Abstract
It has been suggested recently that masticatory muscle size reduction in humans resulted in greater encephalization through decreased compressive forces on the cranial vault. Following this logic, if masticatory muscle size were increased, then a reduction in brain growth should also occur. The present study was designed to test this hypothesis using a myostatin (GDF-8) knockout mouse model. Myostatin is a negative regulator of skeletal muscle growth, and individuals lacking this gene show significant hypermuscularity. Sixty-two (32 wild-type and 30 GDF-8 −/− knockout), 1, 28, 56 and 180 day old CD-1 mice were used. Body and masseter muscle weights were collected following dissection and standardized lateral and dorsoventral cephalographs were obtained. Cephalometric landmarks were identified on the radiographs and cranial volume was calculated. Mean differences were assessed using a two-way ANOVA. KO mice had significantly greater body and masseter weights beginning at 28 days compared to WT controls. No significant differences in cranial volumes were noted between KO and WT. Muscle weight was not significantly correlated with cranial volume in 1, 28, or 180 day old mice. Muscle weights exhibited a positive correlation with cranial volume at 56 days. Results demonstrate that masticatory hypermuscularity is not associated with reduced cranial volume. In contrast, there is abundant data demonstrating the opposite, brain growth determines cranial vault growth and masticatory apparatus only affects ectocranial morphology. The results presented here do not support the hypothesis that a reduction in masticatory musculature relaxed compressive forces on the cranial vault allowing for greater encephalization.
Keywords: Myostatin, hypermuscularity, brain evolution, knockout model
Introduction
Historically it has been suggested that when masticatory muscle reduction took place, it allowed for the possibility of brain expansion. The mechanism was implied to be involved in the selection for smaller teeth or smaller faces (Weidenreich, 1941a; Tobias, 1971; Polanski and Franciscus, 2006). Experimental research, however, has generally held that the growth and morphology of the cranial vault, although influenced by masticatory musculature, is largely determined by the growth of the brain (Moss and Young, 1960; Riesenfeld, 1967; Sun et al., 2004; Sardi et al., 2007). These data are supported by a propensity of research and observation that the human final shape of the cranium is heavily influenced and determined by early expansion of the brain (Weidenreich, 1941b; Moss, 1969; Moss and Salentijn, 1969; Mooney et al., 2002; Richtsmeier et al., 2006).
Musculature, specifically masticatory musculature, has been reported to strongly influence craniofacial growth. Experimentally increasing the loading of the jaws due to masticatory muscle hyperfunction may lead to increased sutural growth and bone apposition (Herring, 1993; Herring and Teng, 2000; Shibazaki et al., 2007; Herring, 2008). These growth alterations appear to occur both by tension and compression (Sun et al., 2004; Herring, 2008). However, compression has also been suggested to cause cessation of growth at the calvarial sutures (Oppenheimer et al., 2009). Cartilage is much less relatively affected by muscle loads compared to sutures and periodontal ligaments (Bjork and Skieller, 1983), and in humans the masticatory muscle phenotype is associated with variations in the vertical growth of the face, but not with sagittal growth (Rowlerson et al., 2005).
Recent work has also shown that the gene Myostatin is a negative regulator of skeletal muscle growth, and that the myostatin sequence has remained highly conserved throughout evolution (Lee, 2007; Tsuchida, 2008a, 2008b). Recently Stedman et al. (2004) suggested that a frameshift mutation in the myosin heavy chain (MYH), specifically Masticatory MHC, may have indirectly had a great influence on brain development during human evolution. These authors suggest that this mutation had significant functional impacts on mastication. Specifically a reduction in the size and contractile force of the masticatory muscles would lead to reduced stress across patent sutures, thus removing an evolutionary constraint on greater encephalization (2004). However, McCollum et al. (2006) (in response to (Stedman et al., 2004), downplayed the role of Masticatory MHC in the evolution of the larger hominid brain, and suggested that the myosin mutation played little, if any, role in the craniofacial and brain evolution of Homo. McCollum et al. (2006) suggested that such an abrupt mutation in Masticatory MHC, and its forced corresponding behavioral shift in diet, would have been detrimental to survival, and thus would have had a low threshold for natural selection.
The Stedman et al. (2004) hypothesis is that a decrease in the masticatory musculature would result in a relaxation of compressive forces on the cranial vault and thus allow an increase in brain size (i.e. cranial volume). Following this logic, it could also be hypothesized that an increase in masticatory musculature should also significantly inhibit or decrease brain size and cranial volume, presumably through an increase in compressive muscular forces on the cranial vault. A knock-out mouse model for myostatin, specifically the GDF-8 sequence (GDF-8 −/−) has been developed (McPherron et al., 1997; Byron et al., 2006; Hamrick et al., 2006a; Hamrick et al., 2006b; Nicholson et al., 2006) which will allow us to test this hypothesis. The GDF-8 knockout model shows significant increase in postnatal skeletal muscle mass, up to twice as in the wild type, control mice. This has important implications for behavior in the knockout mice, specifically concerning jaw musculature, producing relatively greater bite forces than wild type mice (Byron et al., 2004; Byron et al., 2006; Byron et al., 2008). The present study was designed to test the hypothesis that an increase in masticatory musculature should significantly inhibit cranial volume in a hypermuscular murine GDF-8 knock-out model.
