Abstract
Aims/hypothesis
Diabetic retinopathy is characterised by early blood–retina barrier (BRB) breakdown and neurodegeneration. Diabetes causes imbalance of nerve growth factor (NGF), leading to accumulation of the NGF precursor (proNGF), as well as the NGF receptor, p75 neurotrophin receptor (p75NTR), suggesting a possible pathological role of the proNGF–p75NTR axis in the diabetic retina. To date, the role of this axis in diabetes-induced retinal inflammation and BRB breakdown has not been explored. We hypothesised that modulating p75NTR would prevent diabetes- and proNGF-induced retinal inflammation and BRB breakdown.
Methods
Diabetes was induced by streptozotocin in wild-type and p75NTR knockout (p75KO) mice. After 5 weeks, the expression of inflammatory mediators, ganglion cell loss and BRB breakdown were determined. Cleavage-resistant proNGF was overexpressed in rodent retinas with and without p75NTR short hairpin RNA or with pharmacological inhibitors. In vitro, the effects of proNGF were investigated in retinal Müller glial cell line (rMC-1) and primary Müller cells.
Results
Deletion of p75NTR blunted the diabetes-induced decrease in retinal NGF expression and increases in proNGF, nuclear factor κB (NFκB), p-NFκB and TNF-α. Deletion of p75NTR also abrogated diabetes-induced glial fibrillary acidic protein expression, ganglion cell loss and vascular permeability. Inhibited expression or cleavage of p75NTR blunted proNGF-induced retinal inflammation and vascular permeability. In vitro, proNGF induced p75NTR-dependent production of inflammatory mediators in primary wild-type Müller and rMC-1 cultures, but not in p75KO Müller cells.
Conclusions/interpretation
The proNGF–p75NTR axis contributes to retinal inflammation and vascular dysfunction in the rodent diabetic retina. These findings underscore the importance of p75NTR as a novel regulator of inflammation and potential therapeutic target in diabetic retinopathy.
Keywords: BRB breakdown, Diabetic retinopathy, Ganglion loss, IL-1β, Inflammation, Müller cells, NFκB, p75KO, proNGF, TNF-α
Introduction
Diabetic retinopathy, the leading cause of blindness in working-age adults in the USA, is a progressive and potentially devastating vascular-neurodegenerative disease [1]. A growing body of evidence reviewed by various teams [2–4] supports the concept that diabetes-induced oxidative stress disturbs retinal homeostasis by activating glial cells, reducing neurotrophic and prosurvival inputs, and increasing proinflammatory cytokines. Recent findings by us indicate that diabetes causes an imbalance of nerve growth factor (NGF), leading to accumulation of the NGF precursor (proNGF) and the NGF receptor, p75 neurotrophin receptor (p75NTR) [5–7]. Diabetes-induced peroxynitrite also enhances p75NTR expression and tyrosine nitration, which inhibits prosurvival signalling of the NGF receptor, tyrosine receptor kinase A (TRKA) [6, 7]. Thus, diabetes shifts the neurotrophin balance from NGF towards proNGF and receptor activity towards p75NTR, suggesting a possible pathological role of the proNGF–p75NTR axis in the diabetic retina. Although the proNGF–p75NTR axis has been implicated in neurodegenerative diseases [8–10], its role in diabetes-induced retinal inflammation and blood–retina barrier (BRB) breakdown has not been fully elucidated.
The p75NTR receptor, a member of the TNF receptor superfamily [11], has multiple and cell-specific functions, which, as previously reviewed [12–14], are dependent upon the availability of ligands and co-receptors. In the retina, p75NTR is expressed predominantly by Müller cells, which also express the p75NTR co-receptors, sortilin and TRKA [6, 15]. The p75NTR–sortilin complex has a higher affinity for proNGF and is usually pro-apoptotic, while p75NTR–TRKA has increased NGF affinity and is usually pro-survival [13, 16]. p75NTR lacks a catalytic domain [17, 18] and signals through association of effector molecules with the cytoplasmic p75 intracellular domain (p75ICD ) generated by α-secretase-catalysed cleavage of the extracellular domain followed by γ-secretase-catalysed intramembrane proteolysis [19, 20]. The outcome of p75NTR signalling can be beneficial or detrimental depending upon p75NTR co-receptor interactions and the cellular environment [21, 22]. Specifically, the p75 receptor has been involved in nuclear factor κB (NFκB) activation and production of proinflammatory mediators [15, 23, 24]. In the present work, we hypothesised that modulation of p75NTR would prevent diabetes- and proNGF-induced retinal inflammation and BRB breakdown. Molecular and pharmacological approaches were used to examine this hypothesis. A p75NTR knockout (p75KO) mouse model was used to examine diabetes-induced inflammation and preservation of BRB function. The effects of modulating p75NTR expression or activity were examined in a model of proNGF overexpression and in Müller cell cultures.
Methods
Animal preparation
All procedures with animals were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and the Charlie Norwood VA Medical Center Animal Care and Use Committee. We used 30 male Sprague–Dawley rats (~250 g body weight) from Harlan Laboratories (Indianapolis, IN, USA) and 60 male mice (~20 g body weight), including 18 p75KO mice and 42 wild-type (WT) mice. The p75NTR , B6.129S4NgfrtmIjae/J (p75KO, exon III knockout mice [25]) were obtained from Jackson Laboratories (Bar Harbor, Maine, USA) and crossed with C57BL6-J mice (Jackson Laboratories). These mice were crossed and back-crossed to establish a colony of homozygous p75KO and WT breeders that produced the mice used here.
Induction of diabetes
Male 8-week-old WT and p75KO mice (20 g) were randomly assigned to four groups: WT controls, WT diabetic, homozygous knockout control (HOC), homozygous knockout diabetic (HOD). Mice were rendered diabetic by five consecutive intraperitoneal injections of streptozotocin (60 mg/kg) in 0.01 mol/l citrate buffer. Diabetes was initially confirmed by detection of glucose in urine and later by blood glucose over 12.7 mmol/l. Deletion of p75NTR did not alter body weight in control (25.8±0.5 g HOC vs 25.3±0.4 g in WT control) or diabetic groups (24.1±0.6 g HOD vs 24.7±0.8 g in WT diabetic), or glucose levels in controls (7.6±0.8 mmol/l HOC vs 7.7±0.5 mmol/l in WT control) or diabetic groups (14.8±1.1 mmol/l HOD vs 14.4±0.8 mmol/l in WT diabetic).
Overexpression of proNGF in rodent retinas
Stable overexpression of proNGF was achieved via intravitreal injection of proNGF123 plasmid [26] and electroporation as described previously by our group [27]. Rats were randomised into three groups: (1) control group, green fluorescence protein (GFP)-plasmid (pGFP) alone; (2) proNGF group, pGFP–proNGF plus lentiviral particles containing scrambled p75NTR (5000 infectious units (IFU)/eye); and (3) a group receiving pGFP– proNGF plus lentiviral particles containing short hairpin RNA (shRNA) against p75NTR (5000 IFU/eye). Lentiviral particles were obtained from Dharmacon (Thermo Fisher Scientific, Rockford, IL, USA). Animals were killed after 4 weeks. Additional groups of WT mice were randomised into four groups receiving: (1) (control group) pGFP-plasmid alone; (2) pGFP– proNGF; (3) pGFP–proNGF plus 40 ng C30–35, a selective antagonist for p75NTR receptor [28] (kind gift from Uri Saragovi, McGill University, Montreal, QC, Canada); and (4) pGFP–proNGF plus 100 ng γ-secretase inhibitor (compound E) (Calbiochem/EMD Biosciences, Billerica, MA, USA).
Antibodies
The following primary antibodies were used: polyclonal anti-NGF and anti-proNGF (Alomone Labs, Jerusalem, Israel); polyclonal anti-p75NTR (catalogue number 07–476), polyclonal anti-vascular endothelial growth factor (VEGF) and monoclonal anti-GFP antibody (Millipore, Billerica, MA, USA); monoclonal anti-TNF-α, polyclonal anti-IL-1β and polyclonal anti-sortilin (Abcam, Cambridge, MA, USA); monoclonal IgG2 (R&D Systems, Minneapolis, MN, USA); polyclonal anti-glial fibrillary acidic protein (GFAP) and monoclonal anti-vimentin (Thermo Fisher); polyclonal β-actin (Sigma-Aldrich, St Louis, MO, USA); monoclonal anti-brain-specific homeobox/POU domain protein 3A (BRN3A) and polyclonal anti-NFκB-p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and monoclonal anti-phospho-NFκB (p65 subunit) (Cell Signaling, Danvers, MA, USA). Secondary antibodies used were Texas-red- or Oregon-green-conjugated goat anti-mouse or goat anti-rabbit antibodies (Invitrogen, Grand Island, NY, USA), and horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse (Calbiochem/EMD Biosciences).
