Abstract
EpCam is a transmembrane epithelial adhesion molecule present on all non-squamous epithelial cells. It is often overexpressed in certain carcinomas, such as breast and colon, and in dermatology, eg, basal cell carcinoma (BCC). Various monoclonal antibodies have been used to detect EpCam, including BerEP4 and epithelial specific antigen. We compared anti-EpCam clones, BerEP4, and epithelial specific antigen clone VU-1D9. One hundred and twelve lesions were stained with both antibodies. All basal cell carcinomas stained uniformly and strongly positive with both antibodies. Diffuse positive staining was also seen in all trichoepitheliomas and merkel cell carcinomas. Focal positive staining was seen in squamous cell carcinoma and benign sebaceous neoplasms. Clone VU-1D9 was more likely to produce focal positive staining as compared to BerEP4. This focal positive staining of sebaceous neoplasms and squamous cell carcinomas is a potential diagnostic pitfall.
Keywords: EpCam, BerEP4, basal cell carcinoma, squamous cell carcinoma, merkel cell carcinoma
Introduction
EpCAM (CD 326) is a human transmembrane 40 Kd glycoprotein epithelial adhesion molecule. The antigen is located on the cell membrane and within the cytoplasm of all non-squamous epithelial cells.1 This epithelial specific antigen is also present on carcinomas of various organs. EpCAM has roles in cell signaling, proliferation, adhesion, migration, and differentiation. It can also act as an oncogenic signaling molecule via the wnt signaling pathway.2
EpCAM is overexpressed in certain carcinomas including colon, pancreas, and breast.3–5 Anti-EpCAM antibodies are available for both histopathologic diagnostic and therapeutic use in human subjects. Anti-EpCAM monoclonal antibodies have been used as targeted therapy in patients with breast and colon cancer with modest results. Edrecolomab has been used in patients with colorectal carcinoma.6–8 Adecatumumab is a human IgG1 antibody targeting EpCAM in breast cancer patients.9,10
Monoclonal antibodies to epithelial cell antigen have also been used to identify carcinomas immuno-histochemically. In particular, previous studies have looked at the use of these antibodies to discriminate between non-melanoma skin cancers, including basal cell carcinoma and squamous cell carcinoma. Previous studies have shown that anti-EpCAM antibody Ber-EP4 is a sensitive marker of basal cell carcinoma; however, it fails to stain cutaneous squamous cell carcinoma.11–15 The effectiveness of other monoclonal antibody clones directed against similar epithelial cell antigens have not been compared directly.
Anti-EpCAM antibody clones such as Ber-EP4, VU1D9, and AUA1 have been raised against a variety of cell lines. Given the potential diagnostic and therapeutic applications of anti-EpCam antibodies, we undertook a study to investigate the expression of EpCAM in a wider variety of cutaneous neoplasms. We compared cutaneous lesions of basal cell carcinoma with clinical simulators. This study also aims to evaluate differences in immune-histochemical staining seen with Epithelial Antigen clone Ber-EP4 and Epithelial Specific Antigen clone VU-1D9, monoclonal antibodies derived from different cancer cell lines.
Methods
Representative cases were obtained from the dermatopathology files, including 24 basal cell carcinomas and 88 common skin neoplasms. Specimens were obtained from sequential specimens in a daily readout with the addition of fout cases of merkel cell carcinoma and eight trichoepitheliomas obtained by computer search of recent cases. Specimens had been fixed in neutral buffered formalin for 24 hours, processed in a standard 8 hour cycle, and paraffin embedded. Sections were cut at 4 micrometers and de-paraffinized using standard protocols. Antigen retrieval was performed using a low pH 6.0 citrate buffer (Biogenex, San Ramon, CA) at 100 °C for 20 minutes in a Dako PTLink (Dako Corp, Glostrup, Denmark) automated retrieval unit. Endogenous peroxidase was blocked with hydrogen peroxide. Specimens were then incubated for 1 hour with the primary antibodies. We compared antibodies to epithelial antigen Ber-EP4 to MCF-7 human breast carcinoma cell line (prediluted, catalog # Ir637; Dako Corporation, Glostrup, Denmark) with mouse monoclonal antibodies to human epithelial specific antigen clone VU-1D9 (1:100 dilution; small cell lung carcinoma cell line HG9; Leica Biosystems New Castle Upon Tyne, United Kingdom). The antibodies’ staining were then detected using the Dako EnVision flex histochemistry detection kit on a Dako auto-stainer (Dako Corp, Denmark). Sections were counterstained with hematoxalin. Sections were then reviewed by two of the authors independently, one a dermatopathologist (DM) and the other a fourth year medical student (TM), for intensity and distribution of staining. The results are recorded in Table 1.
