Abstract
Background
A number of biomarkers have been proven potentially useful for their ability to indicate bone metastases (BM) in cancer patients. The aim of this study was to investigate the relative utility of a newly developed N-terminal propeptide of collagen type I (PINP) human serum assay for the detection of BM in cancer patients. This assay has a corresponding rat PINP assay which in the future might help in translational science between rodent and human trials.
Methods
Participants were 161 prostate, lung and breast cancer patients stratified by number of BM (Soloway score). PINP was assessed and correlated to number of BM. Additionally, the PINP marker was correlated to bone resorption of young (ALPHA CTX-I)- and aged bone (BETA CTX-I); number of osteoclasts (Tartrate-resistant acid phosphatase 5b, TRACP5B) and osteoclast activity (CTX-I/ TRACP5B).
Results
PINP was significantly elevated in breast- and prostate cancer patients +BM, compared to −BM (P < 0.001), however not in lung cancer patients. A strong linear association was seen between PINP and the number of BMs. Significant elevation of PINP was observed at Soloway scores 1–4 (<0 BM) compared with score 0 (0 BM) (P < 0.001). The correlation between bone resorption of young bone or aged bone and bone formation was highly significant in patients +BM and −BM (P < 0.0001).
Conclusions
Data suggest that the present PINP potentially could determine skeletal involvement in patients with breast or prostate cancer. Correlations suggested that coupling between bone resorption and bone formation was maintained in breast- and prostate cancer patients.
Keywords: biochemical markers, bone resorption, bone formation, osteoclastogenesis, breast cancer, prostate cancer, coupling
Introduction
Several cancer types are able to metastasize to organs secondary to the primary tumor. Breast, prostate, and lung cancers are the primary tumors that most frequently metastasize to the skeleton. A metabolically active bone metastasis (BM) exerts profound effects in the local bone micro-environment, the most significant being the balance between bone resorption and bone formation-being either lytic or sclerotic metastasis.1 This results in hypocalcaemia, which in turn leads to severe bone pain and lower quality of life. Early diagnosis and treatment of BM’s might mitigate these consequences.2
Currently, BM in cancer patients is mainly diagnosed by imaging techniques such as Technetium-99 scintigraphy or x-ray.3,4 Imaging techniques are valuable diagnostic tools. However their accuracy in early diagnosis or feasibility in ongoing close monitoring of patients is limited.3 Even though scintigraphy can give quantitative information on skeletal “hot spots” containing BM’s, this assessment is expensive, invasive, time-consuming, and exposes cancer patients to irradiation, limiting its use for monitoring purposes.4 Easy-to-use and accurate diagnostic tools would be valuable supplements to imaging techniques.
Since biochemical markers of bone turnover can be assessed non-invasively, they could prove clinically practical in providing additional systemic information of bone turnover. A panel of markers may be selected to assess disease stages and skeletal subtype of the metastasis, and thereby provide essential information for choice of treatment. The use of bone turnover markers to detect the presence of BM’s is extensively discussed in the literature. Numerous biomarkers of bone resorption, formation, and osteoclastogenesis have been evaluated for their ability to indicate BM in cancer patients.5–17 Some biomarkers may prove more useful than others for the evaluation of BM’s. Several studies suggest that collagenous markers may be the most reliable markers in general for the presence of BMs.
Bone is a dynamic tissue which is continuously remodeled throughout life, not only to maintain calcium homeostasis but also to repair micro-damage and thus maintain bone quality.18 This continuous remodeling of bone involves cells that strive to achieve a coordinated and balanced resorption of old bone (osteoclasts) and those responsible for adequate formation of new bone (osteoblasts), in a local, coordinated and sequential manner referred to as coupling.19–22 When normal coupling occurs in healthy adult bones the amount of bone formed is equal to that resorbed.19–21,23,24 Uncoupling normally occurs when the balance between formation and resorption is dissociated, such as during normal skeletal growth, or in the pathogenesis of diseases such as osteopetrosis or osteoporosis,25,26 and turnover,18 in some, but not all, osteopetrotic mutations.20,27 In postmenopausal osteoporosis there is an increase in both bone formation and bone resorption, however bone resorption exceeds bone formation leading to a continuous negative bone balance, bone fragility and increased risk of fractures.28,29
The aim of this study was to evaluate the ability of a newly developed bone formation serum assay (PINP) to detect the presence of BM in patients with breast, lung or prostate cancer. This ELISA assay differs from other PINP assays by having a corresponding assay for the assessment of rat serum PINP thus making it possible to evaluate the same epitope30 in both a preclinical cancer model and in clinical studies. Furthermore, we aimed to investigate whether coupling existed between bone formation, bone resorption, and the number of osteoclasts. These analyses were performed by correlating PINP data, indicating bone formation, to the well-established C-telopeptide of collagen type I bone resorption markers ααCTX-I31 (of young bone) and ββCTX-I32 (of aged bone), and TRACP5B as an index for osteoclast numbers.33–35
Materials and Methods
Patients and study design
The study design has been published previously.15 Briefly, the study included 90 breast cancer patients (45 +BM and 45 −BM), 30 lung cancer patients (16 +BM and 14 −BM) and 42 prostate cancer patients (25 +BM and 17 −BM) that were referred to the Cancer Institute Hospital, Tokyo, Japan, between October 2002 and April 2004. All patients underwent bone scans using a radionuclide (Technetium-99m), as well as computer tomography (CT) and/or magnetic resonance imaging (MRI) to verify and quantify the presence of BMs. All patients with skeletal complications were newly diagnosed and none had received therapies known to influence bone turnover in the previous 2 years prior to entry to the study. One breast cancer patient had also been diagnosed with Paget’s disease and was excluded from the analysis.
