ABSTRACT
Staphylococcus aureus is a prominent cause of human infections worldwide and is notorious for its ability to acquire resistance to antibiotics. Methicillin-resistant S. aureus (MRSA), in particular, is endemic in hospitals and is the most frequent cause of community-associated bacterial infections in the United States. Inasmuch as treatment options for severe MRSA infections are limited, there is need for a vaccine that protects against such infections. However, recent efforts to generate a staphylococcal vaccine have met with little success in human clinical trials. These failures are somewhat puzzling, since the vaccine antigens tested promote opsonophagocytosis in vitro and confer protection in animal infection models. One possibility is that the pathogen inhibits (and/or fails to elicit) the development of protective immunity in humans. Indeed, S. aureus produces numerous molecules that can potentially promote immune evasion, including protein A (SpA), an immunoglobulin (Ig)-binding protein present on the bacterial surface and freely secreted into the extracellular environment. SpA binds the Fc region of antibody and the Fab regions of the B-cell receptor, processes that are known to block opsonophagocytosis and cause B-cell death in vitro. In a recent study, Falugi et al. [F. Falugi, H. K. Kim, D. M. Missiakas, and O. Schneewind, mBio 4(5):e00575-13, 2013] showed that vaccination with spa mutant S. aureus strains lacking antibody Fc- and/or Fab-binding capacity protects against subsequent challenge with the USA300 epidemic strain. The findings provide strong support for the idea that SpA promotes S. aureus immune evasion in vivo and form the foundation for a new approach in our efforts to develop a vaccine that prevents severe S. aureus infections.
Commentary
Staphylococcus aureus is a ubiquitous human pathogen and a leading cause of infections worldwide. The pathogen is capable of causing a diversity of syndromes ranging in severity from common skin and soft tissue lesions to highly invasive and systemic disease. The high prevalence of staphylococcal infection is facilitated by the commensal lifestyle of the bacterium, which is frequently associated with the skin and anterior nares of healthy individuals. S. aureus is a predominant cause of nosocomial infections, which often occur in individuals with predisposing risk factors, such as hemodialysis or surgery. Historically, the success of S. aureus as a human pathogen has been influenced by a strong propensity to develop antibiotic resistance, and methicillin-resistant S. aureus (MRSA) now ranks as a leading cause of hospital-associated infections (1). Multidrug-resistant S. aureus strains are endemic in hospitals, and the pathogen has developed mechanisms to negate virtually all antibiotics of clinical value. To further obfuscate S. aureus epidemiology, one of the most notable developments in recent bacterial infectious disease history was the rapid emergence of community-associated MRSA (CA-MRSA). First reported in the 1990s, CA-MRSA rapidly emerged worldwide, and a strain known as USA300 is the most abundant cause of community-associated bacterial infections in the United States (2).
The outlook for new therapeutic options to treat S. aureus is confounded by a paucity of new classes of antimicrobial agents in the drug discovery pipeline (3). Considering the penchant of S. aureus to rapidly develop antibiotic resistance, there is a clearly defined need for an effective vaccine. Unfortunately, the overwhelming majority of attempts to develop a clinically useful vaccine have failed (4). The lack of success is largely attributed to use of conventional strategies directed at enhancing the process of opsonophagocytosis, which is problematic since the vast majority of adults are already endowed with a repertoire of opsonic antibodies and serum complement. Indeed, vaccines comprised of S. aureus surface antigens, such as iron surface determinant B (IsdB) and polysaccharide capsular antigens CP5 and CP8, failed to protect against S. aureus infection according to results from phase III clinical trials (5, 6). Although S. aureus vaccines designed to enhance bacterial uptake by phagocytes have had limited utility, it remains to be determined if alternative vaccine strategies will prove useful. For example, S. aureus secretes several toxins that collectively contribute to pathogenesis, and toxins such as alpha-hemolysin (Hla) are under evaluation as vaccine candidates in early clinical trials.
To gain an enhanced understanding of the mechanisms by which S. aureus causes disease, Falugi et al. investigated the role of SpA in virulence and host immune evasion (7). The authors generated Newman strains with deletion of spa (Δspa) and mutations in the antibody Fc- and/or F(ab′)2-binding domains of spa (spaKK, spaAA, and spaKKAA), and compared the ability of wild-type and mutant strains to circumvent and/or alter innate and adaptive immune responses in the mouse (7). The authors show that (i) the Fc-binding domain of SpA is important for S. aureus survival in mouse blood in vivo and in vitro, (ii) infection or vaccination with the spaKKAA strain elicits a pronounced anti-S. aureus antibody response not present following infection the wild-type strain, and (iii) vaccination or infection with the spaKKAA protects mice from death caused by subsequent USA300 infection. These SpA-mediated phenomena were absent in mice lacking B cells and antibody. One important implication of these findings is that spaKKAA could be used—at least in part—in a vaccine approach for S. aureus infections.