Immunolocalisation studies
Retina sections were fixed using 2% wt/vol. paraformaldehyde and incubated overnight in PBS-containing primary antibody (1:100 dilution) or negative control at 4°C. Sections were blocked for 1 h with 20% vol./vol. goat serum in PBS containing 0.3% vol./vol. Triton X-100 and incubated for 2 h with secondary antibody (1:500) in PBS-containing 0.3% vol./vol. Triton X-100 and 1% wt/vol. BSA. Sections were coverslipped with Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA) and images collected by microscope (AxioObserver.Z1; Zeiss, Jena, Germany).
Quantification of retina ganglion cells
Retina sections containing the optic nerve were counted. BRN3A is a POU-domain transcription factor that is expressed by retinal ganglion cells (RGCs) and compares favourably to fluorogold labelling [29]. Total cells (DAPI-positive) and ganglion cells (BRN3A- and DAPI-positive) in the ganglion cell layer (GCL) were counted and the length of the GCL measured from one ora-serrata to the other. Cell numbers were normalised to length of the GCL in mm.
Determination of blood–retina barrier function
Integrity of the BRB was measured as described by our group [5, 30]. Mice received jugular vein injections of 10 mg/kg BSA-conjugated fluorescein (Invitrogen, Grand Island, NY, USA). After 20 min, animals were killed and blood and retinas collected. Serial imaging of fluorescence in six sections was quantified by microscope (Zeiss). Contralateral retinas were homogenised in RIPA-buffer to detect the fluorescence of retinal lysates using a fluorescent plate reader (BioTek Synergy2, Winooski, VT, USA) (excitation 370 nm, emission 460 nm). Retina fluorescence was normalised to that of serum.
Retinal protein extraction and western blot analysis
Retinas and cells were isolated and homogenised in RIPA buffer [7]. Samples (30 µg protein) were separated by SDS-PAGE. Membranes were probed with primary antibodies in 5% wt/vol. milk or 0.5% wt/vol. BSA, in PBST followed by secondary antibody (1:5000 in 5% wt/vol. milk in PBST). Blots were developed using Pierce-ECL Western Blot (Thermo Fisher) or Immobilon Western Chemiluminescent HRP (Millipore) substrates. Membranes were reprobed with β-actin to confirm equal loading. Films were scanned. Band intensities were quantified using densitometry software (alphaEaseFC, Proteinsimple, Santa Clara, CA, USA) and expressed as relative optical density to β-actin normalised to controls.
Quantitative real-time PCR
A one-step quantitative RT-PCR kit (Invitrogen) was used to amplify 10 ng retinal mRNA as described [6, 7]. PCR primers (electronic supplementary material [ESM] Fig. 1d) were purchased from Integrated DNA Technologies (Coralville, IA, USA). Quantitative PCR was performed (Realplex Master Cycler; Eppendorf, Hamburg, Germany). The expression of TNF-α, NGF or VEGF was normalised to 18S and expressed relative to WT control.
Tissue culture studies with rat Müller cell line
The rat retinal Müller glial cell line (rMC-1), obtained from V. Sarthy (Department of Ophthalmology, Northwestern University, Chicago, IL, USA), has been previously characterised [31]. Cells were grown to confluence for 48 h in normal glucose (5 mmol/l) or high glucose (25 mmol/l) as described previously [6] and treated overnight with 50 ng/ml cleavage-resistant proNGF (Alomone Labs) in the presence or absence of the p75NTR receptor antagonist (20 µmol/l) [28] or 10 µmol/l compound E (γ-secretase inhibitor).
Primary mouse Müller cells
Primary mouse Müller cells (PM) were cultured as described previously [32]. Briefly, eyes from mice at postnatal day 7 were incubated overnight in serum-free DMEM with 0.1% vol./vol. penicillin/streptomycin. Retinas were removed by dissection and plated in 5 mmol/l glucose growth medium (DMEM, 10% vol./vol. FBS, 1% vol./vol. penicillin/streptomycin). The purity of the cultures was verified by immunostaining for the Müller cell marker vimentin. PM were switched to low serum medium (0.5% vol./vol. FBS) overnight, followed by treatment with 100 µmol/l peroxynitrite or 50 ng/ml cleavage-resistant proNGF, or both for 6 h. For long-term studies, cells from WT were treated with the antagonist for p75NTR receptor (20 µmol/l) and compared with untreated WT or p75KO cells. Cells were grown in low-serum medium, which was changed every 2 days, and collected after 6 days.
Determination of TNF-α by ELISA
Levels of TNF-α in PM cell supernatant fractions were determined using ELISA following the manufacturer's protocol (Catalogue number 88–7013-22; eBioscience, San Diego, CA, USA,). Briefly, ELISA plates were coated with the capture antibody and left overnight at 4°C, followed by samples with avidin-horseradish peroxidase. Absorbance was measured at 450 nm (microplate reader; Synergy2-BioTeck).
Data analysis
Results are expressed as mean ± SEM. Data normality was verified by evaluation of the histogram and the Shapiro–Wilk test. For normal data with equal variances, differences between experimental groups were evaluated by ANOVA, followed by the Tukey–Kramer multiple comparison test. Non-parametric data were evaluated by Wilcoxon’s (Mann–Whitney) test, followed by Steel–Dwass all-pairs comparisons. Data analysis was performed using JMP Pro 10.0.1 release 2 software (SAS Institute, Cary, NC) Significance was defined as p<0.05.
Results
Deletion of p75NTR does not cause diabetes-induced changes in sortilin or TRKA expression
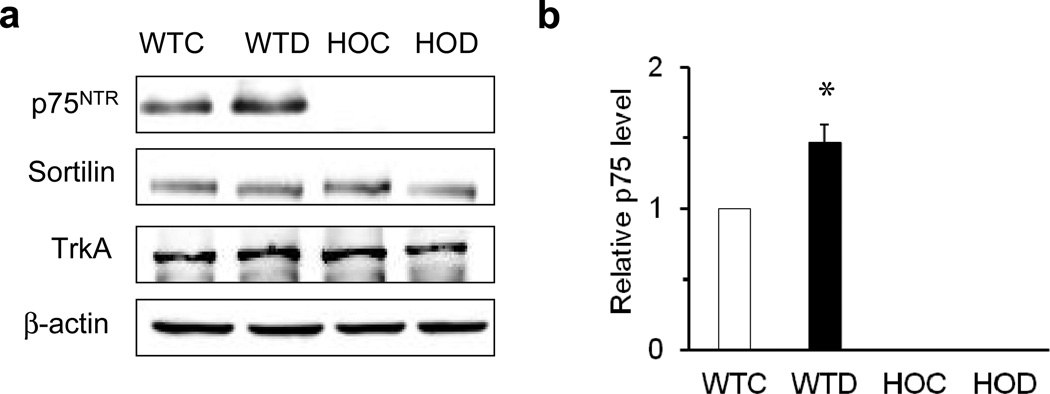
The p75KO mouse model lacks Exon III, which encodes the receptor-binding region [25], and was used to examine to what extent p75NTR modulates diabetes-induced inflammation. Expression of p75NTR and the co-receptors sortilin and TRKA was examined in WT control, WT diabetic, HOC and HOD mice after 4 to 5 weeks of streptozotocin-induced diabetes (Fig. 1a). Diabetes induced a significant 1.5-fold increase in p75NTR expression in WT diabetic compared with WT control mice. p75NTR expression was not detected in HOC and HOD mice (Fig. 1b). No significant differences were found in expression patterns of the p75NTR co-receptors, sortilin or TRKA, in any of the groups.
Fig. 1.
