Abstract
Background
Pulsed electromagnetic field (PEMF) therapy has shown promising therapeutic effectiveness on bone- and cartilage-related pathologies, being also safe for management of knee osteoarthritis.
Aim
The aim of this study was to investigate the clinical efficacy of a PEMF device for management of knee osteoarthritis in elderly patients.
Materials and methods
A total of 33 patients were screened, and 28 patients, aged between 60 and 83 and affected by bilateral knee osteoarthritis, were enrolled in this study. They received PEMF therapy on the right leg for a total of three 30-minute sessions per week for a period of 6 weeks, while the left leg did not receive any treatment and served as control. An intravenous drip containing ketoprofen, sodium clodronate, glucosamine sulfate, calcitonin, and ascorbic acid, for a total volume of 500 mL, was administered during PEMF therapy. At baseline and 3 months post-PEMF therapy, Visual Analog Scale (VAS) was used to assess knee pain and Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) was used to measure knee pain, stiffness and physical function.
Results
Changes in VAS and WOMAC scores were calculated for both knees as baseline minus post-treatment. A two sample Student’s t-test, comparing change in knee-related VAS pain for PEMF-treated leg (49.8 ± 2.03) vs control leg (11 ± 1.1), showed a significant difference in favor of PEMF therapy (P < 0.001). A two sample Student’s t-test comparing change in knee-related WOMAC pain, stiffness, and physical function for PEMF-treated leg (8.5 ± 0.4, 3.5 ± 0.2, 38.5 ± 2.08, respectively) vs control leg (2.6 ± 0.2; 1.6 ± 0.1; 4.5 ± 0.5 respectively), also showed a significant difference in favor of PEMF therapy (P < 0.001). No adverse reactions to therapy were observed.
Conclusion
The present study shows that PEMF therapy improves pain, stiffness and physical function in elderly patients affected by knee osteoarthritis.
Keywords: osteoarthritis, elderly, pulsed electromagnetic field, magnet therapy, knee
Introduction
Osteoarthritis (OA) is a degenerative joint disease frequently affecting the knee and afflicting the constantly increasing elderly population.1,2 Knee OA symptoms include pain, stiffness, and functional limitation, leading to loss of autonomy and poor quality of life in patients affected by this disease.3 Nowadays, various treatment options are available for the management of this condition. They include: nonsteroidal anti-inflammatory drugs (NSAIDs) for pain management;4 bisphosphonates to decrease pain and improve functionality preserving the structural integrity of subchondral bone;5 therapeutic exercise;6 viscosupplementation with hyaluronic acid alone or in combination with bisphosphonates or NSAIDs to improve pain and functional activity7–9 since hyaluronic acid improves articular cartilage degeneration and decreases osteophyte formation, as showed by experimental studies using OA models.10,11 These treatment modalities are effective in reducing pain and inflammation, but their long-term administration is associated with a high incidence of side effects or may not be applicable to the elderly.12 Building upon these foundations, there is an urgent need for alternative therapies for this pathological condition. Pulsed electromagnetic field (PEMF) therapy has proved to be safe and has also shown promising therapeutic effectiveness on bone- and cartilage-related pathologies, including knee and cervical spine OA.13–18
Aim
The aim of this study was to investigate the clinical efficacy of a PEMF device for management of knee OA in elderly patients.
Materials and methods
Patients
A total of 33 patients were screened, and 28 patients, aged between 60 and 83 (69.9 ± 1.5 [mean ± Standard Error of the Mean {SEM}]) and affected by bilateral knee OA, were enrolled in this study. All patients signed the informed consent. The protocol was planned and applied in agreement with the Declaration of Helsinki and was approved by the Institutional Review Board at the Poliambulatorio del Secondo Parere (Modena, Italy), where the procedure was performed.
Inclusion/exclusion criteria
The inclusion criteria for this study were a diagnosis of bilateral knee OA according to the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association,19 recurrent joint pain for at least a year prior to treatment, and daily pain in the knee ≥30 mm, as assessed by a 1–100 mm Visual Analog Scale (VAS). The exclusion criteria were: unilateral knee OA, intra-articular administration of drugs to the affected knees within 6 months before the study, systemic corticosteroid therapy or physiotherapy (iontophoresis with anti-inflammatory drugs, soft [not heating] laser, and ultrasound therapy) in the previous 6 weeks, and knee pain due to malignant, autoimmune and inflammatory pathologies or resulting from defective pathologies of the knee.
