Abstract
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the seventh most common in women. This cancer varies widely in incidence throughout the world, with rising incidence in Egypt. HCC is considered the second most frequent cause of cancer incidence and mortality among men in Egypt. This study aimed to estimate the serum levels of nitric oxide (NO) and glutathione reductase in order to evaluate their role as oxidative status markers in HCC development and progression. For this purpose, serum levels of these parameters were assessed in 50 HCC patients, and 30 cirrhotic patients in addition to 15 healthy subjects as a control group. In the present study, glutathione reductase activity showed a significant increase in HCC as compared to the control group (P= 0.019). On the other hand, no significant difference was observed between the cirrhotic and HCC patients (P= 0.492). Serum NO was significantly higher in patients with HCC than in cirrhotic patients (P= 0.001) or the control group (P= 0.001), with a sensitivity of (74%) and specificity of (88.89%) at a cut-off level of 614.1 μmol/l. While AFP, alpha-fetoprotein, at a cutoff level of 200 ng/ml had a sensitivity of (52%), the specificity was (100%). Indeed, nitric oxide was high in 62.5% of AFP-negative HCC patients. In conclusion, glutathione reductase has no role in HCC diagnosis. However, nitric oxide is a potential diagnostic marker for HCC. The simultaneous determination of serum nitric oxide and AFP gave significant improvement in the detection of HCC patients compared to that of AFP alone.
Keywords: Nitric oxide, Glutathione reductase, Hepatocellular carcinoma, Alpha-fetoprotein
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the seventh most common in women [1]. The incidence of this particular cancer varies throughout the world, with rising incidence in Egypt [2]. In Egypt, HCC was considered as a common malignant tumor, accounting for about 4.7% of chronic liver disease patients with most HCC patients being presented at a late stage in approximately 85% of cases [3].
Detection of HCC at early stages is critical for good clinical outcomes as the prognosis of HCC patients is very poor when diagnosed at late stages. Although serum AFP is the most established tumor marker in HCC and considered as the gold standard to which other markers are compared, it was found to be normal in about 30% of the patients, especially in early stages [4]. Ultrasonography is an important tool for the diagnosis of HCC, however it depends on the operator’s experience, and accordingly the validity of other biomarkers in the diagnosis of HCC needs to be investigated [5].
Nitric oxide (NO) plays an important role in HCC development and its progression. During the pre-HCC period, viral infection (hepatitis B and C) or other unforeseen circumstances may lead to uncontrolled, prolonged, and/or massive production of NO by inducible nitric oxide synthase (iNOS) in the liver [6]. At the same time, NO released by the tumor cells might enhance angiogenesis, which can lead to accelerated growth of the primary tumor, as well as facilitate the process of metastasis [6].
The glutathione antioxidant system in cirrhosis and hepatocellular carcinoma is imbalanced and supports the hypothesis that oxidative stress plays an important role in the development of those liver diseases [7]. Glutathione reductase is highly specific for the oxidized form of glutathione (GSSG) and could be used in the detection of liver injury [8].
Thus, we assessed the diagnostic accuracy of nitric oxide and glutathione reductase activity as biochemical markers of HCC. Added to this we assessed the sensitivity and specificity of nitric oxide as a marker for HCC diagnosis.
Experimental
Subjects
In the present study, we measured serum nitric oxide and glutathione reductase levels in patients with HCC, cirrhotic patients, and healthy controls and evaluated a possible diagnostic value using these markers. From March 2012 to September 2012, 50 patients with HCC (37 males and 13 females; aged 40–90 years with a mean ± SE of 60.7 ± 1.29) were recruited from the Oncology Center, Mansoura University, Mansoura, Egypt. Since all HCC patients in this study have cirrhosis as an underlying liver disorder and in order to nullify the effect of cirrhosis on the level of the studied parameters, a group of 30 cirrhotic patients, (19 males and 11 females; aged 33–80 years with a mean ± SE of 56.4 ± 1.6), without any evidence of HCC was used and selected from the outpatient clinic of the Specialized Medical Hospital, Mansoura University, Mansoura, Egypt. All cases involved in this study were tested for either pathological proof or a typical radiologic pattern on the post-contrast study plus the diagnostic serum AFP. The severity of liver disease was assessed by the Child–Pugh classification [9]. The stage and management were defined according to the Barcelona-Clinic Liver Cancer Group diagnostic and treatment strategy (BCLC) [10]. Patients with other types of malignancy, advanced organ failure, and advanced medical co-morbidity were excluded from the study. A control group comprised of 15 healthy individuals (11 males and 4 females; aged 51–63 years with a mean ± SE of 55.7 ± 1.24) with no apparent evidence of active disease or medical disorders was selected.