Material and Methods
Fifteen (8 wild-type and 7 GDF-8 −/− knockout) 1 day old, fourteen (6 wild-type and 8 GDF-8 −/− knockout) 28 day old (weaning), seventeen (9 wild-type and 8 GDF-8 −/− knockout) 56 day old, and 16 (9 wild-type and 7 GDF-8 −/− knockout), 180 day old male CD-1 mice were utilized (Table 1). Myostatin-deficient mice were produced by deletion of the C-terminal region of the myostatin gene in embryonic stem cells as described by McPherron et al. (1997). All mice were housed together and given food (Harlan TekLad hard rodent chow, Tampa, FL) and water ad libitum. Wild-type control and GDF-8 −/− myostatin-deficient mice were bred and maintained in the Department of Cellular Biology & Anatomy Medical College of Georgia.
Table 1.
Description of Sample
| Group | Age | n |
|---|---|---|
| WT | 1 day | 8 |
| GDF −/− | 7 | |
| WT | 28 day | 6 |
| GDF −/− | 8 | |
| WT | 56 day | 9 |
| GDF −/− | 8 | |
| WT | 180 day | 9 |
| GDF −/− | 7 |
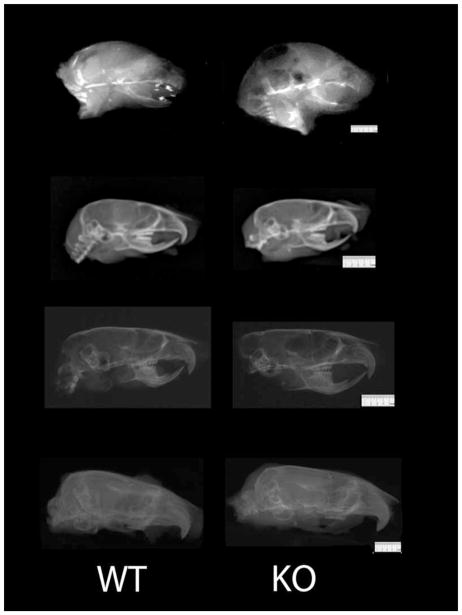
Mice were euthanized at one day postnatal, 28, 56, or 180 days of age at skeletal maturity by CO2 overdose and body weights were immediately recorded. All work was approved by the Institutional Care and Use Committee at Medical College of Georgia. Mouse heads were disarticulated and the masseter muscles were then dissected and weighed to the nearest 0.001g. Masseter muscles muscle are absolutely larger than temporalis and thus easier to identify and easy to dissect in mice. Thus, this muscle was chosen for study. Heads were then fixed in 10% neutral buffered formalin for 24 hours, and then transferred to 70% ethanol for radiographic analysis. Lateral and dorso-ventral radiographs were taken using a Faxitron MX-20 (Faxitron X-Ray Corporation, Lincolnshire, IL) at 35kV for 250 seconds at 5X magnification with X-OMAT V diagnostic film (Kodak, Rochester, NY). Radiographs were scanned on an AGFA DuoScan using AGFA FotoLook 3.2 software (Figure 1).
Figure 1. Lateral Cephalographs.
Representative Wild Type and Myostatin Knockout Mice Lateral Cephalographs from top to bottom: 1 day, 28 days, 56 days, and 180 days post-natal.
Cephalometric landmarks were identified on the radiographs and cranial volume (cranial vault length*width*height) was calculated (Dekaban and Lieberman, 1964; Haack and Meihoff, 1971; Rogers, 1984; Kamdar et al., 2009), by the first author on two occasions. Intra-rater reliability was r= 0.98; p<0.01. Cranial vault length was measured from the most superior point of the parietal bones (Pa) to Nasion (Na). Cranial vault width was measured from the most lateral point of the calvarium (CW1-CW2). Cranial vault height was measured from opisthion (Op) to the frontal-parietal suture (Fp) (Figure 2). Partial correlations were also calculated for masseter weights and cranial volume. The researcher who measured the cephalometrics and masseter weights was blinded to study group. Mean differences were assessed using a two-way ANOVA in SPSS 15 (Chicago, IL). Significance is defined as < 0.05.
Figure 2. Cepahlometric Landmarks.
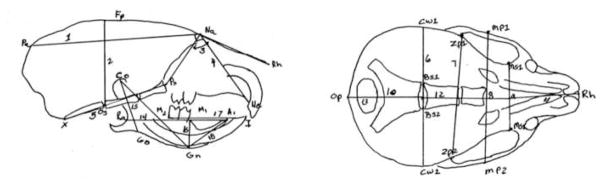
Landmarks of interest are superior point of the parietal bones (Pa), Nasion (Na), the most lateral point of the calvarium (CW1-CW2), opisthion (Op), and frontal-parietal suture (Fp).