Therapeutic regimen
PEMF therapy was performed using the Magnetofield device (F&B International, Parma, Italy). The applicators were held at the sides of the knee by a velcro band. The medical device combines low and high frequencies by means of 2 local devices in the shape of a hemisphere. The low-frequency field releases an intensity between 50 and 100 Gauss. The high-frequency field develops an intensity between 60 and 80 decibel relative to 1 volt (dBV)/meter (m). Low frequency takes the form of a square wave with frequency comprised between 6 and 100 Hz and duty cycle comprised between 30% and 70%. The high frequency also takes the form of a square wave, which is made up of a modulating and a carrier wave (continuous modulation). The modulating wave frequency varies between 100 and 5000 Hz, with duty cycle constant at 50%. The carrier-wave frequency varies between 20 and 30 MHz, with duty cycle at 50%.
In the present study, the patients underwent two consecutive therapeutic regimens: (1) 6÷100 Hz (low frequency) and 500÷2000 Hz (high frequency) for 15 minutes, and (2) 6÷100 Hz (low frequency) and 100÷5000 Hz (high frequency) for 15 minutes. A total of three 30-minute sessions per week for a period of 6 weeks were administered to each patient. The right leg was treated with PEMF therapy, while the left leg did not receive any treatment and was used as control (Figure 1). An intravenous drip, containing ketoprofen (4 mL [160 mg/mL]; Dompè Farmaceutici, Milan, Italy), sodium clodronate (10 mL [30 mg/mL]; Abiogen Pharma, Pisa, Italy), glucosamine sulfate (1 mL [1.5 mg/mL]; Rottapharm, Monza, Italy), calcitonin (1 mL [100 Ui/mL]; Sandoz Industrial Products, Trento, Italy), and ascorbic acid (5 mL [0.2 g/mL]; Bayer, Milan, Italy), was administered while patients were receiving PEMF therapy.
Figure 1.
Patient undergoing PEMF therapy (right leg).
Abbreviation: PEMF, pulsed electromagnetic field.
Assessment of results
VAS and the Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) have been extensively used in clinical investigations to assess pain, stiffness, and physical function in patients affected by knee OA.20–22 In our study, VAS (0–100 mm, 0 = no pain, 100 = maximum pain) and WOMAC (subscore 0–20, 0 = minimum pain, 20 = maximum pain) were used to measure knee-related pain at baseline and at 3 months post-PEMF therapy. Furthermore, WOMAC was also used to determine knee-related stiffness (subscore 0–8, 0 = minimum stiffness, 8 = maximum stiffness) and physical function (subscore 0–68, 0 = minimum physical function, 68 = maximum physical function).
Statistical analysis
All data are represented as the means ± SEM and were analyzed using GraphPad Prism 5.04 (GraphPad Software Inc., San Diego, CA, USA). Changes in VAS and WOMAC scores were calculated for both knees as baseline minus post-treatment. An unpaired two-sample Student’s t-test was used to compare change in knee-related VAS and WOMAC scores for PEMF-treated leg (mean ± SEM) vs control leg (mean ± SEM). P < 0.05 was considered significant.
Results
A total of 28 patients participated in the present study. At baseline, no significant difference was observed in mean VAS and WOMAC pain and mean WOMAC stiffness and physical function between left and right knee. VAS pain in the right knee changed from a baseline of 78.2 ± 1.2 to 28.4 ± 1.2 mm at 3 month follow-up. VAS pain in the control knee changed from a baseline of 78.2 ± 1.9 to 67.2 ± 1.7 mm at 3 month follow-up. In the right knee, WOMAC pain, stiffness, and physical function changed from baseline values of 15.6 ± 0.3, 6.3 ± 0.2, and 54.4 ± 1.8 to 7.1 ± 0.3, 2.8 ± 0.1, and 15.8 ± 0.9 at 3 month follow-up, respectively. In the control knee, WOMAC pain, stiffness, and physical function changed from baseline values of 15.3 ± 0.3, 6.3 ± 0.2, and 54.5 ± 1.8 to 12.9 ± 0.4, 4.7 ± 0.2, and 50.03 ± 1.8 at 3 month follow-up, respectively.
