Abstract
Aims and background
YKL-40 is secreted by several types of tumors. Increased serum YKL-40 levels have been reported in prostate, glioblastoma, breast and colorectal cancers. Determination of YKL-40 levels may serve as a valuable biomarker for the diagnosis and treatment of gastric cancer. The purpose of this study was to determine the serum YKL-40 levels expressed in gastric carcinomas.
Methods
Between 2009 and 2011, we retrospectively reviewed 100 patients with gastric cancer and compared their serum samples to 75 healthy volunteers. YKL-40 levels were determined by an enzyme-linked immunosorbent assay (ELISA).
Results
We found significantly higher serum levels of YKL-40 in patients with gastric cancer compared to the healthy population (P < 0.0001). We also found significant differences in serum YKL-40 levels between female and male patients with gastric cancer (P < 0.01).
Conclusions
YKL-40 is over-expressed in gastric cancer, suggesting a more aggressive phenotype. YKL-40 may be a useful serum biomarker for gastric cancer identification, and future studies should focus on the role of YKL-40 in the tumorigenesis of gastric cancer and responsiveness toward treatment.
Keywords: gastric cancer, YKL-40
Introduction
Gastric cancer is the fourth most common aggressive malignancy.1 Although gastric cancer has a poor prognosis, with a 25% five-year survival rate in the United States,2 there is no standard biomarker for early diagnosis and no consensus on screening programs. As a result, new molecular markers and therapeutic strategies are necessary to design effective diagnostic and therapeutic agents. Degradation or breakdown of the extracellular matrix is the main structural change during the invasion and metastasis of gastric cancer.3
YKL-40 (also called CHI3L1), a member of the mammalian chitinase-like protein family (which has no chitinase activity), is a heparin- and chitin-binding lectin4–6 secreted by activated neutrophils7 macrophages during late stages of differentiation,8,9 arthritic chondrocytes,12 differentiated vascular smooth cells,6 and fibroblast-like synovial cells.10,11
YKL-40 is a growth factor for connective tissue cells and also plays a role in the migration of endothelial cells.5–11 Although the biological function of YKL-40 is unknown, its pattern of expression in normal and various disease stages suggests that it plays a role in the remodeling or degradation of the extracellular matrix.
Recent studies have identified YKL-40 as a biomarker for a variety of cancers.13 A number of studies in patients with solid tumors and hematological malignancies14–21 have shown that elevated serum YKL-40 levels are correlated with many malignant tumors.22
In vivo, elevation of YKL-40 mRNA levels has been demonstrated in tumor-associated macrophages23 and in normal and malignant epithelial cells of the breast.24 The human YKL-40 gene (CHI3LI)8,25 and the crystal structure of the protein26,27 have also been characterized.
The serum levels of YKL-40 in gastric cancer are unknown. We hypothesized that YKL-40 serum levels in patients with gastric cancer might be associated with tumor aggression. The aim of our study was to determine whether YKL-40 levels are elevated in the serum of gastric cancer patients compared to healthy controls and whether YKL-40 could serve as a peripheral biomarker for gastric carcinomas.
Patients and Methods
This study included 100 patients (48 women and 52 men, aged 41–67 years) with recently diagnosed, histologically confirmed gastric adenocarcinomas who were admitted to the Department of General Surgery in Yuzuncu Yil University Medical Faculty, Van, Turkey. The control group consisted of 75 healthy volunteer subjects (35 women and 40 men, aged 40–55 years). Diseases such as infection and asymptomatic early adenocarcinomas and adenomas were ruled out by clinical history, physical examination, and routine laboratory tests, including liver and renal function tests and colonoscopies.
The study protocol was approved by the Research Ethics Committee of the Yuzuncu Yil University Medical Faculty Van, Turkey. Written informed consent was obtained from each individual. Demographic characteristics and clinical data, including symptoms and medications, were obtained via a questionnaire transcribed by trained personnel.
From every patient, 5 ml of blood was taken from the cubital vein in the operating room before the start of the operation. Blood samples were processed into serum aliquots within 3 hr, stored at −70 °C, and analyzed by researchers blind to the clinical parameters and the study endpoints. Serum levels of YKL-40 were determined in duplicate by an ELISA assay (Quidel Corporation, San Diego, CA).26
A biotinylated Fab monoclonal mouse antibody against human YKL-40 (capture antibody) and an alkaline phosphatase-labeled polyclonal rabbit antibody against human YKL-40 (detection antibody) were added to streptavidin-coated microplate wells to determine YKL-40 levels. Bound enzyme activity was detected with p-nitrophenyl phosphate as the substrate. The detection limit of the ELISA is 20 ng/ml, the intra-assay coefficient of variation (during an 11-day period) is <3.7%, and the long-term inter-assay coefficient of variation (during a 5-year period) is <8.6%. The YKL-40 ELISA is useful for the measurement of serum concentrations of YKL-40 in humans.