Results
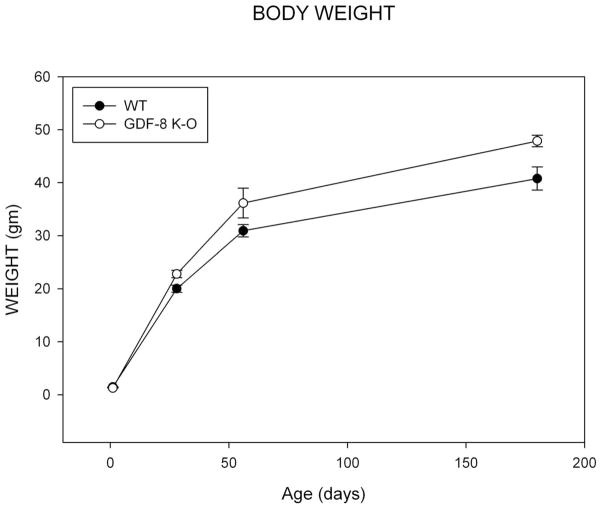
Results for somatic growth show that the mean body weight in 1 day old wild-type (WT) and GDF-8 knockout mice was identical at 1 day of age (Figure 3). The bodyweight begin to deviate at 28 days, weaning, and the knockout mice have greater bodyweights at all later time points. The assumption of normality and homogeneity of variance were met. There was no significant group by age effect for mean body weight (F=2.12; p=ns). There was a significant main effect by age (F=289.55, p<0.001) with bodyweight increasing at each age, p<0.001. There was a significant main effect by group (F=11.79, p=0.001) with the knockout mice exhibiting greater bodyweights.
Figure 3. Bodyweight.
Mean Bodyweight for Wild Type and Myostatin Knockout Mice by Age.
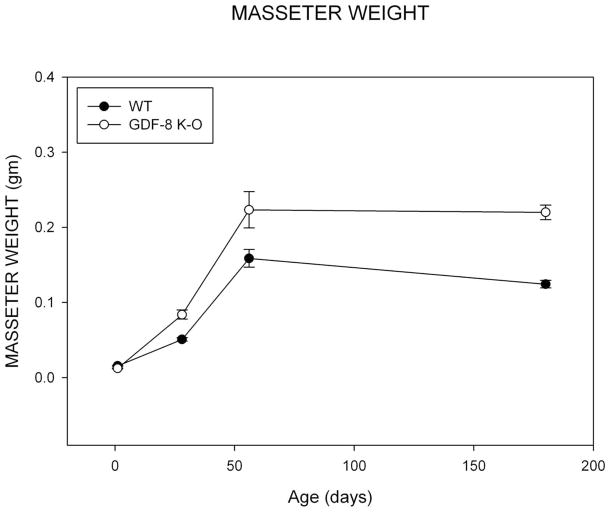
Results for masseter muscle growth revealed that mean masseter muscle weight at 1 day of age was less for GDF-8 knockout mice compared to wild-type control mice (Figure 4). In contrast, the groups begin to deviate for this measure at 28, and show great differences at 56 days of age consistent with the hypermuscular morphology of these knockout mice. The perceived decrease at 180 days of age for mean masseter muscle weights for both groups appears to be due to inter-individual variation in the samples. The assumption of homogeneity of variance was violated for this variable. A reciprocal square root power transformation allowed for this assumption and normality to be met. A significant group by age effect was noted for mean masseter muscle weight (F=26.72; p<0.001). There was a significant main effect by age (F=1190.23, p<0.001). Post-hoc bonferonni analysis revealed significant difference for all comparisons p<0.001, with the exception of 56 to 180 days which did not differ significantly, p=ns. There was a significant main effect by group (F=8.89, p=0.004) with the knockout mice exhibiting greater masseter weights. Relative masseter muscle weights, corrected for body mass, exhibit the same relationship (Appendix 1).
Figure 4. Masseter Muscle.
Mean Masseter Muscle weight for Wild Type and Myostatin Knockout Mice by Age.
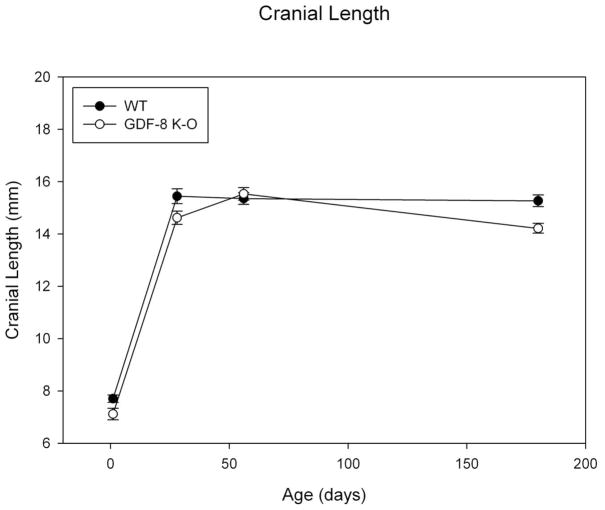
Results for cranial length revealed an increase between day 1 to 28 for both groups where it appears growth was completed for this dimension (Figure 5). There do not appear to be strong differences by group for this variable, however data suggest some variability. The assumptions of normality and homogeneity of variance were met. There was no significant group by age effect for mean cranial length (F=2.64; p=ns). There was a significant main effect by age (F=550.27, p<0.001). Post-hoc bonferonni analysis revealed significant differences for all age comparisons to day 1 (day 1 values being the smallest, p<0.001). In addition, day 56 was significantly different from 180 days, p=0.020, suggesting variation in cranial length in this sample overall. There was a significant main effect by group (F=12.36, p<0.001) with the wild type animals having greater mean cranial length measures.