At 3 month follow-up, knee-related VAS pain significantly improved in PEMF-treated leg (49.8 ± 2.03) if compared with control leg (11 ± 1.1; P < 0.001, Figure 2). At 3 month follow-up, knee-related WOMAC pain, stiffness, and physical function significantly improved in PEMF-treated leg (8.5 ± 0.4, 3.5 ± 0.2 and 38.5 ± 2.08, respectively) if compared with control leg (2.6 ± 0.2, 1.6 ± 0.1 and 4.5 ± 0.5, respectively; P < 0.001, Figure 3). No adverse reactions to therapy were observed.
Figure 2.
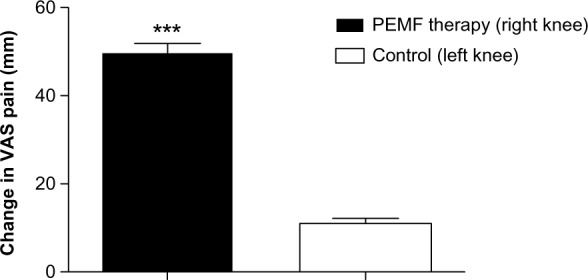
Change in knee-related VAS pain for PEMF-treated leg vs control leg. Data are presented as the means ± SEM.
Note: ***P < 0.001.
Abbreviations: VAS, Visual Analog Scale; PEMF, pulsed electromagnetic field; SEM, Standard Error of the Mean; vs, versus.
Figure 3.
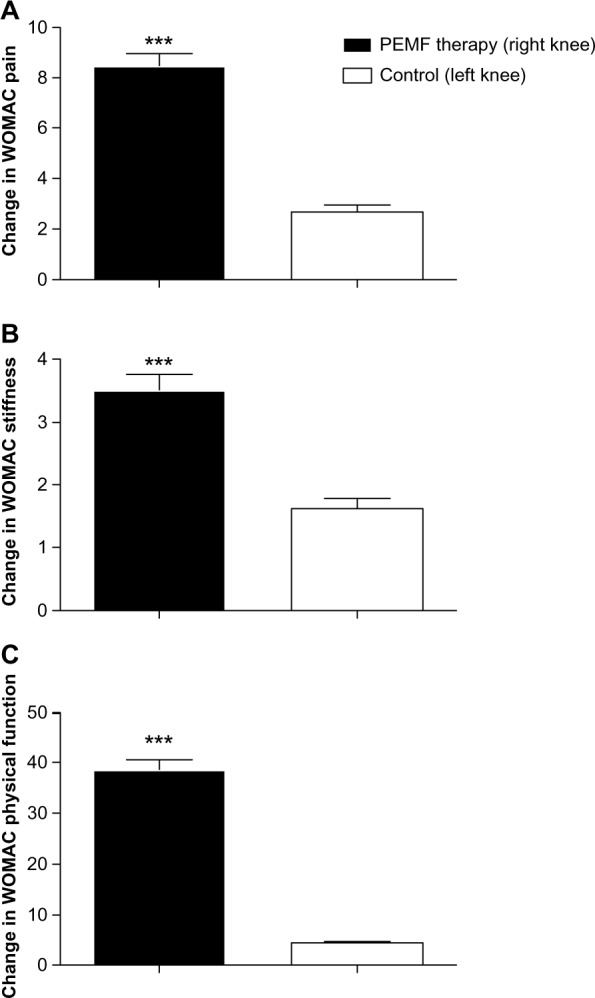
Change in knee-related WOMAC pain (A), stiffness (B), and physical function (C) for PEMF-treated leg vs control leg. Data are presented as the means ± SEM.
Note: ***P < 0.001.
Abbreviations: PEMF, pulsed electromagnetic field; SEM, Standard Error of the Mean; WOMAC, Western Ontario McMaster Universities Osteoarthritis Index; vs, versus.
