Abstract
Patients with a strong family history of breast cancer are often counseled to receive genetic screening for BRCA1 and BRCA2 mutations, the strongest known predictors of breast cancer. A major limitation of genetic testing is the number of inconclusive results due to unclassified BRCA1 and BRCA2 sequence variants. Many known deleterious BRCA1 and BRCA2 mutations affect splicing, and these typically lie near intron/exon boundaries. However, there are also potential internal exonic mutations that disrupt functional exonic splicing enhancer (ESE) sequences, resulting in exon skipping. Using previously established sequence matrices for the scoring of putative ESE motifs, we have systematically examined several BRCA2 mutations for potential ESE disruption mutations. These predictions revealed that BRCA2 T2722R (8393C→G), which segregates with affected individuals in a family with breast cancer, disrupts three potential ESE sites. Reverse-transcriptase polymerase chain reaction analysis confirms that this mutation causes exon skipping, leading to an out-of-frame fusion of BRCA2 exons 17 and 19. This represents the first BRCA2 missense mutation shown to be a predicted deleterious protein-truncating mutation and suggests a potentially useful method for determining the clinical significance of a subset of the many unclassified variants in BRCA1 and BRCA2.
Breast cancer is a leading cause of cancer deaths among women and is expected to claim the lives of >40,000 individuals in the United States in 2002 (see “Breast Cancer Facts and Figures 2001–2002” at the American Cancer Society Web site). The majority of breast cancer cases occur sporadically, but 5%–10% of cases are caused by inherited mutations in the breast cancer–susceptibility genes BRCA1 (MIM 113705) and BRCA2 (MIM 600185). Mutations in BRCA1 and BRCA2 predict probabilities of breast cancer by age 70 years of 45%–87% and 26%–84%, respectively, making these the strongest predictors of breast cancer known (Ford et al. 1994, 1998; Struewing et al. 1997; Thorlacius et al. 1998; Antoniou 2000; Satagopan 2001). Thus, patients with a strong family history of breast and ovarian cancer are counseled to receive genetic testing for mutations in BRCA1 and BRCA2.
In the Breast Cancer Linkage Consortium data set (Ford et al. 1998), linkage to BRCA1 or BRCA2 is found in as many as 84% of patients with breast cancer in families with strong family history. However, in other data sets, the rates of deleterious mutations in BRCA1 or BRCA2 are lower, ranging from 16% to 26% for BRCA1 (Couch et al. 1997; Ganguly et al. 1997; Frank et al. 1998) and from 7% to 13% for BRCA2 (Ganguly et al. 1997; Frank et al. 1998). Mutation rates are even lower when family history is not used in data set–selection criteria (Krainer et al. 1997; Southey and Hopper 1999; Shih et al. 2002). Most reported disease-associated alleles of BRCA1 and BRCA2 are small insertions, deletions, or splice-site mutations that result in protein truncation. Only a small number of amino acid substitutions in either gene have been described as deleterious missense mutations, yet a very large number of different unclassified variant alleles are routinely encountered in clinical and research laboratories. In our recent study (J. D. Fackenthal, L. Sveen, Q. Gao, E. K. Kohlmeir, J. Jensen, C. Adebamowo, T. O. Ogundiran, A. A. Adenipekun, R. Oyesegun, O. Campbell, E. E. U. Akang, S. Das, and O. I. Olopade, unpublished data), 68% of BRCA2 sequences from a hospital-based cohort of Nigerian patients with breast cancer who were age 40 years or younger had variations of some kind, but only 14% could be classified as deleterious alleles or polymorphisms. Likewise, in our clinic-based cohort at the University of Chicago Cancer Risk Clinic, 30/89 (34%) patients tested had BRCA2 alterations of some kind, and 64% of these (16/25 different alleles) were either novel alleles or described as “unclassified” variants in the Breast Cancer Information Core (BIC) Web site (J. D. Fackenthal, L. Sveen, Q. Gao, E. K. Kohlmeir, J. Jensen, C. Adebamowo, T. O. Ogundiran, A. A. Adenipekun, R. Oyesegun, O. Campbell, E. E. U. Akang, S. Das, and O. I. Olopade, unpublished data). These findings are consistent with other reports that show high frequencies of BRCA2 variations but low frequencies of predicted deleterious protein-truncating BRCA2 mutations in patients with breast cancer, especially those of African ancestry (Wagner et al. 1999).