Figure 5. Cranial Length.
Mean Cranial Length measure for Wild Type and Myostatin Knockout Mice by Age.
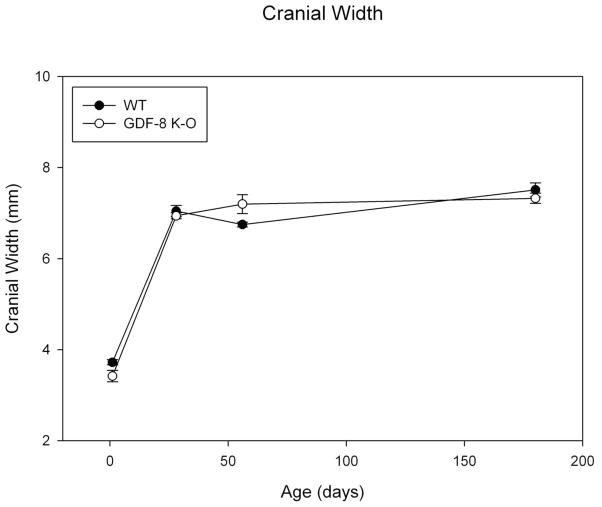
Results for cranial width are similar to that for length with a great increase in this dimension between day 1 and 28 for both groups (Figure 6). There do not appear to be strong group differences for this variable, but again we note variability. The assumptions of normality and homogeneity of variance were met. There was a significant group by age effect noted for mean cranial width (F=3.68; p=0.018). There was a significant main effect by age (F=354.56, p<0.001). Post-hoc bonferonni analysis revealed significant differences for all age comparisons to day 1 (day 1 values being the smallest, p<0.001). In addition, day 56 was significantly different from 180 days, p=0.003, suggesting variation in cranial width in this sample overall. There was no significant main effect by group (F=0.156, p=ns).
Figure 6. Cranial Width.
Mean Cranial Width measure for Wild Type and Myostatin Knockout Mice by Age.
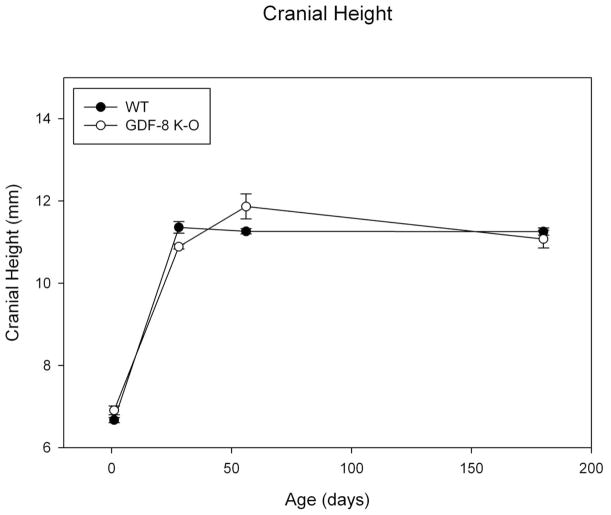
Results for cranial height are similar to that for the other cranial dimensions with a great increase in this dimension between day 1 and 28 for both groups (Figure 7). There do not appear to be strong group differences for this variable, but again we note variability. The assumptions of normality and homogeneity of variance were met. There was a significant group by age effect noted for mean cranial height (F=4.85; p=0.005). There was a significant main effect by age (F=438.95, p<0.001). Post-hoc bonferonni analysis revealed significant differences for all age comparisons to day 1 (day 1 values being the smallest, p<0.001). In addition, day 28 was significantly different from day 56 mean values, p=0.033. There was no significant main effect by group (F=0.196, p=ns).
Figure 7. Cranial Height.
Mean Cranial Height measure for Wild Type and Myostatin Knockout Mice by Age.
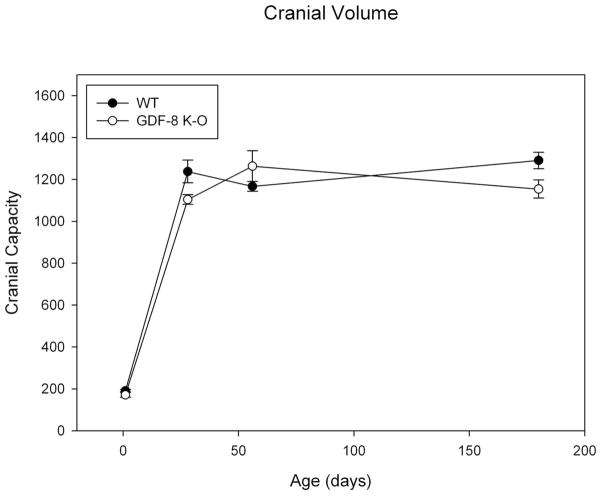
Results for cranial volume revealed that mean cranial volumes greatly increase between 1 and 28 days but appear to level off after 28 days (Figure 8). The assumptions of homogeneity of variance were met. There was a significant group by age effect noted for mean cranial volume (F=4.445, p = 0.008). There was a significant main effect by age (F=354.54, p<0.001). Post-hoc bonferonni analysis revealed significant differences for all age comparisons to day 1 (day 1 values being the smallest, p<0.001). There was no significant main effect by group (F=3.36, p=ns).