Discussion
Experimental studies had previously shown that PEMF therapy produces an anabolic effect on the two key cell types in the skeletal system, ie, osteoblasts and chondrocytes23–26 that are involved in experimental and clinical OA. Furthermore, PEMF therapy possesses healing properties at the cellular level.27–31 In the present study we investigated the efficacy of PEMF therapy for management of knee OA-related pain, stiffness and physical function in elderly patients. We observed a significant improvement in all the above mentioned endpoints at the 3-month follow-up in the knee receiving PEMF therapy, if compared to the control knee without adverse events. Previous studies show contrasting results on the efficacy of PEMF therapy in the management of knee OA-related symptoms. Positive results, consistent with a significant improvement in activities of daily living, stiffness and pain following PEMF therapy, were reported in 83 patients affected by knee OA, if compared with control subjects at 6- and 12-week follow-up following a 6-week therapy.32 This evidence was confirmed by another study involving 34 patients affected by early knee OA, who experienced a 50% decrease in VAS pain starting at day 1 and persisting up to day 42.33 Findings from Fischer and coworkers showed positive results in 71 knee OA patients who underwent low-frequency PEMF therapy for 6 weeks.34 Patients had an increase in mobility and walking distance test, with long-term analgesic and functional effects even at 4 weeks after the end of treatment.34 A significant improvement in WOMAC score was also observed in 75 patients affected by knee OA, who received a 6-week PEMF therapy.35
Trock and colleagues also reported an improvement in pain and functional performance in patients affected by knee OA undergoing PEMF therapy for about 1 month, if compared to control group.15 In opposition to the studies mentioned above, Ozgüçlü and coworkers performed a study involving 40 patients undergoing PEMF therapy for 2 weeks and found no differences between sham and treated group concerning WOMAC pain, stiffness, and physical function scores.36 Ay and Evcik observed a significant improvement in pain in 55 patients affected by knee OA after hot pack/therapeutic ultrasound/PEMF therapy, but this improvement was also present in the sham group after five sessions per week for 2 weeks.37 In our study, we observed a slight decrease in VA S and WOMAC pain, stiffness, and physical function in the control knee likely due to the intravenous drip. Therefore, a therapy combining PEMF therapy and an intravenous drip containing ketoprofen, sodium clodronate, glucosamine sulfate, calcitonin and ascorbic acid may be helpful to provide increased and accelerated relief from knee OA-related symptoms.
Conclusion
PEMF therapy produces a significant benefit in terms of reduction in knee-related pain, stiffness, and physical function in elderly patients with knee OA. Further studies need to be designed to determine effectiveness of PEMF therapy in the long-term follow-up and clarify its mechanism.
Acknowledgments
The authors contributed equally to this work. This article was not supported by grants. Statistical support was provided by the Applied Statistics Lab (ASL) in cooperation with the Center for Clinical and Translational Science (CCTS). The CCTS is supported by grant number UL1TR000117.
Footnotes
Disclosure
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152–162. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iannitti T, Palmieri B. Inflammation and genetics: an insight in the centenarian model. Hum Biol. 2011;83(4):531–559. doi: 10.3378/027.083.0407. [DOI] [PubMed] [Google Scholar]
- 3.Fary RE, Carroll GJ, Briffa TG, Gupta R, Briffa NK. The effectiveness of pulsed electrical stimulation (E-PES) in the management of osteoarthritis of the knee: a protocol for a randomised controlled trial. BMC musculoskeletal disorders. 2008;9:18. doi: 10.1186/1471-2474-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennell KL, Hunter DJ, Hinman RS. Management of osteoarthritis of the knee. BMJ. 2012 Jul 30;345:e4934. doi: 10.1136/bmj.e4934. [DOI] [PubMed] [Google Scholar]
