Abstract
This study tested the hypothesis that the Dahl salt-sensitive (SS) rat has vascular dysfunction due, in part, to the upregulation of the cytochrome P450-4A ω-hydroxylase (CYP4A)/20-hydroxyeicosatetraenoic acid (20-HETE) system. To assess the role of vascular 20-HETE, SS were compared to SS-5BN consomic rats, carrying CYP4A alleles on chromosome 5 from the normotensive Brown Norway introgressed onto the SS genetic background. Cerebral arteries from SS-5BN had less CYP4A protein than arteries from SS rats fed either normal salt (NS, 0.4% NaCl) or high salt (HS, 4.0% NaCl) diet. Acetylcholine (ACh)-induced dilation of middle cerebral arteries (MCA) from SS and SS-5BN was present in MCA of SS-5BN fed either NS or HS diet, but absent in SS. In SS fed either diet, ACh-induced dilation was restored by acute treatment with the CYP4A inhibitor DDMS or the 20-HETE antagonist 20-HEDE. The restored response to ACh in DDMS-treated SS was inhibited by L-NAME and unaffected by indomethacin or MS-PPOH. Vascular relaxation responses to the nitric oxide (NO) donor sodium nitroprusside were intact in both SS and SS-5BN rats and unaffected by the acute addition of DDMS, indicating the vascular dysfunction of the SS rat is due to a reduced bioavailability of NO instead of failure the vascular smooth muscle cells to respond to the vasodilator. Superoxide levels in cerebral arteries of SS-5BN rats (evaluated semi-quantitatively by DHE fluorescence) were lower than those in arteries of SS rats. These findings indicate SS rats have an upregulation of the CYP4A/20-HETE pathway resulting in elevated ROS and reduced NO bioavailability resulting in vascular dysfunction.
Key Words/Phrases: Vascular dysfunction, Salt-sensitive hypertension, Oxidative stress, 20-HETE, CYP4A
INTRODUCTION
Endothelial dysfunction is the failure of the endothelial cell layer to maintain vascular homeostasis resulting in increasing vascular constriction and reduced vascular relaxation to vasodilator stimuli. Increased levels of reactive oxygen species in the endothelium not only compromise NO-dependent vasodilatation, but also overwhelm the antioxidant, anti-inflammatory, and anti-proliferative properties of NO in the vessel wall. Endothelial dysfunction is associated with hypertension [1, 2] and many other cardiovascular diseases,[3] and has been shown to be an early prognosticator of future adverse cardiovascular-related incidents (including myocardial infarction, stroke, and death).[3, 4]
20-Hydroxyeicosatetraenoic acid (20-HETE) is a vasoconstrictor metabolite of arachidonic acid formed in vascular smooth muscle (VSM) cells through the action of cytochrome P450 ω-hydroxylase 4A (CYP4A). The CYP4A/20-HETE system has been implicated in the development of hypertension in humans and animals.[5–8] Studies on human subjects have shown an association between genetic variants in CYP4AF, a human gene that forms 20-HETE, and an increase in both urinary excretion of 20-HETE and mean arterial pressure (MAP).[5, 6] In animal models, spontaneously hypertensive rats (SHR)[7] and androgen-induced hypertensive rats[9] have elevated vascular 20-HETE and high blood pressure (BP) that can be ameliorated with a CYP4A inhibitor. Cyp4a14 knockout mice further demonstrate the role of the CYP4A/20-HETE pathway in the development of hypertension as these mice develop severe hypertension as a result of increased expression of the Cyp4a12 isoform in the kidney and enhanced 20-HETE production.[8]
In addition to the pro-hypertensive effects of 20-HETE, the CYP4A/20-HETE pathway has also been associated with endothelial dysfunction. Ward et al demonstrated an association between elevated urinary 20-HETE excretion and endothelial dysfunction in humans.[10] Similar to reports in hypertensive rat models,[7, 9] Sprague-Dawley (S-D) rats receiving a CYP4A2-carrying adenovirus demonstrate increased BP, reduced acetylcholine (ACh)-induced vascular relaxation, and attenuated nitric oxide (NO) production.[11] In Dahl salt-sensitive (SS) rats, the constriction of skeletal muscle arterioles in response to elevated PO2 is potentiated by increased dietary salt, an effect that can be abolished with 20-HETE inhibition.[12]
The present study tested the hypothesis that an upregulation of the CYP4A/20-HETE system plays a direct role in the vascular dysfunction of the SS rat through the production of reactive oxygen species and the subsequent decrease in NO bioavailability. Earlier studies showed that a three-day high salt (HS) diet causes an upregulation of CYP4A mRNA in SS cremasteric arterioles,[12] and in mesenteric arteries from S-D rats,[13] suggesting an association between increased dietary salt and elevated 20-HETE production in resistance arteries. While it is clear that salt can activate the CYP4A/20-HETE pathway, SS rats also exhibit vascular dysfunction when they are normotensive and maintained on a normal salt diet. As such, increases in the CYP4A/20-HETE system may be independent of dietary sodium.[14–16] In order to determine the direct effects of salt on the CYP4A/20-HETE pathway independent of changes in arterial pressure, the present study evaluated the effect of short-term (three days) elevated dietary salt intake as the Dahl SS rat does not demonstrate salt-induced pressure changes within this limited time frame.[16] In addition, we evaluated vascular function in a novel cosomic rat strain (SS-5BN) carrying CYP4A alleles from the Brown Norway (BN) rat in the Dahl SS genetic background that exhibits dramatic attenuation of salt-sensitivity of blood pressure.[17]
The SS-5BN rat is a strain developed by the Program for Genomic Applications (PGA) group at the Medical College of Wisconsin (MCW) as part of a consomic panel of rats in which single chromosomes from the Brown Norway rat were introgressed individually onto the genetic background of the Dahl SS rat using marker-assisted selection.[18] As reported by Cowley et al, consomic rat strains allow the investigation into the role of specific genes and chromosomes in controlling physiological traits with reduced genetic variability.[18] For this reason, the SS-5BN consomic rat, carrying the CYP4A genes on chromosome 5 from the Brown Norway rat, is an excellent control animal for the investigation of the role of CYP4A and 20-HETE in the vascular dysfunction in the Dahl SS rat. Specifically, the SS-5BN consomic rat has ~95% genetic homology to the Dahl SS rat, but exhibits protection from salt-induced BP elevations.[17] We hypothesized that these rats would also have protection from the vascular dysfunction present in the SS rat due to a decreased contribution of the CYP4A/20-HETE pathway.
METHODS
Experimental Groups
Male SS (SS/JrHsd/Mcwi) and SS-Chr 5BN/Mcwi (SS-5BN) rats 8–10 weeks old were fed either normal salt (NS; 0.4% NaCl; Dyets, Inc., Bethlehem, PA) from weaning or switched to a HS diet (4.0% NaCl; Dyets, Inc.) for 3 days prior to experiments, with water ad libitum. The Medical College of Wisconsin IACUC approved all protocols.
Isolated Vessel Experiments
Animals were anesthetized with intramuscular injection containing (in mg/kg): ketamine (75.0), acepromazine (2.5) and anased (10.0). Middle cerebral arteries (MCA) were cannulated as previously described [19] and internal diameter was measured using television microscopy. Vessels lacking active tone at rest were excluded from study.
Responses to acetylcholine (ACh; 10−10–10−5 mol/L) and sodium nitroprusside (SNP; 10−12–10−4 mol/L) were determined before and after treatment with N-methyl-sulfonyl-12,12-dibromododec-11-enamide (DDMS, 50 µmol/L) or 6(Z), 15 (Z)-20-HEDE (20-HEDE, 1µmol/L). In another experiment, responses to ACh before and after incubation with N(G)-nitro-L- arginine methyl ester (L-NAME; 100 µmol/L) to inhibit nitric oxide synthase, indomethacin (1 µmol/L) to inhibit cyclooxygenase, or N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexanamide (MS-PPOH; 100 mmol/L) to inhibit epoxygenases were recorded in DDMS-treated MCA from SS and control MCA from SS-5BN. Additional vessels were incubated without inhibitors as a time control.