All participants signed approved written consent and the study was performed in accordance with the Helsinki Declaration II and Standards of Good Clinical Practice. The Local Ethical Committee approved the study protocol.
Severity of metastatic bone disease (Soloway score)
The number of BM was recorded and the skeletal load was graded, as previously proposed by Soloway et al. Briefly, Soloway 0 refers to patients without BM, Soloway 1 to patients with fewer than 6 BM, Soloway 2 to patients with 6–20 BM, Soloway 3 to patients with more than 20 but less than a “super scan” defined involvement of more than 75% of the ribs, vertebrae, and pelvic bones; and Soloway 4 to patients with a “super scan”.36
Quantification of bone resorption by serum PINP
Serum samples were collected from all patients and stored at −40 °C until analysis. The concentration of PINP fragments was measured by the newly developed competitive ELISA assay for human N-terminal propetide of collagen type I (Nordic Bioscience, Herlev, Denmark). The assay was run using 20 μl undiluted serum samples in a one-step ELISA 1 hour at 4 °C using a horse radish labeled monoclonal antibody against a 10 amino acid sequence in the N-terminal pro-peptide of collagen type I.30 Mean intra-variation on 10 independent runs was 4.4% on double determinations. Mean inter-variation on 10 independent runs was 5.4% on double determination. Dilution recovery was 90%–114% on 10 normal serum samples representing the entire range of the standard curve.30
Bone resorption and number of osteoclasts
Data on the collagen type I bone resoption markers ββCTX-I32 (assessing resorption of aged collagen type I) and ααCTX-I31 (resorption of newly synthesized collagen type I) and the marker for the number of osteoclasts (TRACP5b) have been published previously by our group15 and these data were used for correlations to the bone formation marker, PINP, detected in serum. The correlation between the CTX-I/TRACP5b ratio, and PINP, was used to indicate resorption activity per osteoclasts versus bone formation.
Statistical analysis
The values of each of the biochemical markers were logarithmically transformed to obtain normality. Comparisons between the level of PINP in patients with different cancer types without BM was performed by analysis of variance (ANOVA) using the General Linear Models Procedure (GLM). The same statistical procedure was used for comparison of PINP levels in patients without and with BM for each cancer type. In the comparison of the PINP levels for each Soloway score against the level in patients without metastasis, the Dunnett’s adjustment of the level of significance was employed to correct for multiple comparisons. Correlations between the biochemical markers were determined using Spearmans Rho. Differences and associations were considered statistically significant if P < 0.05. GRAPH PAD PRISM 5 (Graph Pad Software, La Jolla, CA, USA) was used for calculations.
Results
PINP related to bone cancer type
Demographics of 161 cancer patients stratified according to cancer type and the presence or absence of BM have previously been reported.15 There were no statistically significant differences in age and BMI between patients with or without BM.
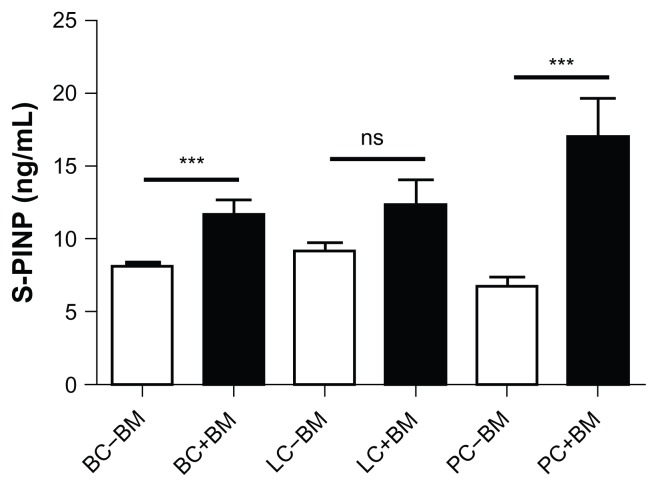
Figure 1 shows the mean values of serum PINP in patients stratified according to cancer type and presence or absence of BM. There was a significantly increased level of PINP, indicating increased bone formation, in breast and prostate cancer patients with BM compared with those without BM. However, PINP was not able to determine the presence of BM in lung cancer patients.
Figure 1.
Bone formation (PINP) marker levels in 161 breast, lung and prostate cancer patients stratified by cancer type and presence (+BM) or absence of bone metastasis (−BM).
Note: Results shown are geometric mean ± SEM.
