tissue-specific expression patterns 108, 110, a clear physiological function has yet to be assigned to any (though MSL10 has been characterized by patch-clamp electrophysiology, see below). The recent characterization of two endoplasmic reticulum-localized MscS homologs from Schizosaccharomyces pombe, Msy1 and Msy2, suggests that these channels may serve as hypoosmotic stress signaling molecules as much as osmotic safety valves 118. msy1- msy2- mutant cells exhibit greater swelling and higher Ca2+ influx upon hypoosmotic shock, and are more likely to subsequently undergo cell death. Consistent with this idea, we have proposed that MSL10 could play a role in hypoosmotic stress signal transduction through membrane depolarization 144.
To conclude, current evidence indicates that members of the MscS superfamily exhibit unique forms of regulation and variations of function. While all are variations on a common theme—action as an osmotic conduit in response to membrane tension—the proteins within this family may have become as diverse as the organism in which they reside. We anticipate that more precise analyses, under diverse growth conditions and at the single cell or organellar level, will reveal the role played by these channels in the osmotic homeostasis of cells and organelles.
IV. Electrophysiological Behavior
Besides EcMscS, many MscS superfamily members have been shown to be mechanosensitive, including five others from E. coli (MscK, YbdG, YnaI, YjeP, and YbiO)59, 60, 103 and three from other bacterial species (TtMscS from Thermobacter tengcongensis73, MscSP from Silicibacter pomeroyi145, MscCG from Corynebacterium glutamicum132 Two MscS homologs from the archaea Methanococcus jannaschii, MscMJ, and MscMJR have been characterized 115, 146, as have two channels from photosynthetic eukaryotes (MSC1 from Chlamydomonas reinhardtii and MSL10 from Arabidopsis thaliana141, 144. Despite striking differences in topology and sometimes very low sequence identity, these channels demonstrate surprisingly conserved behavior in many aspects. Perhaps most striking are their relatively large unitary conductances, often orders of magnitude larger than those recorded from most animal ion channels. Their major characteristics are listed in Table 2 and discussed in further detail below. Not included here are MscS-related channels from B. subtlilis147, Streptococcus faecalis148, and the bCNG family 119.
Table 2.
Single-channel properties of MscS family members
| Species | Name | Unitary conductance | Ion selectivity (PCl : PK) | Number of TMHsc | Identity in the pore-lining domain + upper vestibule to EcMscS, %d | References | |
|---|---|---|---|---|---|---|---|
| Prokaryotes | E. coli | EcMscL a | 3 nS 1 | Non-selective | 2 | - | Sukharev, 1994; Häse, 1995 |
| EcMscS | 1.2 nS 1/350 pS 5 | 1.2–3 | 3 | 100 | Levina, 1999; Sukharev, 2002 | ||
| EcMscK | 1 nS 1 | < EcMscS | 11* | 32 | Martinac, 1987; Li, 2002 | ||
| YjeP | 250–400 pS 1 | NR | 11* | 27 | Edwards, 2012 | ||
| YbdG b | 350–400 pS 1 | NR | 5* | 21 | Schumann, 2010 | ||
| YnaI | ~ 100 pS 1 | NR | 5* | 30 | Edwards, 2012 | ||
| YbiO | ~ 850 pS 1 | NR | 12* | 24 | Edwards, 2012 | ||
| S. pomeroyi | MscSP | 1.04 nS 2 | 1.4 | 3* | 49 | Petrov, 2013 | |
| T. tengcongensis | TtMscS | 134 pS 1 | 8.7 | 3 | 29 | Zhang, 2013 | |
| C. glutamicum | MscCG | 328 pS 2 | 0.3 | 4 | 29 | Börngen, 2010; Becker, 2012 | |
| M. jannashii | MscMJLR | 2 nS 3 | 0.2 | 5* | 41 | Kloda, 2001a,b | |
| MscMJ | 270 pS 3 | 0.16 | 5* | 36 | Kloda, 2001a | ||
| Eukaryotes | C. reinhardtii | MSC1 | 390 pS 4 | 7 | 5* | 32 | Nakayama, 2007 |
| A. thaliana | MSL10 | 103 pS 5 | 5.9 (PCl : PNa) | 6* | 18 | Haswell, 2008; Maksaev, 2012 |
200 mM KCl, 90 mM MgCl2, 10 mM CaCl2, and 5 mM HEPES (pH 7.0)
250 mM KCl, 90 mM MgCl2, and 5 mM HEPES (pH 7.2)
200 mM KCl, 5 mM MgCl2, and 5 mM HEPES (pH 7.2)
200 mM KCl, 40 mM MgCl2, 10 mM CaCl2, 0.1 mM EDTA, and 5 mM HEPES-KOH (pH 7.2)
96 mM NaCl, 2 mM KCl, 2 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES (pH 7.38)
100 mM KCl, 10 mM MgCl2, 10 mM HEPES-KOH (pH 7.4)
MscL is not a MscS homolog, added for reference
Channel activity was only shown for a V229A mutant of YbdG-encoded protein
number of transmembrane helices were predicted via TMHMM 2.0 server.
Alignments were made using Kalign algorithm in Unipro UGENE software
= Predicted
NR = not reported
A. Conductance and Ion Selectivity
The MscS homologs listed in Table 2 all have weak to moderate ionic preferences and single-channel conductances that fall approximately into a 4-fold range. MscSP closely resembles EcMscS in sequence and in channel characteristics 145, and MscK has a conductance close to that of EcMscS 56, 103. However, some variation is observed among the prokaryotic channels, with a smaller conductance typically associated with more selectivity. MscCG has a single-channel conductance of 0.3 nS, about one-third the size of that provided by EcMscS, and prefers cations (PCl− : PK+ = 0.3) 132. YjeP has a similar conductance, and is also likely to have a preference for cations, as this was the early characterization of MscM activity (PCl− : PK+ = 0.4)53, 60. As described above, TtMscS has a single-channel conductance approximately half that of EcMscS and is more strongly anion-selective (PCl− : PK+ = 8.7) 73. MscMJ (270 pS) and MscMJLR (2 nS) both exhibit a similar preference for cations (PCl− : PK+ = 0.16 and 0.2, respectively) 115, 146. Eukaryotic channels MSC1 and MSL10 are quite similar to each other: both have conductances around a third of that of MscS under similar conditions and both show a preference for anions (PCl− : PK+ = 7 and 6, respectively) 141, 144, 149.