- 5.Iannitti T, Rosini S, Lodi D, Frediani B, Rottigni V, Palmieri B. Bisphosphonates: focus on inflammation and bone loss. Am J Ther. 2012;19(3):228–246. doi: 10.1097/MJT.0b013e318247148f. [DOI] [PubMed] [Google Scholar]
- 6.Roos EM, Juhl CB. Osteoarthritis 2012 year in review: rehabilitation and outcomes. Osteoarthritis Cartilage. 2012;20(12):1477–1483. doi: 10.1016/j.joca.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Palmieri B, Rottigni V, Iannitti T. Preliminary study of highly cross-linked hyaluronic acid-based combination therapy for management of knee osteoarthritis-related pain. Drug Des Devel Ther. 2013;7:7–12. doi: 10.2147/DDDT.S37330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iannitti T, Lodi D, Palmieri B. Intra-articular injections for the treatment of osteoarthritis: focus on the clinical use of hyaluronic acid. Drugs R D. 2011;11(1):13–27. doi: 10.2165/11539760-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iannitti T, Rottigni V, Palmieri B. A pilot study to compare two different hyaluronic acid compounds for treatment of knee osteoarthritis. Int J Immunopathol Pharmacol. 2012;25(4):1093–1098. doi: 10.1177/039463201202500426. [DOI] [PubMed] [Google Scholar]
- 10.Iannitti T, Elhensheri M, Bingöl AO, Palmieri B. Preliminary histopathological study of intra-articular injection of a novel highly cross-linked hyaluronic acid in a rabbit model of knee osteoarthritis. J Mol Histol. 2013 Apr;44(2):191–201. doi: 10.1007/s10735-012-9457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, Raitcheva D, Hawes M, Moran N, Yu X, Wang F, Matthews GL. Hylan G-F 20 maintains cartilage integrity and decreases osteophyte formation in osteoarthritis through both anabolic and anti-catabolic mechanisms. Osteoarthritis Cartilage. 2012 Nov;20(11):1336–1346. doi: 10.1016/j.joca.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Braund R, Abbott JH. Recommending NSAIDs and paracetamol: a survey of New Zealand physiotherapists’ knowledge and behaviours. Physiother Res Int. 2011;16(1):43–49. doi: 10.1002/pri.472. [DOI] [PubMed] [Google Scholar]
- 13.Assiotis A, Sachinis NP, Chalidis BE. Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature. J Orthop Surg Res. 2012;7:24. doi: 10.1186/1749-799X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boopalan PR, Arumugam S, Livingston A, Mohanty M, Chittaranjan S. Pulsed electromagnetic field therapy results in healing of full thickness articular cartilage defect. Int Orthop. 2011;35(1):143–148. doi: 10.1007/s00264-010-0994-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trock DH, Bollet AJ, Dyer RH, Jr, Fielding LP, Miner WK, Markoll R. A double-blind trial of the clinical effects of pulsed electromagnetic fields in osteoarthritis. J Rheumatol. 1993;20(3):456–460. [PubMed] [Google Scholar]
- 16.Trock DH, Bollet AJ, Markoll R. The effect of pulsed electromagnetic fields in the treatment of osteoarthritis of the knee and cervical spine. Report of randomized, double blind, placebo controlled trials. J Rheumatol. 1994;21(10):1903–1911. [PubMed] [Google Scholar]
- 17.van Bergen CJ, Blankevoort L, de Haan RJ, et al. Pulsed electromagnetic fields after arthroscopic treatment for osteochondral defects of the talus: double-blind randomized controlled multicenter trial. BMC Musculoskelet Disord. 2009;10:83. doi: 10.1186/1471-2474-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zizic TM, Hoffman KC, Holt PA, et al. The treatment of osteoarthritis of the knee with pulsed electrical stimulation. J Rheumatol. 1995;22(9):1757–1761. [PubMed] [Google Scholar]
- 19.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 20.Iannitti T, Rottigni V, Palmieri B. A pilot study to compare two different hyaluronic acid compounds for treatment of knee osteoarthritis. Int J Immunopathol Pharmacol. 2012;25(4):1093–1098. doi: 10.1177/039463201202500426. [DOI] [PubMed] [Google Scholar]
- 21.Baron G, Tubach F, Ravaud P, Logeart I, Dougados M. Validation of a short form of the Western Ontario and McMaster Universities Osteoarthritis Index function subscale in hip and knee osteoarthritis. Arthritis Rheum. 2007;57(4):633–638. doi: 10.1002/art.22685. [DOI] [PubMed] [Google Scholar]