At the end of the experiment, maximum diameter was determined by adding hydrogen peroxide (H2O2; 1.76 mmol/L) to the superfusate to achieve maximum dilation. Active resting tone (%) was calculated as [(Dmax-Drest)/Dmax]×100, where Dmax is the maximum diameter in the presence of H2O2 and Drest is the resting control diameter.
Western Blot
CYP4A protein expression in cerebral arteries was assessed by Western blotting as previously described [13, 20, 21] using CYP4A1/A2/A3 antibody (Santa Cruz, sc-53247). Expression of eNOS (BD Biosciences, #610296), peNOS (BD Biosciences, #612393), Cu/Zn SOD (Enzo Life Sciences), Mn-SOD (Assay Designs), and EC-SOD (Santa Cruz, sc-3222) were also assessed using Western blotting. Relative intensity of the bands was quantified and normalized to a loading control (β-actin) using a computer-based densitometer system and Image-Quant software (Molecular Dynamics).
Dihydroethidium Fluorescence
Vascular reactive oxygen species (ROS) levels in basilar arteries were assessed semi-quantitatively using dihydroethidium (DHE).[22] Basilar arteries were used as a substitute for MCA, as both vessels demonstrate a NO-dependent dilation to ACh[23] and the larger diameter allows for improved cross sectioning. In these experiments, the arteries were isolated and incubated for 1 hour in PSS heated to 37° with DHE (5 µmol/L) for the final 15 min. The vessels were cut into 10 µm transverse sections and imaged with a Nikon Eclipse TS100 microscope (Nikon, Tokyo, Japan) equipped with a ×20 objective, a 540 nm excitation filter, a 605 nm emission filter (Chroma Technology Corp., Bellows Falls, VT) and QImaging Regiga-2000R digital camera (Surrey, British Columbia, Canada). Fluorescence of multiple images of each artery were quantified using ImageJ software and background fluorescence was subtracted from the fluorescence value of the basilar artery ring. [22]
Statistical Analysis
Data were summarized as mean ± SEM. For comparisons of two groups, an unpaired Student’s t-test was used. For all concentration-response curves, differences between multiple groups at each concentration were determined using a one-way analysis of variance (ANOVA). Differences between individual means following ANOVA were evaluated using a post hoc Student-Newman-Keuls. A probability level of P<0.05 was considered to be statistically significant.
RESULTS
Arterial Blood Pressure and Vessel Characteristics
The Program for Genetic Applications (PGA) at the Medical College of Wisconsin generated SS-5BN and performed phenotyping protocols, including assessment of conscious BP in the parental and consomic strains. SS-5BN consomic rats are protected from salt-induced increases in mean arterial pressure (130±2.6 mmHg) following a three-week HS (8.0% NaCl) diet compared to the SS rat (177±2.5 mmHg). The present study utilized a 3-day HS diet of 4.0% NaCl because SS rats remain normotensive within this time frame [16, 24] and thus blood pressure differences between the two rat strains should not affect our results. Active resting tone (Table 1) was unaffected by HS diet or any of the inhibitors in either strain; the only exception being HS-fed MCA of SS rats treated with 20-HEDE; which may be due to a increased contribution of 20-HETE in SS rats fed HS diet. Nonetheless, the consistency of active resting tone between treatment groups indicates changes in the magnitude of vascular relaxation in different experimental groups are independent of differences in resting tone and do not reflect a pre-existing constriction.
Table 1. Middle Cerebral Artery Diameters and Resting Tone.
Data are summarized as mean ± SEM.
| Experimental Groups | Number (n) |
Maximum Diameter (µm) |
Resting Diameter (µm) |
Active Tone (%) |
|---|---|---|---|---|
| SS NS | 8 | 256±2.9Ψ | 129±11.3 | 50±4.0 |
| SS HS | 8 | 248±5.2 | 152±5.0 | 40±2.4 |
| SS NS + DDMS | 8 | 240±1.8 | 145±8.0 | 39±3.2 |
| SS HS + DDMS | 8 | 238±2.4 | 135±5.6 | 43±2.1 |
| SS NS + 20-HEDE | 6 | 236±4.2 | 145±9.3 | 39±1.9 |
| SS HS + 20-HEDE | 6 | 232±1.9 | 175±6.7Φ | 25±1.3Φ |
| SS NS + DDMS + L-NAME | 8 | 240±1.8 | 140±6.7 | 42±2.7 |
| SS HS + DDMS + L-NAME | 8 | 238±2.4 | 133±6.8 | 44±2.6 |
| SS NS + DDMS + INDO | 6 | 240±1.9 | 143±3.5 | 40±1.1 |
| SS HS + DDMS + INDO | 6 | 237±1.7 | 139±5.1 | 41±2.0 |
| SS NS + DDMS + MS-PPOH | 6 | 233±2.4 | 130±1.9 | 44±1.0 |
| SS HS + DDMS + MS-PPOH | 6 | 233±2.0 | 135±3.8 | 42±1.7 |
| SS-5BN NS | 8 | 265±5.4* | 140±6.6 | 47±2.5 |
| SS-5BN HS | 8 | 260±7.2* | 137±2.6 | 47±1.3 |
| SS-5BN NS + DDMS | 8 | 265±5.4* | 133±4.4 | 49±2.4 |
| SS-5BN HS + DDMS | 8 | 260±7.2* | 139±6.6 | 47±1.3 |
| SS-5BN NS + L-NAME | 6 | 252±4.0 | 131±5.9 | 47±1.5 |
| SS-5BN HS + L-NAME | 6 | 249±4.7 | 132±5.7 | 47±2.7 |
| SS-5BN NS + INDO | 5 | 249±7.6 | 134±6.9 | 46±2.4 |
| SS-5BN HS + INDO | 5 | 246±5.7 | 141±7.7 | 43±3.0 |
Abbreviations are defined as follows: SS, Dahl SS; SS-5BN, SS-Chr 5BN/Mcwi; NS, normal salt; HS, high salt; DDMS, N-methyl-sulfonyl-12,12-dibromododec-11-enamide; 20-HEDE, 6(Z), 15 (Z)-20-HEDE, L-NAME, N (G)-nitro-L- arginine methyl ester; INDO, indomethacin; MS-PPOH, N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexanamide.
P<0.05, significantly different from NS- and HS-fed SS rats in all categories, except NS-fed SS rats without inhibitor.
P<0.05, significantly different from NS-and HS-fed SS rats in the presence of either MS-PPOH or 20-HEDE.
P<0.05, significantly different from every other treatment group in the same category.