Deletion of p75NTR does not cause diabetes-induced changes in sortilin or TRKA expression. (a) Representative blots and (b) densitometric analysis of p75NTR protein levels in retinas of WT control (WTC), WT diabetic (WTD), HOC and HOD mice after 4 to 5 weeks of streptozotocin-induced diabetes. Note (b) that P75NTR was absent in HOC and HOD mice. Protein levels were normalised to β-actin and respective WT controls; n=4, *p<0.05
Deletion of p75NTR eliminates the diabetes-induced imbalance in proNGF and NGF expression
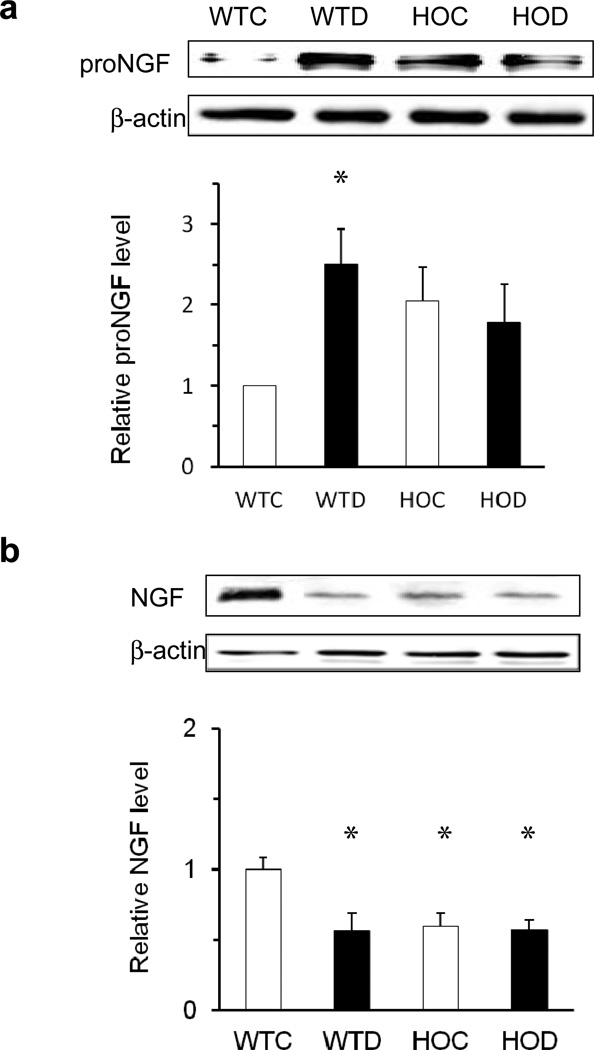
We examined whether deletion of p75NTR eliminates the diabetes-induced proNGF and NGF imbalance observed in experimental and clinical diabetes [5]. Diabetes induced a significant 2.5-fold increase in proNGF (Fig. 2a) and a 0.6-fold decrease in NGF expression (Fig. 2b) in retinas of WT diabetic vs WT control mice, changes not seen in HOD vs HOC mice (Fig. 2a, b). ESM Figure 1a shows a diabetes-induced increase in Ngf mRNA in WT diabetic, but not in HOD or HOC mice, where Ngf expression was similar to that in WT control retinas. Interestingly, basal proNGF protein levels were elevated and NGF levels decreased in p75KO mice vs WT controls (Fig. 2a, b), suggesting that regulation of proNGF levels may involve proteolytic events related to p75NTR signalling, rather than a direct effect on proNgf gene expression. As shown in ESM Fig. 2a–c, deletion or inhibition of p75NTR decreased proNGF expression in cell lysate, but did not alter NGF levels. Deletion of p75NTR, but not inhibition modestly increased proNGF release and decreased NGF release in cell soup, without reaching statistical significance, probably due to small sample size.
Fig. 2.
Deletion of p75NTR eliminates the diabetes-induced imbalance in proNGF and NGF expression. (a) Representative blots and statistical analysis of proNGF and (b) NGF protein levels in retinas of WT control (WTC), WT diabetic (WTD), HOC and HOD mice after 4 to 5 weeks of streptozotocin-induced diabetes. Protein expression is presented as relative optical density of western blot bands normalised to β-actin and respective WT controls; n=6, *p<0.05
Deletion of p75NTR eliminates the diabetes-induced upregulation of inflammatory mediators
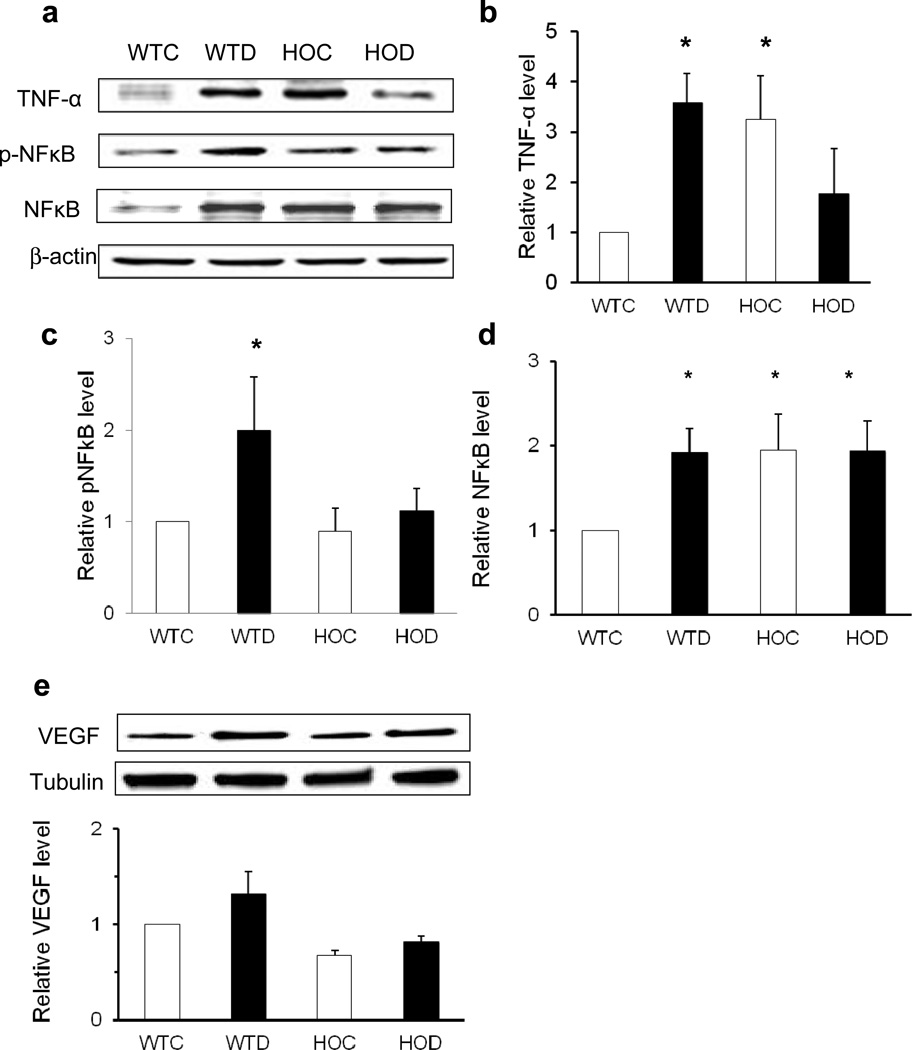
We next examined the effect of deleting p75NTR on the production of diabetes-induced inflammatory mediators. A 3.4-fold increase in 26 kDa membrane-bound TNF-α (Fig. 3a, b), a twofold increase in p-NFĸB (Fig. 3a, c) and a 1.9-fold increase in NFĸB (Fig. 3a, d) were observed in retinas from WT diabetic compared with WT control mice. These effects were absent in retinas from HOD compared with WT control and HOC mice (Fig. 3b, c). Interestingly, basal levels of TNF-α and NFĸB were markedly higher in HOC than in WT control mice (Fig. 3b, d), but not at the mRNA level (ESM Fig. 1b). As shown in ESM Fig. 2a, d, deleting or antagonising the p75NTR receptor increased membrane-bound TNF-α (26 kDa) while decreasing soluble TNF-α (17 kDa) vs untreated WT primary Müller cells. Analysis of VEGF expression in WT diabetic mice showed a modest, but not significant 1.36-fold increase (Fig. 3e) and a 1.27-fold increase in mRNA (ESM Fig. 1c) compared with WT controls. Deletion of p75NTR reduced VEGF in some HOC and HOD retinas, bit did not significantly alter protein or gene expression in these mice.