- 22.Hughes R, Carr A. A randomized, double-blind, placebo-controlled trial of glucosamine sulphate as an analgesic in osteoarthritis of the knee. Rheumatology. 2002;41(3):279–284. doi: 10.1093/rheumatology/41.3.279. [DOI] [PubMed] [Google Scholar]
- 23.Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res. 2004;(419):30–37. doi: 10.1097/00003086-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 24.De Mattei M, Caruso A, Pezzetti F, et al. Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect Tissue Res. 2001;42(4):269–279. doi: 10.3109/03008200109016841. [DOI] [PubMed] [Google Scholar]
- 25.Diniz P, Soejima K, Ito G. Nitric oxide mediates the effects of pulsed electromagnetic field stimulation on the osteoblast proliferation and differentiation. Nitric Oxide. 2002;7(1):18–23. doi: 10.1016/s1089-8603(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 26.Pezzetti F, De Mattei M, Caruso A, et al. Effects of pulsed electromagnetic fields on human chondrocytes: an in vitro study. Calcif Tissue Int. 1999;65(5):396–401. doi: 10.1007/s002239900720. [DOI] [PubMed] [Google Scholar]
- 27.Ciombor DM, Aaron RK, Wang S, Simon B. Modification of osteoarthritis by pulsed electromagnetic field – a morphological study. Osteoarthritis Cartilage. 2003;11(6):455–462. doi: 10.1016/s1063-4584(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 28.De Mattei M, Pasello M, Pellati A, et al. Effects of electromagnetic fields on proteoglycan metabolism of bovine articular cartilage explants. Connect Tissue Res. 2003;44(3–4):154–159. [PubMed] [Google Scholar]
- 29.Fini M, Giavaresi G, Torricelli P, et al. Pulsed electromagnetic fields reduce knee osteoarthritic lesion progression in the aged Dunkin Hartley guinea pig. J Orthop Res. 2005;23(4):899–908. doi: 10.1016/j.orthres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Fini M, Torricelli P, Giavaresi G, et al. Effect of pulsed electromagnetic field stimulation on knee cartilage, subchondral and epyphiseal trabecular bone of aged Dunkin Hartley guinea pigs. Biomed Pharmacother. 2008;62(10):709–715. doi: 10.1016/j.biopha.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Rogachefsky RA, Altman RD, Markov MS, Cheung HS. Use of a permanent magnetic field to inhibit the development of canine osteoarthritis. Bioelectromagnetics. 2004;25(4):260–270. doi: 10.1002/bem.10192. [DOI] [PubMed] [Google Scholar]
- 32.Thamsborg G, Florescu A, Oturai P, Fallentin E, Tritsaris K, Dissing S. Treatment of knee osteoarthritis with pulsed electromagnetic fields: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage. 2005;13(7):575–581. doi: 10.1016/j.joca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Nelson FR, Zvirbulis R, Pilla AA. Non-invasive electromagnetic field therapy produces rapid and substantial pain reduction in early knee osteoarthritis: a randomized double-blind pilot study. Rheumatol Int. doi: 10.1007/s00296-012-2366-8. [Epub March 27, 2012] [DOI] [PubMed] [Google Scholar]
- 34.Fischer G, Pelka RB, Barovic J. [Adjuvant treatment of knee osteoarthritis with weak pulsing magnetic fields. Results of a placebo-controlled trial prospective clinical trial.] Z Orthop Ihre Grenzgeb. 2005;143(5):544–550. doi: 10.1055/s-2005-836830. German. [DOI] [PubMed] [Google Scholar]
- 35.Pipitone N, Scott DL. Magnetic pulse treatment for knee osteoarthritis: a randomised, double-blind, placebo-controlled study. Curr Med Res Opin. 2001;17(3):190–196. doi: 10.1185/0300799039117061. [DOI] [PubMed] [Google Scholar]
- 36.Ozgüçlü E, Cetin A, Cetin M, Calp E. Additional effect of pulsed electromagnetic field therapy on knee osteoarthritis treatment: a randomized, placebo-controlled study. Clin Rheumatol. 2010;29(8):927–931. doi: 10.1007/s10067-010-1453-z. [DOI] [PubMed] [Google Scholar]
- 37.Ay S, Evcik D. The effects of pulsed electromagnetic fields in the treatment of knee osteoarthritis: a randomized, placebo-controlled trial. Rheumatol Int. 2009;29(6):663–666. doi: 10.1007/s00296-008-0754-x. [DOI] [PubMed] [Google Scholar]