B. Inactivation and desensitization
Models of the MscS activation cycle typically include four distinct states: open, closed, inactive and desensitized 56, 98, 100, 150. While the latter three states are distinct, both an inactivated and a desensitized channel will manifest themselves as current decay in patch-clamp recordings under fixed membrane tension. To experimentally distinguish between them one must either apply an additional pulse of pressure beyond what is required to saturate all the channels in the patch, or decrease tension and apply it once again. In the inactive state, the channel cannot make a transition to the open state under any tension, while a desensitized channel could be gated by the application of increased tension. While inactivation and/or desensitization under sustained membrane tension have been reported for MscS expressed in several systems 90, 98, 149, MscSP, MscCG, MscK, MscMJ and MscMJLR do not desensitize 56, 103, 115, 132, 145, 146. MSL10 does not show any significant signs of inactivation 144, while MSC1 inactivates at positive membrane potentials, but not at negative 141. These results leave unclear the physiological relevance of inactivation 62.
Recent insight into the molecular mechanism and physiological relevance of inactivation came from the discovery that, like channel opening, EcMscS inactivation occurs as a transition from the closed state 150. The inactivated state is characterized by an ~8.5 nm2 in-place protein expansion (small compared to the 12–15 nm2 expansion associated with channel opening). During inactivation, the TM1/TM2 hairpin is thought to bend away from TM3, which stays in the closed arrangement 72. The result is a channel conformation wherein TM1and TM2 are sterically restricted from transmitting tension to the pore region, and the channel cannot be gated. This behavior may prevent efflux of important solutes from the cell under slightly hypoosmotic conditions. On the other hand, the lack of inactivation from the open state ensures that the population of channels stays open as long as membrane tension is kept high 150, 151.
C. Hysteresis
Another feature of mechanosensitive channel behavior is hysteresis, or a difference between the tensions required for opening and closing. EcMscS is routinely observed to close at lower tensions than at which it opened when tension is rapidly applied and released. However, this behavior disappears when tension is changed more gradually, and is likely the consequence of a relatively slow closing rate (summarized in 57, 152). The eukaryotic channels MSC1 and MSL10 also show hysteresis. These channels not only close at a lower tension than at which they opened, but a subpopulation of both types of channels often is observed to stay open even after all membrane tension has been released 141, 144. There are no reports of any functional importance attributed to this phenomenon, but the continuous slow depolarization of the membrane due to channels staying open after membrane tension is relieved could result in the gating of depolarization-activated channels and/or the propagation of a systemic signal.
Thus, despite limited sequence identity, the MscS family members so far characterized share similar basic channel characteristics such as conductance and ion selectivity. Other behaviors observed under patch clamp, such as hysteresis and inactivation/desensitization, are more variable and have unclear physiological relevance. One could speculate that the conserved features of these channels reflect their common function (rapid release of osmolytes in response to membrane tension) while their characteristic differences reflect the specific natures of their ecological niches 60. Additional examples may help to determine the functional range of properties that have been selected by evolution.
V. Topological Diversity in the MscS Superfamily
The topological complexity of MscS family members (as described above and illustrated in Figure 4) has been taken to imply regulatory complexity 23, 112, and data suggest that this may indeed be the case. Many members of the MscS family contain N- and C-terminal domains dramatically larger than that of MscS, presenting the possibility of additional functions and regulation sites. For example, the unusually large periplasmic N-terminal region of MscK could regulate channel activity by preventing gating in the absence of high K+ 103, 153. Removal of the N-terminal region of MscK, including TM helices1–9, abolishes K+-dependent gating and promotes its ability to provide protection from hypoosmotic shock 76. Similarly, the presence of an extra TM helix C-terminal to the pore-forming helix is unique to MscCG, and can confer the ability to facilitate glutamate efflux when fused to EcMscS 133. Proteins comprising the bCNG family all encode a large soluble C-terminal domain containing a cyclic nucleotide-binding domain. This domain has been shown to negatively regulate the mechanosensitive channel activity of one of the family members 119, 154.
The eukaryotic MscS family members show topology that is just as diverse. A variety of physiological functions have been attributed to the chloroplast channels MSL2 and MSL3, which contain a C-terminal cytoplasmic domain three times the size of the MscS soluble domain 124, 155. Although the regulatory and functional importance of this domain has yet to be confirmed, preliminary evidence suggests that a highly conserved domain within this region is required for proper subcellular localization and channel function in vivo (E. S. Haswell, unpublished). Class II (plasma membrane- and ER-localized) eukaryotic homologs of MscS, such as MSL10, typically share a common topology of 6 TM regions, large soluble N- and C-termini, and a large cytoplasmic loop between TM helix 4 and 5 110, 118, suggesting that their conserved structure serves a eukaryote-specific function. The large cytoplasmic regions of many Class II proteins suggest a number of possible regulatory mechanisms. For example, Msy1 and Msy2 contain an EF-hand Ca2+-binding motif 156 in the large cytoplasmic loop between TM4 and TM5. Genetic analyses suggest that this region is important for sensing and/or controlling Ca2+ influx as well as contributing to channel function in response to hypoosmotic stress 118.