It is therefore necessary to define these unclassified variants functionally as deleterious missense alleles, low-penetrance alleles, or benign polymorphisms. Unfortunately, no generally accepted functional test for either BRCA1 or BRCA2 exists. To address the clinical relevance of a subset of these alleles, we are examining those base substitutions that might lead to splicing errors resulting in protein truncation. Most BRCA1 and BRCA2 mutations known to affect splicing lie at intron/exon boundaries. However, the Glu1694Ter allele of BRCA1, which carries a G→T transversion within exon 18, causes exon skipping that results in an in-frame splice between exons 17 and 19 (Mazoyer et al. 1998; Liu et al. 2001). This G→T base substitution disrupts a consensus exonic splicing enhancer (ESE) motif that is likely bound by the SF2/ASF serine/arginine-rich (SR) protein, one of several related proteins that bind ESEs to identify exonic sequences during pre-mRNA splicing. In addition, mutations that cause exon skipping without disrupting consensus splice-site sequences have been found in several disease-related genes, including CFTR, FACVIII, FANCC, FBN1, HPRT1, ATP7A, NF1, OAT, WASP, and HEXB, reviewed by Valentine (1998) and Cartegni et al. (2002). In each of these genes, one or more mutations associated with exon skipping disrupts a putative ESE motif, indicating that this may be a prevalent phenomenon in disease-related genes (Liu et al. 2001).
To determine whether other mutations associated with putative ESE motifs in BRCA1 or BRCA2 may be predicted from sequence analysis, we used previously established sequence matrices for scoring likely ESE motifs (Liu et al. 1998, 2000; Cartegni and Krainer 2002; Cartegni et al. 2002). In these studies, sequences with in vitro ESE activity were identified for several SR proteins, and consensus motifs were derived from these sequences. Matrices were then derived that assigned a score for each base in each position of the SR protein-binding site, as shown by Cartegni et al. (2002).
To identify candidates for ESE-disruption alleles, we examined 23 different exonic single-base BRCA2 substitutions identified in the University of Chicago Cancer Risk Clinic, where genetic testing is performed according to institutional-review-board–approved protocols after informed consent is obtained. These alleles were found in 41 individuals in 22 different families. Five of these alleles fell within predicted ESE sequences, and four of these base substitutions were predicted to disrupt ESE function. Of these, only one allele, BRCA2 T2722R, segregated with affected members of a family with breast cancer (family number 98-11). The other alleles occurred in families for which insufficient data were available to ascertain cosegregation or occurred in conjunction with known deleterious mutations that would mask the potential effects of deleterious ESE mutations.
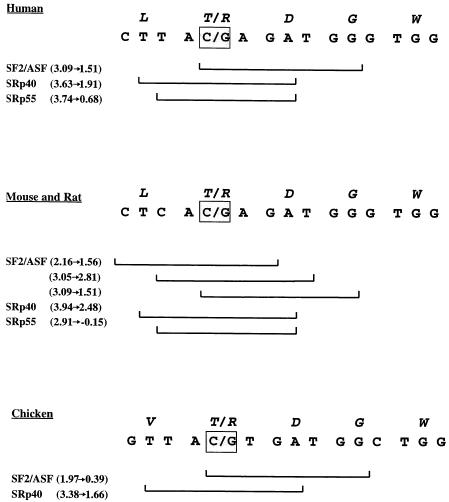
BRCA2 T2722R results from a C→G transition at base 8393 in BRCA2 exon 18. We determined that BRCA2 8393C→G lies within overlapping high-score motifs for three SR proteins: SF2/ASF, SRp40, and SRp55 (fig. 1). In all three cases, the C→G transversion decreases the motif score to a value well below the respective threshold (table 1). If any one of these potential SR protein motifs is a functional ESE, we predict that its disruption would cause an exon-skipping pre-mRNA splicing defect, resulting in an out-of-frame fusion between exons 17 and 19.
Figure 1.
The sequence surrounding the C→G transversion at BRCA2 base 8393 (gray square) in humans, and the homologous regions in mouse, rat, and chicken. BRCA2 T2722R results from a C→G transversion at this base. The top line (italics) in each panel represents the amino acid sequence, and the second line represents the base sequence. The brackets show high-scoring protein-binding motifs for the SR proteins listed on the left. Values in parentheses are the actual scores for the wild-type (left) and mutant (right) sequences for each motif. Note that there are three overlapping high-scoring SF2/ASF motifs for the mouse/rat sequence. The C→G transversion has been detected only in the present study.