Statistical Analysis
Comparisons between the two groups were performed using the Mann-Whitney U-test. The differences were considered statistically significant at P < 0.05. The median average is given as the mean ± standard deviation.
Results
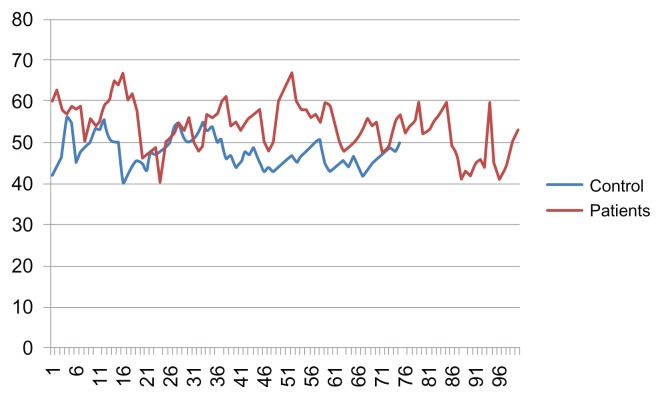
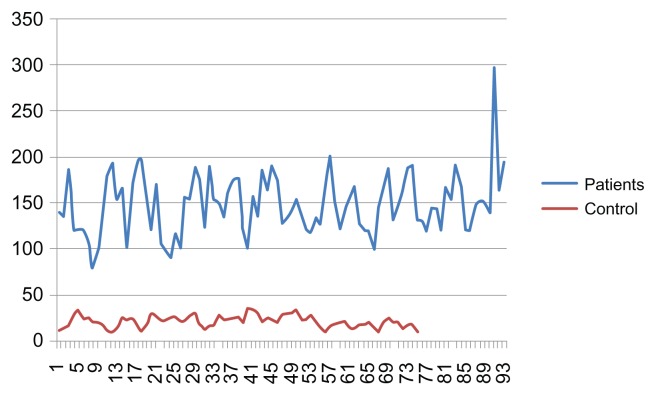
Average values of the YKL-40 serum levels from patients with gastric cancer and from healthy controls are shown in Table 1. The relationships between age and serum YKL-40 levels for patients with gastric cancer and healthy controls are shown in Figures 1 and 2. The median age of the patients who participated in this study was 57 years (range: 41 to 67, mean: 56.8 ± 12.5).
Table 1.
Mean values of YKL-40 levels in patients with gastric cancer and in healthy subjects.
| Gastric cancer patients | Healthy | P-value | |
|---|---|---|---|
| Age | 55.7 ± 14.2 | 52.8 ± 10.9 | P > 0.05 |
| Total YKL-40 (μg/l) | 140 ± 78 | 25 ± 9 | <0.0001 |
Note: Data are presented as the means ± SD.
Figure 1.
Graph showing the age of the healthy controls and patients with gastric cancer Age (y).
Figure 2.
Graph showing the age versus the serum YKL-40 levels of healthy controls and patients with gastric cancer YKL-40 μ/l.
YKL-40 levels were significantly higher in patients with gastric cancer than in healthy individuals (P < 0.0001). Additionally, we found significant differences between the serum YKL-40 levels of male and female patients with gastric cancer (P < 0.01) (Tables 2–4).
Table 2.
Mean values of serum YKL-40 levels grouped by age in healthy subjects.
| Serum YKL-40 levels (μ/l) in healthy controls | |
|---|---|
| <50 years | 15 ± 6 |
| ≥50 years | 20 ± 3 |
| P-value | <0.05 |
Note: Data are presented as the means ± SD.
Table 4.
Mean values of serum YKL-40 levels grouped by gender in patients with gastric cancer and in healthy controls.
| Healthy controls | Gastric cancer patients | |
|---|---|---|
| Female | 17 ± 5 | 99 ± 31 |
| Male | 23 ± 7 | 124 ± 20 |
| P-value | <0.05 | <0.01 |
Note: Data are means ± SD.
Discussion
Gastric cancer is the second leading cause of cancer-related deaths worldwide. In the majority of cases, it is not diagnosed until it reaches an advanced stage, and no efficient therapeutic modality has been suggested to overcome the problem of treatment resistance. Gastric adenocarcinomas involve a complex network of molecular alterations throughout their carcinogenesis.