PINP related to the extent of metastatic bone disease
Since this assay carried little value in diagnosing BM in lung cancer patients, this subpopulation was excluded from further analyses. Accordingly, data from breast and prostate cancer patients were pooled together for subsequent analyses (n = 132). The demographic data for patients was stratified according to Soloway score. No linear associations were found between Soloway score and the demographic characteristics of patients.15
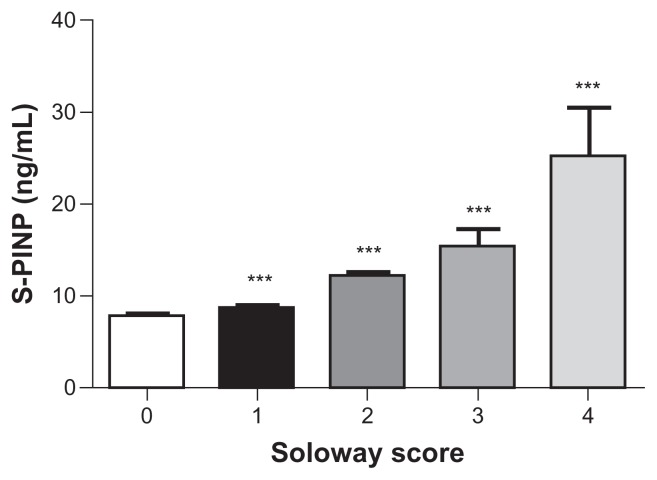
Figure 2 shows associations between Soloway score and the mean PINP values. The marker indicated significant linear increases with advancing severity of the metastatic involvement of the skeletal system. PINP was highly significantly increased at all Soloway scores 1, 2, 3 and 4 compared with Soloway score 0 (ie, no BM) (P < 0.001).
Figure 2.
Bone formation (PINP) marker levels in 132 breast and prostate cancer patients stratified according to the extent of metastatic bone disease described by the Soloway score 0 (−BM), and 1–4 (+BM). Number of patients within each Soloway score: 0 (n = 45); 1 (n = 20); 2 (n = 13); 3 (n = 7) and 4 (n = 5).
Notes: Results shown are geometric mean ± SEM. ***P < 0.001 indicated highly significant elevated level in +BM patients compared to −BM, ns = non significant elevation in +BM patients as compared with −BM.
The coupling between bone formation and bone resorption in breast and prostate cancer patients
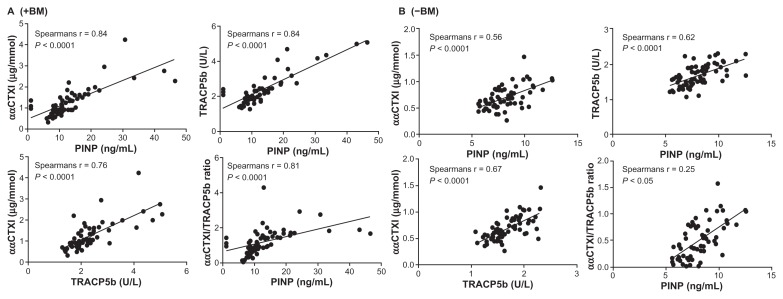
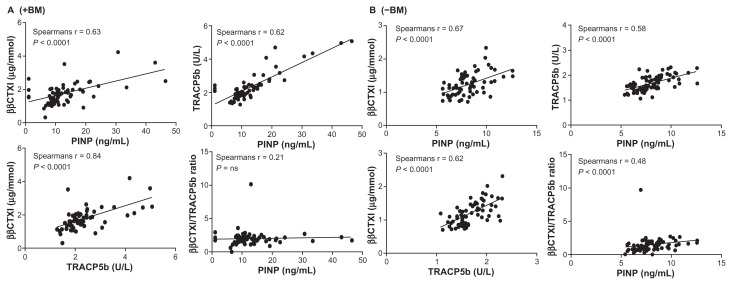
Patients with prostate or breast cancer were pooled and stratified by +BM and −BM for correlation calculations. Correlations were performed using both the ααCTX-I resorption marker as a measure of osteoclast resorption of newly synthesized collagen type I (Fig. 3) and the ββCTX-I resorption marker as a measure of osteoclast resorption of aged synthesized collagen type I37 (Fig. 4). A significant correlation was observed for all our correlation tests (P < 0.0001) in both +BM patients (Figs. 3A and 4A) and −BM patients (Figs. 3B and 4B) except for ββCTX-I/TRACP5b ratio vs. PINP in patients with BM. Data indicated that bone formation, as assessed by PINP vs. resorption of young or old bone (ααCTX-I or ββCTX-I); bone formation (PINP) vs. number of osteoclasts (TRACP5b); resorption of young or old bone (ααCTX-I or ββCTX-I) vs. number of osteoclasts (TRACP5b) and the activity of osteoclasts (ααCTX-I or ββCTX-I/ TRACP5b) vs. bone formation (PINP) all are associated in breast and prostate cancer patients both with or without BM. In patients with BM Spearman correlations were strongest when using the resorption marker ααCTX-I rather than ββCTX-I.
Figure 3.
Correlations between ααCTX-I and PINP; ααCTX-I and TRAC5b; TRACP5b and PINP; ααCTX-I/TRACP5b ratio and PINP in patients +BM (A) and −BM (B). A significant correlation was observed for all cases. A Spearman Rho correlation (Spearman r) is stated for each correlation in the respective figures.
Note: ***P < 0.0001 indicated highly significant elevated levels for Soloway score 1,2,3,4 compared to score 0.
Figure 4.
Correlations between ββCTX-I and PINP; ββCTX-I and TRAC5b; TRACP5b and PINP; ββCTX-I/TRACP5b ratio and PINP in patients +BM (A) and −BM (B). A significant correlation was observed for all cases. Spearman Rho correlation (r) is stated for each correlation in the respective figures.