Effect of CYP4A Inhibition and 20-HETE Antagonism on Vascular Relaxation
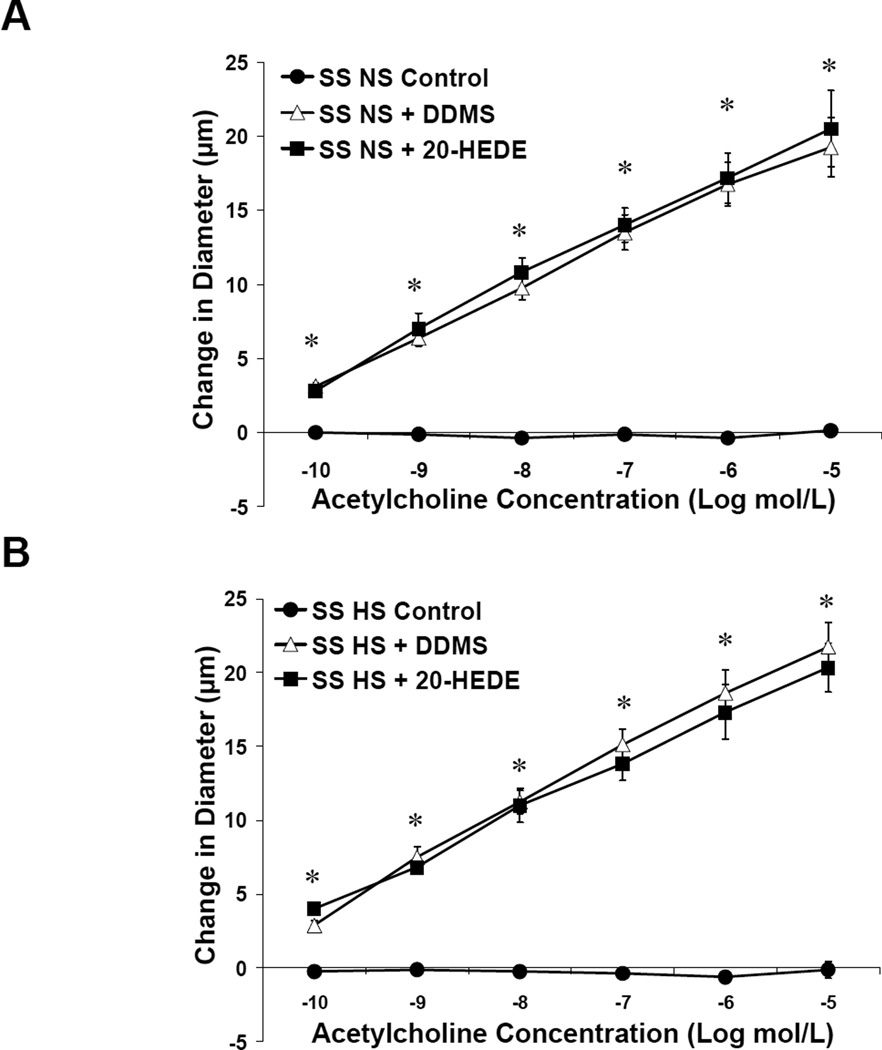
Middle cerebral arteries from NS-fed (Figure 1A) or HS-fed (Figure 1B) SS rats failed to dilate in response to the endothelium-dependent vasodilator ACh. Vascular relaxation to ACh was restored by inhibiting 20-HETE production with DDMS in SS rats fed either NS or HS diet, and also by treatment with the 20-HETE antagonist 20-HEDE.
Figure 1.
Response to acetylcholine in MCA of SS fed normal salt (NS; A) or high salt (HS; B) ± acute addition of DDMS or 20-HEDE. Data are expressed as mean change in diameter from baseline (µm) ± SEM. *P<0.05, significant difference from response in the absence of inhibitor, same diet.
Mechanisms of the Restored Vascular Relaxation
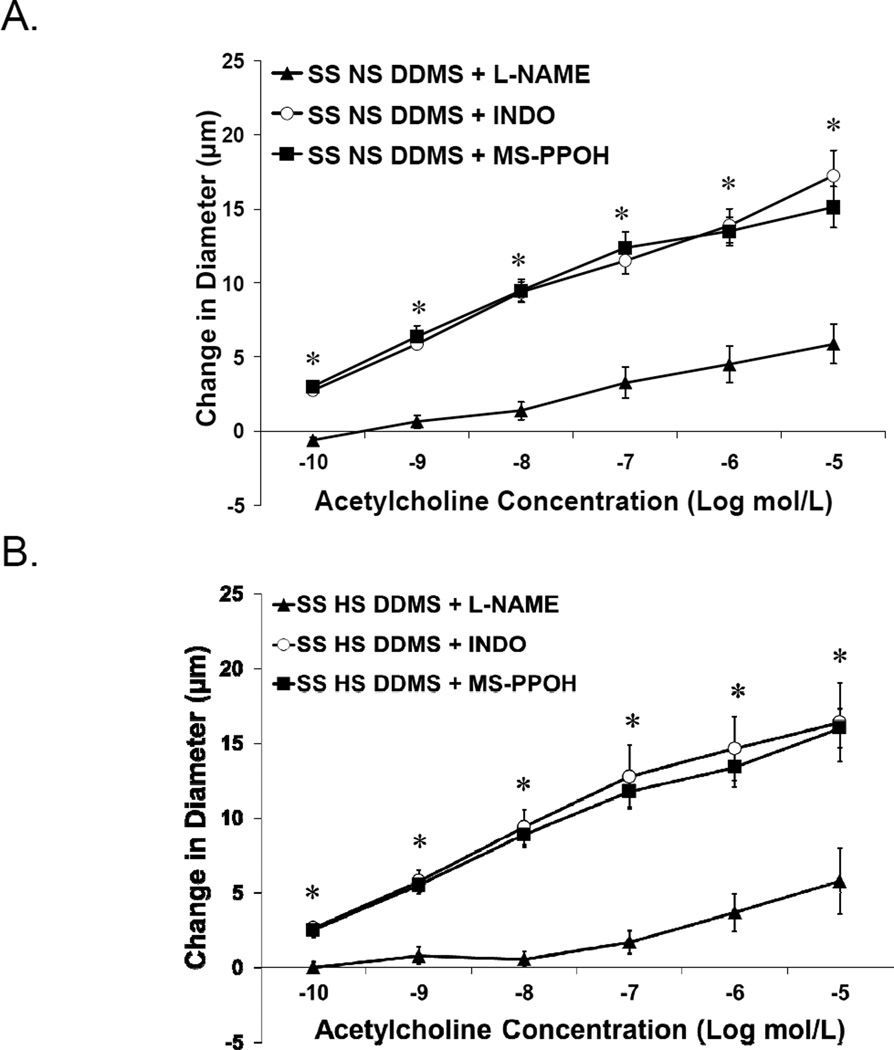
The responses of DDMS-treated MCA from SS rats (± the NOS inhibitor L-NAME; the cyclooxygenase inhibitor indomethacin; or the epoxygenase inhibitor MS-PPOH) are presented in Figure 2. The restored dilation to ACh in DDMS-treated MCA from SS rats fed NS (Figure 2A) or HS diet (Figure 2B) was eliminated by L-NAME, demonstrating a NO-dependent response. Vessel responses to ACh did not utilize either the cyclooxygenase or epoxygenase pathways, as their respective inhibitors did not affect the magnitude of vasodilation.
Figure 2.
Response to acetylcholine in DDMS-treated MCA of SS fed normal salt (NS; A) or high salt (HS; B) diet ± L-NAME, indomethacin or MS-PPOH (n=8 for all groups). Data are expressed as mean change in diameter from baseline (µm) ± SEM. *P<0.05, significant difference from L-NAME-treated arteries.