Fig. 3.
Deletion of p75NTR eliminates diabetes-induced upregulation of inflammatory mediators after 4 to 5 weeks of streptozotocin-induced diabetes. (a, e) Representative blots and protein expression of TNF-a (b), p-NFκB (c), NFκB (d) and VEGF (e) in retinas of WT control (WTC), WT diabetic (WTD), HOC and HOD mice at the diabetes duration mentioned. Protein expression is presented as relative optical density of western blot bands normalised to (3-actin and respective WT controls; n=4–6, *p<0.05
Silencing of p75NTR expression blunts proNGF-induced glial activation and inflammation
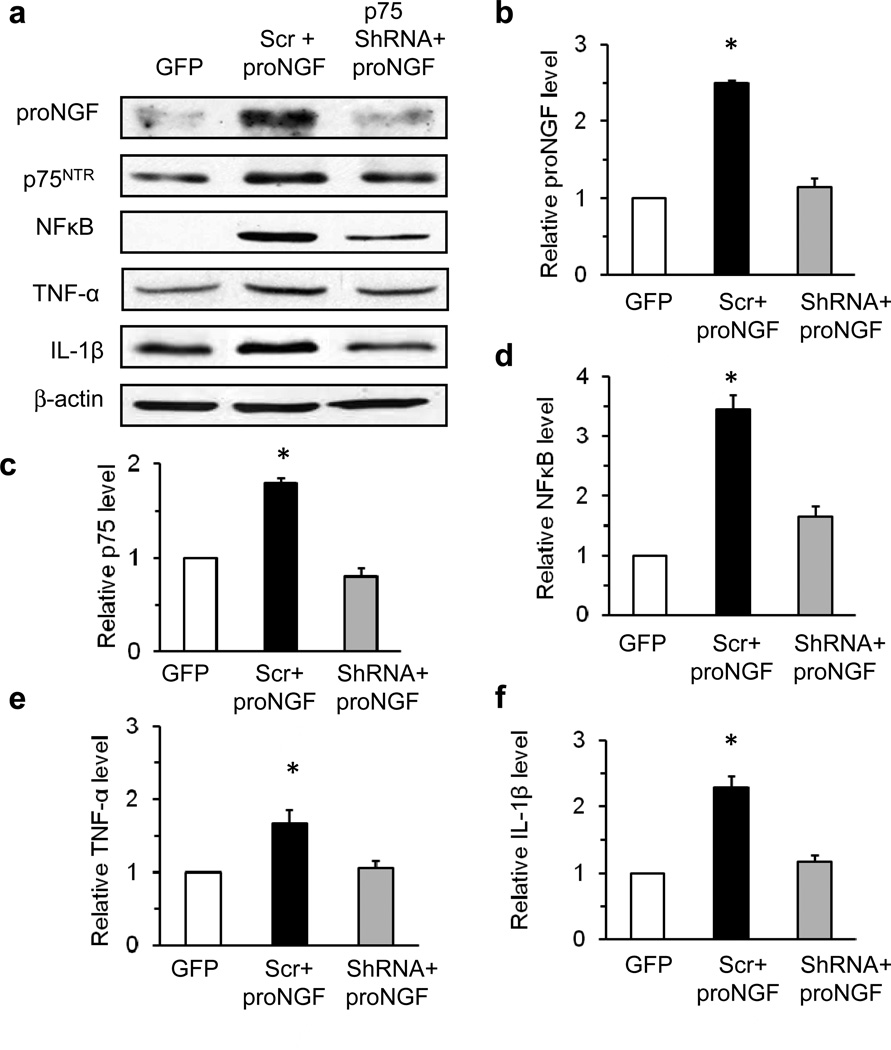
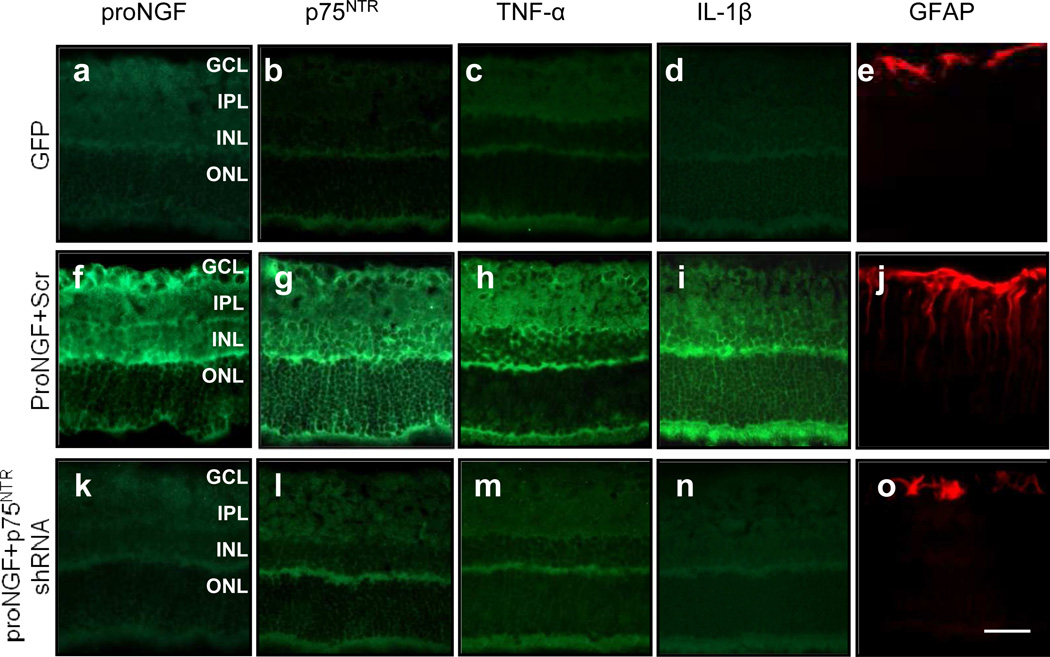
The above results suggested that deletion of p75NTR eliminates the diabetes-induced imbalance in proNGF. To evaluate to which extent proNGF–p75NTR stimulation induces retinal inflammation independently of the diabetic milieu, overexpression of cleavage-resistant proNGF and knockdown of p75NTR using shRNA were performed in healthy rat retinas. Representative blots (Fig. 4a) and statistical analysis show that overexpression of cleavage-resistant proNGF plus scrambled shRNA induced a 2.5-fold increase in proNGF (Fig. 4b), a 1.8-fold increase in p75 (Fig. 4c), a 3.6-fold increase in NFκB (Fig. 4d), a 1.7-fold increase in TNF-α (Fig. 4e) and a 2.3-fold increase in IL-1β (Fig. 4f). Knockdown of p75NTR reduced proNGF-induced inflammatory responses to control levels. Immunohistochemical analysis showed that, relative to GFP controls (Fig. 5a–e), overexpression of proNGF induced expression of proNGF, p75NTR , TNF-α, IL-1β and GFAP (Fig. 5f–j). This was localised to inner layers of the retina and the outer limiting membrane, which are areas occupied by Müller glial cells. GFAP staining was typical of activated Müller cells, appearing robustly in the GCL and extending radially into the retina. Knockdown of p75NTR by p75 NTR shRNA reduced the increased expression of proNGF, p75NTR , TNF-α, IL-1β and GFAP (Fig. 5k–o) to GFP control levels.
Fig. 4.
Silencing p75NTR expression blunts proNGF-induced inflammation. (a) Representative blots and protein expression levels of proNGF (b), p75NTR (c), NFκB (d), TNF-α (e) and IL-1β (f) in retinas removed 4 weeks after intravitreal injections of pGFP (control), scrambled shRNA plus cleavage-resistant proNGF (Scr+proNGF), and p75NTR shRNA plus cleavage-resistant proNGF (p75shRNA+proNGF). Protein expression is presented as relative optical density of western blot bands normalised to β-actin and GFP controls; n=4, *p<0.05
Fig. 5.