Blood Sampling
Fasting blood samples were collected from all patients and control groups and were subsequently divided into two portions. The first portion was collected in tubes containing ethylenediaminetetraacetic acid (EDTA) and used for blood picture investigation within 5 hours. The second portion was collected in a monovette without additives. This blood was left to clot for 20–30 min at room temperature, followed by centrifugation at 1500 rpm for 10 min. The serum was then transferred to a polypropylene tube and if the analysis was not performed immediately, the samples were frozen and maintained at −80°C until use.
Analysis of Biochemical Parameters
Serum α-fetoprotein was measured using a commercially available ELISA kit from (DiaMetra Company). The serum nitric oxide level was measured using a commercially available kit from (R&D systems Kit, USA). The serum glutathione reductase activity was measured using a commercially available kit from (Biodiagnostic and research reagents, Egypt).
Statistical Analysis
For descriptive statistics, the frequency and percentage were calculated for qualitative variables, the mean values ± standard error (SE) and range were used for quantitative variables. For comparison between the two groups, Student‘s t-test was used. For comparison between more than two groups, the ANOVA test was used. For correlation, Pearson correlation was used. For comparison of qualitative variables, the chi square test was used. If the assumptions of the chi square test were violated, Fisher’s exact correction was used. For the calculation of specificity and sensitivity of a given marker, the cut-off level is determined according to the following equation (cut-off level = mean of control +2 S.D). Statistical computations were done on a personal computer using the computer software SPSS version 13 (Chicago, IL, USA). Statistical significance was taken at P< 0.05.
Results and Discussion
The characteristics of cirrhotic and HCC patients’ groups are summarized in Table 1, while tumor characteristics of HCC patients are given in Table 2. HCC, cirrhotic, and control groups were matched regarding age (P= 0.08).
Tab. 1.
Characteristics of the studied patient groups
| HCC (n=50) | Cirrhosis (n=30) | P | |
|---|---|---|---|
| Sex | |||
|
| |||
| Male | 37 (74%) | 19 (63.33%) | 0.3 |
| Female | 13 (26%) | 11 (36.67%) | |
|
| |||
| Child-Pugh classification | |||
|
| |||
| A | 21(42%) | 9 (30%) | 0.4 |
| B | 18(36%) | 11 (36.7%) | |
| C | 11(22%) | 10 (33.3%) | |
|
| |||
| Ascitis | |||
|
| |||
| No | 16 (32%) | 14 (48.27%) | 0.15 |
| Yes | 34 (68%) | 15 (51.72%) | |
n…number of patients.
Tab. 2.
Tumor-related findings of the HCC patient group
| HCC (n=50) | |
|---|---|
| No. of lesions | |
|
| |
| Single | 16 (32%) |
| Two lesions | 15 (30%) |
| Three lesions | 11 (22%) |
| Multifocal | 8 (16%) |
|
| |
| Metastasis | |
|
| |
| Absent | 31 (62%) |
| Present | 19 (38%) |
|
| |
| BCLC | |
|
| |
| A | 1 (2%) |
| B | 17 (34%) |
| C | 20 (40%) |
| D | 12 (24%) |
|
| |
| Performance status | |
|
| |
| 0 | 2 (4%) |
| 1 | 22 (44%) |
| 2 | 18 (36%) |
| 3 | 8 (16%) |
|
| |
| Portal vein | |
|
| |
| Patent | 37 (74%) |
| Thrombosed | 13 (26%) |
n…number of patients.