Table 1.
Staining patterns.
| Biopsy proven lesion | Samples | Staining: epithelial antigen clone Ber-EP4 | Staining: epithelial specific antigen clone VU-1D9 |
|---|---|---|---|
| Basal cell carcinoma | 24 | 24 diffusely positive | 24 diffusely positive |
| Trichoepithelioma | 8 | 8 diffusely positive | 8 diffusely positive |
| Actinic keratosis | 12 | 12 negative | 12 negative |
| Squamous cell carcinoma in situ | 7 | 7 negative | 4 negative 3 focal positive staining in lower half of epidermis |
| Squamous cell carcinoma | 11 | 10 negative 1 focally positive with basal layer staining |
9 negative 1 focally positive 1 diffusely positive |
| Seborrheic keratosis | 16 | 16 negative | 16 negative |
| Lichen planus like keratosis | 6 | 6 negative | 6 negative |
| Nevi | 11 | 11 negative | 11 negative |
| Hemangioma | 2 | 2 negative | 2 negative |
| Inverted follicular keratosis | 5 | 5 negative | 5 negative |
| Sebaceous adenoma/hyperplasia | 6 | 5 negative 1 focal positive |
4 negative 2 focal positive |
| Merkel cell carcinoma | 4 | 4 diffusely positive | 4 diffusely positive |
Results
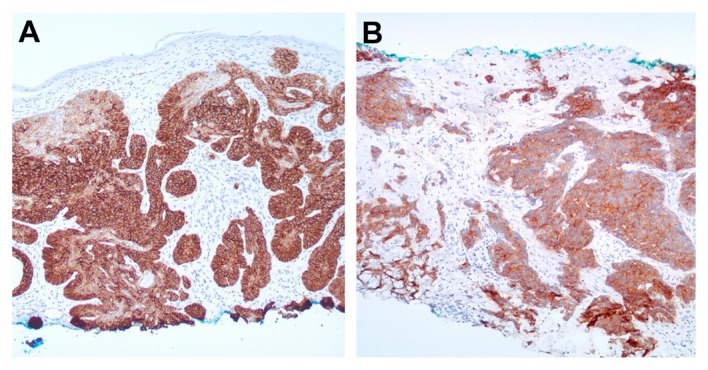
Two separate investigators determined the staining pattern of 112 biopsy slides. All basal cell carcinoma (24/24), merkel cell carcinoma (4/4), and trichoepithelioma (8/8) slides stained diffusely positive with both of the epithelial specific antibodies tested (Epithelial Antigen clone Ber-EP4 and Epithelial Specific Antigen clone VU-1D9) (Figs. 1A and 2B). Actinic keratosis (12/12), seborrheic keratosis (16/16), lichen planus like keratosis (6/6), hemangiomas (2/2), and inverted follicular keratosis (5/5) showed no staining by either monoclonal antibody (with the exception of germinative follicular cells) or eccrine duct cells.
Figure 1.
(A and B) Basal cell carcinoma and merkel cell carcinoma: diffusely positive staining of neoplastic cells (Ber-EP4; 40x).
Figure 2.