Discussion
We evaluated a newly developed PINP assay in a clinical study of breast and prostate cancer patients with and without BM. Furthermore, we investigated the coupling between bone resorption and bone formation related to number of osteoclasts. Only one small study on coupling in prostate cancer patients has previously been conducted, which included administration of gonadotropin-releasing hormone agonist treatment.38 In these patients coupling between bone resorption and formation was observed.
Our main findings were: 1) Elevated levels of this serum PINP assay was found in prostate- and breast cancer patients with BM compared to patients without BM, however not in lung cancer patients; 2) This PINP assay was increased according to number of BM when stratified by the Soloway score with significant increase at score 2, 3 and 4; 3) There was significant correlations between bone formation (PINP), resorption (CTX-I) and number of osteoclasts (TRACP5B) in prostate- and breast cancer patients with BM.
The occurrence of tumor cells in bone tissue have a strong impact on the mechanisms of bone turnover due to the secretion soluble factors such as hormones, cytokines and growth factors which directly or indirectly stimulate osteoclast and osteoblast proliferation and function.1,39 This cell-cell interaction is commonly referred to as the vicious cycle. Breast cancer metastases are predominately osteolytic (70%–85%)39 whereas the majority of prostate cancer metastases are osteoblastic (65%)40 although mixed lesions exists in both cancer types.41 These differences provide the rationale for investigating different biomarkers in evaluating BM in patients with different types of cancer. The results may be useful for tailoring treatment for individual patients.
Comparison of PINP assays and their relation to bone metastases
In the present paper we evaluated a newly developed PINP assay for the detection of BM in prostate-, lung- and breast cancer patients. This human serum PINP assay differs from other PINP assays42–44 by the fact that it has a parallel PINP assay for30 corresponding epitope in the rat PINP sequence, and thus offers potential value in translational science between rat cancer models and human studies. We found that the PINP levels assessed by this human serum PINP assay, was significantly higher in patients with BM than in patients without BM in the case of prostate and breast cancers but not lung cancer. There was however a trend towards higher levels in lung cancer patients with BM compared to without BM so the lack of difference may be due to the low number of lung cancer patients.
Data stratified according to the Soloway score indicated that the levels assess by this PINP assay was correlated to the number of BM. Levels were highly significantly elevated at all Soloway scores, indicating that this assay is very sensitive for the detection of BM. However, we do not have data available to determine whether it is more sensitive that scintigraphy.
By the use of different PINP assays, other groups have evaluated PINP in relation to bone metastases.45–55 In relation to our study a group showed that the levels assessed by another PINP assay were significantly elevated in breast cancer patients with BM compared to those without BM, and correlated to the number of BM’s.55 A PINP assay was evaluated in 64 prostate cancer patients stratified by −BM; −BM/+lymph node metastases or +BM. Here PINP was elevated in the −BM/+lymph node metastases and +BM groups when compared to the −BM group.56 Interestingly, PINP was even elevated 8 months prior to detection of the first BM by scintigraphy, indicating that PINP is a powerful marker for very early diagnosis of BM which might not be detected by other means. Similar findings have been presented by Koizumi et al57 who showed PINP was elevated in prostate cancer patients with BM compared to those without BM. Nevertheless, PINP was the best discriminator, among three markers, of the extent of disease. Finally, Jung et al28 who analyzed 10 different serum markers and found that PINP was significantly elevated in patients with BM compared to without BM. All these findings correspond to our evaluation of this newly developed human serum PINP assay.
PINP compared to other bone formation marker for the detection of BM
Other markers of bone formation such as bone alkaline phosphates (BALP), osteocalcin (OC), have been evaluated for their ability to describe bone metastases indicating that some bone formation markers are and some are not elevated in prostate- and breast cancer patients.58–60 Recently, a group demonstrated that BSAP was elevated in prostate cancer patients with BM and correlated to the Extent of disease (EOD), whereas OC did not.60 Similar data for OC have been published by Hegele A et al59 showing that OC was not elevated in prostate cancer patients with BM. It may be difficult to directly compare different bone formation markers since they all are of a different nature. PINP is a collagenous marker; OC is a noncollagenous marker;61 bone alkaline phosphatase is an enzyme found on the surface of osteoblasts.62 The expression of the collagen type I gene is known to be expressed prior to the alkaline phosphatase genes and alkaline phosphatase prior to the OC genes indicating that collagen type I derived markers may be most helpful for early detection of BM.63
Different assays assessing different epitopes of the same bone formation protein may further complicate the picture. Thus, several statements in the bone field have been made with regards to the relevance of assessing either the monomeric or trimeric form of the PINP peptide.64,65 Further research is needed to understand why measurements of the same protein by different assessment technologies produce different outcomes, and the possible pathological rationale for the discrepancies.
Coupling between osteoclasts and osteoblasts
Even in BM of the osteolytic nature, highly increased levels of bone formation parameters have been identified in the vicious cycle.1,39,66 This emphasizes that more research into the coupling between bone resorption and bone formation may be important in detecting different types of BM.
Interestingly, the coupling between bone resorption and bone formation was maintained in these prostate- and breast cancer patients with BM or without BM when analyzed separately with no differentiation between cancer types (data not shown). Thus we pooled the data to increase the statistical power. Bone formation was significantly related to bone resorption and the number of osteoclasts; in the same manner bone resorption was significantly related to number of osteoclasts.