BCLC…Barcelona-Clinic Liver Cancer Group diagnostic and treatment strategy.
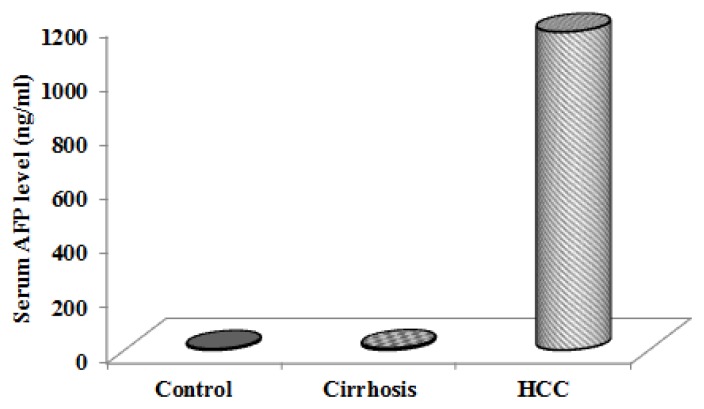
The distinction between HCC and cirrhosis has become challenging because regenerative nodules may mimic tumors in cirrhotic livers, and also because of elevated serum levels of AFP in patients with cirrhosis [11]. We found a significant increase in the serum concentration of α-fetoprotein in HCC patients as compared to patients with liver cirrhosis and the control group. Similar results were obtained by many studies [12–14] (Figure 1). Although AFP is a serological marker currently available for the detection of hepatocellular carcinoma, its poor sensitivity and specificity renders it unsatisfactory for this purpose and suggests the need for novel biomarkers for the detection of early HCC [15].
Fig. 1.
Serum α-fetoprotein (AFP) level in cirrhotic, hepatocellular carcinoma (HCC) patients and control group.
NO is a small, potent lipophilic gas with divergent biological activities that seems to play an important role in modulating tissue injury and carcinogenesis [16]. Serum nitrite/nitrate levels act as an index for in vivo NO production in patients with HCC [17]. Inducible nitric oxide synthase (iNOS) is related to a high output pathway for NO production which contributes to tumor cell angiogenesis as well as the invasion and metastasis of HCC [18]. Therefore, we aimed to measure the serum concentration NO in patients with liver cirrhosis and HCC to evaluate its activity as a tumor marker for liver malignancies.
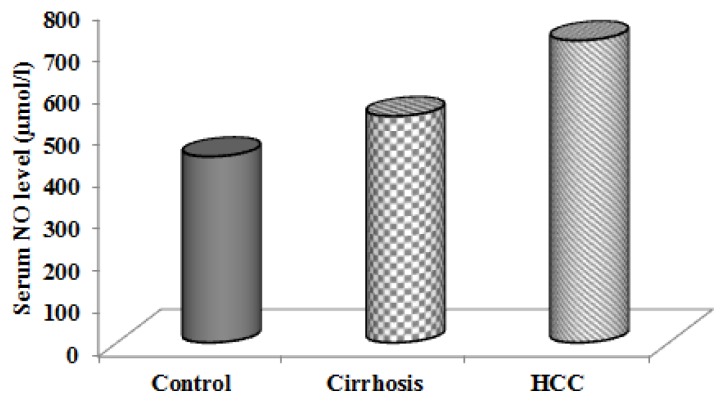
Interestingly, as shown in Table 3, our study found that HCC patients showed significantly higher NO levels than patients with cirrhosis, and significantly higher than normal controls (Figure 2). This was in agreement with Mansurova, Osman, and Abd El Moety and Abd El Moety [19–21]. These data point to the potential value of nitric oxide in the diagnosis of HCC. As shown in Table 4, at a cut-off level of 200 ng/ml, AFP had a sensitivity of 52%, specificity of 100%, positive predictive value of 100%, and a negative predictive value of 65.2%. Other studies have shown similar results with the specificity of AFP close to 100%, but at a cost to the sensitivity which falls below 45% [22]. The diagnostic performance of nitric oxide in HCC patients at a cut-off of 614.1 μmol/l showed that the sensitivity, specificity, positive predictive value, and negative predictive value were (74%, 88.89%, 88.1%, and 75.5%, respectively).