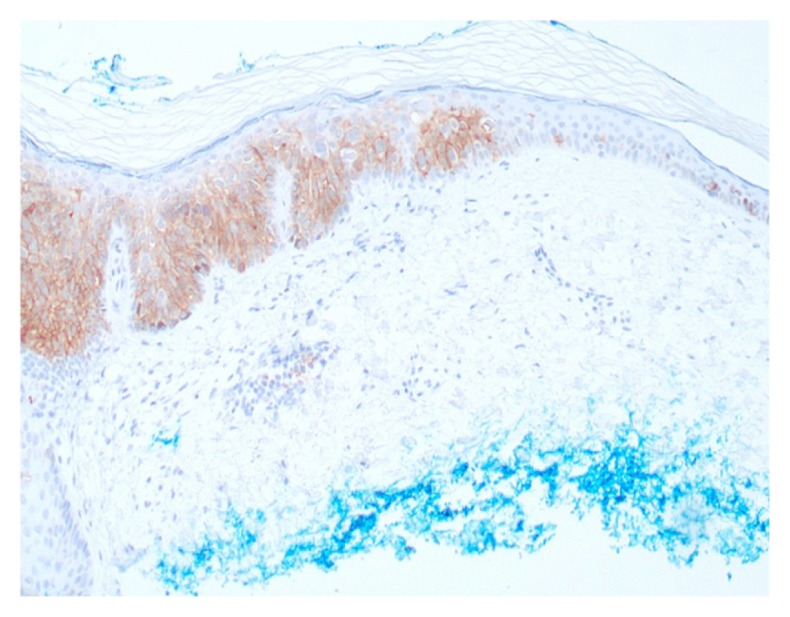
Squamous cell carcinoma in situ—focally positive staining in lower half of epidermis (Epithelial specific antigen; 40x).
Of the seven cases of squamous cell carcinoma in situ, three showed focal staining by Epithelial Specific Antigen clone VU-1D9 in the lower half of the epidermis (Fig. 2). This was not seen in the same cases stained with Ber-EP4. The remaining cases of squamous cell carcinoma in situ did not stain with either monoclonal antibody.
Of the eleven cases of squamous cell carcinoma stained for Ber-EP4, 10 did not show positive staining. One case had focally positive staining of the basal layer of the epidermis. Of the eleven cases of squamous cell carcinoma stained for Epithelial Specific Antigen, one was diffusely positive, and another contained focally positive staining in the lower half of the epidermis; the remaining slides did not show positive staining (Fig. 3).
Figure 3.
(A) Squamous cell carcinoma with diffusely positive staining (Epithelial specific antigen; 40x). (B) Squamous cell carcinoma with positive basal layer staining (Ber-EP4; 40x).
No nevi showed positive staining for Ber-EP4 (11/11) or Epithelial Specific Antigen. Benign sebaceous tumors, including 3 sebaceous hyperplasias and 3 sebaceous adenoma, showed focal positive staining in 1 case stained by Ber-EP4 and 2 cases stained by epithelial specific antigen. These results are summarized in Table 1.
Discussion
Monoclonal antibodies directed against epithelial specific antigen (EpCAM; CD326) have been used both for the diagnosis and treatment of carcinomas of various organs. Previous studies have shown a lack of cutaneous squamous cell carcinoma immune-histochemical staining by Ber-EP4 (11–15). Our study found that 8/9 (89%) cases of squamous cell carcinoma did not stain for the epithelial antigen Ber-EP4. However, one case demonstrated focally positive basal layer staining. Epithelial specific antigen clone VU-1D9 did not stain 7/9 cases (78%), but stained one case focally positive and one case diffusely positive.
Other authors have reported negative staining of squamous cell carcinoma in situ with epithelial antigen clone Ber-Ep4. Tope et al showed 8/8 cases of squamous intraepithelial neoplasia to be negative for Ber-EP4 staining; this was recapitulated by Ansai et al in 10/10 cases.16,17 Consistent with these reports, the present study found that 7/7 cases of Bowen’s disease did not stain immune-histochemically for epithelial antigen clone Ber-EP4. However, 3/7 cases stained for epithelial specific antigen clone VU-1D9, with focal positive staining in the lower half of the epidermis. Thus, this anti EpCAM antibody, derived from a small cell lung carcinoma cell line (HG9) may be less specific for basal cell carcinoma. This focal positive staining pattern using antibodies to Epithelial specific antigen (VU-1D9) may represent a potential diagnostic pitfall.