Effect of Brown Norway CYP4A Alleles on Vascular Relaxation
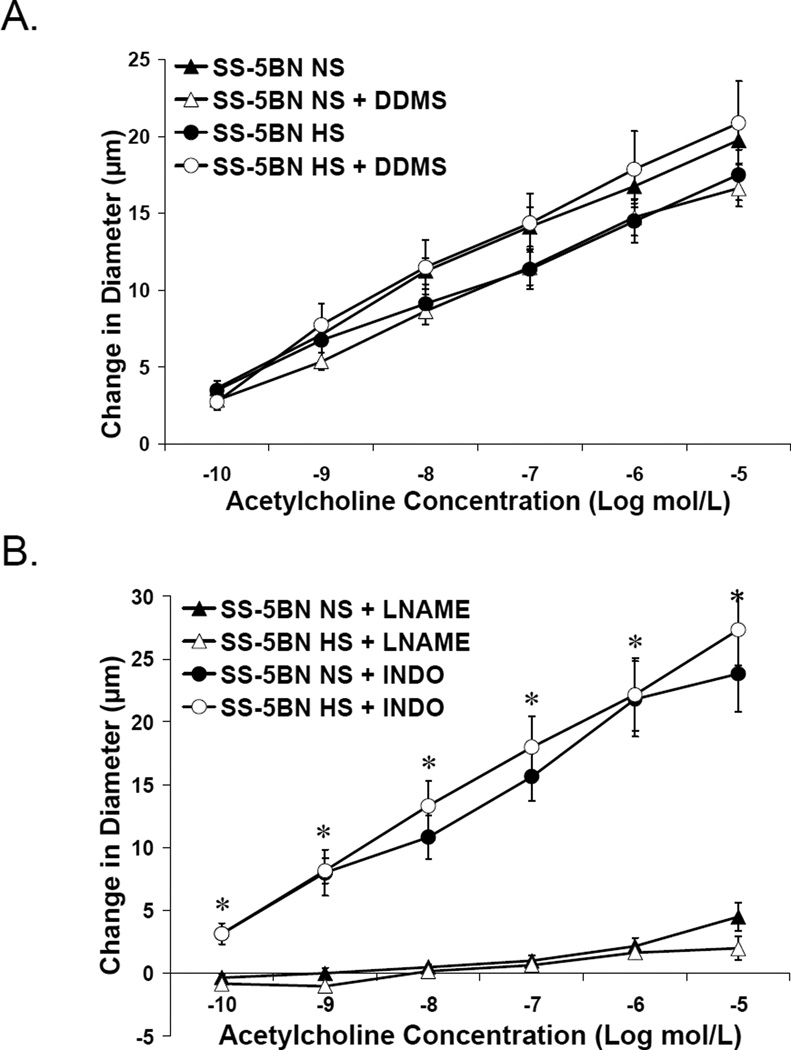
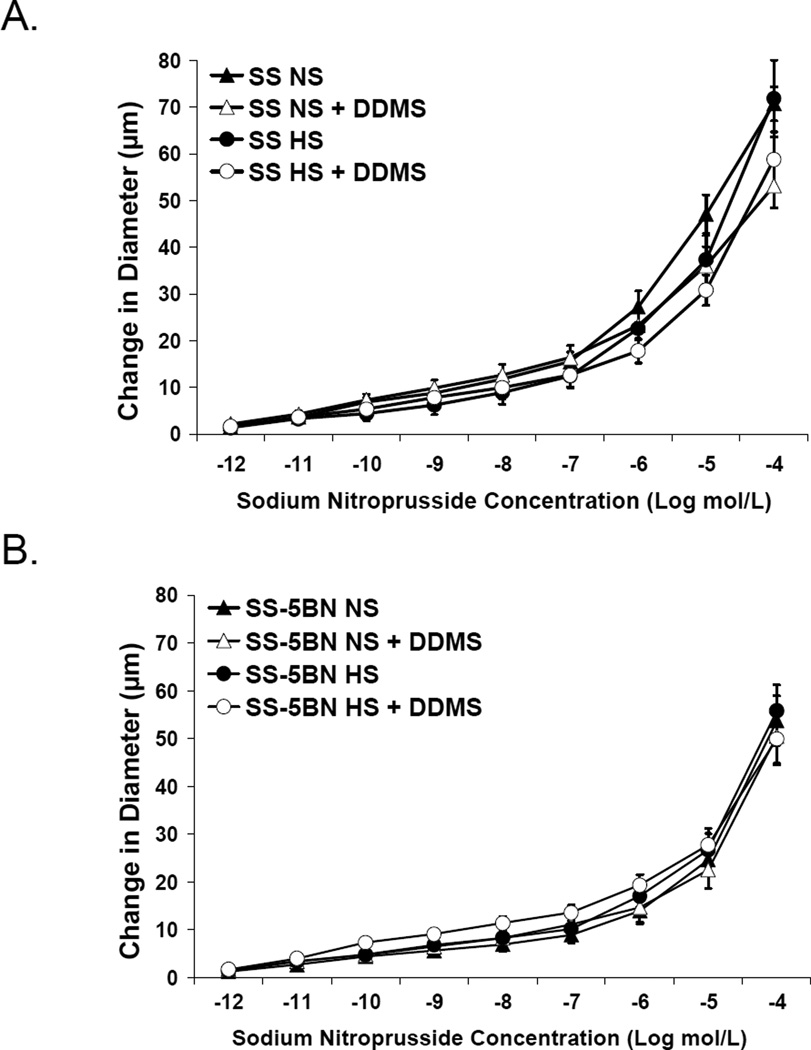
Introgressing CYP4A genes from the BN rat into the SS genetic background restored vascular responses to ACh in SS-5BN fed either NS or HS diet; and this dilation was unaffected by DDMS (Figure 3A). The ACh-induced response in these vessels is NO-dependent, as the response was blocked by L-NAME and unaffected by indomethacin (Figure 3B). Time control experiments (not shown) demonstrated no effect of the incubation period on vascular responses to ACh in any group. Middle cerebral arteries from both SS and SS-5BN rats fed NS and HS diet showed robust dilation to the NO donor sodium nitroprusside, with no additional effect of DDMS (Figure 4); showing the vascular dysfunction in the SS rat is not due to a failure of VSM cells to respond to NO.
Figure 3.
Response to acetylcholine in MCA of SS-5BN fed normal salt (NS) or high salt (HS) ± acute addition of DDMS (A) or either L-NAME or indomethacin (B). Data are expressed as mean change in diameter from baseline (µm) ± SEM. *P<0.05, significant difference from L-NAME-treated vessels.
Figure 4.
Response to sodium nitroprusside (10−12 – 10−5 M) in isolated middle cerebral arteries of Dahl SS rats (A) and SS-5BN consomic rats (B) fed normal salt (NS) or high salt (HS) diet (n=12 in all groups) ± acute addition of DDMS (50 µM) to the perfusate and superfusate. Data are expressed as mean change in diameter from baseline (µm) ± SEM.
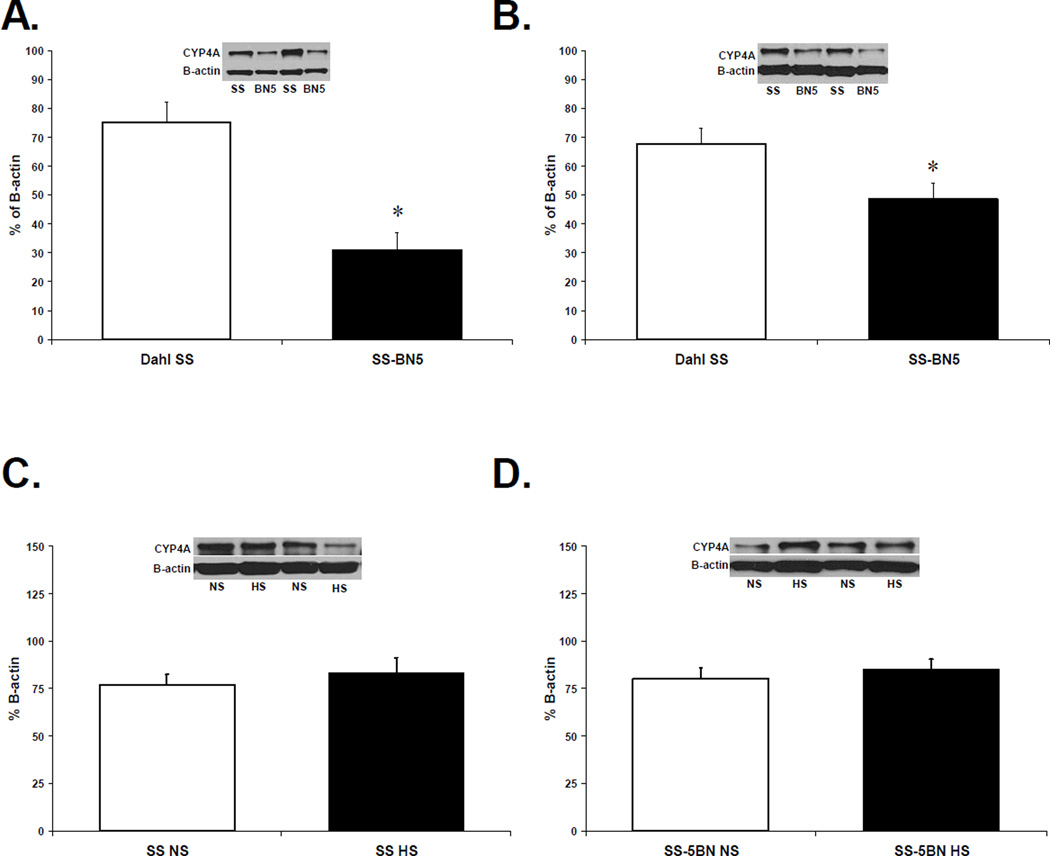
CYP4A Enzyme Expression in Cerebral Arteries from SS and SS-5BN rats
CYP4A protein expression was significantly lower in cerebral arteries from SS-5BN rats compared to arteries from SS rats fed either normal salt (Figure 5A) or high salt (Figure 5B). CYP4A expression was unaffected by changes in dietary salt intake in either animal strain (Figure 5C and 5D).