FUTURE DIRECTIONS
As we hope we have demonstrated above, these are exciting times for scientists who study mechanosensitive ion channels. Every new detail regarding the structure, the physiological function, and the biophysical parameters that govern the gating mechanism of EcMscS adds to our understanding of E. coli biology, and helps elaborate an important model system for the study of mechanosensitivity. Prokaryotic homologs of MscS provide additional examples of the ways in which various bacteria might exploit the membrane tension sensor and osmotic safety valve provided by a MscS family member. The suggestion that more diverged MscS families may have additional regulatory mechanisms overlaid onto a conserved mechanosensitive channel core is particularly interesting in this regard 119. The coming years should also bring a greater understanding of the role played by the diverse eukaryotic family of MscS homologs. Eukaryotic cells respond to osmotic stress differently than bacteria, inducing cell signaling pathways in addition to releasing osmolytes 21. Studies of the yeast Msy1 and Msy2 suggest that they might play a role in both of these responses 118; further investigation will establish this point. New discoveries are also likely as some of the technical challenges associated with the study of mechanosensitive channels are overcome. Approaches to investigate osmoregulation and osmotic stress response in single cells and organelles may reveal more subtle phenotypes than can be detected in a bacterial culture or from a whole-plant phenotype. The development of fluorescent biosensors that report on ion flux, pH, transmembrane voltage, and membrane tension could produce unexpected insights into the function of MscS-like mechanosensitive channels in their endogenous cellular context.
Acknowledgments
Our work on MscS-like channels in plants and microbes is supported by grants from the National Science Foundation (MCB-1253103) and the National Institutes of Health (R01 GM084211-01). We gratefully acknowledge the discoveries and insights of our colleagues and apologize to those whose work we could not include due to size limits.
ABBREVIATIONS
- MS
mechanosensitive
- MscS
mechanosensitive channel of small conductance
- MscL
mechanosensitive channel of large conductance
- MscM
mechanosensitive channel of mini conductance
- MscMJ
mechanosensitive channel of Methanococcus jannaschii,
- MscMJLR
mechanosensitive channel of Methanococcus jannaschii of large conductance and rectifying
- MSL
MscS-Like
- Msy
MscS from yeast
- MscCG
mechanosensitive channel of Corynebacterium glutamicum
- MscSP
mechanosensitive channels of Silicibacter pomeroyi
- TM
transmembrane
- pS
picosiemens
- nS
nanosiemens
- pA
picoampere
- PCl−
permeability to Cl− ions
- MSC
mechanosensitive channel
- EPR
electron paramagnetic resonance
- MD
molecular dynamics
References
- 1.Gadsby DC. Ion channels versus ion pumps: the principal difference, in principle. Nat Rev Mol Cell Biol. 2009;10:344–352. doi: 10.1038/nrm2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hille B. Ion channels of excitable membranes. 3. Sinauer; Sunderland, Mass: 2001. [Google Scholar]
- 3.Ringer S. Regarding the Action of Hydrate of Soda, Hydrate of Ammonia, and Hydrate of Potash on the Ventricle of the Frog’s Heart. J Physiol. 1882;3:195–202. 196. doi: 10.1113/jphysiol.1882.sp000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Kakimoto Y, Toda K, Naruse K. Mechanobiology in cardiac physiology and diseases. J Cell Mol Med. 2013;17:225–232. doi: 10.1111/jcmm.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kullmann DM. Neurological channelopathies. Annu Rev Neurosci. 2010;33:151–172. doi: 10.1146/annurev-neuro-060909-153122. [DOI] [PubMed] [Google Scholar]
- 6.Nilius B, Voets T. The puzzle of TRPV4 channelopathies. EMBO Rep. 2013;14:152–163. doi: 10.1038/embor.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kung C, Blount P. Channels in microbes: so many holes to fill. Mol Microbiol. 2004;53:373–380. doi: 10.1111/j.1365-2958.2004.04180.x. [DOI] [PubMed] [Google Scholar]
- 8.Hedrich R. Ion channels in plants. Physiol Rev. 2012;92:1777–1811. doi: 10.1152/physrev.00038.2011. [DOI] [PubMed] [Google Scholar]
- 9.Wayne R. The Excitability of Plant-Cells - with a Special Emphasis on Characean Internodal Cells. Botanical Review. 1994;60:265–367. doi: 10.1007/BF02960261. [DOI] [PubMed] [Google Scholar]
- 10.Cole KS, Curtis HJ. Electric Impedance of Nitella during Activity. J Gen Physiol. 1938;22:37–64. doi: 10.1085/jgp.22.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole KS, Curtis HJ. Electric Impedance of the Squid Giant Axon during Activity. J Gen Physiol. 1939;22:649–670. doi: 10.1085/jgp.22.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamada T. Some Observations on Potential Differences Across the Ectoplasm Membrane of Paramecium. J Exp Bot. 1934;11:94–102. [Google Scholar]
- 13.Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 14.Booth IR. Bacterial Ion Channels. In: Setlow JK, editor. Genetic Engineering. Kluwer Academic/Plenum Publishers; 2003. pp. 91–111. [DOI] [PubMed] [Google Scholar]
- 15.Ward JM, Maser P, Schroeder JI. Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol. 2009;71:59–82. doi: 10.1146/annurev.physiol.010908.163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veley KM, Haswell ES. Plastids and pathogens: mechanosensitive channels and survival in a hypoosmotic world. Plant Signal Behav. 2012;7:668–671. doi: 10.4161/psb.19991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinac B, Saimi Y, Kung C. Ion channels in microbes. Physiol Rev. 2008;88:1449–1490. doi: 10.1152/physrev.00005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noskov SY, Roux B. Ion selectivity in potassium channels. Biophys Chem. 2006;124:279–291. doi: 10.1016/j.bpc.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Sukharev SI, Martinac B, Arshavsky VY, Kung C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys J. 1993;65:177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blatz AL, Magleby KL. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986;323:718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- 21.Sukharev S, Corey DP. Mechanosensitive channels: multiplicity of families and gating paradigms. Science STKE. 2004;2004 doi: 10.1126/stke.2192004re4. [DOI] [PubMed] [Google Scholar]