Consistent with our findings, previous investigators have described positive epithelial antigen immune-histochemical staining of basal cell carcinomas in all (100%) samples studied.11–14,16,18–22 Most recently, Ansai et al reported two studies with slightly lower Ber-EP4 positivity seen in basal cell carcinoma, the first with 8/10 (80%) positive, and the second with 30/31 (97%) positive.15,17
All trichoepitheliomas stained diffusely positive for both epithelial antigens studied. This finding is consistent with previous reports of Ber-EP4 positivity in follicular neoplasms such as trichoepithelioma.12,13,17–19,22 and trichoblastoma.17,23 Thus, these lesions are not reliably differentiated using the monoclonal antibodies studied and their diagnosis relies heavily on microscopic features. Our study also confirmed the previously reported staining of merkel cell carcinomas.12
Our study confirmed findings by Ansai et al,17 which showed no immune-histochemical staining of these epithelial antigens within seborrheic keratoses. Actinic keratoses included in our study failed to stain for EpCAM with both antibodies studied, which is consistent with previous reports.16,17
Our study found that immune-histochemical staining for monoclonal antibodies to Epithelial Antigen clone Ber-EP4 may be more specific for basal cell carcinoma than that of Epithelial-specific antigen clone VU-1D9. Out of 112 cases, the former stained 100% of basal cell carcinomas, merkel cell carcinomas, and trichoepitheliomas, and did not stain 97% (74/76) of other skin lesions. The latter positively stained 100% of basal cell carcinomas, merkel cell carcinomas and trichopitheliomas, but also showed at least focal positive staining of 10% (7/76) of lesions tested.
The present study was the first to analyze epithelial antigen immune-histochemical staining of lichen planus like keratoses, nevi, hemangiomas, and inverted follicular keratoses. These lesions often need to be differentiated clinically from basal cell carcinoma. None of these lesions (0/24) stained with epithelial antigen clone Ber-EP4. The specificity and accuracy of BerEP4 antibody for cutaneous basal cell carcinoma shown in this study serves as a fundamental proof of concept for the clinical application of this antibody both as a potential in-vivo diagnostic probe and potentially as a therapeutic agent.
Footnotes
Author Contributions
Conceived and designed the experiments: DRM, BD. Analyzed the data: DRM, BD, TMM. Wrote the first draft of the manuscript: TMM. Contributed to the writing of the manuscript: DRM, BD, TMM. Agree with manuscript results and conclusions: DRM, BD, TMM. Jointly developed the structure and arguments for the paper: DRM, BD, TMM. Made critical revisions and approved final version: DRM, BD, TMM. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
References
- 1.Latza U, Niedobitek G, Schwarting R, Nekarda H, Stein H. Ber-EP4: new monoclonal antibody which distinguishes epithelia from mesothelial. J Clin Pathol. 1990;43(3):213–9. doi: 10.1136/jcp.43.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maetzel D, Denzel S, Mack B, et al. Nuclear signalling by tumour- associated antigen EpCAM. Nat Cell Biol. 2009;11(2):162–71. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle PA, Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96(3):417–23. doi: 10.1038/sj.bjc.6603494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Went P, Vasei M, Bubendorf L, et al. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer. 2006;94(1):128–35. doi: 10.1038/sj.bjc.6602924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35(1):122–8. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Punt CJ, Nagy A, Douillard JY, et al. Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet. 2002;360(9334):671–7. doi: 10.1016/S0140-6736(02)09836-7. [DOI] [PubMed] [Google Scholar]
- 7.Fields AL, Keller A, Schwartzberg L, et al. Adjuvant therapy with the monoclonal antibody Edrecolomab plus fluorouracil-based therapy does not improve overall survival of patients with stage III colon cancer. J Clin Oncol. 2009;27(12):1941–7. doi: 10.1200/JCO.2008.18.5710. [DOI] [PubMed] [Google Scholar]