Overexpression of proNGF induced glial Müller activation and inflammation. Immunohistochemical analysis of retinas was performed 4 weeks after intravitreal injections of pGFP alone (control) (a–e), proNGF plus scrambled p75NTR shRNA (proNGF+Scr) (f–j) and proNGF plus p75NTR shRNA (proNGF+p75shRNA) (k-o). Retinal sections were examined from four different rats in each group. Scale bar 50 µm. INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer
Deletion of p75NTR eliminates diabetes-induced glial activation and ganglion cell loss
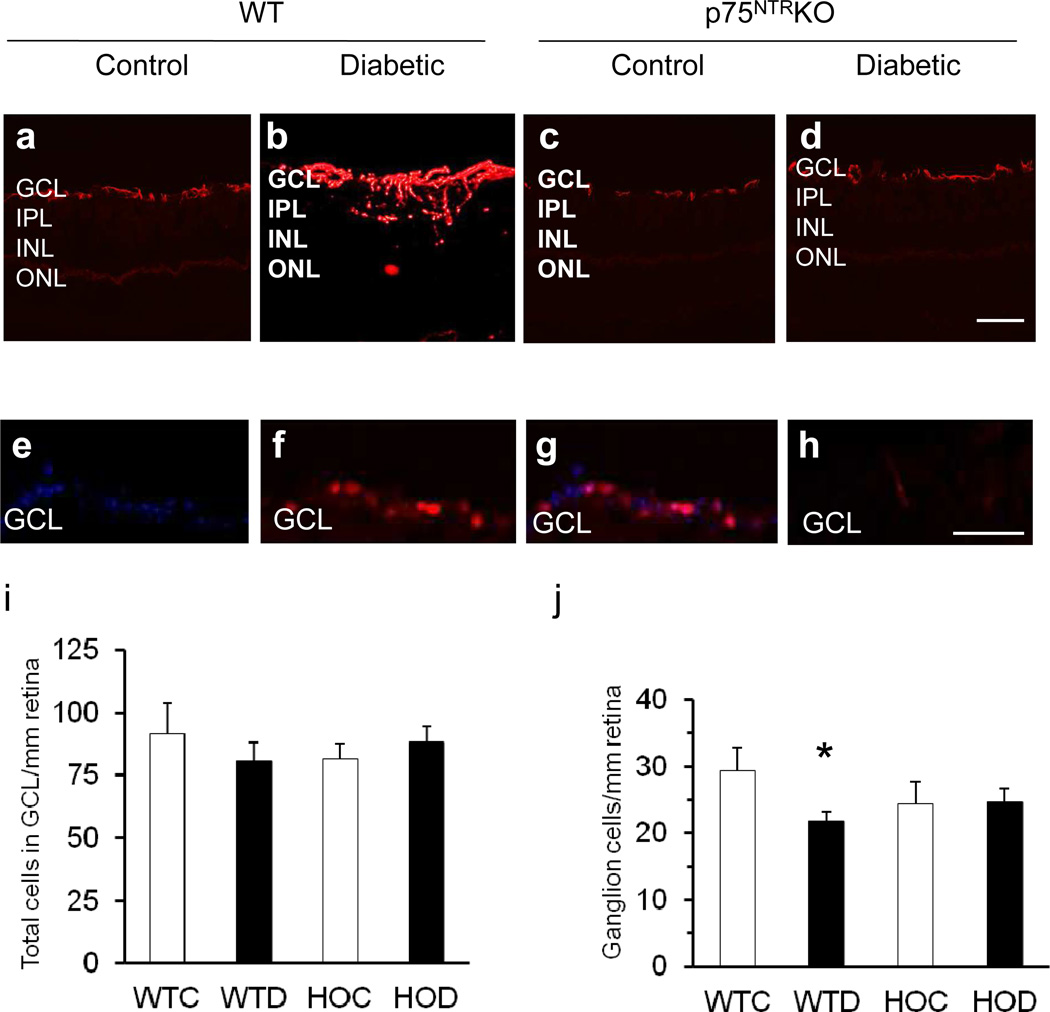
To confirm that deletion of p75NTR rendered p75KO mice insensitive to diabetes-induced inflammation, we next examined GFAP expression and ganglion cell numbers after 4 to 5 weeks of streptozotocin-induced diabetes. Representative micrographs (Fig. 6a–d) show that elevated GFAP (red) was present in WT diabetic (Fig. 6b), but not in WT control (Fig. 6a), HOC or HOD mice (Fig. 6c, d). Neuronal death, especially ganglion cell loss, is also characteristic of the diabetic retina [33]. RGCs were identified as BRN3A-positive cells (Fig. 6f) that co-localised with DAPI-positive nuclei (Fig. 6g). Mouse IgG2 as negative isotype control showed minimal staining (Fig. 6h). Subsequent analysis of these counts showed no difference in total cell number in the GCLs of WT control, WT diabetic, HOC or HOD mice (Fig. 6i), but a significant 25% decrease in BRN3A-positive ganglion cells in WT diabetic compared with WT control retinas (Fig. 6j). No difference in BRN3A-positive ganglion cell numbers was observed between HOC and HOD retinas (Fig. 6j). Although basal numbers of BRN3A ganglion cells were reduced in HOC and HOD mice, this difference was not significant.
Fig. 6.
Deletion of p75NTR eliminates diabetes-induced glial activation and ganglion cell loss. (a–d) Representative micrographs (scale bars 50 µm) showing GFAP expression (red) and (e–h) BRN3 A expression (red), with statistical analysis of total cells in (i) the GCL and (j) ganglion cells in retinas of WT control (WTC), WT diabetic (WTD), HOC and HOD mice after 4 to 5 weeks of streptozotocin-induced diabetes; n=6–8, *p<0.05. INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer
Modulation of p75NTR prevents diabetes- and proNGF-induced BRB breakdown
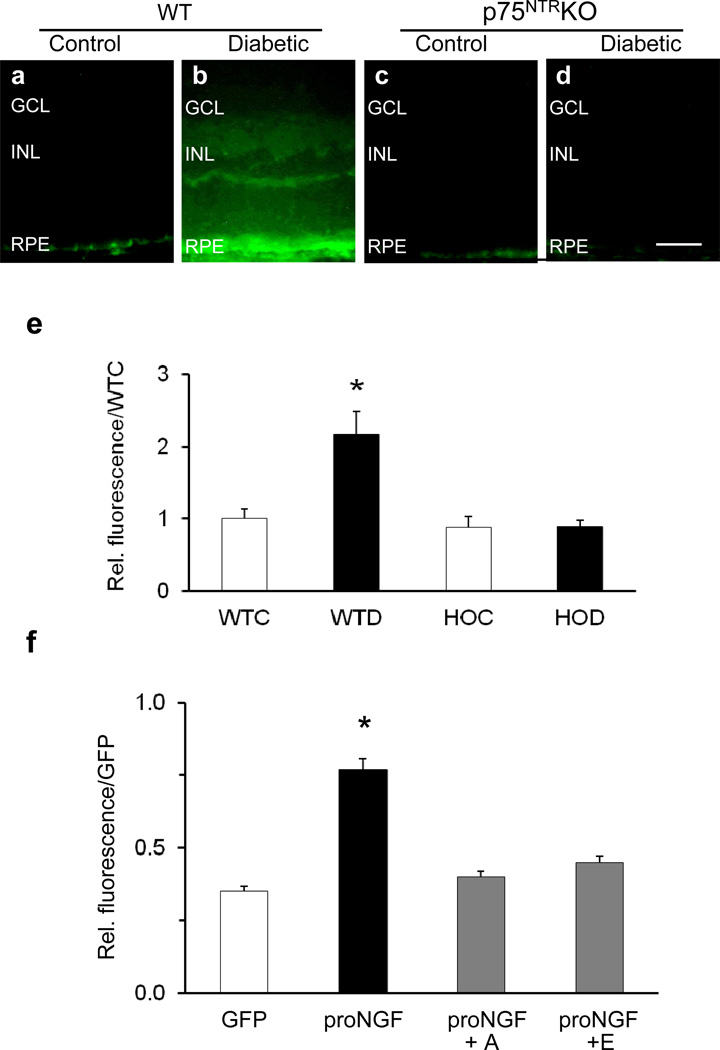
Representative micrographs (Fig. 7a–d) show increased extravasation of BSA-conjugated green fluorescence in WT diabetic (Fig. 7b), but not in WT control (Fig. 7a), HOC (Fig. 7c) or HOD retinas (Fig. 7d). This effect was associated with a twofold increase in fluorescence of retinal lysate from WT diabetic compared with WT controls, with no increase in HOC or HOD mice (Fig. 7e). In an alternative model, overexpression of cleavage-resistant proNGF in WT mice also caused a twofold increase in BRB breakdown as assessed by extravasation of BSA-fluorescein (Fig. 7f). This effect was abrogated by the selective antagonist for p75NTR receptor (40 ng/eye) or by the selective γ-secretase inhibitor (100 ng/eye).