- 22.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 23.Haswell ES, Phillips R, Rees DC. Mechanosensitive channels: what can they do and how do they do it? Structure. 2011;19:1356–1369. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anishkin A, Akitake B, Kamaraju K, Chiang CS, Sukharev S. Hydration properties of mechanosensitive channel pores define the energetics of gating. J Phys Condens Matter. 2010;22:454120. doi: 10.1088/0953-8984/22/45/454120. [DOI] [PubMed] [Google Scholar]
- 25.Wiggins P, Phillips R. Membrane-protein interactions in mechanosensitive channels. Biophys J. 2005;88:880–902. doi: 10.1529/biophysj.104.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balleza D. Mechanical properties of lipid bilayers and regulation of mechanosensitive function: from biological to biomimetic channels. Channels (Austin) 2012;6:220–233. doi: 10.4161/chan.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markin VS, Sachs F. Thermodynamics of mechanosensitivity. Phys Biol. 2004;1:110–124. doi: 10.1088/1478-3967/1/2/007. [DOI] [PubMed] [Google Scholar]
- 29.Gullingsrud J, Schulten K. Lipid bilayer pressure profiles and mechanosensitive channel gating. Biophys J. 2004;86:3496–3509. doi: 10.1529/biophysj.103.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 31.Martinac B, Adler J, Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 32.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K(+) channels TREK-1 and TRAAK. J Biol Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- 33.Eijkelkamp N, Quick K, Wood JN. Transient Receptor Potential Channels and Mechanosensation. Annu Rev Neurosci. 2013 doi: 10.1146/annurev-neuro-062012-170412. [DOI] [PubMed] [Google Scholar]
- 34.Bounoutas A, Chalfie M. Touch sensitivity in Caenorhabditis elegans. Pflugers Arch. 2007;454:691–702. doi: 10.1007/s00424-006-0187-x. [DOI] [PubMed] [Google Scholar]
- 35.Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- 36.Anishkin A, Kung C. Stiffened lipid platforms at molecular force foci. Proc Natl Acad Sci U S A. 2013;110:4886–4892. doi: 10.1073/pnas.1302018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamill OP. Twenty odd years of stretch-sensitive channels. Pflugers Archiv-Eur J Physiol. 2006;453:333–351. doi: 10.1007/s00424-006-0131-0. [DOI] [PubMed] [Google Scholar]
- 38.Sachs F. Stretch-activated ion channels: what are they? Physiology. 2010;25:50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–329. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 40.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol - London. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang XC, Sachs F. Characterization of stretch-activated ion channels in Xenopus oocytes. J Physiol. 1990;431:103–122. doi: 10.1113/jphysiol.1990.sp018322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lansman JB, Hallam TJ, Rink TJ. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? Nature. 1987;325:811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- 43.Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987;330:66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- 44.Erxleben C. Stretch-activated current through single ion channels in the abdominal stretch receptor organ of the crayfish. J Gen Physiol. 1989;94:1071–1083. doi: 10.1085/jgp.94.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinac B. 3.5 billion years of mechanosensory transduction: Structure and Function of Mechanosensitive Channels in Prokaryotes. In: Hamill OP, editor. Mechanosensitive Ion Channels. Elsevier; 2007. pp. 26–57. [Google Scholar]
- 46.Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 47.Sukharev S, Sachs F. Molecular force transduction by ion channels: diversity and unifying principles. J Cell Sci. 2012;125:3075–3083. doi: 10.1242/jcs.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haswell E, Monshausen GB. A Force of Nature: Molecular Mechanisms of Mechanotransduction in Plants. Journal of Experimental Botany. 2013 doi: 10.1093/jxb/ert204. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci USA. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blount P, Sukharev SI, Moe PC, Martinac B, Kung C. Mechanosensitive channels of bacteria. Methods Enzymol. 1999;294:458–482. doi: 10.1016/s0076-6879(99)94027-2. [DOI] [PubMed] [Google Scholar]
- 51.Ruthe HJ, Adler J. Fusion of bacterial spheroplasts by electric fields. Biochim Biophys Acta. 1985;819:105–113. doi: 10.1016/0005-2736(85)90200-7. [DOI] [PubMed] [Google Scholar]
- 52.Cui C, Smith DO, Adler J. Characterization of mechanosensitive channels in Escherichia coli cytoplasmic membrane by whole-cell patch clamp recording. J Memb Biol. 1995;144:31–42. doi: 10.1007/BF00238414. [DOI] [PubMed] [Google Scholar]
- 53.Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Memb Biol. 1996;151:175–187. doi: 10.1007/s002329900068. [DOI] [PubMed] [Google Scholar]
- 54.Berrier C, Coulombe A, Houssin C, Ghazi A. A patch-clamp study of ion channels of inner and outer membranes and of contact zones of E. coli, fused into giant liposomes. Pressure-activated channels are localized in the inner membrane. FEBS Lett. 1989;259:27–32. doi: 10.1016/0014-5793(89)81486-3. [DOI] [PubMed] [Google Scholar]