Figure 8. Cranial Capacity.
Mean Cranial Capacity measure for Wild Type and Myostatin Knockout Mice by Age.
No significant relationships were noted between masseter muscle weight and cranial volume at 1 (r= 0.381; p>0.05), 28 (r=0.233; p>0.05), or 180 days of age (r= −0.458; p>0.05) for either group. There was a significant positive correlation at 56 days (r=.589, p=0.043).
Discussion
Myostatin is expressed early in development and as somite condensations become topographically organized it becomes restricted to the myotome layer. In fetal stages, transcripts are found throughout developing muscle and have a profound effect on the regulation of differentiation pathways for mesenchymal calls. When myostatin activity is blocked, more cells are committed to myoblast and myotube formation, which results in significantly elevated numbers of muscle fibers in adult muscle. In some muscles this increase in fiber number may be as much as 90 percent (McPherron et al., 1997; McPherron and Lee, 1997).
The results presented here do not support the hypothesis that an increase in musculature, specifically masticatory musculature, inhibits the expansion of the calvarial dimensions. Data presented here show marked differences in somatic growth as measured by bodyweight and masticatory musculature as measured by gross masseter muscle weight for the myostatin knockout mice. It is only at birth where the knockout mice exhibit smaller somatic and musculature growth measures. We have previously observed that in the myostatin deficient model as having a lengthened hyperplastic period of muscle development, allowing for increased proliferation of myoblast and myotube populations. The model, thus has a later onset for the muscular hypertrophic stage, by weaning, allowing for greater growth potential due to increased numbers of myofibers (Vecchione et al., 2010). We do not observe corresponding differences to cranial length, width, height, or overall cranial volume measures at any age. Our data suggest that the hypermuscular myostatin knockout mice have similar calvarial growth patterns as wild type mice.
What is interesting about these data is they provide some temporal information concerning neural expansion for these mice, as well as data on muscle development. It appears that calvarial expansion is completed by about 28 days, here weaning, in these models. We observe variation in calvarial measures after 28 days indicative of inter-individual variation. Bodyweight appears to increase at each time interval out to 180 days. However, we observe that masseter muscle weight only exhibits increases to 56 days, suggesting that in these mice calvarial growth and development is completed prior to somatic growth similar to that observed in all mammals.
These results suggest that there is no causal relationship between masticatory muscularity and cranial volume (i.e., brain size) as predicted by Stedman et al., (2004). In contrast, there is abundant data demonstrating just the opposite, that brain growth determines cranial vault growth and morphology and that the masticatory apparatus only affects ectocranial morphology including such structures as sutural interdigitation and the position of the temporal lines on the cranial vault (Moss and Young, 1960; Riesenfeld, 1967; Byron et al., 2004; Sun et al., 2004; Richtsmeier et al., 2006; Sardi et al., 2007; Vecchione et al., 2007; Byron et al., 2008; Byron, 2009; Vecchione et al., 2010; Mooney and Richtmeier, In Press). It has been shown that removal of the temporalis and masseter muscles, although limiting growth in the length of the skull and other postcranial dimensions due to its detrimental effect on mastication and dietary restrictions, did not significantly affect overall brain size (Riesenfeld, 1967). In addition, antero-posterior growth restrictions could be mediated by dietary supplements, suggesting that cranial form is determined early in the width dimension, and that brain size was relatively unaffected by surgical manipulation or differential dietary conditions (Riesenfeld, 1967; Riesenfeld, 1973).
These findings suggest that a removal of lateral compressive forces on the cranium (by removal of the masticatory musculature) did not influence brain size, which parallels our results that increasing compressive forces (from masticatory hypermusculature) also had no influence on cranial volume and brain size. These findings suggest independence in the growth of these two regions. Byron (2006), also showed that in CD-1 mice, skull width achieved maximal growth significantly earlier in age compared to suture interdigitation, and concluded that “mechanical forces resulting from mastication play a more pivotal role in patterning cranial sutures than encephalization” (p. 560). Byron et al (2004) also showed that increased musculature in the GDF-8 −/− knock out mouse model, with its resulting increased bite forces, did affect bone formation along the cranial sutures, creating increased sutural interdigitation as a result. Vecchione et al. (2007; 2010), also in the GDF-8 −/− knockout mouse model, reported significantly different craniofacial morphology in the hypermuscular model compared to wild-type controls. In particular, they noted significantly shorter cranial vault length, a brachycephalic phenotype, shorter maxillary length, and a longer mandibular body length in myostatin deficient mice. These findings were probably a result of altered biomechanical masticatory stress in the knockout mice. Byron et al. (2008) demonstrated that the GDF-8 −/− knockout mice possess an anterosuperiorly and dorsoventrally compressed temporal bone as part of the brachycephalic phenotype and Vecchione et al., (2010) found significantly shorter craniofacial lengths in the knockout model which suggested that muscle function plays a significant role in the ontogeny of craniofacial growth. However, these authors also suggest that their model demonstrates that hypermuscularity does have a significant influence over adult skeletal phenotype, but at least for craniofacial growth, early changes in cellular growth and differentiation have an equal impact on final form.