Tab. 3.
Comparison of studied parameters in HCC vs. cirrhotic patients and control group (mean ± SE)
| Control (n=15) | Cirrhosis (n=30) | HCC (n=50) | P | Between group comparison | P | |
|---|---|---|---|---|---|---|
| AFP (ng/ml) | 4.17 ± 0.53 | 7 ± 2 | 1168 ± 307*# | 0.004 | HCC vs. Control HCC vs. Cirrhosis Cirrhosis vs. Control |
<0.001 <0.001 0.12 |
| Nitric oxide (μmol/l) | 442.2 ± 29.06 | 537.96 ± 20.98 | 718.04 ± 36.53*# | <0.0001 | HCC vs. control HCC vs. cirrhosis Cirrhosis vs. control |
0.001 0.001 0.626 |
| Glutathione reductase (U/l) | 10.82 ± 0.99 | 20.01 ± 2.27 | 24.62 ± 2.3* | 0.019* | HCC vs. control HCC vs. cirrhosis Cirrhosis vs. control |
0.019 0.492 0.243 |
n…number of patients;
…significant difference as compared with the control group at p< 0.05;
…significant difference as compared with the cirrhotic group at p<0.05
Fig. 2.
Serum nitric oxide level in cirrhotic, hepatocellular carcinoma (HCC) patients and control group.
Tab. 4.
Sensitivity and specificity values for AFP and nitric oxide in HCC patients vs. cirrhotic patients and control group.
| Markers | HCC (n=50) | Control and Cirrhosis (n=45) | Sensitivity | Specificity | +ve predictive | −ve value |
|---|---|---|---|---|---|---|
| AFP < 200 | 24 | 45 | 52% | 100% | 100% | 65.2% |
| AFP > 200 | 26 | 0 | ||||
| NO < 614.1 | 13 | 40 | ||||
| NO > 614.1 | 37 | 5 | 74% | 88.89% | 88.1% | 75.5% |
n…number of patients.
As shown in Table 5, 24 out of 50 patients with HCC (48%) were AFP-negative. Notably, 62.5% (15/24) of these AFP-negative HCC patients were NO-positive. Thus, we found that the combined determination of AFP and nitric oxide had a sensitivity of 82% in the determination of HCC patients. Moreover, the simultaneous determinations of serum AFP and serum NO concentrations gave significant improvements in HCC detection compared with AFP alone and this was consistent with Moriyama [23]. Consequently, nitric oxide can be used as a complementary marker to serum AFP concentrations to detect HCC. However, due to the small sample size, these data need to be validated with a larger sample size.
Tab. 5.
AFP and NO values in HCC patients.
… above and below the suggested diagnostic cutoff value.
Hepatocytes produce NO in response to several inflammatory stimuli. Tumor cells themselves are able to produce large amounts of NO due to induced expression of iNOS, which may prevail in rapidly growing tumors [24]. Thus, there is a possibility that NO production by hepatic tissue is accelerated in patients with HCC [17]. Increased NO generation is well-recognized as an essential step initiating neoplastic transformation [25]. Added to this, NO plays an important role in HCC development and its progression [26].
A possible explanation for increased serum NO levels in HCC is that NO is reactively induced by the hepatic tissue surrounding HCC by three independent mechanisms: (1) tumor cells directly stimulate macrophages and Kupffer cells to produce NO, (2) HCC produces a variety of cytokines that may stimulate hepatocytes to produce NO, and (3) a marked deterioration of liver function in HCC patients may be associated with increased portosystemic shunting and further development of hyperdynamic circulation, leading to an increase in NO production [6].