- 55.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 56.Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sukharev S, Akitake B, Anishkin A. The Bacterial Mechanosensitive Channel MscS: Emerging Principles of Gating and Modulation. In: Hamill OP, editor. Mechanosensitive Ion Channels. Elsevier; 2007. [Google Scholar]
- 58.Sukharev S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys J. 2002;83:290–298. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schumann U, Edwards MD, Rasmussen T, Bartlett W, van West P, Booth IR. YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc Natl Acad Sci USA. 2010;107:12664–12669. doi: 10.1073/pnas.1001405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edwards MD, Black S, Rasmussen T, Rasmussen A, Stokes NR, Stephen TL, Miller S, Booth IR. Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels (Austin) 2012;6:272–281. doi: 10.4161/chan.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood JM, Bremer E, Csonka LN, Kraemer R, Poolman B, van der Heide T, Smith LT. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:437–460. doi: 10.1016/s1095-6433(01)00442-1. [DOI] [PubMed] [Google Scholar]
- 62.Booth IR, Blount P. The MscS and MscL families of mechanosensitive channels act as microbial emergency release valves. J Bacteriol. 2012;194:4802–4809. doi: 10.1128/JB.00576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Csonka LN, Epstein W. Osmoregulation. In: Neidhardt FC, Curtiss R, editors. Escherichia coli and Salmonella : cellular and molecular biology. 2. ASM Press; Washington, D.C: 1996. [Google Scholar]
- 64.Britten RJ, Mc CF. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perozo E, Kloda A, Cortes DM, Martinac B. Site-directed spin-labeling analysis of reconstituted Mscl in the closed state. J Gen Physiol. 2001;118:193–206. doi: 10.1085/jgp.118.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinac B. Mechanosensitive channels in prokaryotes. Cell Physiol Biochem. 2001;11:61–76. doi: 10.1159/000047793. [DOI] [PubMed] [Google Scholar]
- 67.Iscla I, Blount P. Sensing and responding to membrane tension: the bacterial MscL channel as a model system. Biophys J. 2012;103:169–174. doi: 10.1016/j.bpj.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kloda A, Petrov E, Meyer GR, Nguyen T, Hurst AC, Hool L, Martinac B. Mechanosensitive channel of large conductance. Int J Biochem Cell Biol. 2008;40:164–169. doi: 10.1016/j.biocel.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Naismith JH, Booth IR. Bacterial mechanosensitive channels--MscS: evolution’s solution to creating sensitivity in function. Annu Rev Biophys. 2012;41:157–177. doi: 10.1146/annurev-biophys-101211-113227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lai JY, Poon YS, Kaiser JT, Rees DC. Open and shut: crystal structures of the dodecylmaltoside solubilized mechanosensitive channel of small conductance from Escherichia coli and Helicobacter pylori at 4.4 A and 4.1 A resolutions. Protein Sci. 2013;22:502–509. doi: 10.1002/pro.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 72.Steinbacher S, Bass RB, Strop P, Rees DC. Structures of the prokaryotic mechanosensitive channels MscL and MscS. In: Hamill OP, editor. Mechanosensitive Ion Channels, Part A. 2007. pp. 1–24. [Google Scholar]
- 73.Zhang X, Wang J, Feng Y, Ge J, Li W, Sun W, Iscla I, Yu J, Blount P, Li Y, Yang M. Structure and molecular mechanism of an anion-selective mechanosensitive channel of small conductance. Proc Natl Acad Sci U S A. 2012;109:18180–18185. doi: 10.1073/pnas.1207977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dorwart MR, Wray R, Brautigam CA, Jiang YX, Blount P. S. aureus MscL Is a pentamer in vivo but of variable stoichiometries in vitro: Implications for detergent-solubilized membrane proteins. Plos Biol. 2010;8 doi: 10.1371/journal.pbio.1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carugo O, Argos P. Protein-protein crystal-packing contacts. Protein Sci. 1997;6:2261–2263. doi: 10.1002/pro.5560061021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller S, Bartlett W, Chandrasekaran S, Simpson S, Edwards M, Booth IR. Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 2003;22:36–46. doi: 10.1093/emboj/cdg011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller S, Edwards MD, Ozdemir C, Booth IR. The closed structure of the MscS mechanosensitive channel. Cross-linking of single cysteine mutants. J Biol Chem. 2003;278:32246–32250. doi: 10.1074/jbc.M303188200. [DOI] [PubMed] [Google Scholar]
- 78.Vasquez V, Sotomayor M, Cortes DM, Roux B, Schulten K, Perozo E. Three-dimensional architecture of membrane-embedded MscS in the closed conformation. J Mol Biol. 2008;378:55–70. doi: 10.1016/j.jmb.2007.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anishkin A, Sukharev S. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys J. 2004;86:2883–2895. doi: 10.1016/S0006-3495(04)74340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beckstein O, Sansom MSP. The influence of geometry, surface character, and flexibility on the permeation of ions and water through biological pores. Phys Biol. 2004;1:42–52. doi: 10.1088/1478-3967/1/1/005. [DOI] [PubMed] [Google Scholar]
- 81.Belyy V, Anishkin A, Kamaraju K, Liu NL, Sukharev S. The tension-transmitting ‘clutch’ in the mechanosensitive channel MscS. Nat Struct Mol Biol. 2010;17:451–U492. doi: 10.1038/nsmb.1775. [DOI] [PubMed] [Google Scholar]
- 82.Sotomayor M, Schulten K. Molecular dynamics study of gating in the mechanosensitive channel of small conductance MscS. Biophys J. 2004;87:3050–3065. doi: 10.1529/biophysj.104.046045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spronk SA, Dougherty DA, Lester HA. Hydration of the pore of the mechanosensitive channel of small conductance (MscS) studied by molecular dynamics. Biophys J. 2005;88:149A–149A. [Google Scholar]