Fig. 7.
Modulation of p75NTR prevents diabetes- and proNGF-induced BRB breakdown. (a–d) Representative micrographs (scale bar 50 µm) and statistical analysis (e) of retinal vascular permeability assessed by extravasation of BSA-conjugated fluorescein after 4 to 5 weeks of streptozotocin-induced diabetes in WT control (WTC), WT diabetic (WTD), HOC and HOD mice. (f) Statistical analysis of proNGF-induced retinal vascular permeability assessed after 1 week in the presence of the p75NTR -specific antagonist (A) (40 ng) or the γ-secretase inhibitor compound E (E) (100 ng) compared with GFP controls; n=4–6, *p<0.05. INL, inner nuclear layer; RPE, retinal pigmented epithelial layer
Blocking of p75NTR action and p75NTR deletion prevent proNGF-mediated inflammation in Müller cells
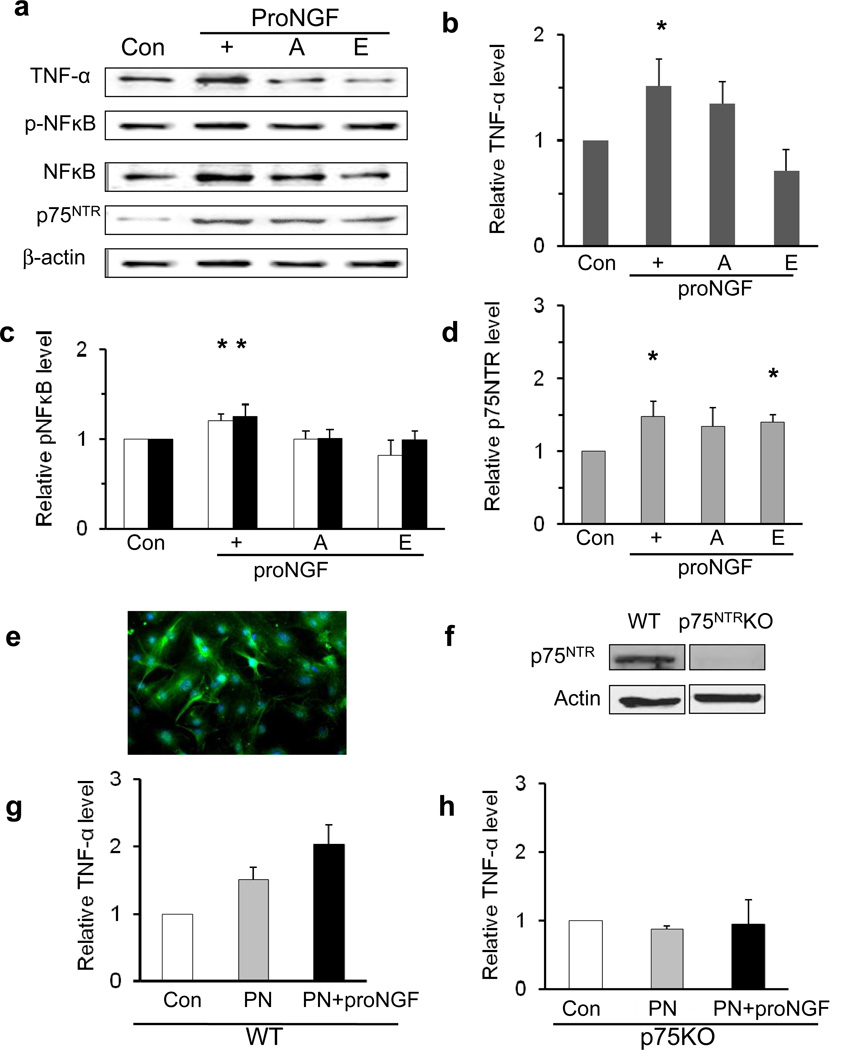
We and others have demonstrated p75NTR co-localisation of proNGF in Müller cells [5, 6, 15]. Previously we reported that high glucose significantly stimulates p75NTR expression in rMC-1 [6]. Here, blocking the action or cleavage of p75NTR was evaluated in rMC-1 cultures maintained in high glucose plus mutant-proNGF. Representative blots (Fig. 8a) and statistical analyses (Fig. 8b–d) show that exposure of rMC-1 cells to proNGF further induced p75NTR expression (1.6-fold) compared with high glucose cultures alone (Fig. 8d). The proNGF-induced elevation of p75NTR was accompanied by a significant 1.6-fold increase in TNF-α (Fig. 8b) and a small, but significant 1.3-fold increase in p-NFκB and NFκB (Fig. 8c). These effects were markedly reduced by co-treatment with compound E (γ-secretase inhibitor). Interestingly, exposure of cells to high glucose and proNGF plus the p75NTR antagonist or compound E also resulted in upregulation of p75NTR expression vs controls (Fig. 8d). Finally we investigated whether deletion of p75NTR rendered Müller cells insensitive to the proinflammatory effects of proNGF. Cultures of primary Müller cells were isolated [32] and characterised by robust expression of the Müller cell marker vimentin (Fig. 8e). Western blot confirmed the lack of p75NTR expression in p75KO Müller cells (Fig. 8f). To mimic the pro-oxidative milieu of the diabetic retina, primary Müller cells were cultured in 100 µmol/l peroxynitrite and stimulated with mutant proNGF. Treatment of WT Müller cells with peroxynitrite alone or in combination with proNGF induced release of TNF-α into the supernatant fraction (Fig. 8g). In contrast, treatment of p75KO-Müller cells with peroxynitrite alone or in combination with proNGF showed no significant change in secreted TNF-α compared with p75KO controls (Fig. 8h).
Fig. 8.
Modulation of p75NTR action or expression prevents proNGF-mediated inflammation in Müller cells. (a) Representative blots and densitometric analysis of TNF-α (b), p-NFκB (black columns) and NFκB (white columns) (c), and p75NTR (d) protein levels in rMC-1 cells grown for 2 days in 25 mmol/l glucose (control) and treated for 16 h with cleavage-resistant proNGF (50 ng/ml) in the presence or absence of the p75 antagonist (A) (20 µmol/l) or compound E (E) (10 µmol/l), which is an inhibitor of γ-secretase-catalysed p75 cleavage. (n=4–7, *p<0.05) (e) Primary Müller cells showed robust expression of the Müller cell marker vimentin (green) in most cells. (f) Expression of p75NTR in cultures of WT Müller cells was absent in p75KO Müller cells. (g) Statistical analysis of TNF-α in supernatant fractions from primary WT Müller cells and (h) p75KO Müller cells exposed to 100 µmol/l peroxynitrite (PN) alone and in combination 26 with 50 ng/ml cleavage-resistant pro-NGF, compared with control cells in 5 mmol/l glucose media alone (n=3)
Discussion
Retinal inflammation, as reviewed by others [34, 35], is increasingly recognised as an important factor in the pathogenesis of diabetic retinopathy. The present results demonstrate the importance of p75NTR in diabetes- and proNGF-induced retinal inflammation and BRB breakdown. First, deletion of p75NTR blunted diabetes-induced retinal inflammation, glial activation, ganglion cell loss and vascular permeability. Second, inhibition of p75NTR or its cleavage blocked proNGF-induced expression of inflammatory mediators both in vivo in a rodent model that overexpressed cleavage-resistant proNGF and in vitro in Müller cells.