Table 1.
BRCA2 8393C→G Disruption of Three High-Score ESE Motifs
| SR Protein | MotifThreshold | Wild-TypeScore | C→GScore |
| SF2/ASF | 1.956 | 3.09 | 1.51 |
| SRp40 | 2.670 | 3.63 | 1.91 |
| SRp55 | 2.676 | 3.74 | .68 |
To test directly whether the 8393C→G transversion disrupts functional ESE activity, we performed an RT-PCR assay using blood cell mRNA from patient III-2 (fig. 2). Ten milliliters of patient blood was diluted with 6 ml of PBS, was added to 3 ml of Ficoll (Amersham/Pharmacia Biotechnology), and was then spun for 15 min at 2,800 rpm at 18°C. B-lymphocytes were collected from the interface, counted, and checked for viability. The cells were washed with PBS several times, were resuspended in FBS (BioWhittaker), were added to a glycerol/Roswell Park Memorial Institute (RPMI; Invitrogen) buffer, and were frozen in liquid nitrogen. Thawed cells were processed using the Absolutely RNA RT-PCR Miniprep Kit, according to instructions provided by Stratagene. The RT-PCR was performed using the SuperScript One-Step RT-PCR with Platinum Taq, according to instructions provided by Life Technologies. Primers to exons flanking exon 18 were used: forward, TGGAAACTGGCAGCTATGG; and reverse, CCTCCTGAATTTTAGTGAATAAGGCTTCTAGTCTC. The thermocycling profile was as follows: RT reaction (50°C for 30 min, 94°C for 2 min), followed by a touchdown three-step PCR (95°C for 30 s; 57°C for 30 s, drop 0.5°C per cycle; 72°C for 45 s) for 14 cycles, followed by a three-step PCR (95°C for 30 s, 50°C for 30 s, 72°C for 45 s) for 35 cycles.
Figure 2.
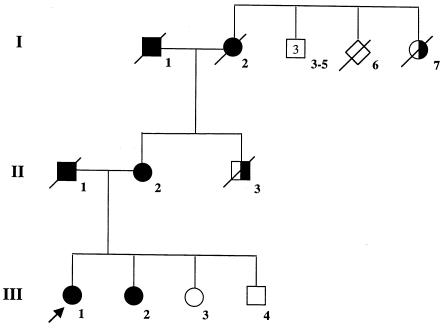
Family 98-11. Circles denote female individuals, squares denote male individuals, and diamonds denote individuals for whom sex was not reported. Slash marks indicate individuals who have died. The arrow indicates the proband. Blackened shapes denote affected individuals, unblackened shapes denote unaffected individuals, and half-blackened shapes denote individuals in whom the disease was reported but not confirmed. Causes of death are as follows: individual II-1, stage 4 non–small cell lung cancer; individual II-3, liver cancer; individual I-1, acute myocardial infarction; individual I-2, ovarian cancer; and individual I-7, unknown.
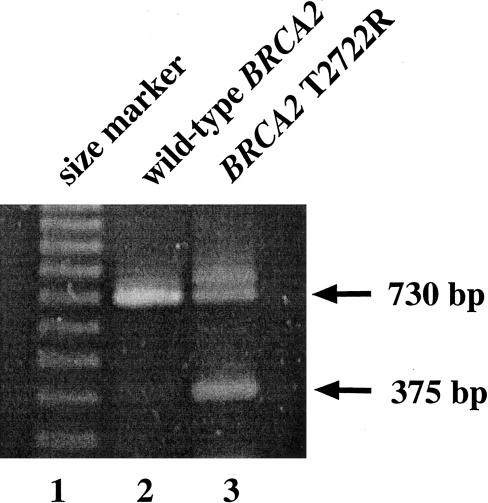
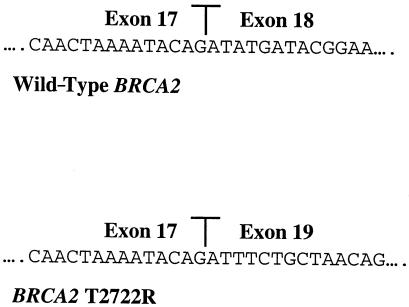
The expected full-length RT-PCR product is 730 bp, whereas the predicted product from mRNA that excludes exon 18 is 375 bp. Figure 3 shows the results of an RT-PCR using wild-type BRCA2 mRNA, which produces only a 730-bp fragment, whereas the same reaction using mRNA from the heterozygous patient III-2 yields both the full-length and the predicted truncated fragment. To show that this fragment represents an exon 18–skipping event, we cloned the PCR fragment and sequenced three independent isolates in both orientations. All three contained the predicted splice junction between BRCA2 exons 17 and 19 (fig. 4). The 3′ end of exon 17 falls between the second and third positions of codon 2659, and the 5′ end of exon 19 begins with the first base in codon 2778. The out-of-frame splice junction between exons 17 and 19 is predicted to add 42 novel codons to the 3′ end of codon 2659, followed by a stop codon. The predicted truncation removes 927 wild-type amino acids from the carboxy terminus of the BRCA2 protein and lies well upstream of other truncating mutations known to be functionally null (Tavtigian et al. 1996; Peto et al. 1999). Unfortunately, there was insufficient patient material available to demonstrate the truncated protein with western analysis.