- 84.Wang W, Black SS, Edwards MD, Miller S, Morrison EL, Bartlett W, Dong C, Naismith JH, Booth IR. The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science. 2008;321:1179–1183. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edwards MD, Li Y, Kim S, Miller S, Bartlett W, Black S, Dennison S, Iscla I, Blount P, Bowie JU, Booth IR. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat Struct Mol Biol. 2005;12:113–119. doi: 10.1038/nsmb895. [DOI] [PubMed] [Google Scholar]
- 86.Anishkin A, Kamaraju K, Sukharev S. Mechanosensitive channel MscS in the open state: Modeling of the transition, explicit simulations, and experimental measurements of conductance. J Gen Physiol. 2008;132:67–83. doi: 10.1085/jgp.200810000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasquez V, Sotomayor M, Cordero-Morales J, Schulten K, Perozo E. A structural mechanism for MscS gating in lipid bilayers. Science. 2008;321:1210–1214. doi: 10.1126/science.1159674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schiemann O, Prisner TF. Long-range distance determinations in biomacromolecules by EPR spectroscopy. Q Rev Biophys. 2007;40:1–53. doi: 10.1017/S003358350700460X. [DOI] [PubMed] [Google Scholar]
- 89.Pliotas C, Ward R, Branigan E, Rasmussen A, Hagelueken G, Huang H, Black SS, Booth IR, Schiemann O, Naismith JH. Conformational state of the MscS mechanosensitive channel in solution revealed by pulsed electron-electron double resonance (PELDOR) spectroscopy. Proc Natl Acad Sci U S A. 2012;109:E2675–2682. doi: 10.1073/pnas.1202286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akitake B, Anishkin A, Liu N, Sukharev S. Straightening and sequential buckling of the pore-lining helices define the gating cycle of MscS. Nat Struct Mol Biol. 2007;14:1141–1149. doi: 10.1038/nsmb1341. [DOI] [PubMed] [Google Scholar]
- 91.Nomura T, Sokabe M, Yoshimura K. Lipid-protein interaction of the MscS mechanosensitive channel examined by scanning mutagenesis. Biophys J. 2006;91:2874–2881. doi: 10.1529/biophysj.106.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malcolm HR, Heo YY, Elmore DE, Maurer JA. Defining the role of the tension sensor in the mechanosensitive channel of small conductance. Biophys J. 2011;101:345–352. doi: 10.1016/j.bpj.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Booth IR, Rasmussen T, Edwards MD, Black S, Rasmussen A, Bartlett W, Miller S. Sensing bilayer tension: bacterial mechanosensitive channels and their gating mechanisms. Biochem Soc Trans. 2011;39:733–740. doi: 10.1042/BST0390733. [DOI] [PubMed] [Google Scholar]
- 94.Schumann U, Edwards MD, Li C, Booth IR. The conserved carboxy-terminus of the MscS mechanosensitive channel is not essential but increases stability and activity. FEBS Lett. 2004;572:233–237. doi: 10.1016/j.febslet.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 95.Rasmussen A, Rasmussen T, Edwards MD, Schauer D, Schumann U, Miller S, Booth IR. The role of tryptophan residues in the function and stability of the mechanosensitive channel MscS from Escherichia coli. Biochem. 2007;46:10899–10908. doi: 10.1021/bi701056k. [DOI] [PubMed] [Google Scholar]
- 96.Nomura T, Sokabe M, Yoshimura K. Interaction between the cytoplasmic and transmembrane domains of the mechanosensitive channel MscS. Biophys J. 2008;94:1638–1645. doi: 10.1529/biophysj.107.114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koprowski P, Grajkowski W, Isacoff EY, Kubalski A. Genetic screen for potassium leaky small mechanosensitive channels (MscS) in Escherichia coli: recognition of cytoplasmic beta domain as a new gating element. J, Biol Chem. 2011;286:877–888. doi: 10.1074/jbc.M110.176131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akitake B, Anishkin A, Sukharev S. The “dashpot” mechanism of stretch-dependent gating in MscS. J Gen Physiol. 2005;125:143–154. doi: 10.1085/jgp.200409198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koprowski P, Kubalski A. Voltage-independent adaptation of mechanosensitive channels in Escherichia coli protoplasts. J Membr Biol. 1998;164:253–262. doi: 10.1007/s002329900410. [DOI] [PubMed] [Google Scholar]
- 100.Grajkowski W, Kubalski A, Koprowski P. Surface changes of the mechanosensitive channel MscS upon its activation, inactivation, and closing. Biophys J. 2005;88:3050–3059. doi: 10.1529/biophysj.104.053546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Machiyama H, Tatsumi H, Sokabe M. Structural changes in the cytoplasmic domain of the mechanosensitive channel MscS during opening. Biophys J. 2009;97:1048–1057. doi: 10.1016/j.bpj.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sotomayor M, van der Straaten TA, Ravaioli U, Schulten K. Electrostatic properties of the mechanosensitive channel of small conductance MscS. Biophys J. 2006;90:3496–3510. doi: 10.1529/biophysj.105.080069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 2002;21:5323–5330. doi: 10.1093/emboj/cdf537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sotomayor M, Vasquez V, Perozo E, Schulten K. Ion conduction through MscS as determined by electrophysiology and simulation. Biophys J. 2007;92:886–902. doi: 10.1529/biophysj.106.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Edwards MD, Bartlett W, Booth IR. Pore mutations of the Escherichia coli MscS channel affect desensitization but not ionic preference. Biophys J. 2008;94:3003–3013. doi: 10.1529/biophysj.107.123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gamini R, Sotomayor M, Chipot C, Schulten K. Cytoplasmic domain filter function in the mechanosensitive channel of small conductance. Biophys J. 2011;101:80–89. doi: 10.1016/j.bpj.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cox CD, Nomura T, Ziegler CS, Campbell AK, Wann KT, Martinac B. Selectivity mechanism of the mechanosensitive channel MscS revealed by probing channel subconducting states. Nat Commun. 2013;4:2137. doi: 10.1038/ncomms3137. [DOI] [PubMed] [Google Scholar]