Figure 5.
Western blots comparing CYP4A protein (50 kDa) expression in cerebral arteries from (A) normal salt (NS)-fed SS vs. SS-5BN, (B) high salt (HS)-fed SS and SS-5BN, (C) NS vs. HS in SS rats, and (D) NS vs. HS in SS-5BN rats (n=8 for all groups). Data are presented as percent (%) of β-actin expression. *P<0.05, significant difference from cerebral vessels from SS, same diet.
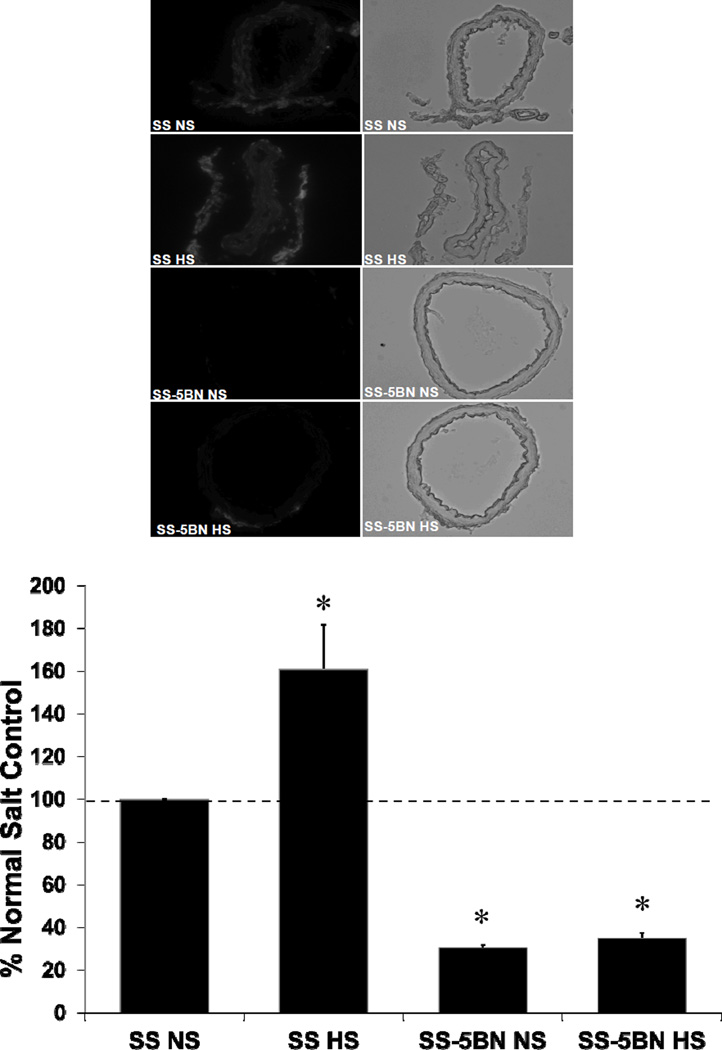
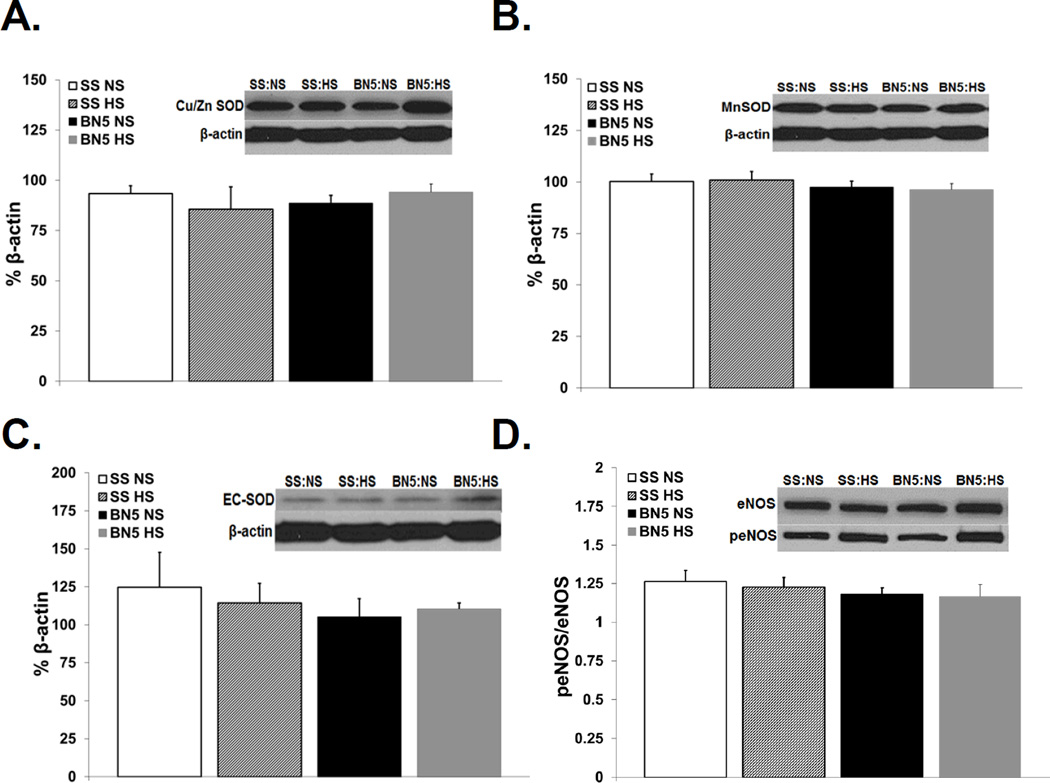
ROS Levels, SOD Expression, and eNOS Expression
Consistent with the hypothesis that the vascular effects of 20-HETE are mediated via oxidative stress, ROS levels evaluated semiquantitatively via DHE fluorescence were significantly higher in basilar arteries of SS rats compared to those of SS-5BN rats fed the same diet. ROS levels in HS-fed SS rats were also significantly higher than those in SS rats fed NS diet (Figure 6). Western blotting revealed no difference in antioxidant protein expression (Mn-SOD, Cu/Zn SOD, and EC-SOD; Figures 7A, 7B, and 7C, respectively) or in peNOS/eNOS expression (Figure 7C) in any of our treatment groups; suggesting the vascular dysfunction in SS rats compared to SS-5BN rats is not due to alterations in antioxidant defenses or in the activity of eNOS.
Figure 6.
ROS levels evaluated semiquantitatively via DHE fluorescence in basilar arteries from normal salt (NS) and high salt (HS)-fed SS and SS-5BN (n=6 for all groups). (A) Representative fluorescent and bright field images of basilar artery cross-sections (10µm thickness) from SS and SS-5BN. (B) Vascular ROS levels expressed as fluorescence units in DHE-treated basilar artery cross-sections. Data are expressed % of raw fluorescence units in NS-fed SS arteries ± SEM. *P<0.05, significant difference from control, NS-fed SS.
Figure 7.