Encephalization does appear to have a large genetic component. Genes like microcephalin (MCPH1. known for cell damage response) and abnormal spindle-like microcephaly-associated (ASPM, known for its regulation of neural cell proliferation) both appear to be more recent single nucleotide polymorphisms from the human lineage (Tang, 2006). Other theories suggest that dietary changes led to somatic growth and metabolic changes during the process of brain enlargement during human evolution (Foley and Lee, 1991; Leonard et al., 2003; Leonard et al., 2007). This is based on the observation that within primates, brain size is positively correlated with dietary quality. Humans are relatively undermuscled compared to other primates and display a large amount of fat, particularly during the ages of active encephalization. It is suggested this fat supplies the addition energy necessary for human encephalization and reduces energy requirements throughout the rest of the body (Leonard et al., 2003).
McCollum et al. (2006) suggested that the Masticatory MHC mutation played little, if any, role in the craniofacial and brain evolution of Homo. An abrupt mutation in Masticatory MHC, and its forced corresponding behavioral shift in diet, would have been detrimental to survival, and thus would have had a low threshold for natural selection. These authors (McCollum et al., 2006) also suggested that Stedman et al. (2004) overlooked the observation that muscles can upregulate, downregulate, or switch their expression from type I to type II fibers or vice versa in response to changing physiological conditions. Stedman et al. (2004) ignored muscular response to aging, hormones levels, and diet, which could cause large intra-specific variation and larger inter-specific variation. In addition, human brain growth is 80–95% completed by the eruption of the first permanent tooth (Tobias, 1971; Enlow and Hans, 1996), making masticatory forces an unlikely candidate to inhibit the rapidly expanding neurocranium.
This study was limited by the cross sectional nature of its design. GDF8 was used as a proxy for Masticatory MHC due to availability of the model and lack of a better homologous gene in mice, and a resulting hypermuscular morphology. MYH may have direct effects on brain growth and development, but probably not through control of muscle size.
Conclusion
The results presented here do not support the hypothesis that a reduction in masticatory musculature relaxed compressive forces on the cranial vault and allowed the brain to grow bigger. Given the results of our experimental design, we reject the hypothesis that masticatory forces restrict brain growth. Alternative hypotheses should still be explored.
Acknowledgments
This work was supported in part by NIH grant# AR 049717. This paper was presented in part at the 37th General Session of the American Association of Dental Research (AADR), Dallas April, 2008, and the 77th Annual Meeting of the American Association of Physical Anthropologists (AAPA), Columbus April 2008.
Appendix
| Group | Age | Bodyweight | p value | Masseter Weight | p value | Relative Masseter Weight | p value |
|---|---|---|---|---|---|---|---|
| WT | 1 | 1.4704 +/− 0.0276 | p=0.001 | 0.0156 +/−0.0006 | p=0.001 | 0.0107 +/−0.0005 | p=0.067 |
| KO | 1 | 1.2879 +/−0.0346 | 0.0123 +/−0.0005 | 0.0095 +/−0.0003 | |||
| WT | 28 | 20.0200 +/−0.6721 | p=0.016 | 0.0508 +/−0.0024 | p=0.001 | 0.0026 +/−0.0002 | p=0.001 |
| KO | 28 | 22.7925 +/−0.6895 | 0.0839 +/−0.0061 | 0.0037 +/−0.0002 | |||
| WT | 56 | 30.9289 +/−1.1583 | p=0.092 | 0.1588 +/−0.0118 | p=0.025 | 0.0051 +/−0.0003 | p=0.027 |
| KO | 56 | 36.1500 +/−2.7983 | 0.2234 +/−0.0241 | 0.0061 +/−0.0003 | |||
| WT | 180 | 40.7767 +/−2.1862 | p=0.019 | 0.1244 +/−0.0050 | p<0.001 | 0.0031 +/−0.0002 | p=0.001 |
| KO | 180 | 47.8657 +/−1.0860 | 0.2200 +/−0.0098 | 0.0059 +/−0.0002 |
Contributor Information
James Cray, Jr., Department of Surgery, Division of Plastic and Reconstructive Surgery, University of Pittsburgh and Pediatric Craniofacial Biology Laboratory, Children’s Hospital of Pittsburgh
Jared Kneib, University of Pittsburgh School of Dental Medicine
Lisa Vecchione, Pittsburgh Cleft-Craniofacial Research Center, Department of Surgery, Division of Plastic Surgery and Department of Orthodontics & Dentofacial Orthopedics, University of Pittsburgh, Pittsburgh, PA 15260.