- 108.Pivetti CD, Yen MR, Miller S, Busch W, Tseng YH, Booth IR, Saier MH., Jr Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kloda A, Martinac B. Common evolutionary origins of mechanosensitive ion channels in Archaea, Bacteria and cell-walled Eukarya. Archaea. 2002;1:35–44. doi: 10.1155/2002/419261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haswell ES. MscS-like proteins in plants. In: Hamill OP, editor. Mechanosensitive Ion Channels, Part A. 2007. pp. 329–359. [Google Scholar]
- 111.Balleza D, Gomez-Lagunas F. Conserved motifs in mechanosensitive channels MscL and MscS. Eur Biophys J. 2009;38:1013–1027. doi: 10.1007/s00249-009-0460-y. [DOI] [PubMed] [Google Scholar]
- 112.Malcolm HR, Maurer JA. The mechanosensitive channel of small conductance (MscS) superfamily: not just mechanosensitive channels anymore. Chembiochem. 2012;13:2037–2043. doi: 10.1002/cbic.201200410. [DOI] [PubMed] [Google Scholar]
- 113.Porter BW, Zhu YJ, Webb DT, Christopher DA. Novel thigmomorphogenetic responses in Carica papaya: touch decreases anthocyanin levels and stimulates petiole cork outgrowths. Ann Bot. 2009;103:847–858. doi: 10.1093/aob/mcp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prole DL, Taylor CW. Identification and analysis of putative homologues of mechanosensitive channels in pathogenic protozoa. PLoS One. 2013;8:e66068. doi: 10.1371/journal.pone.0066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kloda A, Martinac B. Molecular identification of a mechanosensitive channel in archaea. Biophys J. 2001;80:229–240. doi: 10.1016/S0006-3495(01)76009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sheetz MP, Sable JE, Dobereiner HG. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu Rev Biophys Biomol Struct. 2006;35:417–434. doi: 10.1146/annurev.biophys.35.040405.102017. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y, Hamill OP. On the discrepancy between whole-cell and membrane patch mechanosensitivity in Xenopus oocytes. J Physiol. 2000;523(Pt 1):101–115. doi: 10.1111/j.1469-7793.2000.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakayama Y, Yoshimura K, Iida H. Organellar mechanosensitive channels in fission yeast regulate the hypo-osmotic shock response. Nat Commun. 2012;3:1020. doi: 10.1038/ncomms2014. [DOI] [PubMed] [Google Scholar]
- 119.Malcolm HR, Elmore DE, Maurer JA. Mechanosensitive behavior of bacterial cyclic nucleotide gated (bCNG) ion channels: Insights into the mechanism of channel gating in the mechanosensitive channel of small conductance superfamily. Biochem Biophys Res Commun. 2012;417:972–976. doi: 10.1016/j.bbrc.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 120.Stokes NR, Murray HD, Subramaniam C, Gourse RL, Louis P, Bartlett W, Miller S, Booth IR. A role for mechanosensitive channels in survival of stationary phase: regulation of channel expression by RpoS. Proc Natl Acad Sci USA. 2003;100:15959–15964. doi: 10.1073/pnas.2536607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huisman GW, Siegele DA, Zambran MM, Kolter R. Morphological and Physiological Changes during Stationary Phase. In: Neidhardt FC, Curtiss R, editors. Escherichia coli and Salmonella : cellular and molecular biology. 2. Vol. 2. ASM Press; Washington, D.C: 1996. p. xx.p. 2822. [Google Scholar]
- 122.Romantsov T, Battle AR, Hendel JL, Martinac B, Wood JM. Protein localization in Escherichia coli cells: comparison of the cytoplasmic membrane proteins ProP, LacY, ProW, AqpZ, MscS, and MscL. J Bacteriol. 2010;192:912–924. doi: 10.1128/JB.00967-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilson M, Haswell E. A role for mechanosensitive channels in chloroplast and bacterial fission. Plant Signal Behav. 2012;7:157–160. doi: 10.4161/psb.18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilson ME, Jensen GS, Haswell ES. Two mechanosensitive channel homologs influence division ring placement in Arabidopsis chloroplasts. Plant Cell. 2011;23:2939–2949. doi: 10.1105/tpc.111.088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McLaggan D, Jones MA, Gouesbet G, Levina N, Lindey S, Epstein W, Booth IR. Analysis of the kefA2 mutation suggests that KefA is a cation-specific channel involved in osmotic adaptation in Escherichia coli. Mol Microbiol. 2002;43:521–536. doi: 10.1046/j.1365-2958.2002.02764.x. [DOI] [PubMed] [Google Scholar]
- 126.Moe PC, Blount P, Kung C. Functional and structural conservation in the mechanosensitive channel MscL implicates elements crucial for mechanosensation. Mol Microbiol. 1998;28:583–592. doi: 10.1046/j.1365-2958.1998.00821.x. [DOI] [PubMed] [Google Scholar]
- 127.Wahome PG, Setlow P. Growth, osmotic downshock resistance and differentiation of Bacillus subtilis strains lacking mechanosensitive channels. Arch Microbiol. 2008;189:49–58. doi: 10.1007/s00203-007-0292-z. [DOI] [PubMed] [Google Scholar]
- 128.Hoffmann T, Boiangiu C, Moses S, Bremer E. Responses of Bacillus subtilis to hypotonic challenges: physiological contributions of mechanosensitive channels to cellular survival. Appl Environ Microbiol. 2008;74:2454–2460. doi: 10.1128/AEM.01573-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hermann T. Industrial production of amino acids by coryneform bacteria. J Biotechnol. 2003;104:155–172. doi: 10.1016/s0168-1656(03)00149-4. [DOI] [PubMed] [Google Scholar]
- 130.Nottebrock D, Meyer U, Kramer R, Morbach S. Molecular and biochemical characterization of mechanosensitive channels in Corynebacterium glutamicum. FEMS Microbiol Lett. 2003;218:305–309. doi: 10.1111/j.1574-6968.2003.tb11533.x. [DOI] [PubMed] [Google Scholar]
