Abstract
Hypertension is a major risk factor for increased cardiovascular events with accelerated sympathetic nerve activity implicated in the pathogenesis and progression of disease. Blood pressure is not adequately controlled in many patients, despite the availability of effective pharmacotherapy. Novel procedure- as well as device-based strategies, such as percutaneous renal sympathetic nerve denervation, have been developed to improve blood pressure in these refractory patients. Renal sympathetic denervation not only reduces blood pressure but also renal as well as systemic sympathetic nerve activity in such patients. The reduction in blood pressure appears to be sustained over 3 years after the procedure, which suggests absence of re-innervation of renal sympathetic nerves. Safety appears to be adequate. This approach may also have potential in other disorders associated with enhanced sympathetic nerve activity such as congestive heart failure, chronic kidney disease and metabolic syndrome.
This review will focus on the current status of percutaneous renal sympathetic nerve denervation, clinical efficacy and safety outcomes and prospects beyond refractory hypertension.
Keywords: denervation, hypertension, renal
Introduction
Treatment-resistant hypertension is commonly found in both community-based samples as well as databases from large scale clinical trials [1]. The general definition of treatment-resistant hypertension is that of a patient who is unable to reach guideline recommended target blood pressure despite three or more antihypertensive drug classes at the highest tolerated doses, with one of those drugs being a diuretic [1, 2]. Most recent analysis of the NHANES dataset, a large community-based cohort in the US, suggests that 8.9% of hypertensives meet the strict criteria for treatment-resistant hypertension [3]. A recent study by de la Sierra et al. [4] used ambulatory blood pressure monitoring (ABPM) to identify patients with resistant hypertension and found that out of 68 045 treated patients 8295 (12.2% of the database) had resistant hypertension defined as office blood pressure ≥140 and/or 90 mmHg while being treated with more than three antihypertensive drugs. After ABPM, 62.5% of patients were classified as true resistant hypertensives, the remaining 37.5% having white coat resistance Therefore, true resistant hypertension appears to be evident in around 8–9% of a treated hypertensive population.
Other analyses, e.g. from major clinical trials of antihypertensive therapies, suggest that the percentage may be even higher. In any event, it is clear that given the extremely high overall prevalence of hypertension in the adult population (expected to increase further over the next few decades, particularly in emerging countries), treatment-resistant hypertension represents a major health problem.
Causes of treatment-resistant hypertension are multiple but include secondary causes of hypertension, interfering substances, poor compliance and adherence as well as therapeutic inertia [5]. Use of inappropriate antihypertensive drug combinations is a prominent cause [5]. Furthermore, some patients are intolerant to single or multiple antihypertensive drug therapies due to intolerable adverse events.
There have been very few properly conducted randomized control trials of add-on antihypertensive therapy in the treatment-resistant hypertension setting. One agent that has been studied is the mineralocorticoid receptor antagonist (MRA), spironolactone. In the ASPIRANT study [6], a significant reduction in both 24 h ambulatory and office systolic blood pressure recordings (−9.8 and −5.4 mmHg, respectively) were noted with spironolactone (n = 59) compared with placebo (n = 56), additional to maximized background antihypertensive therapy. These findings suggest that an MRA add-on should at least be considered in patients who are resistant to standard antihypertensive agents provided that renal function is relatively well preserved and serum potassium closely monitored.
If all of the above considerations have been exhausted in the management of the patient there now exists the potential for procedure/device-based approaches to treatment-resistant hypertension. This review will focus on renal sympathetic nerve ablation as one such procedure-based approach. Before discussing this approach, the pathophysiology underlying the sympathetic contribution to hypertension will be considered.
Sympathetic nervous system in hypertension
The contribution of sympathetic activation to the genesis and progression of hypertension has been well recognized for many decades [7]. This contribution appears to be particularly prominent in younger hypertensives. Smith et al. [8] demonstrated a step-up in muscle sympathetic nerve activity from normal to high-normal blood pressure, to white coat to borderline to then established hypertension, with or without left ventricular hypertrophy. These data are supported by other measures of sympathetic activation in man, specifically spillover of norepinephrine into plasma. In a study of renal spillover from the kidneys, a significant increase was noted in patients with essential hypertension in comparison with normotensive controls [9]. This increase was particularly prominent in those hypertensives aged 20 to 39 years.