Figure 3.
RT-PCR products from patient mRNA with wild-type BRCA2 (lane 2) or heterozygous for BRCA2 T2722R (lane 3).
Figure 4.
Sequence of the exon 17/exon 18 junction in wild-type BRCA2 (top) and of the exon17/exon 19 junction from the cloned 375-bp RT-PCR product from the BRCA2 T2722R heterozygote (bottom).
BRCA2 exon 18 is 355 bp long, and position 8393 lies in the middle of this sequence (base 189). The 8393C→G transversion therefore does not directly disrupt an intron/exon splice site (Padgett et al. 1986; Shapiro and Senapathy 1987), and no other base substitutions were detected in BRCA2 in this family. Premature stop codons often trigger nonsense-mediated mRNA decay (NMD) (Maquat 1995; Zhang and Maquat 1997; Zhang et al. 1998; Sun et al. 2001). If NMD did affect the truncated BRCA2 T2722R mRNA, it did not reduce message abundance below the level of detection.
Since the RT-PCR amplification reaction shown in figure 3 likely reached saturation kinetics, we are unable to determine the relative abundance of wild-type and truncated mRNA species. We propose that the BRCA2 8393C→G transversion disrupts a functional ESE, creating a misspliced message predicted to encode a truncated, nonfunctional protein. However, these data do not allow us to determine which of the three SR protein motifs is the functionally relevant sequence. Indeed, although it is unlikely that each motif can be recognized simultaneously, because of the overlap between them, it is possible that each motif is important in a different cell type, depending on the relative expression levels of SF2/ASF, SRp40, and SRp55 (Zahler et al. 1993; Hanamura et al. 1998). It is worth noting that putative SF2/ASF, SRp40, and SRp55 binding sites occur in BRCA2 homologs from mouse (GenBank accession number XM_124706) and rat (GenBank accession number NM_031542) at the position homologous to human BRCA2 8393 (fig. 1). Indeed, the mouse/rat sequence at this position carries three overlapping sites for SF2/ASF, one of which is not reduced below the threshold value by a hypothetical C→G transversion similar to the human BRCA2 T2722R allele. In the homologous BRCA2 sequence in chicken (GenBank accession number AY083934), putative binding sites for SF2/ASF and SRp40 are detected, but no SRp55 binding site is detected using motif scores derived in human systems. Although we cannot predict the SR protein-binding requirements for each tissue in each species, we suggest that the conservation of the SF2/ASF and SRp40 high-scoring motifs may reflect a conserved role in BRCA2 splicing regulation.