Our study found a significant positive correlation between serum nitric oxide levels and ascites in HCC patients (P= 0.026), which was in agreement with Mirodzhov [27]. On the other hand, there was an insignificant difference between serum NO in patients suffering from portal vein invasion compared to those without portal vein invasion. Consequently, no correlation was found between serum NO and an increasing stage of BCLC. No correlation between the concentrations of serum NO and serum AFP was found in patients with HCC and this was consistent with Moriyama [23]. In agreement with Moriyama [23], the assay for nitrate / nitrites is inexpensive and simple to perform; it may be a beneficial tool for screening HCC. In addition, the determination of nitrite / nitrate might be useful for the diagnosis of HCC.
Glutathione reductase is a homodimeric flavoprotein which catalyzes the reduction of oxidized glutathione (GSSG) to the reduced form (GSH) in the presence of NADPH as a reducing cofactor [28]. Glutathione reductase has a function in diverse cellular phenomena, including the defensive response against free radicals and reactive oxygen species (ROS), as well as protein and DNA biosyntheses via maintainence of a high ratio of GSH/GSSG [29].
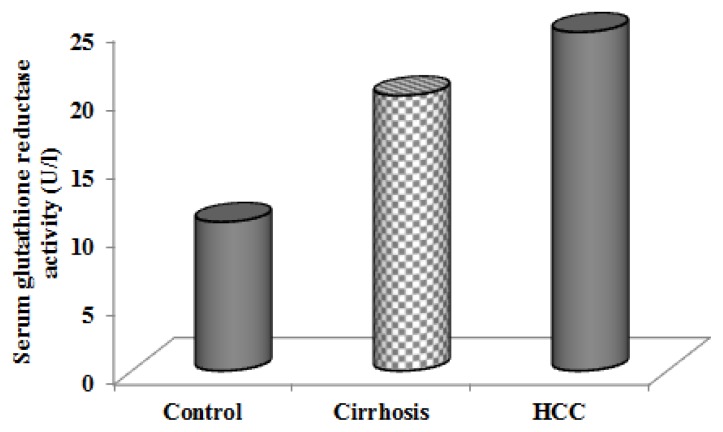
As shown in Table 3, our findings showed that glutathione reductase activity was increased significantly in HCC patients with respect to the control (Figure 3). This was consistent with Czeczot [7], Scibior [30], and Tsai [31]. Also, the levels for liver cirrhosis tended to be higher than in controls, but the differences were not statistically significant.
Fig. 3.
Serum glutathione reductase activity in cirrhotic, hepatocellular carcinoma (HCC) patients and control group.
The increased activity of glutathione reductase in the serum of patients with HCC may be a compensatory up-regulation [31]. Also, this increase in glutathione reductase is probably a function of the increased detoxification capacity as an adaptive response for oxidative stress [30].
Glutathione reductase was highly specific for GSSG and could be used in the detection of liver injury. So the increased activity of glutathione reductase could be explained by the fact that the increased production of reactive oxygen metabolites might be actively scavenged by GSH, resulting in the formation of GSSG, which is rapidly converted to GSH by glutathione reductase [8].
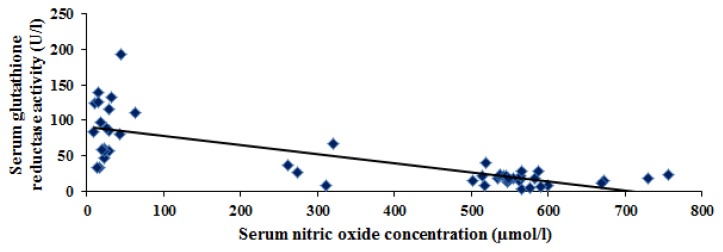
A significant negative correlation was found between serum nitric oxide and glutathione reductase activity in cirrhotic patients (r= −0.424, p= 0.02) (Figure 4). In vivo inhibition of glutathione reductase activity by both exogenous and endogenous NO has also been reported [32]. Glutathione reductase maintains intracellular GSH levels and protects cells from oxidative stress and cytotoxic chemicals. Several reports suggest that RNS derived from NO decrease intracellular GSH levels [33]. Since glutathione reductase is responsible for keeping intracellular GSH levels constant, its inactivation by nitrosocompounds is a likely mechanism for this change [34]. This may explain the significant negative correlation between NO and glutathione reductase in cirrhotic patients.