Various factors influence the biology of gastric cancer.2 To our knowledge, this study represents the first report on the novel serum biomarker YKL-40 in patients with gastric cancer. All patients with gastric cancer had significantly higher serum concentrations of YKL-40 compared to the healthy population.
The YKL-40 ELISA is useful for the measurement of serum (or EDTA-treated plasma) YKL-40 concentrations in humans,29 but not in other species, such as bovine, swine, rabbit, mouse, and rabbit. The detection limit of the ELISA is 20 ng/ml.22
The median serum concentration of YKL-40 in healthy adults was 43 μg/l (90th percentile = 95 μg/l; 95th percentile = 124 μg/l).22,28 By quantitatively measuring the elevated serum levels of YKL-40 using an ELISA assay, we demonstrated that the production of YKL-40 is a crucial event in gastric carcinogenesis.
In 1995, we reported increased serum levels of YKL-40 in some patients with metastatic breast cancer.29 Recent studies have found elevated serum levels of YKL-40 in patients with several types of localized or advanced solid cancers.14–20 These cancer patients were scored as having elevated serum YKL-40 levels if their serum YKL-40 levels were higher than the age-adjusted upper 95th percentile confidence limit of serum YKL-40 levels in healthy subjects.28–30 Preoperative serum levels of YKL-40 were elevated in 19% of patients with primary breast cancer, and patients with metastases to axillary lymph nodes had higher serum YKL-40 levels compared to lymph-node-negative patients.31 Preoperative YKL-40 serum levels from patients with colorectal cancer were elevated in 26% of the patients, and there was an association between serum YKL-40 levels and Dukes’ stage; 16% of the patients with Dukes’ A, 26% of patients with Dukes’ B, 19% of patients with Dukes’ C, and 39% of patients with Dukes’ D had elevated preoperative serum YKL-40 levels.32 In patients with small-cell lung cancer, 22% of patients with local disease and 40% of patients with extended disease had elevated serum YKL-40 levels.15 Forty-three percent of patients with metastatic prostate cancer,14 83% of patients with metastatic renal cell cancer33–35 and 45% of patients with metastatic malignant melanoma12 had elevated serum YKL-40 levels. In patients with glioblastoma, the serum YKL-40 levels were related to tumor grade and burden; 72% of patients with glioblastoma multiforme and 57% of patients with lower grade gliomas had high serum YKL-40 levels.21 Additionally, we found higher serum YKL-40 levels in patients with gastric cancer. Because of the activity of YKL-40 in the carcinogenesis pathway, serum YKL-40 levels may represent a useful, cost-effective, and noninvasive biomarker for the early detection of gastric cancer. Further studies with larger sample sizes are required to establish the use of serum YKL-40 levels for this purpose.
The biological function of YKL-40 in cancer remains unknown. It has been suggested that YKL-40 may play a role in the proliferation and differentiation of tumor cells, the prevention of apoptosis, the stimulation of angiogenesis, the remodeling of extracellular tissue, and the stimulation of fibroblasts surrounding the tumor, although in vivo proof of these hypotheses has yet to be obtained.35 YKL-40 is not produced by fibroblasts, but it is a growth factor for fibroblasts, synovial cells, and chondrocytes.9 Some studies have demonstrated that the increased serum YKL-40 levels found in various cancer patients reflect YKL-40 secretion from a subset of tumors with a more aggressive phenotype.
The oncogenic function of YKL-40 might be associated with its activation of the Akt pathway.36 Recent studies have sought to determine which member of the Akt pathway is activated by YKL-40 in gastric cancer. YKL-40 induces mitogen-activated protein kinase (MAP) and PI3K signaling cascades in fibroblasts, leading to the phosphorylation of both the extracellular signal-regulated kinase (ERK)-1/2 MAP kinase- and protein kinase B (AKT)-mediated signaling cascades,36 which are associated with the control of mitogenesis. Aberrant activation of the PI3 K/Akt pathways has been reported in several cancers. Activation of Akt kinase is necessary for many events of the metastatic pathway, including the escape of cells from the tumor’s original environment, entrance into and departure from the circulation, activation of proliferation, blockage of apoptosis, and activation of angiogenesis.36
Not all cancer patients in this study had elevated serum YKL-40 levels compared to healthy age-matched controls, suggesting either that not all tumors secrete YKL-40 or that the protein is secreted at a low level in these patients. Cancer cells that secrete YKL-40 may have a different phenotype than cancer cells that do not express and secrete YKL-40; YKL-40 expression may therefore reflect differences in the biology of various cancer types. Serum concentrations of YKL-40 were independent of serum carcino-embryonic antigen (CEA) in patients with colorectal cancer,20 serum CA-125 and CA15-3 in patients with ovarian cancer,19 serum HER2 in patients with metastatic breast cancer,14 serum prostate-specific antigen (PSA) in patients with metastatic prostate cancer,17 and serum lactate dehydrogenase (LDH) in patients with small-cell lung cancer,18 indicating that serum YKL-40 reflects other aspects of tumor growth and metastasis than these tumor markers.22
This study shows that serum concentrations of YKL-40 have a high sensitivity for gastric cancer, and determination of serum YKL-40 can be used as a test for the presence of gastric cancer. We demonstrated that over-expression of serum YKL-40 was frequently detected in patients with gastric cancer.