Distribution of efferent and afferent sympathetic nerves
Renal efferent sympathetic activity
The kidneys are extensively innervated by renal sympathetic efferent nerves, i.e. nerves transmitting from the central nervous system and acting upon the kidney [10]. The functional effect of these renal sympathetic efferents relates to modulation of autonomic control of the kidney. Specifically, renin secretion is activated by β1-adrenoceptor stimulation, enhanced tubular sodium reabsorption by α1b-adrenoceptors and reduced renal blood flow via α1a-adrenoceptors [11] (Figure 1). Thus, the functional consequences of this innervation are critical to renal control of regulatory hormones, modulation of total body volume status and effects on the pressure-natriuresis curve. Elegant pre-clinical in vivo experiments by DiBona et al. [12] have established that for a given renal perfusion pressure, renal sympathetic denervation shifts the diuresis and natriuresis curves to the left, i.e. an increase in water and sodium excretion for the same renal perfusion pressure is achieved in the denervated compared with the innervated animal.
Figure 1.
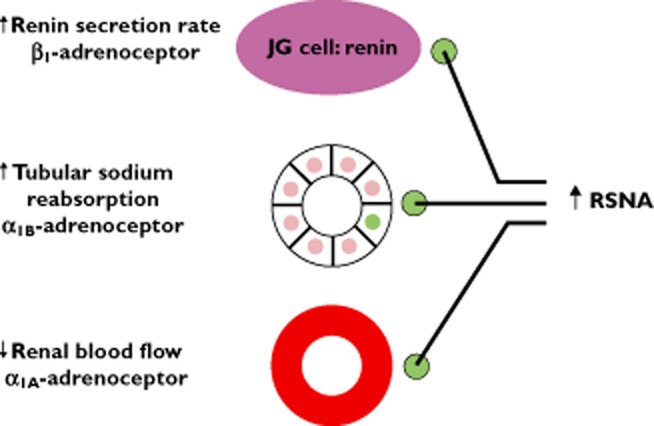
Effect of renal sympathetic efferents on renal function. RSNA = renal sympathetic nerve activity, JG = juxtaglomerular
Based on these pathophysiological considerations, abrogation or disruption of renal sympathetic efferents represents an attractive therapeutic target in the management of hypertension (and potentially other disorders characterized by altered renal sympathetic nerve activity). This has been supported through the pre-clinical literature in various low and high renin models of hypertension in animals [13].
Renal afferent sympathetic activity
The regions proximate to the kidney are highly innervated by mechano-sensitive and chemo-sensitive nerve receptors [14]. Renal afferent nerves transmit this information to the central sympathetic nervous system which in turn modulates activity of key organs including heart, kidney and vasculature (Figure 2). These findings are supported by rhizotomy experiments in animals demonstrating ability to reduce blood pressure in animals with renal disease [15]. These animals demonstrate increased central catecholamine concentrations compared with healthy controls and such increases are abrogated by renal afferent denervation. Similarly, in renal transplant patients denervation via nephrectomy of the non-functioning kidney reduced both renal sympathetic efferent activity and blood pressure [16].
Figure 2.
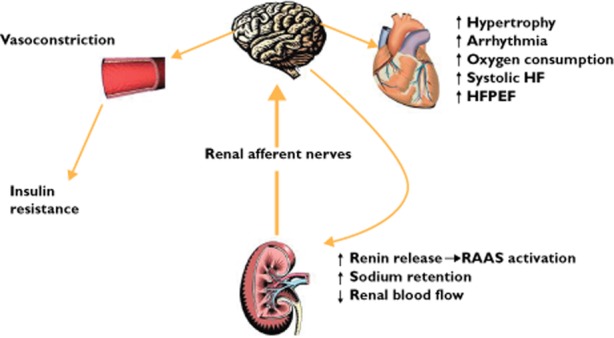
Effect of renal afferents on central nervous system and in turn other key organs. HF = heart failure; HFEPF = heart failure with preserved ejection fraction; RAAS = renin-angiotensin-aldosterone
Surgical sympathetic denervation in the management of hypertension
In the era preceding the emergence of modern antihypertensive pharmacotherapy surgical denervation was perhaps the only effective approach to treating patients with significant elevations in blood pressure. Case series comparing this surgical approach with medical therapies (such as existed) demonstrated a roughly 50% improvement in survival with denervation for the same starting blood pressure values [17]. The magnitude of the blood pressure reduction able to be achieved appeared to correlate with the starting pre-operative mean blood pressure. However, these early and rather crude approaches to unselected sympathetic denervation were accompanied by significant adverse events limiting their clinical utility. In particular, patients experienced impotence, incontinence and, almost invariably, orthostatic hypotension, essentially rendering them unable to achieve upright posture for significant periods of time [18].
Percutaneous and minimally invasive approaches to renal sympathetic denervation
A number of newer approaches have been developed to achieve specifically renal sympathetic denervation, but to avoid the complications of the earlier surgical approaches, as outlined above. Most of these approaches focus on the sympathetic nerve plexus that surrounds the main trunk of each renal artery. These nerves reside within the adventitia of the main artery or immediately adjacent. These novel approaches include various approaches to radiofrequency (RF) energy application, use of ultrasound waves, direct injection of neurotoxins such as guanethidine and even extracorporeal approaches that are completely non-invasive.