Fig. 4.
Significant negative correlation between serum nitric oxide concentration (μmol/l) and serum glutathione reductase activity (U/l) in cirrhotic patients (r= −0.424, p= 0.02).
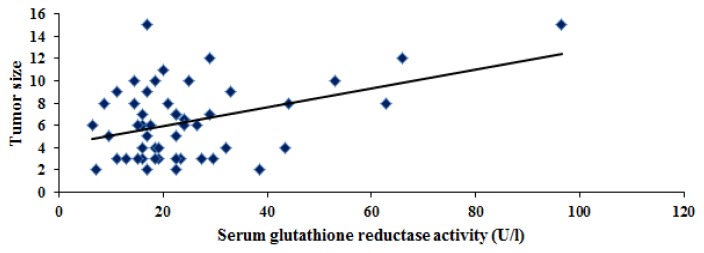
On the other hand, this study elucidated no correlation between serum glutathione reductase activity and serum AFP. However, a significant correlation between serum glutathione reductase activity and tumor size was found in HCC patients (r= 0.413, p= 0.003) (Figure 5). Further studies are needed to evaluate whether the presence of high levels of the antioxidant relieves tumors from oxidative stress allows them to reach larger sizes.
Fig. 5.
Significant positive correlation between serum glutathione reductase (U/l) and tumor size in HCC patients (r= 0.413, p= 0.003).
Conclusion
This study demonstrated that serum nitric oxide and glutathione reductase levels are significantly elevated in patients with HCC. So we can conclude that:
Nitric oxide is a promising diagnostic marker for HCC that may aid in screening and detection of HCC.
The combined estimation of nitric oxide and AFP increases the sensitivity of detection and diagnosis of HCC to 82%.
Glutathione reductase is not a diagnostic marker for HCC. However, it correlates significantly with tumor size.
These findings should be validated in further studies recruiting a larger number of patients.
Footnotes
Authors’ Statements
Competing Interests
The authors declare no conflict of interest.
Informed Consent, Ethical Approvals
The Institutional and National Ethical Guides for Experiments on Human Subjects were followed and informed consent was obtained. See ‘Experimental’ for details.
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(3):1118–1127. doi: 10.1056/NEJMra1001683. http://dx.doi.org/10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Lehman EM, Wilson ML. Epidemiology of hepatitis viruses among hepatocellular carcinoma cases and healthy people in Egypt: a systematic review and meta-analysis. Int J Cancer. 2009;124:690–697. doi: 10.1002/ijc.23937. http://dx.doi.org/10.1002/ijc.23937. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Gafar Y, Sleem H, Tawfik M. Percutaneous ethanol injection in large size and multiple HCC: two years follow-up in 165 patients. Med J Cairo Univ. 2002;70(Suppl II):299–304. [Google Scholar]
- 4.Stefaniuk P, Cianciara J, Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2010;16:418–424. doi: 10.3748/wjg.v16.i4.418. http://dx.doi.org/10.3748/wjg.v16.i4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, Khin MW, Koo WH Asian Oncology Summit. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. http://dx.doi.org/10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 6.Hon WM, Lee KH, Khoo HE. Nitric oxide in liver diseases: friend, foe, or just passerby? Ann N Y Acad Sci. 2002;962:275–295. doi: 10.1111/j.1749-6632.2002.tb04074.x. http://dx.doi.org/10.1111/j.1749-6632.2002.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 7.Czeczot H, Scibior D, Skrzycki M, Podsiad M. Glutathione and GSH-dependent enzymes in patients with liver cirrhosis and hepatocellular carcinoma. Acta Biochim Pol. 2006;53:237–242. http://www.ncbi.nlm.nih.gov/pubmed/16404476. [PubMed] [Google Scholar]