- 8.Niedzwiecki D, Bertagnolli MM, Warren RS, et al. Documenting the natural history of patients with resected stage II adenocarcinoma of the colon after random assignment to adjuvant treatment with edrecolomab or observation: results from CALGB 9581. J Clin Oncol. 2011;29(23):3146–52. doi: 10.1200/JCO.2010.32.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M, Scheulen ME, Dittrich C, et al. An open-label, randomized phase II study of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in patients with metastatic breast cancer. Ann Oncol. 21(2):275–82. doi: 10.1093/annonc/mdp314. [DOI] [PubMed] [Google Scholar]
- 10.Münz M, Murr A, Kvesic M, et al. Side-by-side analysis of five clinically tested anti-EpCAM monoclonal antibodies. Cancer Cell Int. 2010;10:44. doi: 10.1186/1475-2867-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tellechea O, Reis JP, Domingues JC, Baptista AP. Monoclonal antibody Ber EP4 distinguishes basal-cell carcinoma from squamous-cell carcinoma of the skin. Am J Dermatopathol. 1993;15(5):452–5. doi: 10.1097/00000372-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez FJ, Burchette JL, Jr, Grichnik JM, Hitchcock MG. Ber-EP4 immunoreactivity in normal skin and cutaneous neoplasms. Mod Pathol. 1995;8(8):854–8. [PubMed] [Google Scholar]
- 13.Swanson PE, Fitzpatrick MM, Ritter JH, Glusac EJ, Wick MR. Immunohistologic differential diagnosis of basal cell carcinoma, squamous cell carcinoma, and trichoepithelioma in small cutaneous biopsy specimens. J Cutan Pathol. 1998;25(3):153–9. doi: 10.1111/j.1600-0560.1998.tb01708.x. [DOI] [PubMed] [Google Scholar]
- 14.Beer TW, Shepherd P, Theaker JM. Ber EP4 and epithelial membrane antigen aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology. 2000;37(3):218–323. doi: 10.1046/j.1365-2559.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 15.Ansai S, Takeichi H, Arase S, Kawana S, Kimura T. Sebaceous carcinoma: an immunohistochemical reappraisal. Am J Dermatopathol. 2011;33(6):579–87. doi: 10.1097/DAD.0b013e31820a2027. [DOI] [PubMed] [Google Scholar]
- 16.Tope WD, Nowfar-Rad M, Kist DA. Ber-EP4-positive phenotype differentiates actinic keratosis from superficial basal cell carcinoma. Dermatol Surg. 2000;26(5):415–8. doi: 10.1046/j.1524-4725.2000.99287.x. [DOI] [PubMed] [Google Scholar]
- 17.Ansai S, Takayama R, Kimura T, Kawana S. Ber-EP4 is a useful marker for follicular germinative cell differentiation of cutaneous epithelial neoplasms. J Dermatol. 2012;39(8):688–92. doi: 10.1111/j.1346-8138.2011.01494.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith KJ, Williams J, Corbett D, Skelton H. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25(4):464–71. doi: 10.1097/00000478-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Krahl D, Sellheyer K. Monoclonal antibody Ber-EP4 reliably discriminates between microcystic adnexal carcinoma and basal cell carcinoma. J Cutan Pathol. 2007;34(10):782–7. doi: 10.1111/j.1600-0560.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 20.Sramek B, Lisle A, Loy T. Immunohistochemistry in ocular carcinomas. J Cutan Pathol. 2008;35(7):641–6. doi: 10.1111/j.1600-0560.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishida M, Kushima R, Okabe H. Immunohistochemical demonstration of D2-40 in basal cell carcinomas of the skin. J Cutan Pathol. 2008;35(10):926–30. doi: 10.1111/j.1600-0560.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 22.Shimanovich I, Krahl D, Rose C. Trichoadenoma of Nikolowski is a distinct neoplasm within the spectrum of follicular tumors. J Am Acad Dermatol. 2010;62(2):277–83. doi: 10.1016/j.jaad.2009.06.086. [DOI] [PubMed] [Google Scholar]
- 23.Kist D, Perkins W, Christ S, Zachary CB. Anti-human epithelial antigen (Ber-EP4) helps define basal cell carcinoma masked by inflammation. Dermatol Surg. 1997;23(11):1067–70. doi: 10.1111/j.1524-4725.1997.tb00449.x. [DOI] [PubMed] [Google Scholar]