Western blot demonstrating protein expression of (A) Cu/Zn SOD, (B) MnSOD, and(C) EC-SOD in cerebral vessels from Dahl SS (SS) and SS-5BN consomic rats on either a normal salt (NS) or high salt (HS) diet. Data are presented as a % of β-actin. (D) Western blot demonstrating protein expression of eNOS and peNOS in cerebral vessels from Dahl SS (SS) and SS-5BN consomic rats on either a normal salt (NS) or high salt (HS) diet. Data are presented as a ratio of % β-actin of peNOS to % β-actin of eNOS. (n=4 for all groups)
DISCUSSION
The present study shows that multiple interventions that interrupt the CYP4A/20-HETE pathway ameliorate the severe vascular dysfunction that exists in cerebral arteries of Dahl SS rats fed either normal or high salt diet. Specifically, pharmacological interventions that either inhibit the catalytic function of CYP4A enzymes or antagonize the actions of 20-HETE both restored vascular relaxation in cerebral resistance arteries of Dahl SS rats. In a similar fashion, genetic substitution of chromosome 5 carrying the CYP4A alleles from the BN rat onto the genetic background of the SS rat (the SS-5BN consomic rat) reduced the influence of this pathway by decreasing vascular CYP4A protein expression, and this was accompanied by a restoration of endothelium-dependent vascular relaxation in response to ACh. One limitation of the current study is the inability of current methodology, which assesses 20-HETE production under optimum conditions of PO2 and substrate availability, to evaluate differences in 20-HETE production under normal physiological conditions. As such, it is also conceivable that differences in CYP4A enzyme activity (in addition to differences in the expression of CYP4A enzymes in the arteries) may also exist in the two strains. Nonetheless, the findings of the current study suggest that upregulation of the CYP4A/20-HETE pathway plays a direct role in the endothelial dysfunction that exists in the SS rat fed NS or HS diet. In this regard, the beneficial effects of chromosomal substitution and pharmacological interruption of the CYP4A/20-HETE pathway on vascular function in rats fed NS diet are especially important because they indicate that the CYP4A/20-HETE pathway may play an important role in contributing to vascular dysfunction in SS rats, even prior to the onset of hypertension.
Previous studies have shown that increased levels of ROS are important contributors to vascular dysfunction in SS rats.[22, 25] For example, cerebral vascular relaxation in response to ACh and reduced PO2 in SS rats fed either NS or HS diet can be restored by scavenging superoxide with tempol.[22, 25] In the present study, we utilized DHE fluorescence to evaluate vascular ROS levels. While this approach has its limitations compared to other methods that provide a better quantitative evaluation of superoxide levels per se, e.g. HPLC determination of 2-hydroxyethidium that is more feasible in other tissues [26–28], it still provides a valuable, albeit semi-quantitative, estimation of vascular ROS levels. In these experiments, we found genetic manipulation of CYP4A protein expression via chromosome substitution in the SS-5BN consomic rat led to a decrease in vascular oxidant stress concomitant with a restoration of endothelium-dependent vasodilation to ACh in animals fed either normal or high salt diet. The latter findings suggest that impaired endothelial function in the SS rats and the beneficial effects of inhibiting 20-HETE production on vascular reactivity are secondary to a reduction in ROS. The latter interpretation is consistent with published findings showing that 20-HETE can increase vascular ROS via several mechanisms including: 1) the normal catalytic process of 20-HETE formation by CYP4A enzymes [29–31]; 2) 20-HETE-induced NADPH oxidase activation [9, 32–34]; and 3), uncoupling of eNOS [35, 36]. Because eNOS expression and phosphorylation of eNOS were similar in SS and SS-5BN rats and were unaffected by HS diet, the most likely explanation for our findings is that reduced 20-HETE levels and/or CYP4A enzyme activity restores NO bioavailability by preventing the destruction of NO by superoxide anions, rather than via any effect on eNOS expression or activation.
Current sequence analysis (rgd.mcw.edu) has identified a nonsynonymous single nucleotide polymorphism predicted to be damaging in the CYP4A8 allele of the SS rat that may affect expression of the other CYP4A isoforms. A similar phenomenon has been observed in the cyp4a14 knockout mouse,[8] in which the knockout of the cyp4a14 gene results in increased ω-hydroxylase activity,[37] increased urinary excretion of 20-HETE, and elevated mean arterial pressure due to the secondary upregulation of the cyp4a12 gene.[8, 37] This raises the possibility that one or more of the other CYP4A isoforms is upregulated in the Dahl SS rat due to a non-functional CYP4A8 allele. This could provide a possible explanation for the elevated CYP4A protein expression in cerebral arteries from Dahl SS rats fed either normal or high salt diet compared to arteries from SS-5BN consomic rats, which do not carry the mutation.
Multiple studies have shown the Dahl SS rat is susceptible to heightened oxidative stress due to reduced antioxidant defense mechanisms, specifically in the myocardium, [38] kidney, [39, 40] and vasculature. [22] In this study, there were no differences in expression of any of the SOD enzymes between SS and SS-5BN rats fed either diet. Due to the pro-oxidant capabilities of 20-HETE, it is likely that the upregulation of the CYP4A/20-HETE pathway operating in conjunction with reduced antioxidant defenses in the SS genetic background leads to increased vascular oxidant stress in SS rats compared to SS-5BN consomic rats. We believe that the most likely reason for the restoration of endothelium-dependent vascular relaxation in SS-5BN consomic rat is the reduced expression of CYP4A enzymes resulting in lower levels of ROS that do not overpower antioxidant defense mechanisms that are compromised in the SS genetic background due, at least in part, to the chronically low ANG II levels resulting from impaired regulation of the renin allele (found on chromosome 13).[22, 25]
It is important to discuss the contrasting roles of 20-HETE in the pathogenesis of hypertension in the vasculature compared to the kidney, with specific reference to these two rat strains. In renal physiology, 20-HETE is generally considered to be anti-hypertensive due to its tubular effects promoting natriuresis. [41, 42] Deficiencies in renal 20-HETE production have been associated with sodium retention and salt-sensitive hypertension in both human [43] and animal studies. [44–46] In fact, Dahl SS rats have inadequate 20-HETE production in the outer medulla [47], which can be ameliorated by introgressing Brown Norway chromosome 5 into the SS genetic background (the SS-5BN consomic rat).[48] Those observations contrast with the increased expression of CYP4A enzyme protein in the vasculature that we observed in the present study. This is likely due to local differences in the regulation of CYP4A gene expression in the kidney and vasculature. For example, it has been shown that human hypertensive subjects have significantly higher amounts of plasma 20-HETE but a significantly lower urinary excretion of 20-HETE, an indirect indicator of renal 20-HETE production. Local control over 20-HETE synthesis may be a result of differences in the expression of certain receptors responsible for CYP4A enzyme activation within the kidney versus the vasculature (e.g. receptors for either angiotensin II or endothelin-1) [49] or due to local differences in signal transduction pathways. Regardless of these potential underlying mechanisms, our laboratory and others have consistently demonstrated that elevated 20-HETE production in the vasculature is deleterious and contributes to the development and progression of hypertension. [11, 13, 50]
One very striking observation in the present study is that high salt diet failed to eliminate endothelium dependent dilation to acetylcholine in arteries of the SS-5BN consomic rats. This finding is in contrast to the ability of high salt diet to eliminate endothelium-dependent dilation in arteries of salt insensitive Sprague-Dawley rats,[51] mice,[52] and SS-13BN consomic rats carrying a normally functioning renin allele from the Brown Norway rat in the SS genetic background.[24] This raises the crucially important question of the relative role of CYP4A/20-HETE pathway in contributing to salt-induced vascular impairment vs. cerebral vascular dysfunction independent of salt intake (e.g. in SS rats). Studies in mesenteric arteries of Sprague Dawley rats have shown that high salt diet upregulates CYP4A mRNA and protein expression, and that inhibiting CYP4A restores vascular relaxation response to reduced PO2 [13]. By contrast, the present study shows no difference in CYP4A protein expression in the MCA of SS rats fed either NS or HS diet; and pharmacological blockade of the CYP4A/20-HETE pathway with either DDMS or 20-HEDE restores vascular function in both NS- and HS-fed SS rats. The latter findings indicate that CYP4A enzymes contribute to overall vascular dysfunction in the SS rat, independent of dietary salt intake. This observation may have important significance in light of previous studies [14, 15, 24, 25] demonstrating that vascular relaxation mechanisms are lost in MCA of SS, even when the animals are normotensive and maintained on a NS diet.