To determine whether the exon-skipping phenotype associated with BRCA2 T2722R segregates with cancer, we examined the occurrence of this allele in members of family 98-11 (fig. 2). The proband (III-1) was diagnosed at age 38 years with an intraductal and invasive left breast cancer. Histopathological analysis revealed a poorly differentiated ductal carcinoma of intermediate grade that was estrogen-receptor positive, progesterone-receptor negative, and had a DNA index of 1.77 and an S-phase fraction of 10.9%. In 1998, prior to definitive surgery, the patient underwent genetic testing consisting of complete sequencing of BRCA1 and BRCA2 at Myriad Genetics Laboratory. BRCA2 T2722R was reported and submitted to the BIC. At the time of analysis, this finding was inconclusive. Nevertheless, the patient elected to undergo a bilateral mastectomy, a total abdominal hysterectomy, and bilateral salpingo-oophorectomy, because of her extensive family history of breast/ovarian cancer. The proband’s sister (III-2) received diagnoses of breast cancer at age 36 years and of ovarian cancer at age 39 years. Their mother (II-2) received a diagnosis of breast cancer at age 53 years, and her mother (I-2) was found to have ovarian cancer at age 53 years. This family had other members with breast cancer (I-7 and not shown), as well as lung cancer (II-1 and not shown), liver cancer (II-3 and not shown), colon cancer (I-1), stomach cancer (not shown), and brain tumors (not shown). The presence of early-onset breast cancer, multiple breast cancers among first-degree relatives, and ovarian cancers, all in the same family, is a strong indicator of a hereditary breast cancer syndrome (Frank et al. 1998; Malone et al. 1998) and are consistent with a deleterious mutation in either BRCA1 or BRCA2. BRCA2 T2722R segregated with affected family members II-2, III-1, and III-2, consistent with the hypothesis that BRCA2 T2722R is a deleterious allele. Unfortunately, the deleterious nature of this allele cannot be demonstrated more rigorously by loss-of-heterozygosity analysis of breast tumors from affected individuals, because no tumors are available from this family. Since this allele has never been detected previously and is likely to be very rare, it would not be practical to attempt to determine its relative frequency in a large cohort of affected and unaffected populations.
To our knowledge, these results provide the first evidence for a BRCA2 missense mutation whose deleterious effect could be due to disruption of a functional ESE. Unlike the previously described BRCA1 E1694X ESE-disrupting mutation (Mazoyer et al. 1998), BRCA2 T2722R was initially thought to be an amino acid substitution variant, not a suspected truncating allele.
Deleterious mutations in BRCA1 and BRCA2 are found in 27%–47% of patients with breast cancer who have strong family histories of the disease, depending on the selection criteria (Ganguly et al. 1997; Frank et al. 1998; Lancaster et al. 1998). The remaining familial breast cancers may be associated with mutations in an as-yet-undiscovered BRCA3 gene or may arise from complex genetic interactions between susceptibility genes with limited penetrance and genetic modifiers. However, it remains possible that a number of the cases reported as unclassified variants for BRCA1 or BRCA2 actually represent deleterious mutations. Indeed, one study showed that only 63% of the families with breast cancer that show linkage to BRCA1 were associated with definite deleterious mutations in BRCA1 (Ford et al. 1998). One possible reason for this discrepancy may be that inherited genomic rearrangements involving the BRCA1 gene cannot be detected by direct sequencing. Indeed, several studies have reported BRCA1 deletions and frameshift-generating duplications that cannot be detected by conventional PCR-based assays (Petrij-Bosch et al. 1997; Puget et al. 1997, 1999; Swensen et al. 1997; Montagna et al. 1999; Unger et al. 2000). Nonetheless, we propose that another potential reason for the underreporting of deleterious BRCA1/2 mutations may be the presence of unrecognized ESE-disrupting mutations among unclassified BRCA1/2 variants. We anticipate that the use of predictive models for splicing mutations, coupled with RT-PCR and/or protein analysis (when available), will allow proper characterization of the biological relevance of some of these alleles. This approach, which does not require a functional assay for the altered protein, may also allow reclassification of some “unclassified variants” when additional family members are not available for testing.
Predicted changes in protein primary structure often help elucidate protein functional defects, especially when genetic changes result in protein truncation or amino acid substitutions in well-characterized functional domains. The work presented here suggests that one should consider the possibility of splicing defects and codon changes when predicting the effects of base substitutions. Because ESE sequences participate in exon definition during splicing, a predicted silent base change could in fact be a truncating mutation. Likewise, a predicted nonsense or frameshifting mutation could result in an in-frame junction between flanking exons, generating a nonnull allele.
Previous studies have shown that mutated ESEs in other genes cause a limited amount of exon skipping (Shiga 1997; Stickeler 2001; Moseley 2002). This suggests that ESE mutations that result in significant protein truncations or deletions may not always be functionally null but may represent alleles with limited penetrance. As discussed above, we cannot preclude the possibility that exon 18 from BRCA2 T2722R pre-mRNAs are occasionally spliced correctly and are included in mature mRNAs. If, as we suspect, BRCA2 T2722R has a deleterious effect, this could therefore result partly from a loss of full-length BRCA2 mRNA and partly from a protein-disrupting effect of the threonine-to-arginine amino acid substitution. Indeed, as shown in figure 1, both the putative ESE sequences and the amino acids that they encode are conserved between human, mouse, rat, and chicken. We cannot determine, from available data, whether the selective pressure to retain these sequences acts at the level of splicing regulation, amino acid identity, or both.