ααCTX-I as a resorption marker
Levels of ααCTX-I may possibly originate mainly from bone metastases due to their local high bone turnover characteristic, thus generating large quantities of newly formed collagen type I matrix. Viewing the Spearman Rho coefficients it was note-worthy that in patients with BM, a higher correlation was observed between PINP/TRACP5b (r = 0.84) and PINP/ααCTX-I (r = 0.84) than was observed for ααCTX-I/TRACP5b (r = 0.76). This indicates that the number of osteoclasts and bone formation is more tightly coupled than the number of osteoclasts and bone resorption. Furthermore, the mean resorption of young bone per osteoclast (ααCTX-I/TRACP5b) was well correlated to PINP in patients with BM (r = 0.81) indicating that the resorption activity per osteoclast was not elevated in these patients. However, further investigations are needed for such a conclusion and should be regarded as speculation. In patients without BM a similar picture was seen. Spearman correlations were similar between all remodeling parameters, indicating that all were just as tightly coupled with regards to resorption of young bone.
ββCTX-I as a resorption marker
Levels of ββCTX-I may originate mainly as a mean of the total turnover of the skeleton due to the high amount of isomerized collagen type I in normally remodeled bone matrix. The Spearman Rho coefficients showed that ββCTX, PINP and TRACP were just as strongly correlated in patients with and without BM. However, the mean resorption activity on aged bone per osteoclast was not correlated to bone formation in patients with bone metastases also indicating that bone formation is more tightly coupled to number of osteoclasts and not their activity in patients with bone metastases.
These data are somewhat in alignment with research suggesting that it is rather the presence of osteoclasts and not resorptive activity that is important for bone formation.34 Compelling evidence for this is the fact that, in the normal adult skeleton, bone formation is almost exclusively initiated in areas having undergone resorption19–22,67 indicating local signaling events between osteoclasts and osteoblasts. Consistent with this, the number of nuclei in osteoclasts has been shown to correlate to the number of osteoblasts.68 Recently osteoclasts themselves were demonstrated to secrete bone anabolic signals.69 This also led to speculation that resorbing osteoclasts do not always secrete the anabolic signal70 implying that the anabolic signal is not derived exclusively during resorption of the bone matrix. Further strengthening this new view of the coupling between bone resorption and bone formation, was that bone resorption was less correlated to bone formation in cancer patients overall.
Limitations
An increasing number of publications have shown that biomarkers are potential candidates for a more dynamic evaluation of BM in breast- and prostate cancer patients.5–17 However, since biomarkers often are assessed systemically they do not necessarily reflect the local environment of a BM site, but rather are means of the total bone remodeling in the entire skeleton. They also do not necessarily reflect local coupling between bone formation and bone resorption, but throughout the body.
There are important differences in the pathogenesis of osteolytic bone metastases and osteoblastic bone metastases,1,39–41 that most likely affect coupling between bone resorption and bone formation. Further investigations are needed to understand how the two different types of metastases affect coupling. The power of this study was not high enough to add to that discussion.
Another limitation is that this was a cross-sectional study and the statistical analysis was based on an assumption that the individual variation in biomarker levels in patients was low. Future analysis should be performed in a longitudinal study.
In conclusion, the present study evaluated a newly developed human serum PINP and its ability to describe bone metastases. Furthermore, it provided additional evidence that PINP markers may be useful for obtaining valuable systemic information about the development of BM in breast- and prostate cancer patients.
Acknowlegdement
We acknowledge the funding from the Danish “Ministry of Science, Technology and Science” and the Danish science foundation (“Den Danske Forskningsfond”).
Footnotes
- – Diana J. Leeming developed the PINP assay and did the measurements, calculation of data, as well as prepared most of the manuscript
- – Kim Henriksen and Morten A. Karsdal guided in the evaluations of the assay and during writing
- – Inger Byrjalsen guided during the assay development and helped with the statistical calculations
- – Chen Zhang produced the monoclonal antibody for this newly developed PINP assay under guidance of Diana Leeming and Per Qvist
- – Per Qvist guided during the selection of the PINP target and production of the monoclonal antibody
- – Vibeke Barkholt guided the characterization of the antibodies