In conclusion, this study demonstrated that YKL-40 is expressed in gastric cancer. Further studies investigating serum YKL-40 expression may provide valuable information about its role in gastric cancer. Additionally, serum concentrations of YKL-40 may be useful in the early diagnosis of gastric cancer.
Table 3.
Mean values of serum YKL-40 levels grouped by age in patients with gastric cancer.
| Serum YKL-40 levels (μ/l) in patients with gastric cancer | |
|---|---|
| <50 years | 87 ± 35 |
| ≥50 years | 132 ± 29 |
| P-value | <0.001 |
Note: Data are presented as the means ± SD.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Samantaray S, Sharma R, Chattopadhyaya TK, et al. Increased expression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2004;130:37–44. doi: 10.1007/s00432-003-0500-4. [DOI] [PubMed] [Google Scholar]
- 4.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–10. [PubMed] [Google Scholar]
- 5.Renkema GH, Boot RG, Au FL, et al. Chitotriosidase, a chitinase, and the 39 kda human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251:504–9. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- 6.Shackelton LM, Mann DM, Millis AJ. Identification of a 38 kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem. 1995;270:13076–83. doi: 10.1074/jbc.270.22.13076. [DOI] [PubMed] [Google Scholar]
- 7.Volck B, Price PA, Johansen JS, et al. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Physicians. 1998;110:351–60. [PubMed] [Google Scholar]
- 8.Rehli M, Krause SW, Anderseen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3LI), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–5. doi: 10.1006/geno.1997.4778. [DOI] [PubMed] [Google Scholar]
- 9.Boot RG, van Achterberg TA, van Aken BE, et al. Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol. 1999;19:687–94. doi: 10.1161/01.atv.19.3.687. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa KC, Millis AJ. Gp-38k (CHI3LI) is a novel adhesion and migration factor for vascular cells. Exp Cell Res. 2003;287:79–87. doi: 10.1016/s0014-4827(03)00069-7. [DOI] [PubMed] [Google Scholar]
- 11.Nyirkos P, Golds EE. Human synovial cells secrete a 39 kDa protein similar to a bovine mammary protein expressed during the non-lactating period. Biochem J. 1990;269:265–8. doi: 10.1042/bj2690265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Ceuninck F, Gaufillier S, Bonnaud A, Sabatini M, Lesur C, Pastoureau P. YKL-40 induces proliferative events in cultured chondrcytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem Biophys Res Commun. 2001;285:926–31. doi: 10.1006/bbrc.2001.5253. [DOI] [PubMed] [Google Scholar]
- 13.Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15:194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- 14.Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9:4423–34. [PubMed] [Google Scholar]
- 15.Schmidt H, Johansen JS, Gehl J, Geertsen PF, Fode K, von der Maase H. Elevated serum level of YKL-40 is an independent prognostic factor for poor survival in patients with metastatic melanoma. Cancer. 2006;106:1130–9. doi: 10.1002/cncr.21678. [DOI] [PubMed] [Google Scholar]
- 16.Tanwar MK, Gilbert MR, Holland EC. Gene expression microarray analysis reveals YKL-40 to be a potential serum marker for malignant character in human glioma. Cancer Res. 2002;62:4364–8. [PubMed] [Google Scholar]
- 17.Brasso K, Christensen IJ, Johansen JS, et al. Prognostic value of PINP, bone alkaline phosphotase, CTX-1, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66:503–13. doi: 10.1002/pros.20311. [DOI] [PubMed] [Google Scholar]
- 18.Johansen JS, Drivsholm L, Price PA, Christensen IJ. High serum YKL-40 level in patients with small cell lung cancer is related to early death. Lung Cancer. 2004;46:333–40. doi: 10.1016/j.lungcan.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Dupont J, Tanwar MK, Thaler HT, Fleisher M, et al. Early detection and prognosis of ovarian cancer using serum YKL-40. J Clin Oncol. 2004;22:3330–9. doi: 10.1200/JCO.2004.09.112. [DOI] [PubMed] [Google Scholar]