Craig Byron, Department of Biology, Mercer University, Macon GA 31207
Gregory M. Cooper, Department of Surgery, Division of Plastic and Reconstructive Surgery, University of Pittsburgh and Pediatric Craniofacial Biology Laboratory, Children’s Hospital of Pittsburgh and Department of Engineering and Oral Biology, University of Pittsburgh, Pittsburgh PA 15260
Joseph E. Losee, Pittsburgh Cleft-Craniofacial Research Center, Department of Surgery, Division of Plastic Surgery, University of Pittsburgh, Pittsburgh, PA 15260
Michael I. Siegel, Department of Anthropology, Department of Orthodontics, University of Pittsburgh, Pittsburgh PA 15260
Mark W. Hamrick, Department of Cellular Biology & Anatomy, Medical College of Georgia, Augusta, GA, 30912
James J. Sciote, Department of Orthodontics, Temple University, Philadelphia, PA 19140
Mark P. Mooney, Department of Oral Biology, Departments of Anthropology, Department of Surgery, Division of Plastic Surgery, and Department of Orthodontics, University of Pittsburgh, Pittsburgh PA 15260
Bibliography
- Bjork A, Skieller V. Normal and abnormal growth of the mandible. A synthesis of longitudinal cephalometric implant studies over a period of 25 years. Eur J Orthod. 1983;5:1–46. doi: 10.1093/ejo/5.1.1. [DOI] [PubMed] [Google Scholar]
- Byron C, Maness H, Yu J, Hamrick M. Enlargement of the Temporalis Muscle and Alterations in the Lateral Cranial Vault. Int Comp Biol. 2008;48:338–344. doi: 10.1093/icb/icn020. [DOI] [PubMed] [Google Scholar]
- Byron CD. Cranial suture morphology and its relationship to diet in Cebus. J Hum Evol. 2009;57:649–655. doi: 10.1016/j.jhevol.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Byron CD, Borke J, Yu J, Pashley D, Wingard CJ, Hamrick M. Effects of increased muscle mass on mouse sagittal suture morphology and mechanics. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:676–684. doi: 10.1002/ar.a.20055. [DOI] [PubMed] [Google Scholar]
- Byron CD, Hamrick MW, Wingard CJ. Alterations of temporalis muscle contractile force and histological content from the myostatin and Mdx deficient mouse. Arch Oral Biol. 2006;51:396–405. doi: 10.1016/j.archoralbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dekaban A, Lieberman JE. Calculation of Cranial Capacity from Linear Dimensions. Anat Rec. 1964;150:215–219. doi: 10.1002/ar.1091500302. [DOI] [PubMed] [Google Scholar]
- Enlow D, Hans M. Essentials of Facial Growth. Ann Arbor: Needham Press; 1996. [Google Scholar]
- Foley RA, Lee PC. Ecology and energetics of encephalization in hominid evolution. Philos Trans R Soc Lond B Biol Sci. 1991;334:223–231. doi: 10.1098/rstb.1991.0111. discussion 232. [DOI] [PubMed] [Google Scholar]
- Haack DC, Meihoff EC. A method for estimation of cranial capacity from cephalometric roentgenograms. Am J Phys Anthropol. 1971;34:447–452. doi: 10.1002/ajpa.1330340316. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Pennington C, Webb CN, Isales CM. Resistance to body fat gain in ‘double-muscled’ mice fed a high-fat diet. Int J Obes (Lond) 2006a;30:868–870. doi: 10.1038/sj.ijo.0803200. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2006b;21:477–483. doi: 10.1359/JBMR.051203. [DOI] [PubMed] [Google Scholar]
- Herring SW. Epigenetic and functional influences on skull growth. In: Hanken J, Hall B, editors. The Skull. Chicago: University of Chicago Press; 1993. pp. 153–206. [Google Scholar]
- Herring SW. Mechanical influences on suture development and patency. Front Oral Biol. 2008;12:41–56. doi: 10.1159/0000115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Teng S. Strain in the braincase and its sutures during function. Am J Phys Anthropol. 2000;112:575–593. doi: 10.1002/1096-8644(200008)112:4<575::AID-AJPA10>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar MR, Gomez RA, Ascherman JA. Intracranial volumes in a large series of healthy children. Plast Reconstr Surg. 2009;124:2072–2075. doi: 10.1097/PRS.0b013e3181bcefc4. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS ONE. 2007;2:e789. doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WR, Robertson ML, Snodgrass JJ, Kuzawa CW. Metabolic correlates of hominid brain evolution. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:5–15. doi: 10.1016/s1095-6433(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Snodgrass JJ, Robertson ML. Effects of brain evolution on human nutrition and metabolism. Annu Rev Nutr. 2007;27:311–327. doi: 10.1146/annurev.nutr.27.061406.093659. [DOI] [PubMed] [Google Scholar]
- McCollum MA, Sherwood CC, Vinyard CJ, Lovejoy CO, Schachat F. Of muscle-bound crania and human brain evolution: the story behind the MYH16 headlines. J Hum Evol. 2006;50:232–236. doi: 10.1016/j.jhevol.2005.10.003. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney M, Richtmeier J. Cranial Sutures and Calvaria: Normal Development and Craniosynostosis. In: Mao J, Nah D, editors. Craniofacial Growth and Development. New York: Blackwell Munksgund Publishing; In Press. [Google Scholar]