By far the most advanced and best investigated of these strategies is that of percutaneous RF ablation [10] (Figure 3). This procedure involves cannulation of the femoral artery and subsequent placement of the tip of the catheter in the distal renal artery where energy is applied targeting adjacent sympathetic nerve trunks. The catheter is then withdrawn 1–2 cm and circumferentially rotated with further RF energy applications performed in this way, such that 4–6 on average (often more) are applied to the individual renal artery. The same procedure then occurs in the contralateral main renal artery.
Figure 3.
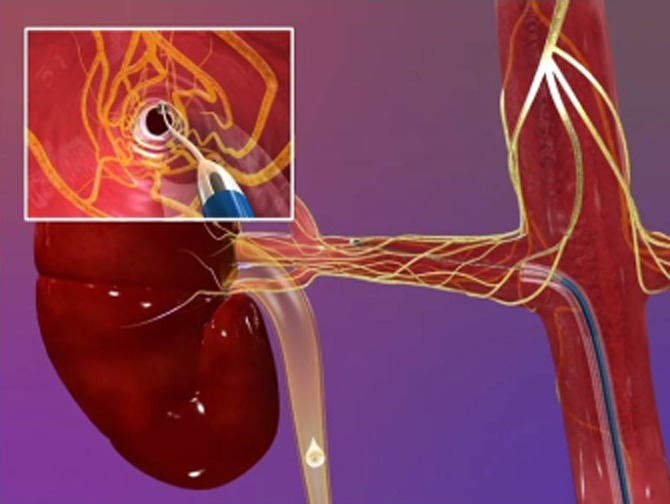
Medtronic/Ardian percutaneous approach to renal sympathetic denervation. Via a femoral artery approach, the distal tip of the catheter is placed (as per Figure) in the distal portion of the renal artery and initial radiofrequency energy applied
Safety data
Initial studies focused primarily on safety of the procedure [19]. From the very first procedure it was noted that diffuse visceral pain occurred in concert with the application of RF energy. These findings suggest that somatic afferent C-fibres travel with the sympathetic nerves which were the targets of the ablation. Subsequent to this observation, patients now routinely receive prophylactic intravenous analgesia and/or sedation. Imaging studies including magnetic resonance angiography (MRA) and computerized tomography (CTA) have indicated absence of atherosclerotic responses to the RF energy application in denervated arteries. This imaging was undertaken both early (1–2 weeks post-procedure) and late (approximately 6 months post-procedure) in these early studies.
The initial safety experience described one episode of renal artery dissection during the catheter procedure (but before application of RF energy) which was subsequently successfully stented. There have also been a number of cases of impaired haemostasis in the groin but at a rate consistent with other arterial cannulation procedures involving the femoral artery.
A theoretical concern is that of renal artery stenosis. No such adverse events have been reported in the Symplicity experience thus far. Nevertheless, and as mentioned, alternative technologies have been developed in an attempt to minimize this and other potential local complications. These include use of multi-electrode catheters to minimize time of catheter in the vessel and ultrasound based approaches to minimize endothelial damage. Furthermore, there have been no cases of vessel thrombosis or kidney embolization reported.
Another safety concern has been that of potential of worsening of renal function itself. This has not been observed in early studies or indeed in the published Symplicity HTN 2 trial [20] vs. a control group.
Efficacy results
Symplicity hypertension I study
Symplicity HTN1 [19] was a 12 months evaluation of safety and blood pressure-lowering efficacy (without a control group) as a first-in-man experience with the denervation procedure. Inclusion criteria involved patients with a systolic blood pressure greater than 160 mmHg despite three or more antihypertensive medications including a diuretic or confirmed intolerance to medications. Furthermore, estimated glomerular filtration rate (eGFR) was required to be >45 ml min−1 1.73 m−2. The key exclusion criteria included known secondary causes of hypertension, type I diabetes mellitus, central sympatholytic drug use and critically evidence of renovascular abnormalities, including renal artery stenosis, prior renal procedure and/or dual renal arteries.
Enrolled patients in fact tended to have considerably poorer blood pressure control than the entry criteria cut-off demanded. Mean blood pressure was over 170 mmHg systolic and 100 mmHg diastolic. This was despite on average, five different antihypertensive drug classes being used in an attempt to control blood pressure. Almost all patients were taking an ACE inhibitor and/or angiotensin receptor blocker as well as diuretics, 69% were receiving calcium channel blockers and 75% β-adrenoceptor blockers.