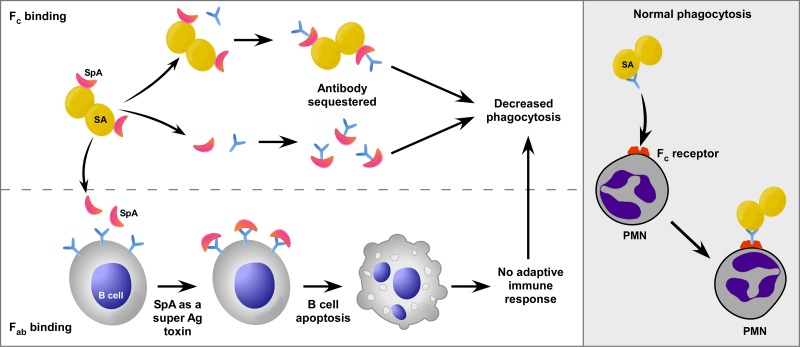
SpA has long been known to bind inhibit opsonophagocytosis in vitro (8), and the ability of the protein to block phagocytosis is dependent on the presence of host antibody (9). In addition to its Fc-binding capacity, SpA binds Fab regions of the B-cell receptor (membrane-anchored IgM) (10, 11), and in doing so, it functions as a B-cell superantigen that induces programmed cell death (12) (Fig. 1). Thus, SpA can potentially alter the innate and adaptive immune responses to S. aureus. In their original studies of the ability of SpA to inhibit phagocytosis, Dossett et al. proposed that SpA “may play a role in the pathogenesis of staphylococcal infections.” (8). This idea is borne out in the work by Falugi and colleagues (7).
FIG 1 .
Mechanisms of SpA-mediated immune evasion. (Left panel) SpA (red crescent shape) present on the surface of S. aureus (SA) or SpA that is freely secreted binds the Fc region of antibody (Ab), thereby preventing normal phagocytosis (right panel). Alternatively, SpA binds the Fab regions of the B-cell receptor (lower left panel), which induces B-cell death and prevents the production of antibody specific for S. aureus. Ag, antigen; PMN, polymorphonuclear leukocyte.
The mechanisms for SpA-mediated virulence seem clear—i.e., inhibition of opsonophagocytosis mediated by specific antibody and inhibition of B-cell responses that lead to production of opsonic and neutralizing antibodies (Fig. 1). Although these processes likely contribute to the success of S. aureus as a human pathogen (as Falugi et al. suggest [7]), several other factors—some highlighted by the authors—must be considered. First, whether humans have protective immunity to S. aureus is a question that remains to be resolved. The observation that a select group of individuals succumb to recurrent infections provides strong support to the idea that at least some people lack or fail to develop immunity to S. aureus infections. On the other hand, most adults have been exposed to S. aureus or other staphylococci, and 30% of noninstitutionalized individuals are asymptomatically colonized by the bacterium (13), but the vast majority fail to develop—or have never had—serious invasive S. aureus infections. Also, many skin and soft tissue infections resolve without treatment. These findings appear at variance with the idea that humans fail to develop immunity or lack immunity (which includes innate immunity) to S. aureus.
Indeed, the innate immune system is widely regarded as the primary defense against S. aureus infections in humans. Previous studies have demonstrated that S. aureus cells (including USA300) opsonized with normal human serum or those present in human blood are bound and/or ingested rapidly by phagocytes in vitro (14, 15). Consistent with these findings, David Rogers first reported that S. aureus is rapidly cleared from the bloodstream of rabbits by neutrophils, which ultimately traffic the pathogen to distal tissues (16). Whether antibody is required for phagocytosis of S. aureus has also been brought into question in past studies. For example, early work by Shayegani and Kapral demonstrated that S. aureus can be ingested by leukocytes in the absence of antibodies under conditions now known to prime neutrophils for enhanced phagocytosis (17). Notably, adherence primes neutrophils for enhanced functions, such as phagocytosis, and the vast majority of community-associated S. aureus infections are those of skin and soft tissue—conditions under which phagocytes are adherent. Thus, although specific antistaphylococcal antibodies can promote phagocytosis in vitro and in mouse infection models, the relative importance of such antibodies in the protection of humans from infection remains to be determined.