Several putative ESE sequences have been found in exons where they have been sought systematically, raising the possibility of functional redundancy. This may diminish the potential exon-skipping effect of a mutation in any one ESE. However, in cases where 3–10 putative ESE sequences occur within a single exon, a single ESE-disrupting base substitution can lead to efficient exon skipping (Liu et al. 2001; Cartegni and Krainer 2002). This suggests that, at least in come cases, individual ESEs may be critical for splicing even when other ESEs are present in the same exon. Although it is difficult to predict the prevalence of ESE-disrupting mutations among known BRCA1 or BRCA2 sequence variants, our recent study of a Nigerian cohort with breast cancer suggests that it might be highly prevalent (J. D. Fackenthal, L. Sveen, Q. Gao, E. K. Kohlmeir, J. Jensen, C. Adebamowo, T. O. Ogundiran, A. A. Adenipekun, R. Oyesegun, O. Campbell, E. E. U. Akang, S. Das, and O. I. Olopade, unpublished data). In that study, 5 of 20 (25%) BRCA2 exonic base substitutions identified in a cohort from Nigeria with early-onset breast cancer represented potential ESE disruptions (no RNA samples are available from these patients for RT-PCR analysis). Future work will determine whether ESE-disrupting mutations in BRCA1 and BRCA2 explain genetic susceptibility to breast cancer in additional families with variants of uncertain significance.
Acknowledgments
We are grateful to Donna L. Fackenthal, for her assistance with DNA sequencing; Lise Sveen, for critical reading of this manuscript; and the family that participated in this study. This work was supported by a grant from the Falk Medical Trust, as well as by grants from the National Women’s Cancer Research Alliance and the U.S. Army Department of Defense (to O.I.O), and by National Center for Research Resources grant MO1 RR00055 (to the University of Chicago Clinical Research Center). O.I.O. is a Doris Duke Distinguished Clinical Scientist. L.C. and A.R.K. were supported, in part, by National Cancer Institute grant CA13106.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- American Cancer Society: Breast Cancer Facts and Figures 2001–2002, http://www.cancer.org/eprise/main/docroot/stt/content/STT_1x_Breast_Cancer_Facts_and_Figures_2001-2002
- Breast Cancer Information Core, http://www.nhgri.nih.gov/Intramural_research/Lab_transfer/Bic/
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for BRCA2 from mouse [accession number XM_124706], rat [accession number NM_031542], and chicken [accession number AY083934])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for BRCA1 [MIM 113705] and BRCA2 [MIM 600185])
References
- Antoniou AC (2000) Risk models for familial ovarian and breast cancer. Genet Epidemiol 18:173–190 [DOI] [PubMed] [Google Scholar]
- Cartegni L, Chew S, Krainer A (2002) Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet 3:285–300 [DOI] [PubMed] [Google Scholar]
- Cartegni L, Krainer AR (2002) Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat Genet 30:377–384 [DOI] [PubMed] [Google Scholar]
- Couch FJ, DeShano ML, Blackwood MA, Calzone K, Stopfer J, Campeau L, Ganguly A, Rebbeck T, Weber BL (1997) BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 336:1409–1415 [DOI] [PubMed] [Google Scholar]
- Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE (1994) Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 343:692–695 [DOI] [PubMed] [Google Scholar]
- Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, et al (1998) Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 62:676–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank TS, Manley SA, Olopade OI, Cummings S, Garber JE, Bernhardt B, Antman K, et al (1998) Sequence analysis of BRCA1 and BRCA2: correlation of mutations with family history and ovarian cancer risk. J Clin Oncol 16:2417–2425 [DOI] [PubMed] [Google Scholar]
- Ganguly A, Leahy K, Marshall AM, Dhulipala R, Godmilow L, Ganguly T (1997) Genetic testing for breast cancer susceptibility: frequency of BRCA1 and BRCA2 mutations. Genet Test 1:85–90 [DOI] [PubMed] [Google Scholar]