- Mooney M, Siegel M, Smith T, Burrows A. Evolutionary Changes in the Cranial Vault and Base: Establishing the Primate Form. In: Mooney M, Siegel M, editors. Understanding Craniofacial Anomalies: The Etiopathogenesis of Craniosynostosis and Facial Clefting. New York: John Wiley and Sons; 2002. pp. 275–293. [Google Scholar]
- Moss ML. The differential roles of periosteal and capsular functional matrices in oro-facial growth. Rep Congr Eur Orthod Soc. 1969:193–205. [PubMed] [Google Scholar]
- Moss ML, Salentijn L. The capsular matrix. Am J Orthod. 1969;56:474–490. doi: 10.1016/0002-9416(69)90209-7. [DOI] [PubMed] [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Nicholson EK, Stock SR, Hamrick MW, Ravosa MJ. Biomineralization and adaptive plasticity of the temporomandibular joint in myostatin knockout mice. Arch Oral Biol. 2006;51:37–49. doi: 10.1016/j.archoralbio.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Oppenheimer AJ, Rhee ST, Goldstein SA, Buchman SR. Force-induced craniosynostosis in the murine sagittal suture. Plast Reconstr Surg. 2009;124:1840–1848. doi: 10.1097/PRS.0b013e3181bf806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanski JM, Franciscus RG. Patterns of craniofacial integration in extant Homo, Pan, and Gorilla. Am J Phys Anthropol. 2006;131:38–49. doi: 10.1002/ajpa.20421. [DOI] [PubMed] [Google Scholar]
- Richtsmeier JT, Aldridge K, DeLeon VB, Panchal J, Kane AA, Marsh JL, Yan P, Cole TM., 3rd Phenotypic integration of neurocranium and brain. J Exp Zoolog B Mol Dev Evol. 2006;306:360–378. doi: 10.1002/jez.b.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenfeld A. Biodynamics of Head Form and Cranio-Facial Relationships. Homo. 1967;18:233–251. [Google Scholar]
- Riesenfeld A. The effect of extreme temperatures and starvation on the body proportions of the rat. Am J Phys Anthropol. 1973;39:427–459. doi: 10.1002/ajpa.1330390311. [DOI] [PubMed] [Google Scholar]
- Rogers S. The Human Skull. Springfield, IL: Thomas; 1984. [Google Scholar]
- Rowlerson A, Raoul G, Daniel Y, Close J, Maurage CA, Ferri J, Sciote JJ. Fiber-type differences in masseter muscle associated with different facial morphologies. Am J Orthod Dentofacial Orthop. 2005;127:37–46. doi: 10.1016/j.ajodo.2004.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi ML, Ventrice F, Ramirez Rozzi F. Allometries throughout the late prenatal and early postnatal human craniofacial ontogeny. Anat Rec (Hoboken) 2007;290:1112–1120. doi: 10.1002/ar.20581. [DOI] [PubMed] [Google Scholar]
- Shibazaki R, Dechow PC, Maki K, Opperman LA. Biomechanical strain and morphologic changes with age in rat calvarial bone and sutures. Plast Reconstr Surg. 2007;119:2167–2178. 2179–2181. doi: 10.1097/01.prs.0000260705.70329.38. [DOI] [PubMed] [Google Scholar]
- Stedman HH, Kozyak BW, Nelson A, Thesier DM, Su LT, Low DW, Bridges CR, Shrager JB, Minugh-Purvis N, Mitchell MA. Myosin gene mutation correlates with anatomical changes in the human lineage. Nature. 2004;428:415–418. doi: 10.1038/nature02358. [DOI] [PubMed] [Google Scholar]
- Sun Z, Lee E, Herring SW. Cranial sutures and bones: growth and fusion in relation to masticatory strain. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:150–161. doi: 10.1002/ar.a.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL. Molecular genetic determinants of human brain size. Biochem Biophys Res Commun. 2006;345:911–916. doi: 10.1016/j.bbrc.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Tobias P. The Brain in Hominid Evolution. New York: Columbia University Press; 1971. [Google Scholar]
- Tsuchida K. Myostatin inhibition by a follistatin-derived peptide ameliorates the pathophysiology of muscular dystrophy model mice. Acta Myol. 2008a;27:14–18. [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K. Targeting myostatin for therapies against muscle-wasting disorders. Curr Opin Drug Discov Devel. 2008b;11:487–494. [PubMed] [Google Scholar]
- Vecchione L, Byron C, Cooper GM, Barbano T, Hamrick MW, Sciote JJ, Mooney MP. Craniofacial morphology in myostatin-deficient mice. J Dent Res. 2007;86:1068–1072. doi: 10.1177/154405910708601109. [DOI] [PubMed] [Google Scholar]
- Vecchione L, Miller J, Byron C, Cooper G, Barbano T, Cray J, Jr, Losee J, Hamrick M, Sciote J, Mooney M. Age Related Changes in Craniofacial Morphology in GDF-8 (Myostatin) Deficient Mice. Anat Rec A Discov Mol Cell Evol Biol. 2010;291:32–41. doi: 10.1002/ar.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenreich F. The brain and it’s role in the phylogenetic transformation of the human skull. Trans Am Philos Soc. 1941a;31:321–442. [Google Scholar]
- Weidenreich F. The brain and its role inthe phylogenetic transformation of the human skull. Trans American Philosophical Society. 1941b;31:321–442. [Google Scholar]