Diabetic retinopathy is characterised by early BRB breakdown, which causes loss of vision through macular oedema and/or vitreoretinal neovascularisation. However, as previously reviewed [36], current treatments to prevent macular oedema are invasive and of limited efficacy. Understanding the mechanisms behind early increases in diabetes-induced vascular permeability is essential for developing novel and effective therapeutic targets for diabetic retinopathy. A large body of evidence demonstrates a clear role for diabetes-induced oxidative stress, resulting in glial activation and release of proinflammatory cytokines including VEGF, TNF-α and IL-1β [30, 34, 37–40]. In addition to these known inflammatory mediators, the present study provides compelling support for an upstream role of the proNGF–p75NTR axis in diabetes-induced retinal inflammation. Our studies show that silencing p75NTR expression abrogated proNGF-induced increases in TNF-α, NFκB, IL-1β and glial activation (Figs 4, 5), and that modulation of p75NTR activity by a p75NTR antagonist or its cleavage using the γ-secretase inhibitor prevented proNGF-induced BRB breakdown in vivo (Fig. 7). Deletion of p75NTR blunted diabetes-induced alterations in proNGF and NGF protein expression (Fig. 2), and diabetes-induced increases in TNF-α, p-NFκB and VEGF (Fig. 3). Interestingly, deletion of p75NTR caused basal alterations in levels of proNGF, TNF-α and total NFκB (Fig. 3), but not in the corresponding mRNA levels, suggesting that protein alteration could be attributed to post-translational protein processing in these KO mice. Moreover, our results show that deleting or antagonising the p75NTR receptor in primary Müller cells increased membrane-bound TNF-α (26 kDa) while decreasing soluble TNF-α (17 kDa) compared with WT. Deletion of p75NTR tended to increase release of proNGF and decrease release of NGF in cell soup compared with WT Müller cells. Thus the p75NTR receptor was shown to play an important role in the regulation of proNGF and NGF levels, and in the relationship between membrane-bound and soluble TNF-α. Most importantly, the differences in TNF-α expression patterns may explain the dichotomy between the insensitivity of p75KO retinas to diabetes-induced vascular permeability and the 18 occurrence of cell death, despite the higher levels of membrane-bound TNF-α and altered proNGF and NGF expression. The notion that deletion of p75NTR alters proteolytic activity, resulting in accumulation of proNGF and membrane-bound TNF-α is supported by recent research on Alzheimer’s disease showing that interaction of the p75NTR extracellular tail domain with β-amyloid inhibited the accumulation of aggregates and subsequent protein expression patterns without altering gene expression [41].
Although NGF-induced upregulation of p75NTR has been shown to occur through TRKA signalling pathways [42], to our knowledge, p75NTR regulation of proNGF has not been fully elucidated. Our previous studies showed a positive correlation between proNGF and p75NTR levels in human and experimental models of diabetes [5, 6], and in a model of proNGF overexpression [27]. Here, we show that knocking-down expression of p75NTR downregulates proNGF expression, supporting the positive link between the ligand and receptor. In contrast to the elevated p75NTR expression in WT diabetic mice, sortilin levels were similar in all four groups. These results are consistent with our previous findings in diabetic rat retinas [6] and in a model of proNGF overexpression [27]. Although approximately 10% of sortilin protein is located at the plasma membrane where it can act as a p75NTR co-receptor, most sortilin is located in the cytoplasm, where it aids trafficking of proteins to the plasma membrane [16]. Thus, increased sortilin at the plasma membrane could participate in proNGF–p75NTR signalling without a detectable change in total sortilin expression.
Despite altered basal protein levels in p75KO mice, our data confirm previous findings that breakdown of BRB can occur early on in streptozotocin-induced diabetes [5, 30, 39, 43]. Deletion of p75NTR rendered p75KO mice insensitive to glial activation, increased vascular permeability and diabetes-induced ganglion cell loss, all of which are hallmarks of early diabetic retinopathy. Although the 25% loss of BRN3 A-positive ganglion cells in WT diabetic mice is higher than the 7% loss reported by others [44] after 6 weeks of streptozotocin-induced diabetic in C57BL6 mice, our total GCL counts and percentage of BRN3A-positive cells in WT control mice are comparable to published reports [45, 46]. Diabetes-induced ganglion cell loss also varies with the duration of diabetes, insulin supplementation and counting method. Thus Martin et al report a 25% loss of total cells in the GCL after 14 weeks of streptozotocin-induced diabetes in C57BL6 mice without insulin supplementation [47], while Barber and co-workers report a significant 10% loss of neuronal cells in the GCL layer in mice after 7.5 months of streptozotocin-induced diabetes with insulin supplementation [33]. Although p75KO mice appear to have fewer BRN3A cells in the GCL than WT controls, this difference was not statistically significant.
Our current findings suggest a pathway by which proNGF-stimulated cleavage of p75NTR activates NFκB with subsequent production of inflammatory mediators such as TNF-α. These results are consistent with work by others, in which p75NTR -induced expression of IL-1β and TNF-α was attributed to activation and nuclear translocation of NFκB [15, 19, 23]. The p75NTR receptor, similar to Notch and amyloid precursor protein, undergoes γ-secretase catalysed intramembrane proteolysis [17] to liberate the intracellular domain (p75ICD ), which can recruit several intracellular binding proteins that dictate specific signalling pathways [19]. The p75NTR intracellular domain can recruit a complex of proteins, including IκB-kinase-β, which, as previously reviewed [48], phosphorylates IκB, resulting in release and activation of NFκB. While the involvement of the p75ICD in NFκB activation has been reported by others [18], the exact molecular mechanisms are still under study. One possibility is that p75ICD associates with TNF receptor-associated factor-6 [49] to mediate activation of NFκB. Alternatively, p75ICD may regulate NFκB activation directly by translocating to the nucleus, where it can act as a transcription enhancer [17–20]. The protective effects of inhibiting p75NTR or its cleavage (p75NTR receptor antagonist and compound E) could be attributed, partly, to decreased retinal inflammation, as demonstrated by their abrogation of increased TNF-α and NFκB protein expression in proNGF-stimulated rMC-1 cells (Fig. 8b, c). Our finding that diabetic insults, including peroxynitrite and proNGF, increased secretion of TNF-α in cultures of WT primary Müller, but not in p75KO Müller cells (Fig. 8g, h) also demonstrates Müller cell involvement in the p75NTR-mediated inflammatory response. In summary, the deletion of p75NTR not only eliminated diabetes-induced glial activation and inflammation, but also prevented ganglion cell loss and BRB breakdown, suggesting that p75NTR inhibition could be a therapeutic target for the early stages of diabetic retinopathy.
Supplementary Material
Acknowledgments
Funding
This work was supported by a Career Development Award from the Juvenile Diabetes Research Foundation (2-2008-149), RO1-EY022408 to ABE and a Grant from Vision Discovery Institute, Georgia Reagents University to ABE. Further support came from a pre-doctoral fellowship from American Heart Association (MAA), a post-doctoral fellowship from American Heart Association (BAM) and a post-doctoral fellowship from Islamic Development Bank (MFE).