- – Mitsuru Koizumi collected the clinical samples and carried out the bone imaging to determine number of bone metastases
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. Diana J. Leeming, Kim Henriksen, Inger Byrjalsen, Per Qvist, Chen Zhang and Morten Karsdal are full-time employees of Nordic Bioscience. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Mundy GRGT. Pathophysiology of bone metastases. In: Rubens RDMG, editor. Cancer and the skeletons. London (UK): Martin Dunitz Ltd; 2000. pp. 43–64. [Google Scholar]
- 2.Brown JE, Cook RJ, Major P, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 3.Dotan ZA. Bone imaging in prostate cancer. Nat Clin Pract Urol. 2008;5:434–44. doi: 10.1038/ncpuro1190. [DOI] [PubMed] [Google Scholar]
- 4.Clamp A, Danson S, Nguyen H, Cole D, Clemons M. Assessment of therapeutic response in patients with metastatic bone disease. Lancet Oncol. 2004;5:607–16. doi: 10.1016/S1470-2045(04)01596-7. [DOI] [PubMed] [Google Scholar]
- 5.Garnero P, Buchs N, Zekri J, Rizzoli R, Coleman RE, Delmas PD. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer. 2000;82:858–64. doi: 10.1054/bjoc.1999.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulrich U, Rhiem K, Schmolling J, et al. Cross-linked type I collagen C- and N-telopeptides in women with bone metastases from breast cancer. Arch Gynecol Obstet. 2001;264:186–90. doi: 10.1007/s004040000105. [DOI] [PubMed] [Google Scholar]
- 7.Kiuchi K, Ishikawa T, Hamaguchi Y, et al. Cross-linked collagen C- and N-telopeptides for an early diagnosis of bone metastasis from breast cancer. Oncol Rep. 2002;9:595–8. [PubMed] [Google Scholar]
- 8.Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Cancer. 2000;88:2919–26. doi: 10.1002/1097-0142(20000615)88:12+<2919::aid-cncr7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Jung K, Lein M, Stephan C, et al. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int J Cancer. 2004;111:783–91. doi: 10.1002/ijc.20314. [DOI] [PubMed] [Google Scholar]
- 10.Ebert W, Muley T, Herb KP, Schmidt-Gayk H. Comparison of bone scintigraphy with bone markers in the diagnosis of bone metastasis in lung carcinoma patients. Anticancer Res. 2004;24:3193–201. [PubMed] [Google Scholar]
- 11.Chao TY, Yu JC, Ku CH, et al. Tartrate-resistant acid phosphatase 5b is a useful serum marker for extensive bone metastasis in breast cancer patients. Clin Cancer Res. 2005;11:544–50. [PubMed] [Google Scholar]
- 12.Lipton A, Ali SM, Leitzel K, et al. Serum osteoprotegerin levels in healthy controls and cancer patients. Clin Cancer Res. 2002;8:2306–10. [PubMed] [Google Scholar]
- 13.Costa L, Demers LM, Gouveia-Oliveira A, et al. Prospective evaluation of the peptide-bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. J Clin Oncol. 2002;20:850–6. doi: 10.1200/JCO.2002.20.3.850. [DOI] [PubMed] [Google Scholar]
- 14.Voorzanger-Rousselot N, Juillet F, Mareau E, Zimmermann J, Kalebic T, Garnero P. Association of 12 serum biochemical markers of angiogenesis, tumour invasion and bone turnover with bone metastases from breast cancer: a crossectional and longitudinal evaluation. Br J Cancer. 2006;95:506–14. doi: 10.1038/sj.bjc.6603285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leeming DJ, Koizumi M, Byrjalsen I, Li B, Qvist P, Tanko LB. The relative use of eight collagenous and noncollagenous markers for diagnosis of skeletal metastases in breast, prostate, or lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15:32–8. doi: 10.1158/1055-9965.EPI-05-0492. [DOI] [PubMed] [Google Scholar]
- 16.Chung YC, Ku CH, Chao TY, Yu JC, Chen MM, Lee SH. Tartrate-resistant acid phosphatase 5b activity is a useful bone marker for monitoring bone metastases in breast cancer patients after treatment. Cancer Epidemiol Biomarkers Prev. 2006;15:424–8. doi: 10.1158/1055-9965.EPI-04-0842. [DOI] [PubMed] [Google Scholar]
- 17.Ali SM, Demers LM, Leitzel K, et al. Baseline serum NTx levels are prognostic in metastatic breast cancer patients with bone-only metastasis. Ann Oncol. 2004;15:455–9. doi: 10.1093/annonc/mdh089. [DOI] [PubMed] [Google Scholar]
- 18.Baron R. Anatomy and Biology of Bone Matrix and Cellular Elements, Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington DC (USA): American Society for Bone and Mineral Research; 2003. [Google Scholar]
- 19.Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature. 1965;206:489–90. doi: 10.1038/206489a0. [DOI] [PubMed] [Google Scholar]
- 20.Parfitt AM. The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res. 1982;4:1–6. doi: 10.1016/0221-8747(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi H, Epker B, Frost Hm. Resorption Precedes Formative Activity. Surg Forum. 1964;15:437–8. [PubMed] [Google Scholar]
- 22.Sarnsethsiri P, Hitt OK, Eyring EJ, Frost HM. Tetracycline-based study of bone dynamics in pycnodysostosis. Clin Orthop Relat Res. 1971;74:301–12. doi: 10.1097/00003086-197101000-00035. [DOI] [PubMed] [Google Scholar]
- 23.Martin TJ. Hormones in the coupling of bone resorption and formation. Osteoporos Int. 1993;3( Suppl 1):121–5. doi: 10.1007/BF01621884. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura M, Udagawa N, Matsuura S, et al. Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology. 2003;144:5441–9. doi: 10.1210/en.2003-0717. [DOI] [PubMed] [Google Scholar]