One possible explanation for a role of the CYP4A/20-HETE pathway in vascular dysfunction independent of dietary salt in SS rats is a contribution of reactive oxygen species to the regulation of CYP4A enzyme expression. A plausible mechanism by which reactive oxygen species could exert a regulatory influence over the CYP4A/20-HETE pathway is indirect, via the effect of superoxide on nitric oxide. Superoxide anion reacts with NO at a rate three times faster than the interaction between superoxide and superoxide dismutase, which as a major effect on basal levels of nitric oxide. [53] Nitric oxide inhibits CYP4A activity by forming a ferrous-nitrosyl complex at the heme binding site of the CYP4A enzyme,[54] and has also been shown to decrease CYP4A protein expression. [55, 56] In the present study, both CYP4A protein expression and ROS levels are increased in arteries from both NS- and HS-fed SS rats compared to the SS-5BN consomic rats. Previous studies indicate that elevated ROS levels in arteries of Dahl SS rats reduce the bioavailability of NO within the vasculature. [14, 25] As a result, the inhibitory influence of NO on the CYP4A/20-HETE pathway would be absent in arteries of Dahl SS rats, which could explain the upregulation of the CYP4A/20-HETE pathway independent of dietary salt intake in Dahl SS rats.
Consomic rat models provide an experimental animal with nearly identical genetic homology to the target animal (SS) while substituting genetic material from a normotensive rat strain (BN). However, there are still limitations to the conclusions that can be drawn from data collected from these animals, as chromosome 5 carries BN alleles other than the CYP4A genes which may contribute to the observed differences in the SS vs. SS-5BN consomic rat in the present study. Nonetheless, the SS-5BN consomic rat is still an excellent animal model to test the present hypothesis that CYP4A enzyme and 20-HETE contribute to vascular dysfunction in the SS rat, as they demonstrate a significant reduction in the salt-sensitivity of blood pressure [17] and lack the vascular dysfunction observed in their close genetic counterpart, the SS rat. The improved vascular function observed in SS-5BN mirrors the restored vasodilator responses in MCA from SS in the presence of the CYP4A inhibitor DDMS and the 20-HETE antagonist 20-HEDE. As noted above, cerebral arteries from SS-5BN also have a significantly lower expression of CYP4A protein, supporting the hypothesis that the restoration of endothelium-dependent vascular relaxation in cerebral arteries of the SS-5BN rats reflects a reduced contribution of the CYP4A/20-HETE system to vascular regulation.
Acknowledgments
Sources of Funding: Financial support provided by the Robert A. Welch Foundation (GL 625910) and NIH (GM31278, DK38226) to JRF and NIH (HL65289; HL72920, and HL92026) to JHL
Abbreviations
- 20-HEDE
6(Z), 15 (Z)-20-HEDE
- 20-HETE
20-Hydroxyeicosatetraenoic Acid
- ACh
Acetylcholine
- ANG II
Angiotensin II
- BN
Brown Norway
- Cu/Zn SOD
Cu/Zn Superoxide Dismutase (SOD1)
- CYP
Cytochrome P450
- Dahl SS
Dahl Salt-Sensitive
- EC-SOD
Extracellular Superoxide Dismutase (SOD3)
- eNOS
Endothelial Nitric Oxide Synthase
- HS
High Salt
- L-NAME
N(G)-nitro-L-arginine methyl ester
- MCA
Middle Cerebral Artery
- Mn-SOD
Mn-Superoxide Dismutase (SOD2)
- MS-PPOH
N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexanamide
- NO
Nitric Oxide
- NS
Normal Salt
- PEG-SOD
Superoxide dismutase–polyethylene glycol
- ROS
Reactive Oxygen Species
- S-D
Sprague-Dawley
- SNP
Sodium Nitroprusside
- SOD
Superoxide Dismutase
- SS-5BN
SS-Chr 5BN/Mcwi
Footnotes
Conflicts of Interest/Disclosure: None
REFERENCES
- 1.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 2.Linder L, Kiowski W, Buhler FR, Luscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;81:1762–1767. doi: 10.1161/01.cir.81.6.1762. [DOI] [PubMed] [Google Scholar]
- 3.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 4.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Zhao Y, Nie D, Shi J, Fu L, Li Y, Yu D, Lu J. Association of a functional cytochrome P450 4F2 haplotype with urinary 20-HETE and hypertension. J. Am. Soc. Nephrol. 2008;19:714–721. doi: 10.1681/ASN.2007060713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, Croft KD. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension. 2008;51:1393–1398. doi: 10.1161/HYPERTENSIONAHA.107.104463. [DOI] [PubMed] [Google Scholar]
- 7.Levere RD, Martasek P, Escalante B, Schwartzman ML, Abraham NG. Effect of heme arginate administration on blood pressure in spontaneously hypertensive rats. J. Clin. Invest. 1990;86:213–219. doi: 10.1172/JCI114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 10.Ward NC, Rivera J, Hodgson J, Puddey IB, Beilin LJ, Falck JR, Croft KD. Urinary 20-hydroxyeicosatetraenoic acid is associated with endothelial dysfunction in humans. Circulation. 2004;110:438–443. doi: 10.1161/01.CIR.0000136808.72912.D9. [DOI] [PubMed] [Google Scholar]
- 11.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ. Res. 2006;98:962–969. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Schmidt JR, Roman RJ, Anjaiah S, Falck JR, Lombard JH. Modulation of vascular O2 responses by cytochrome 450-4A omega-hydroxylase metabolites in Dahl salt-sensitive rats. Microcirculation. 2009;16:345–354. doi: 10.1080/10739680802698007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Roman RJ, Falck JR, de la Cruz L, Lombard JH. Effects of high-salt diet on CYP450-4A omega-hydroxylase expression and active tone in mesenteric resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1557–H1565. doi: 10.1152/ajpheart.00755.2004. [DOI] [PubMed] [Google Scholar]
- 14.Durand MJ, Moreno C, Greene AS, Lombard JH. Impaired Relaxation of Cerebral Arteries in the Absence of Elevated Salt Intake in Normotensive Congenic Rats Carrying the Dahl Salt-Sensitive Renin Gene. Am. J. Physiol. Heart Circ. Physiol. 2010;299:1865–1874. doi: 10.1152/ajpheart.00700.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drenjancevic-Peric I, Weinberg BD, Greene AS, Lombard JH. Restoration of cerebral vascular relaxation in renin congenic rats by introgression of the Dahl R renin gene. Am. J. Hypertens. 2010;23:243–248. doi: 10.1038/ajh.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raffai G, Wang J, Roman RJ, Anjaiah S, Weinberg B, Falck JR, Lombard JH. Modulation by cytochrome P450-4A omega-hydroxylase enzymes of adrenergic vasoconstriction and response to reduced PO in mesenteric resistance arteries of Dahl salt-sensitive rats. Microcirculation. 2010;17:525–535. doi: 10.1111/j.1549-8719.2010.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW., Jr Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am. J. Physiol. Renal Physiol. 2008;295:F837–F842. doi: 10.1152/ajprenal.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowley AW, Jr, Liang M, Roman RJ, Greene AS, Jacob HJ. Consomic rat model systems for physiological genomics. Acta. Physiol. Scand. 2004;181:585–592. doi: 10.1111/j.1365-201X.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- 19.Fredricks KT, Liu Y, Lombard JH. Response of extraparenchymal resistance arteries of rat skeletal muscle to reduced PO2. Am. J. Physiol. 1994;267:H706–H715. doi: 10.1152/ajpheart.1994.267.2.H706. [DOI] [PubMed] [Google Scholar]
- 20.Kunert MP, Roman RJ, Alonso-Galicia M, Falck JR, Lombard JH. Cytochrome P-450 omega-hydroxylase: a potential O(2) sensor in rat arterioles and skeletal muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1840–H1845. doi: 10.1152/ajpheart.2001.280.4.H1840. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Maier KG, Roman RJ, De La Cruz L, Zhu J, Henderson L, Lombard JH. Expression of cytochrome P450-4A isoforms in the rat cremaster muscle microcirculation. Microcirculation. 2004;11:89–96. doi: 10.1080/10739680490266225. [DOI] [PubMed] [Google Scholar]