Abbreviations
- BRB
Blood–retina barrier
- BRN3A
Brain-specific homeobox/POU domain protein 3 A
- GCL
Ganglion cell layer
- GFAP
Glial fibrillary acidic protein
- GFP
Green fluorescence protein
- HOC
Homozygous knockout control
- HOD
Homozygous knockout diabetic
- IFU
Infectious units
- NFκB
Nuclear factor κB
- NGF
Nerve growth factor
- p75ICD
p75 intracellular domain
- p75KO
p75NTR knockout
- p75NTR
p75, neurotrophin receptor
- pGFP
GFP plasmid
- PM
Primary mouse Müller cells
- proNGF
NGF precursor
- RGCs
Retinal ganglion cells
- rMC-1
Retinal Müller glial cell line
- shRNA
Short hairpin RNA
- TRKA
Tyrosine receptor kinase A
- VEGF
Vascular endothelial growth factor
- WT
Wild-type
Footnotes
Contribution statement
BAM, MMHA, SM, MFE and MAA performed experiments, analysed data and reviewed the manuscript. HUS provided p75NTR antagonist and technical expertise that was necessary for data acquisition, and critically edited the manuscript. ABE and BAM conceived the hypothesis and wrote/revised the manuscript. All authors approved the final version of the manuscript.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Klein R, Klein BE, Moss SE, Wong TY. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2006;104:98–107. [PMC free article] [PubMed] [Google Scholar]
- 2.Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher EL, Phipps JA, Wilkinson-Berka JL. Dysfunction of retinal neurons and glia during diabetes. Clin Exp Optom. 2005;88:132–145. doi: 10.1111/j.1444-0938.2005.tb06686.x. [DOI] [PubMed] [Google Scholar]
- 4.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47(Suppl 2):S253–S262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 5.Ali TK, Al-Gayyar MM, Matragoon S, et al. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 2011;54:657–668. doi: 10.1007/s00125-010-1935-1. [DOI] [PubMed] [Google Scholar]
- 6.Al-Gayyar MM, Matragoon S, Pillai BA, Ali TK, Abdelsaid MA, El-Remessy AB. Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor expression in a rat model of diabetes [corrected] Diabetologia. 2011;54:669–680. doi: 10.1007/s00125-010-1994-3. [DOI] [PubMed] [Google Scholar]
- 7.Ali TK, Matragoon S, Pillai BA, Liou GI, El-Remessy AB. Peroxynitrite mediates retinal neurodegeneration by inhibiting NGF survival signal in experimental and human diabetes. Diabetes. 2008;57:889–898. doi: 10.2337/db07-1669. [DOI] [PubMed] [Google Scholar]
- 8.Hempstead BL. Commentary: Regulating proNGF action: Multiple targets for therapeutic intervention. Neurotox Res. 2009;16:255–260. doi: 10.1007/s12640-009-9054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 10.Harrington AW, Leiner B, Blechschmitt C, et al. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17:3261–3270. doi: 10.1038/sj.onc.1202568. [DOI] [PubMed] [Google Scholar]
- 12.Cragnolini AB, Friedman WJ. The function of p75NTR in glia. Trends Neurosci. 2008;31:99–104. doi: 10.1016/j.tins.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez CF. Jekyll-Hyde neurotrophins: the story of proNGF. Trends Neurosci. 2002;25:284–286. doi: 10.1016/s0166-2236(02)02169-0. [DOI] [PubMed] [Google Scholar]
- 14.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 15.Lebrun-Julien F, Bertrand MJ, de Backer O, et al. ProNGF induces TNFalpha-dependent death of retinal ganglion cells through a p75NTR non-cell-autonomous signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3817–3822. doi: 10.1073/pnas.0909276107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nykjaer A, Lee R, Teng KK, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 17.Frade JM. Nuclear translocation of the p75 neurotrophin receptor cytoplasmic domain in response to neurotrophin binding. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1407–1411. doi: 10.1523/JNEUROSCI.3798-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:5425–5436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002;3:673–684. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- 20.Zampieri N, Xu CF, Neubert TA, Chao MV. Cleavage of p75 neurotrophin receptor by alpha-secretase and gamma-secretase requires specific receptor domains. The Journal of biological chemistry. 2005;280:14563–14571. doi: 10.1074/jbc.M412957200. [DOI] [PubMed] [Google Scholar]
- 21.Kenchappa RS, Zampieri N, Chao MV, et al. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–232. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Jung KM, Tan S, Landman N, et al. Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. The Journal of biological chemistry. 2003;278:42161–42169. doi: 10.1074/jbc.M306028200. [DOI] [PubMed] [Google Scholar]
- 23.Carter BD, Kaltschmidt C, Kaltschmidt B, et al. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 24.Bhakar AL, Roux PP, Lachance C, Kryl D, Zeindler C, Barker PA. The p75 neurotrophin receptor (p75NTR) alters tumor necrosis factor-mediated NF-kappaB activity under physiological conditions, but direct p75NTR-mediated NF-kappaB activation requires cell stress. The Journal of biological chemistry. 1999;274:21443–21449. doi: 10.1074/jbc.274.30.21443. [DOI] [PubMed] [Google Scholar]
- 25.Lee KF, Li E, Huber LJ, et al. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 26.Pagadala PC, Dvorak LA, Neet KE. Construction of a mutated pro-nerve growth factor resistant to degradation and suitable for biophysical and cellular utilization. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17939–17943. doi: 10.1073/pnas.0604139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matragoon S, Al-Gayyar MM, Mysona BA, et al. Electroporation-mediated gene delivery of cleavage-resistant pro-nerve growth factor causes retinal neuro- and vascular degeneration. Mol Vis. 2012;18:2993–3003. [PMC free article] [PubMed] [Google Scholar]
- 28.LeSauteur L, Wei L, Gibbs BF, Saragovi HU. Small peptide mimics of nerve growth factor bind TrkA receptors and affect biological responses. The Journal of biological chemistry. 1995;270:6564–6569. doi: 10.1074/jbc.270.12.6564. [DOI] [PubMed] [Google Scholar]
- 29.Nadal-Nicolas FM, Jimenez-Lopez M, Sobrado-Calvo P, et al. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Investigative ophthalmology & visual science. 2009;50:3860–3868. doi: 10.1167/iovs.08-3267. [DOI] [PubMed] [Google Scholar]
- 30.Al-Shabrawey M, Rojas M, Sanders T, et al. Role of NADPH oxidase in retinal vascular inflammation. Investigative ophthalmology & visual science. 2008;49:3239–3244. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Muller cell line. Investigative ophthalmology & visual science. 1998;39:212–216. [PubMed] [Google Scholar]
- 32.Mysona B, Dun Y, Duplantier J, Ganapathy V, Smith SB. Effects of hyperglycemia and oxidative stress on the glutamate transporters GLAST and system xc- in mouse retinal Muller glial cells. Cell Tissue Res. 2009;335:477–488. doi: 10.1007/s00441-008-0742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali TK, El-Remessy AB. Diabetic retinopathy: current management and experimental therapeutic targets. Pharmacotherapy. 2009;29:182–192. doi: 10.1592/phco.29.2.182. [DOI] [PubMed] [Google Scholar]
- 37.Poulaki V, Qin W, Joussen AM, et al. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. 2002;109:805–815. doi: 10.1172/JCI13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nwariaku FE, Chang J, Zhu X, et al. The role of p38 map kinase in tumor necrosis factor-induced redistribution of vascular endothelial cadherin and increased endothelial permeability. Shock. 2002;18:82–85. doi: 10.1097/00024382-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes. 2007;56:2380–2387. doi: 10.2337/db06-1694. [DOI] [PubMed] [Google Scholar]
- 40.El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol. 2006;168:235–244. doi: 10.2353/ajpath.2006.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YJ, Wang X, Lu JJ, et al. p75NTR regulates Abeta deposition by increasing Abeta production but inhibiting Abeta aggregation with its extracellular domain. J Neurosci. 2011;31:2292–2304. doi: 10.1523/JNEUROSCI.2733-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rankin SL, Guy CS, Rahimtula M, Mearow KM. Neurotrophin-induced upregulation of p75NTR via a protein kinase C-delta-dependent mechanism. Brain research. 2008;1217:10–24. doi: 10.1016/j.brainres.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 43.Huang H, Gandhi JK, Zhong X, et al. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investigative ophthalmology & visual science. 2011;52:1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y, Mao D, Chen X, et al. Decrease in retinal neuronal cells in streptozotocin-induced diabetic mice. Molecular vision. 2012;18:1411–1420. [PMC free article] [PubMed] [Google Scholar]
- 45.Shupe JM, Kristan DM, Austad SN, Stenkamp DL. The eye of the laboratory mouse remains anatomically adapted for natural conditions. Brain, behavior and evolution. 2006;67:39–52. doi: 10.1159/000088857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain V, Ravindran E, Dhingra NK. Differential expression of Brn3 transcription factors in intrinsically photosensitive retinal ganglion cells in mouse. The Journal of comparative neurology. 2012;520:742–755. doi: 10.1002/cne.22765. [DOI] [PubMed] [Google Scholar]
- 47.Martin PM, Roon P, van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Investigative ophthalmology & visual science. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- 48.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khursigara G, Orlinick JR, Chao MV. Association of the p75 neurotrophin receptor with TRAF6. The Journal of biological chemistry. 1999;274:2597–2600. doi: 10.1074/jbc.274.5.2597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.