- 25.Goltzman D. Discoveries, drugs and skeletal disorders. Nat Rev Drug Discov. 2002;1:784–96. doi: 10.1038/nrd916. [DOI] [PubMed] [Google Scholar]
- 26.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–49. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 27.Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med. 2004;351:2839–49. doi: 10.1056/NEJMra040952. [DOI] [PubMed] [Google Scholar]
- 28.Rodan GA. Mechanical loading, estrogen deficiency, and the coupling of bone formation to bone resorption. J Bone Miner Res. 1991;6:527–30. doi: 10.1002/jbmr.5650060602. [DOI] [PubMed] [Google Scholar]
- 29.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526–36. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 30.Leeming DJ, Larsen DV, Zhang C, et al. Enzyme-linked immunosorbent serum assays (ELISAs) for rat and human N-terminal pro-peptide of collagen type I (PINP)—assessment of corresponding epitopes. Clin Biochem. 2010;43:1249–56. doi: 10.1016/j.clinbiochem.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Cloos PA, Lyubimova N, Solberg H, et al. An immunoassay for measuring fragments of newly synthesized collagen type I produced during metastatic invasion of bone. Clin Lab. 2004;50:279–89. [PubMed] [Google Scholar]
- 32.Bonde M, Qvist P, Fledelius C, Riis BJ, Christiansen C. Immunoassay for quantifying type I collagen degradation products in urine evaluated. Clin Chem. 1994;40:2022–5. [PubMed] [Google Scholar]
- 33.Henriksen K, Tanko LB, Qvist P, Delmas PD, Christiansen C, Karsdal MA. Assessment of osteoclast number and function: application in the development of new and improved treatment modalities for bone diseases. Osteoporos Int. 2007;18:681–5. doi: 10.1007/s00198-006-0286-8. [DOI] [PubMed] [Google Scholar]
- 34.Karsdal MA, Martin TJ, Bollerslev J, Christiansen C, Henriksen K. Are nonresorbing osteoclasts sources of bone anabolic activity? J Bone Miner Res. 2007;22:487–94. doi: 10.1359/jbmr.070109. [DOI] [PubMed] [Google Scholar]
- 35.Janckila AJ, Yam LT. Biology and Clinical Significance of Tartrate-Resistant Acid Phosphatases: New Perspectives on an Old Enzyme. Calcif Tissue Int. 2009 doi: 10.1007/s00223-009-9309-8. [DOI] [PubMed] [Google Scholar]
- 36.Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195–202. doi: 10.1002/1097-0142(19880101)61:1<195::aid-cncr2820610133>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 37.Leeming DJ, Henriksen K, Byrjalsen I, et al. Is bone quality associated with collagen age? Osteoporos Int. 2009 doi: 10.1007/s00198-009-0904-3. [DOI] [PubMed] [Google Scholar]
- 38.Miyaji Y, Saika T, Yamamoto Y, et al. Effects of gonadotropin-releasing hormone agonists on bone metabolism markers and bone mineral density in patients with prostate cancer. Urology. 2004;64:128–31. doi: 10.1016/j.urology.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 40.Keller ET, Cicuttini FM. The Biology of Skeletal Metastases. Boston: Kluwer Academic Publishers; 2004. [Google Scholar]
- 41.O’Keefe RJ, Guise TA. Molecular mechanisms of bone metastasis and therapeutic implications. Clin Orthop Relat Res. 2003:S100–4. doi: 10.1097/01.blo.0000093847.72468.2f. [DOI] [PubMed] [Google Scholar]
- 42.Melkko J, Kauppila S, Niemi S, et al. Immunoassay for intact aminoterminal propeptide of human type I procollagen. Clin Chem. 1996;42:947–54. [PubMed] [Google Scholar]
- 43.Linkhart SG, Linkhart TA, Taylor AK, Wergedal JE, Bettica P, Baylink DJ. Synthetic peptide-based immunoassay for amino-terminal propeptide of type I procollagen: application for evaluation of bone formation. Clin Chem. 1993;39:2254–8. [PubMed] [Google Scholar]
- 44.Orum O, Hansen M, Jensen CH, et al. Procollagen type I N-terminal propeptide (PINP) as an indicator of type I collagen metabolism: ELISA development, reference interval, and hypovitaminosis D induced hyperparathyroidism. Bone. 1996;19:157–63. doi: 10.1016/8756-3282(96)00165-2. [DOI] [PubMed] [Google Scholar]
- 45.Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008;19:329–37. doi: 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]
- 46.Luftner D, Jozereau D, Schildhauer S, et al. PINP as serum marker of metastatic spread to the bone in breast cancer patients. Anticancer Res. 2005;25:1491–9. [PubMed] [Google Scholar]
- 47.Luftner D, Richter A, Geppert R, Wernecke KD, Possinger K. Normalisation of biochemical markers of bone formation correlates with clinical benefit from therapy in metastatic breast cancer. Anticancer Res. 2003;23:1017–26. [PubMed] [Google Scholar]
- 48.Lein M, Miller K, Wirth M, et al. Bone turnover markers as predictive tools for skeletal complications in men with metastatic prostate cancer treated with zoledronic acid. Prostate. 2009;69:624–32. doi: 10.1002/pros.20917. [DOI] [PubMed] [Google Scholar]
- 49.Brasso K, Christensen IJ, Johansen JS, et al. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66:503–13. doi: 10.1002/pros.20311. [DOI] [PubMed] [Google Scholar]