Despite these caveats, the work by Falugi et al. (7), coupled with earlier studies by the same group (18), represents a significant step forward in our efforts to design a staphylococcal vaccine. SpA has been considered an S. aureus virulence molecule for decades, but the application of nontoxigenic SpA as a vaccine antigen is an innovative and refreshing approach. One of the long-standing problems with S. aureus vaccines and vaccine antigens is that protection can be generated in mice, but such protection has failed to translate successfully to humans. Thus, if the protection generated in mice can be translated to humans, then this approach has tremendous potential for success.
ACKNOWLEDGMENTS
S.D.K. and F.R.D. are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank Anita Mora (NIAID) for assistance with graphic illustration.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
Citation Kobayashi SD, DeLeo FR. 2013. Staphylococcus aureus protein A promotes immune suppression. mBio 4(5):e00764-13. doi:10.1128/mBio.00764-13.
REFERENCES
- 1. Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance (ABCs) MRSA Investigators 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 2.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, Net EMERGEncy ID. Study Group; 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674. [DOI] [PubMed] [Google Scholar]
- 3. Projan SJ. 2008. Whither antibacterial drug discovery? Drug Discov. Today 13:279–280 [DOI] [PubMed] [Google Scholar]
- 4. Jansen KU, Girgenti DQ, Scully IL, Anderson AS. 2013. Vaccine review. Staphylococcus aureus vaccines: problems and prospects. Vaccine 31:2723–2730 [DOI] [PubMed] [Google Scholar]
- 5. Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, Chan IS, McNeely TB, Kartsonis NA, Guris D, Onorato MT, Smugar SS, DiNubile MJ, Sobanjo-ter Meulen A. 2013. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309:1368–1378 [DOI] [PubMed] [Google Scholar]
- 6. Projan SJ, Nesin M, Dunman PM. 2006. Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Curr. Opin. Pharmacol. 6:473–479 [DOI] [PubMed] [Google Scholar]
- 7. Falugi F, Kim HK, Missiakas DM, Schneewind O. 2013. The role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. mBio 4(5):e00575-13. 10.1128/mBio.00575-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dossett JH, Kronvall G, Williams RC, Quie PG. 1969. Antiphagocytic effects of staphylococcal protein A. J. Immunol. 103:1405–1410 [PubMed] [Google Scholar]
- 9. Peterson PK, Verhoef J, Sabath LD, Quie PG. 1977. Effect of protein A on staphylococcal opsonization. Infect. Immun. 15:760–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romagnani S, Giudizi MG, del Prete G, Maggi E, Biagiotti R, Almerigogna F, Ricci M. 1982. Demonstration on protein A of two distinct immunoglobulin-binding sites and their role in the mitogenic activity of Staphylococcus aureus Cowen I on human B cells. J. Immunol. 129:596–602 [PubMed] [Google Scholar]
- 11. Graille M, Stura EA, Corper AL, Sutton BJ, Taussig MJ, Charbonnier JB, Silverman GJ. 2000. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: Structural basis for recognition of B-cell receptors and superantigen activity. Proc. Natl. Acad. Sci. U. S. A. 97:5399–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodyear CS, Silverman GJ. 2003. Death by a B cell superantigen. In vivo VH-targeted apoptotic supraclonal B cell deletion by a staphylococcal toxin. J. Exp. Med. 197:1125–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, Jensen BJ, Killgore G, Tenover FC, Kuehnert MJ. 2008. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J. Infect. Dis. 197:1226–1234 [DOI] [PubMed] [Google Scholar]
- 14. Rogers DE, Tompsett R. 1952. The survival of staphylococci within human leukocytes. J. Exp. Med. 95:209–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Saïd-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175:3907–3919 [DOI] [PubMed] [Google Scholar]
- 16. Rogers DE. 1956. Studies on bacteremia. I. Mechanisms relating to the persistence of bacteremia in rabbits following the intravenous injection of staphylococci. J. Exp. Med. 103:713–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shayegani MG, Kapral FA. 1962. The immediate fate of staphylococci after phagocytosis. J. Gen. Microbiol. 29:625–636 [DOI] [PubMed] [Google Scholar]
- 18. Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. 2010. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J. Exp. Med. 207:1863–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]