- 20.Cintin C, Johansen JS, Christensen IJ, Price PA, Sorensen S, Nielsen HJ. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer. 2002;95:267–74. doi: 10.1002/cncr.10644. [DOI] [PubMed] [Google Scholar]
- 21.Johnsen JS. High serum concentration of YKL-40 is associated with short survival in patients with acute myeloid leukemia. Clin Cancer Res. 2005;11:8644–52. doi: 10.1158/1078-0432.CCR-05-1317. [DOI] [PubMed] [Google Scholar]
- 22.Johansen JS, Schultz NA, Jensen BV. Plasma YKL-40: a potential new cancer biomarker? Future Oncol. 2009 Sep;5(7):1065–82. doi: 10.2217/fon.09.66. Review. [DOI] [PubMed] [Google Scholar]
- 23.Junker JN, Johansen JS, Andersen CB, Kristjansen PE. Expression of YKL-40 by peritumoral macropahges in human small cell lung cancer. Lung Cancer. 2005;48:223–31. doi: 10.1016/j.lungcan.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Roslind A, Johansen JS, Junker JN, et al. YKL-40 expression in benign and malignant lesions of the breast: a methodological study. Appl Immunohistochem Mol Morphol. doi: 10.1097/01.pai.0000213146.77772.6a. [DOI] [PubMed] [Google Scholar]
- 25.Rehli M, Niller HH, Ammon C, et al. Transcriptional regulation of CHI3LI, a marker gene for late stages of macrophage differentiation. J Biol Chem. 2003;278:44058–67. doi: 10.1074/jbc.M306792200. [DOI] [PubMed] [Google Scholar]
- 26.Houston DR, Recklies AD, Krupa JC, van Aalten DM. Structure and ligand-induced conformational change of the 39 kDa glycoprotein from human articular chondrocytes. J Biol Chem. 2003;278:30206–12. doi: 10.1074/jbc.M303371200. [DOI] [PubMed] [Google Scholar]
- 27.Fusetti F, Pijining T, Kalk KH, Bos E, Dijkstra BW. Crystla structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem. 2003;278:37753–60. doi: 10.1074/jbc.M303137200. [DOI] [PubMed] [Google Scholar]
- 28.Johansen JS, Hvolris J, Hansen M, Backer V, Lorenzen I, Price PA. Serum YKL-40 levels in healthy children and adults. Comparison with serum synovial fluid levels of YKL-40 in patients with osteoarthritis or trauma of the knee joint. Br J Rheumatol. 1996;35:553–9. doi: 10.1093/rheumatology/35.6.553. [DOI] [PubMed] [Google Scholar]
- 29.Harvey S, Weisman M, O’Dell J, et al. Chondrex: new marker of joint disease. Clin Chem. 1998;44:509–16. [PubMed] [Google Scholar]
- 30.Johansen JS, Cintin C, Jorgensen M, Kamby C, Price PA. Serum YKL-40 a new potential marker of prognosis and location of metastases of patients with recurrent breast cancer. Eur J Cancer. 1995;31A:1437–42. doi: 10.1016/0959-8049(95)00196-p. [DOI] [PubMed] [Google Scholar]
- 31.Johansen JS, Christensen IJ, Riisbro R, et al. High serum YKL-40 levels in patients with primary breast cancer is related to short recurrence free survival. Breast Cancer Res Treat. 2003;80:15–21. doi: 10.1023/A:1024431000710. [DOI] [PubMed] [Google Scholar]
- 32.Cintin C, Johansen JS, Christensen IJ, Price PA, Sorensen S, et al. Serum YKL-40 and colorectal cancer. Br J Cancer. 1999;79:1494–9. doi: 10.1038/sj.bjc.6690238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geertsen P, Johansen JS, von der Maase H, Jensen BV, Price PA. High pretreatment serum level of YKL-40 is related to short survival in patients with advanced renal cell carcinoma treated with high dose continuous intravenous infusion of IL-2 meeting proceedings of American Society for Clinical Oncology ASCO. 2003;22 abstract 1603. [Google Scholar]
- 34.Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15(2):194–9. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- 35.Shao R, Hamel K, Petersen L, et al. YKL-40, a secreted glycoprotein, promotes tumor angipgenesis. Oncogene. 2009 doi: 10.1038/onc.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Recklies AD, White C, Ling H. The chitinase-3 like protein human cartilage glycoprotein 39 stimulates proliferation of human connective tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signaling pathways. Biochem J. 2002;365:119–26. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]