- 50.Koizumi M, Yonese J, Fukui I, Ogata E. Metabolic gaps in bone formation may be a novel marker to monitor the osseous metastasis of prostate cancer. J Urol. 2002;167:1863–6. [PubMed] [Google Scholar]
- 51.Ramankulov A, Lein M, Kristiansen G, Loening SA, Jung K. Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate. 2007;67:330–40. doi: 10.1002/pros.20540. [DOI] [PubMed] [Google Scholar]
- 52.Klepzig M, Jonas D, Oremek GM. Procollagen type 1 amino-terminal propeptide: a marker for bone metastases in prostate carcinoma. Anticancer Res. 2009;29:671–3. [PubMed] [Google Scholar]
- 53.Body JJ, Lichinitser M, Tjulandin S, Garnero P, Bergstrom B. Oral ibandronate is as active as intravenous zoledronic acid for reducing bone turnover markers in women with breast cancer and bone metastases. Ann Oncol. 2007;18:1165–71. doi: 10.1093/annonc/mdm119. [DOI] [PubMed] [Google Scholar]
- 54.McCloskey E, Paterson A, Kanis J, Tahtela R, Powles T. Effect of oral clodronate on bone mass, bone turnover and subsequent metastases in women with primary breast cancer. Eur J Cancer. 2010;46:558–65. doi: 10.1016/j.ejca.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Pollmann D, Siepmann S, Geppert R, Wernecke KD, Possinger K, Luftner D. The amino-terminal propeptide (PINP) of type I collagen is a clinically valid indicator of bone turnover and extent of metastatic spread in osseous metastatic breast cancer. Anticancer Res. 2007;27:1853–62. [PubMed] [Google Scholar]
- 56.Koopmans N, de Jong IJ, Breeuwsma AJ, van d V. Serum bone turnover markers (PINP and ICTP) for the early detection of bone metastases in patients with prostate cancer: a longitudinal approach. J Urol. 2007;178:849–53. doi: 10.1016/j.juro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 57.Koizumi M, Yonese J, Fukui I, Ogata E. The serum level of the aminoterminal propeptide of type I procollagen is a sensitive marker for prostate cancer metastasis to bone. BJU Int. 2001;87:348–51. doi: 10.1046/j.1464-410x.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- 58.Berruti A, Dogliotti L, Gorzegno G, et al. Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. Clin Chem. 1999;45:1240–7. [PubMed] [Google Scholar]
- 59.Hegele A, Wahl HG, Varga Z, et al. Biochemical markers of bone turnover in patients with localized and metastasized prostate cancer. BJU Int. 2007;99:330–4. doi: 10.1111/j.1464-410X.2006.06604.x. [DOI] [PubMed] [Google Scholar]
- 60.Zafeirakis AG, Papatheodorou GA, Limouris GS. Clinical and imaging correlations of bone turnover markers in prostate cancer patients with bone only metastases. Nucl Med Commun. 2009 doi: 10.1097/MNM.0b013e328335a5ed. [DOI] [PubMed] [Google Scholar]
- 61.Garnero P, Grimaux M, Demiaux B, Preaudat C, Seguin P, Delmas PD. Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J Bone Miner Res. 1992;7:1389–98. doi: 10.1002/jbmr.5650071206. [DOI] [PubMed] [Google Scholar]
- 62.Sassi ML, Eriksen H, Risteli L, et al. Immunochemical characterization of assay for carboxyterminal telopeptide of human type I collagen: loss of antigenicity by treatment with cathepsin K. Bone. 2000;26:367–73. doi: 10.1016/S8756-3282(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 63.Koizumi M, Ogata E. Bone metabolic markers as gauges of metastasis to bone: a review. Ann Nucl Med. 2002;16:161–8. doi: 10.1007/BF02996296. [DOI] [PubMed] [Google Scholar]
- 64.Koivula MK, Ruotsalainen V, Bjorkman M, et al. Difference between total and intact assays for N-terminal propeptide of type I procollagen reflects degradation of pN-collagen rather than denaturation of intact propeptide. Ann Clin Biochem. 2010;47:67–71. doi: 10.1258/acb.2009.009110. [DOI] [PubMed] [Google Scholar]
- 65.Brandt J, Krogh TN, Jensen CH, Frederiksen JK, Teisner B. Thermal instability of the trimeric structure of the N-terminal propeptide of human procollagen type I in relation to assay technology. Clin Chem. 1999;45:47–53. [PubMed] [Google Scholar]
- 66.Siclari VA, Guise TA, Chirgwin JM. Molecular interactions between breast cancer cells and the bone microenvironment drive skeletal metastases. Cancer Metastasis Rev. 2006;25:621–33. doi: 10.1007/s10555-006-9023-1. [DOI] [PubMed] [Google Scholar]
- 67.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Thompson ER, Baylink DJ, Wergedal JE. Increases in number and size of osteoclasts in response to calcium or phosphorus deficiency in the rat. Endocrinology. 1975;97:283–9. doi: 10.1210/endo-97-2-283. [DOI] [PubMed] [Google Scholar]
- 69.Karsdal MA, Neutzsky-Wulff AV, Dziegiel MH, Christiansen C, Henriksen K. Osteoclasts secrete non-bone derived signals that induce bone formation. Biochem Biophys Res Commun. 2008;366:483–8. doi: 10.1016/j.bbrc.2007.11.168. [DOI] [PubMed] [Google Scholar]
- 70.Howard GA, Bottemiller BL, Turner RT, Rader JI, Baylink DJ. Parathyroid hormone stimulates bone formation and resorption in organ culture: evidence for a coupling mechanism. Proc Natl Acad Sci U S A. 1981;78:3204–8. doi: 10.1073/pnas.78.5.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]