- 8.Mahdy KA, Abd-El-Shaheed A, Khadr ME, El-Shamy KA. Antioxidant status and lipid peroxidation activity in evaluating hepatocellular damage in children. East Mediterr Health J. 2009;15:842–852. http://www.ncbi.nlm.nih.gov/pubmed/20187535. [PubMed] [Google Scholar]
- 9.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. http://dx.doi.org/10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115–S120. doi: 10.1002/lt.20034. http://dx.doi.org/10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 11.Ressom HW, Xiao JF, Tuli L, Varghese RS, Zhou B, Tsai TH, Ranjbar MR, Zhao Y, Wang J, Di Poto C, Cheema AK, Tadesse MG, Goldman R, Shetty K. Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Anal Chim Acta. 2012;743:90–100. doi: 10.1016/j.aca.2012.07.013. http://dx.doi.org/10.1016/j.aca.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Wahab M, Mostafa M, Sabry M, el-Farrash M, Yousef T. Aflatoxins as a risk factor for hepatocellular carcinoma in Egypt, Mansoua Gastroenterology Center study. Hepatogastroenterology. 2008;55:1754–1759. http://www.ncbi.nlm.nih.gov/pubmed/19102385. [PubMed] [Google Scholar]
- 13.Kikuchi LO, Paranaguà-Vezozzo DC, Chagas AL, Mello ES, Alves VA, Farias AQ, Pietrobon R, Carrilho FJ. Nodules less than 20 mm and vascular invasion are predictors of survival in small hepatocellular carcinoma. J Clin Gastroenterol. 2009;43:191–195. doi: 10.1097/MCG.0b013e31817ff199. http://dx.doi.org/10.1097/MCG.0b013e31817ff199. [DOI] [PubMed] [Google Scholar]
- 14.Metwaly HA, Al-Gayyar MM, Eletreby S, Ebrahim MA, El-Shishtawy MM. Relevance of serum levels of interleukin-6 and syndecan-1 in patients with hepatocellular carcinoma. Sci Pharm. 2012;80:179–188. doi: 10.3797/scipharm.1110-07. http://dx.doi.org/10.3797/scipharm.1110-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teofănescu I, Gologan E, Stefănescu G, Bălan G. Surveillance of cirrhosis for hepatocellular carcinoma--clinical validation of new serological biomarkers for improved diagnosis. Rev Med Chir Soc Med Nat Iasi. 2010;114:39–46. http://www.ncbi.nlm.nih.gov/pubmed/20509274. [PubMed] [Google Scholar]
- 16.Moety HAAE, Moety AAE, Sayed PE. Evaluation of serum nitric oxide before and after local radiofrequency thermal ablation for hepatocellular carcinoma. Alexandria J Med. 2013;49:67–73. http://dx.doi.org/10.1016/j.ajme.2012.08.002. [Google Scholar]
- 17.Moriyama A, Masumoto A, Nanri H, Tabaru A, Unoki H, Imoto I, Ikeda M, Otsuki M. High plasma concentrations of nitrite/nitrate in patients with hepatocellular carcinoma. Am J Gastroenterol. 1997;92:1520–1523. http://www.ncbi.nlm.nih.gov/pubmed/9317076. [PubMed] [Google Scholar]
- 18.Peng JP, Zheng S, Xiao ZX, Zhang SZ. Inducible nitric oxide synthase expression is related to angiogenesis, bcl-2 and cell proliferation in hepatocellular carcinoma. J Zhejiang Univ Sci. 2003;4:221–227. doi: 10.1631/jzus.2003.0221. http://dx.doi.org/10.1631/jzus.2003.0221. [DOI] [PubMed] [Google Scholar]
- 19.Mansurova FKh, Mutikhova KhSh, Olimova SO. Lipid peroxidation and anti-oxidative protection in patients with chronic type C hepatitis. Klin Med (Mosk) 2005;83:39–42. http://www.ncbi.nlm.nih.gov/pubmed/15984581. [PubMed] [Google Scholar]
- 20.Osman HG, Gabr OM, Lotfy S, Gabr S. Serum levels of bcl-2 and cellular oxidative stress in patients with viral hepatitis. Indian J Med Microbiol. 2007;25:323–329. doi: 10.4103/0255-0857.37333. http://www.ncbi.nlm.nih.gov/pubmed/18087079. [DOI] [PubMed] [Google Scholar]