- 131.Nakamura J, Hirano S, Ito H, Wachi M. Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce L-glutamic acid production. Appl Environ Microbiol. 2007;73:4491–4498. doi: 10.1128/AEM.02446-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Borngen K, Battle AR, Moker N, Morbach S, Marin K, Martinac B, Kramer R. The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim Biophys Acta. 2010;1798:2141–2149. doi: 10.1016/j.bbamem.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 133.Becker M, Borngen K, Nomura T, Battle AR, Marin K, Martinac B, Kramer R. Glutamate efflux mediated by Corynebacterium glutamicum MscCG, Escherichia coli MscS, and their derivatives. Biochim Biophys Acta. 2013;1828:1230–1240. doi: 10.1016/j.bbamem.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 134.Hashimoto K, Murata J, Konishi T, Yabe I, Nakamatsu T, Kawasaki H. Glutamate is excreted across the cytoplasmic membrane through the NCgl1221 channel of Corynebacterium glutamicum by passive diffusion. Biosci Biotechnol Biochem. 2012;76:1422–1424. doi: 10.1271/bbb.120366. [DOI] [PubMed] [Google Scholar]
- 135.Nakayama Y, Yoshimura K, Iida H. A gain-of-function mutation in gating of Corynebacterium glutamicum NCgl1221 causes constitutive glutamate secretion. Appl Environ Microbiol. 2012;78:5432–5434. doi: 10.1128/AEM.01310-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kakuda T, Koide Y, Sakamoto A, Takai S. Characterization of two putative mechanosensitive channel proteins of Campylobacter jejuni involved in protection against osmotic downshock. Vet Microbiol. 2012;160:53–60. doi: 10.1016/j.vetmic.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 137.Tan MW, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Osanai T, Sato S, Tabata S, Tanaka K. Identification of PamA as a PII-binding membrane protein important in nitrogen-related and sugar-catabolic gene expression in Synechocystis sp. PCC 6803. J Biol Chem. 2005;280:34684–34690. doi: 10.1074/jbc.M507489200. [DOI] [PubMed] [Google Scholar]
- 139.Osanai T, Tanaka K. Keeping in touch with PII: PII-interacting proteins in unicellular cyanobacteria. Plant Cell Physiol. 2007;48:908–914. doi: 10.1093/pcp/pcm072. [DOI] [PubMed] [Google Scholar]
- 140.Rowe I, Elahi M, Huq A, Sukharev S. The mechanoelectrical response of the cytoplasmic membrane of Vibrio cholerae. J Gen Physiol. 2013;142:75–85. doi: 10.1085/jgp.201310985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakayama Y, Fujiu K, Sokabe M, Yoshimura K. Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc Natl Acad Sci U S A. 2007;104:5883–5888. doi: 10.1073/pnas.0609996104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haswell ES, Meyerowitz EM. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol. 2006;16:1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 143.Veley KM, Marshburn S, Clure CE, Haswell ES. Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Curr Biol. 2012;22:408–413. doi: 10.1016/j.cub.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maksaev G, Haswell ES. MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc Natl Acad Sci U S A. 2012;109:19015–19020. doi: 10.1073/pnas.1213931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Petrov E, Palanivelu D, Constantine M, Rohde PR, Cox CD, Nomura T, Minor DL, Jr, Martinac B. Patch-clamp characterization of the MscS-like mechanosensitive channel from Silicibacter pomeroyi. Biophys J. 2013;104:1426–1434. doi: 10.1016/j.bpj.2013.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kloda A, Martinac B. Structural and functional differences between two homologous mechanosensitive channels of Methanococcus jannaschii. EMBO J. 2001;20:1888–1896. doi: 10.1093/emboj/20.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Szabo I, Petronilli V, Zoratti M. A patch-clamp study of Bacillus subtilis. Biochim Biophys Acta. 1992;1112:29–38. doi: 10.1016/0005-2736(92)90250-p. [DOI] [PubMed] [Google Scholar]
- 148.Szabo I, Petronilli V, Zoratti M. A patch-clamp investigation of the Streptococcus faecalis cell membrane. J Memb Biol. 1993;131:203–218. doi: 10.1007/BF02260109. [DOI] [PubMed] [Google Scholar]
- 149.Maksaev G, Haswell ES. Expression and characterization of the bacterial mechanosensitive channel MscS in Xenopus laevis oocytes. J Gen Physiol. 2011;138:641–649. doi: 10.1085/jgp.201110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kamaraju K, Belyy V, Rowe I, Anishkin A, Sukharev S. The pathway and spatial scale for MscS inactivation. J Gen Physiol. 2011;138:49–57. doi: 10.1085/jgp.201110606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boer M, Anishkin A, Sukharev S. Adaptive MscS gating in the osmotic permeability response in E. coli: the question of time. Biochemistry. 2011;50:4087–4096. doi: 10.1021/bi1019435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Belyy V, Kamaraju K, Akitake B, Anishkin A, Sukharev S. Adaptive behavior of bacterial mechanosensitive channels is coupled to membrane mechanics. J Gen Physiol. 2010;135:641–652. doi: 10.1085/jgp.200910371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li C, Edwards MD, Jeong H, Roth J, Booth IR. Identification of mutations that alter the gating of the Escherichia coli mechanosensitive channel protein, MscK. Mol Microbiol. 2007;64:560–574. doi: 10.1111/j.1365-2958.2007.05672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Caldwell DB, Malcolm HR, Elmore DE, Maurer JA. Identification and experimental verification of a novel family of bacterial cyclic nucleotide-gated (bCNG) ion channels. Biochim Biophys Acta. 2010;1798:1750–1756. doi: 10.1016/j.bbamem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 155.Jensen GS, Haswell ES. Functional analysis of conserved motifs in the mechanosensitive channel homolog MscS-Like2 from Arabidopsis thaliana. PLoS One. 2012;7:e40336. doi: 10.1371/journal.pone.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lewit-Bentley A, Rety S. EF-hand calcium-binding proteins. Curr Opin Struct Biol. 2000;10:637–643. doi: 10.1016/s0959-440x(00)00142-1. [DOI] [PubMed] [Google Scholar]