- Hanamura A, Caceres JF, Mayeda A, Franza BR, Krainer AR (1998) Regulated tissue-specific expression of antagonistic pre-mRNA splicing factors. RNA 4:430–444 [PMC free article] [PubMed] [Google Scholar]
- Krainer M, Silva AS, FitzGerald MG, Shimada A, Ishioka C, Kanamaru R, MacDonald DJ, Unsal H, Finkelstein DM, Bowcock A, Isselbacher KJ, Haber DA (1997) Differential contributions of BRCA1 and BRCA2 to early-onset breast cancer. N Engl J Med 336:1416–1421 [DOI] [PubMed] [Google Scholar]
- Lancaster JM, Carney ME, Gray J, Myring J, Gumbs C, Sampson J, Wheeler D, France E, Wiseman R, Harper P, Futreal PA (1998) BRCA1 and BRCA2 in breast cancer families from Wales: moderate mutation frequency and two recurrent mutations in BRCA1. Br J Cancer 78:1417–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, Cartegni L, Zhang MQ, Krainer AR (2001) A mechanism for exon skipping caused by nonsense or missense mutations in BRCA1 and other genes. Nat Genet 27:55–58 [DOI] [PubMed] [Google Scholar]
- Liu HX, Chew SL, Cartegni L, Zhang MQ, Krainer AR (2000) Exonic splicing enhancer motif recognized by human SC35 under splicing conditions. Mol Cell Biol 20:1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, Zhang M, Krainer AR (1998) Identification of functional exonic splicing enhancer motifs recognized by individual SR proteins. Genes Dev 12:1998–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone KE, Daling JR, Thompson JD, O'Brien CA, Francisco LV, Ostrander EA (1998) BRCA1 mutations and breast cancer in the general population: analyses in women before age 35 years and in women before age 45 years with first-degree family history. JAMA 279:922–929 [DOI] [PubMed] [Google Scholar]
- Maquat LE (1995) When cells stop making sense: effects of nonsense codons on RNA metabolism in vertebrate cells. RNA 1:453–465 [PMC free article] [PubMed] [Google Scholar]
- Mazoyer S, Puget N, Perrin-Vidoz L, Lynch HT, Serova-Sinilnikova OM, Lenoir GM (1998) A BRCA1 nonsense mutation causes exon skipping. Am J Hum Genet 62:713–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna M, Santacatterina M, Torri A, Menin C, Zullato D, Chieco-Bianchi L, D'Andrea E (1999) Identification of a 3 kb Alu-mediated BRCA1 gene rearrangement in two breast/ovarian cancer families. Oncogene 18:4160–4165 [DOI] [PubMed] [Google Scholar]
- Moseley CT (2002) An exon splice enhancer mutation causes autosomal dominant GH deficiency. J Clin Endocrinol Metab 87:847–852 [DOI] [PubMed] [Google Scholar]
- Padgett RA, Grabowski PJ, Konarska MM, Seiler S, Sharp PA (1986) Splicing of messenger RNA precursors. Ann Rev Biochem 55:1119–1150 [DOI] [PubMed] [Google Scholar]
- Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, Easton DF, Evans C, Deacon J, Stratton MR (1999) Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 91:943–949 [DOI] [PubMed] [Google Scholar]
- Petrij-Bosch A, Peelen T, van Vliet M, van Eijk R, Olmer R, Drusedau M, Hogervorst FB, Hageman S, Arts PJ, Ligtenberg MJ, Meijers-Heijboer H, Klijn JG, Vasen HF, Cornelisse CJ, van't Veer LJ, Bakker E, van Ommen GJ, Devilee P (1997) BRCA1 genomic deletions are major founder mutations in Dutch breast cancer patients. Nat Genet 17:341–345 (erratum 17:503 [1997]) [DOI] [PubMed] [Google Scholar]
- Puget N, Stoppa-Lyonnet D, Sinilnikova OM, Pages S, Lynch HT, Lenoir GM, Mazoyer S (1999) Screening for germ-line rearrangements and regulatory mutations in BRCA1 led to the identification of four new deletions. Cancer Res 59:455–461 [PubMed] [Google Scholar]
- Puget N, Torchard D, Serova-Sinilnikova OM, Lynch HT, Feunteun J, Lenoir GM, Mazoyer S (1997) A 1-kb Alu-mediated germ-line deletion removing BRCA1 exon 17. Cancer Res 57:828–831 [PubMed] [Google Scholar]
- Satagopan JM (2001) The lifetime risks of breast cancer in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Gynecol Oncol 80:395–398 [PubMed] [Google Scholar]