- 22.Durand MJ, Lombard JH. Introgression of the Brown Norway renin allele onto the Dahl salt-sensitive genetic background increases Cu/Zn SOD expression in cerebral arteries. Am. J. Hypertens. 2011;24:563–568. doi: 10.1038/ajh.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayhan WG. Impairment of endothelium-dependent dilatation of basilar artery during chronic hypertension. Am. J. Phys. 1990;259:H1455–H1462. doi: 10.1152/ajpheart.1990.259.5.H1455. [DOI] [PubMed] [Google Scholar]
- 24.Drenjancevic-Peric I, Frisbee JC, Lombard JH. Skeletal muscle arteriolar reactivity in SS.BN13 consomic rats and Dahl salt-sensitive rats. Hypertension. 2003;41:1012–1015. doi: 10.1161/01.HYP.0000067061.26899.3E. [DOI] [PubMed] [Google Scholar]
- 25.Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension. 2005;45:687–691. doi: 10.1161/01.HYP.0000154684.40599.03. [DOI] [PubMed] [Google Scholar]
- 26.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radical Biol. Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 28.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am. J. Physiol. Cell. Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 29.Bondy SC, Naderi S. Contribution of hepatic cytochrome P450 systems to the generation of reactive oxygen species. Biochem. Pharmacol. 1994;48:155–159. doi: 10.1016/0006-2952(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 30.Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic. Biol. Med. 1998;24:1324–1330. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 31.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ. Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 32.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler. Thromb. Vasc. Biol. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Q, Han Y, Bao Y, Li W, Li X, Shen X, Wang X, Yao F, O'Rourke ST, Sun C. 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. Am. J. Physiol Heart C. 2010;299:H1109–H1117. doi: 10.1152/ajpheart.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hama-Tomioka K, Kinoshita H, Azma T, Nakahata K, Matsuda N, Hatakeyama N, Kikuchi H, Hatano Y. The role of 20-hydroxyeicosatetraenoic acid in cerebral arteriolar constriction and the inhibitory effect of propofol. Anesth. Analg. 2009;109:1935–1942. doi: 10.1213/ANE.0b013e3181bd1ebe. [DOI] [PubMed] [Google Scholar]
- 35.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, Schwartzman ML. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1018–H1026. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 36.Cheng J, Wu CC, Gotlinger KH, Zhang F, Falck JR, Narsimhaswamy D, Schwartzman ML. 20-hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IkappaB kinase-dependent endothelial nitric-oxide synthase uncoupling. J. Pharmacol. Exp. Ther. 2010;332:57–65. doi: 10.1124/jpet.109.159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fidelis P, Wilson L, Thomas K, Villalobos M, Oyekan AO. Renal function and vasomotor activity in mice lacking the Cyp4a14 gene. Exp. Biol. Med. (Maywood) 2010;235:1365–1374. doi: 10.1258/ebm.2010.009233. [DOI] [PubMed] [Google Scholar]
- 38.Somova LI, Nadar A, Gregory M, Khan N. Antioxidant status of the hypertrophic heart of Dahl hypertensive rat as a model for evaluation of antioxidants. Methods. Find. Exp. Clin. Pharmacol. 2001;23:5–12. doi: 10.1358/mf.2001.23.1.619173. [DOI] [PubMed] [Google Scholar]
- 39.Meng S, Roberts LJ, 2nd, Cason GW, Curry TS, Manning RD., Jr Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. American journal of physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R732–R738. doi: 10.1152/ajpregu.00346.2001. [DOI] [PubMed] [Google Scholar]
- 40.Manning RD, Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am. J. Nephrol. 2005;25:311–317. doi: 10.1159/000086411. [DOI] [PubMed] [Google Scholar]
- 41.Stec DE, Flasch A, Roman RJ, White JA. Distribution of cytochrome P-450 4A and 4F isoforms along the nephron in mice. Am. J. Physiol. Renal. 2003;284:F95–F102. doi: 10.1152/ajprenal.00132.2002. [DOI] [PubMed] [Google Scholar]
- 42.Escalante B, Erlij D, Falck JR, McGiff JC. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science. 1991;251:799–802. doi: 10.1126/science.1846705. [DOI] [PubMed] [Google Scholar]
- 43.Laffer CL, Gainer JV, Waterman MR, Capdevila JH, Laniado-Schwartzman M, Nasjletti A, Brown NJ, Elijovich F. The T8590C polymorphism of CYP4A11 and 20-hydroxyeicosatetraenoic acid in essential hypertension. Hypertension. 2008;51:767–772. doi: 10.1161/HYPERTENSIONAHA.107.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stec DE, Deng AY, Rapp JP, Roman RJ. Cytochrome P4504A genotype cosegregates with hypertension in Dahl S rats. Hypertension. 1996;27:564–568. doi: 10.1161/01.hyp.27.3.564. [DOI] [PubMed] [Google Scholar]
- 45.Roman RJ, Hoagland KM, Lopez B, Kwitek AE, Garrett MR, Rapp JP, Lazar J, Jacob HJ, Sarkis A. Characterization of blood pressure and renal function in chromosome 5 congenic strains of Dahl S rats. Am. J. Physiol. Renal Physiol. 2006;290:F1463–F1471. doi: 10.1152/ajprenal.00360.2005. [DOI] [PubMed] [Google Scholar]
- 46.Zou AP, Drummond HA, Roman RJ. Role of 20-HETE in elevating loop chloride reabsorption in Dahl SS/Jr rats. Hypertension. 1996;27:631–635. doi: 10.1161/01.hyp.27.3.631. [DOI] [PubMed] [Google Scholar]
- 47.Ma YH, Schwartzman ML, Roman RJ. Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am. J. Physiol. 1994;267:R579–R589. doi: 10.1152/ajpregu.1994.267.2.R579. [DOI] [PubMed] [Google Scholar]
- 48.Williams JM, Fan F, Murphy SR, Schreck C, Lazar J, Jacob HJ, Roman RJ. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl Salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R1209–R1218. doi: 10.1152/ajpregu.00604.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai IJ, Croft KD, Puddey IB, Beilin LJ, Barden A. 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H1194–H1200. doi: 10.1152/ajpheart.00733.2010. [DOI] [PubMed] [Google Scholar]
- 50.Kaide J, Wang MH, Wang JS, Zhang F, Gopal VR, Falck JR, Nasjletti A, Laniado-Schwartzman M. Transfection of CYP4A1 cDNA increases vascular reactivity in renal interlobar arteries. Am. J. Physiol. Renal Physiol. 2003;284:F51–F56. doi: 10.1152/ajprenal.00249.2002. [DOI] [PubMed] [Google Scholar]
- 51.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1124–H1133. doi: 10.1152/ajpheart.00835.2002. [DOI] [PubMed] [Google Scholar]
- 52.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1550–R1556. doi: 10.1152/ajpregu.00703.2006. [DOI] [PubMed] [Google Scholar]
- 53.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 54.Sun CW, Alonso-Galicia M, Taheri MR, Falck JR, Harder DR, Roman RJ. Nitric oxide-20-hydroxyeicosatetraenoic acid interaction in the regulation of K+ channel activity and vascular tone in renal arterioles. Circ. Res. 1998;83:1069–1079. doi: 10.1161/01.res.83.11.1069. [DOI] [PubMed] [Google Scholar]
- 55.Oyekan AO, Youseff T, Fulton D, Quilley J, McGiff JC. Renal cytochrome P450 omega-hydroxylase and epoxygenase activity are differentially modified by nitric oxide and sodium chloride. J. Clin. Invest. 1999;104:1131–1137. doi: 10.1172/JCI6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tunctan B, Yaghini FA, Estes A, Malik KU. Inhibition by nitric oxide of cytochrome P450 4A activity contributes to endotoxin-induced hypotension in rats. Nitric Oxide. 2006;14:51–57. doi: 10.1016/j.niox.2005.09.006. [DOI] [PubMed] [Google Scholar]