- 21.Moety AAAE, Moety HAE. Evaluation of nitric oxide as a novel diagnostic marker for hepatocellular carcinoma. Alexandria Journal of Medicine. 2011;47:31–35. http://dx.doi.org/10.1016/j.ajme.2011.04.001. [Google Scholar]
- 22.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. http://dx.doi.org/10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 23.Moriyama A, Tabaru A, Unoki H, Abe S, Masumoto A, Otsuki M. Plasma nitrite/nitrate concentrations as a tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2000;296:181–191. doi: 10.1016/s0009-8981(00)00260-6. http://dx.doi.org/10.1016/S0009-8981(00)00260-6. [DOI] [PubMed] [Google Scholar]
- 24.Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. http://dx.doi.org/10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 25.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. http://dx.doi.org/10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. http://dx.doi.org/10.1016/S0092-8674(04)00131-X. [DOI] [PubMed] [Google Scholar]
- 27.Mirodzhov GK, Avezov SA, Giiasov MM, Abdullaeva ZM. The role of interleukin-6 and nitric oxide in pathogenesis of portal hypertension and decompensation of liver cirrhosis. Klin Med (Mosk) 2012;90:47–49. http://www.ncbi.nlm.nih.gov/pubmed/22567940. [PubMed] [Google Scholar]
- 28.Kim SJ, Jung HJ, Hyun DH, Park EH, Kim YM, Lim CJ. Glutathione reductase plays an anti-apoptotic role against oxidative stress in human hepatoma cells. Biochimie. 2010;92:927–932. doi: 10.1016/j.biochi.2010.03.007. http://dx.doi.org/10.1016/j.biochi.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. http://dx.doi.org/10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 30.Scibior D, Skrzycki M, Podsiad M, Czeczot H. Glutathione level and glutathione-dependent enzyme activities in blood serum of patients with gastrointestinal tract tumors. Clin Biochem. 2008;41:852–588. doi: 10.1016/j.clinbiochem.2008.03.005. http://dx.doi.org/10.1016/j.clinbiochem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Tsai SM, Lin SK, Lee KT, Hsiao JK, Huang JC, Wu SH, Ma H, Wu SH, Tsai LY. Evaluation of redox statuses in patients with hepatitis B virus-associated hepatocellular carcinoma. Ann Clin Biochem. 2009;46:394–400. doi: 10.1258/acb.2009.009029. http://dx.doi.org/10.1258/acb.2009.009029. [DOI] [PubMed] [Google Scholar]
- 32.Butzer U, Weidenbach H, Gansauge S, Gansauge F, Beger HG, Nussler AK. Increased oxidative stress in the RAW 264.7 macrophage cell line is partially mediated via the S-nitrosothiol-induced inhibition of glutathione reductase. FEBS Lett. 1999;445:274–278. doi: 10.1016/s0014-5793(99)00139-8. http://dx.doi.org/10.1016/S0014-5793(99)00139-8. [DOI] [PubMed] [Google Scholar]
- 33.Luperchio S, Tamir S, Tannenbaum SR. NO-induced oxidative stress and glutathione metabolism in rodent and human cells. Free Radic Biol Med. 1996;21:513–519. doi: 10.1016/0891-5849(96)00219-5. http://dx.doi.org/10.1016/0891-5849(96)00219-5. [DOI] [PubMed] [Google Scholar]
- 34.Boese M, Keese MA, Becker K, Busse R, Mülsch A. Inhibition of glutathione reductase by dinitrosyl-iron-dithiolate complex. J Biol Chem. 1997;272:21767–21773. doi: 10.1074/jbc.272.35.21767. http://dx.doi.org/10.1074/jbc.272.35.21767. [DOI] [PubMed] [Google Scholar]