- Shapiro MB, Senapathy P (1987) RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res 15:7155–7174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga N (1997) Disruption of the splicing enhancer sequence within exon 27 of the dystrophin gene by a nonsense mutation induces partial skipping of the exon and is responsible for Becker muscular dystrophy. J Clin Invest 100:2204–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HA, Couch FJ, Nathanson KL, Blackwood MA, Rebbeck TR, Armstrong KA, Calzone K, Stopfer J, Seal S, Stratton MR, Weber BL (2002) BRCA1 and BRCA2 mutation frequency in women evaluated in a breast cancer risk evaluation clinic. J Clin Oncol 20:994–999 [DOI] [PubMed] [Google Scholar]
- Southey MC, Hopper JL (1999) BRCA1 mutations and other sequence variants in a population-based sample of Australian women with breast cancer. Population-based estimate of the average age-specific cumulative risk of breast cancer for a defined set of protein-truncating mutations in BRCA1 and BRCA2. Australian Breast Cancer Family Study. Br J Cancer 79:34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickeler E (2001) The RNA binding protein YB-1 binds A/C-rich exon enhancers and stimulates splicing of the CD44 alternative exon v4 modulation of soluble CD44 concentrations by hormone and anti-hormone treatment in gynecological tumor cell lines. EMBO J 20:3821–3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA (1997) The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 336:1401–1408 [DOI] [PubMed] [Google Scholar]
- Sun X, Li X, Moriarty PM, Henics T, LaDuca JP, Maquat LE (2001) Nonsense-mediated decay of mRNA for the selenoprotein phospholipid hydroperoxide glutathione peroxidase is detectable in cultured cells but masked or inhibited in rat tissues. Mol Biol Cell 12:1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen J, Hoffman M, Skolnick MH, Neuhausen SL (1997) Identification of a 14 kb deletion involving the promoter region of BRCA1 in a breast cancer family. Hum Mol Genet 6:1513–1517 [DOI] [PubMed] [Google Scholar]
- Tavtigian SV, Simard J, Rommens J, Couch F, Shattuck ED, Neuhausen S, Merajver S, et al (1996) The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet 12:333–337 [DOI] [PubMed] [Google Scholar]
- Thorlacius S, Struewing JP, Hartge P, Olafsdottir GH, Sigvaldason H, Tryggvadottir L, Wacholder S, Tulinius H, Eyfjord JE (1998) Population-based study of risk of breast cancer in carriers of BRCA2 mutation. Lancet 352:1337–1339 [DOI] [PubMed] [Google Scholar]
- Unger MA, Nathanson KL, Calzone K, Antin-Ozerkis D, Shih HA, Martin AM, Lenoir GM, Mazoyer S, Weber BL (2000) Screening for genomic rearrangements in families with breast and ovarian cancer identifies BRCA1 mutations previously missed by conformation-sensitive gel electrophoresis or sequencing. Am J Hum Genet 67:841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine CR (1998) The association of nonsense codons with exon skipping. Mutat Res 411:87–117 [DOI] [PubMed] [Google Scholar]
- Wagner TM, Hirtenlehner K, Shen P, Moeslinger R, Muhr D, Fleischmann E, Concin H, Doeller W, Haid A, Lang AH, Mayer P, Petru E, Ropp E, Langbauer G, Kubista E, Scheiner O, Underhill P, Mountain J, Stierer M, Zielinski C, Oefner P (1999) Global sequence diversity of BRCA2: analysis of 71 breast cancer families and 95 control individuals of worldwide populations. Hum Mol Genet 8:413–423 (erratum 8:717–719 [1999]) [DOI] [PubMed] [Google Scholar]
- Zahler AM, Neugebauer KM, Lane WS, Roth MB (1993) Distinct functions of SR proteins in alternative pre-mRNA splicing. Science 260:219–222 [DOI] [PubMed] [Google Scholar]
- Zhang J, Maquat LE (1997) Evidence that translation reinitiation abrogates nonsense-mediated mRNA decay in mammalian cells. EMBO J 16:826–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Sun X, Qian Y, LaDuca JP, Maquat LE (1998) At least one intron is required for the nonsense-mediated decay of triosephosphate isomerase mRNA: a possible link between nuclear splicing and cytoplasmic translation. Mol Cell Biol 18:5272–5283 [DOI] [PMC free article] [PubMed] [Google Scholar]