The key blood pressure results of Symplicity HTN1 were a 27/17 mmHg reduction in blood pressure compared with baseline at the 12 months end of the formal study evaluation period. This was supported by limited ABPM data that included an increase in patients shifting from non-dipper to dipper status with the procedure. However the magnitude of the ABPM response to denervation in this study (and Symplicity HTN-2) was substantially less than that of office blood pressure falls, suggesting a white coat component may be contributory to the observed office response.
The key mechanistic question was whether sympathetic denervation had in fact been achieved in the kidney. This had been demonstrated pre-clinically where an 85% reduction in total renal norepinephrine content was observed in the percutaneously denervated kidneys of studied animals (data on file, Ardian). The magnitude of that reduction was similar to that achieved with conventional surgical approaches. In man, evidence of renal denervation was observed with a substantial reduction in renal norepinephrine spillover rate in a published case study where blood pressure was also decreased and muscle sympathetic nerve activity (indicative of efferent sympathetic output) was also progressively reduced out to 12 months [21]. Sympathetic nerve activity reduction following renal denervation has now been confirmed in a larger series [22]. However, it remains uncertain at the time of the procedure whether denervation has been successfully achieved, as there are as yet no simple clinical tools to address this question.
The Symplicity HTN-1 experience has now been extended out to 36 months in a larger cohort than the initial published 12 month experience [23]. One hundred and fifty-three patients have been followed in this way but with only 24 actually reaching the 36 months follow-up visit. Nonetheless, the mean reduction in blood pressure persisted, with a mean 33/19 mmHg reduction compared with baseline at 36 months (Figure 4). This is consistent with the surgical experience where many years of improved blood pressure control are observed following the surgical intervention.
Figure 4.
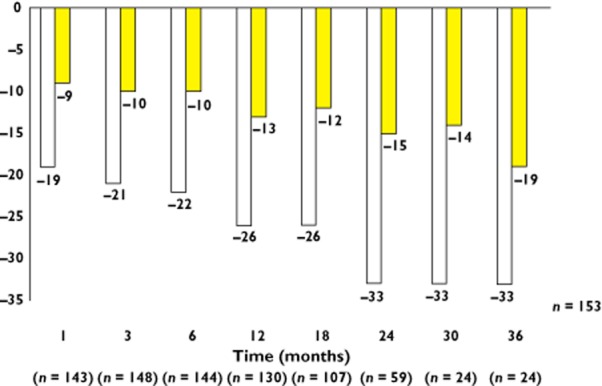
Expanded 36 month blood pressure results from the Symplicity HTN-1 study.  , systolic BP;
, systolic BP;  , diastolic BP
, diastolic BP
Also of interest were the percent blood pressure responders over the 36 months follow-up period. Responders were nominally defined as an office systolic blood pressure reduction of >10 mmHg vs. baseline. At 12 months post-procedure, only 79% had achieved this response in the Symplicity HTN-1 expanded cohort. However, by 36 months all study patients had in fact achieved a ‘response’. This raises the important issue of what physiological mechanisms may be in play post-denervation regarding achievement of a late (but not early) benefit. Possibilities include progressive vascular remodelling, resetting of the baroreflex and/or alterations in renal blood flow and sodium excretory status, all of which may take some time to ‘reset’.
Furthermore, analysis of key subgroups failed to reveal patients with particularly large blood pressure responses (or non-responses). In particular, age greater or less than 65 years, presence or absence of diabetes mellitus, impaired or preserved renal function or high or low heart rate resulted in no heterogeneity in blood pressure response. Thus, a particular subgroup of patients who may particularly benefit or not benefit from the procedure regarding blood pressure response cannot be ascertained from baseline patient characteristics. However, given the limited numbers of patients in this analysis it is clear that much larger numbers, e.g. from a global registry, will be required to tease out fully this important clinical question. Ultimately, pre-procedure measures beyond those that are clinically routine may need to be elucidated (or developed) to improve patient selection and minimize ‘non-responders’.
Symplicity hypertension 2 study
Symplicity HTN-2 [20] used very similar entry criteria to Symplicity HTN-I [19]. The key differences were that there was a 2 week observation period at the end of which baseline systolic blood pressure measures were required to remain above 160 mmHg. In this way concerns about regression to the mean and the Hawthorne effect (as per Symplicity HTN-1) could at least be partially overcome. Patients who meet blood pressure criteria then underwent anatomical screening via MRA, CTA or duplex scanning and if the renal arteries were found to be appropriate for intervention they were then randomized to a control or treatment group with a 6 month primary end point assessment of safety and efficacy. The initial 6 month results demonstrated acceptable safety and a 32/12 mmHg reduction from baseline in the denervation group (n = 49) compared with a 1/0 mmHg increase in blood pressure in the control group (n = 51). This was achieved despite more patients decreasing their medication and fewer patients increasing their medication in the denervation group compared with the control group.
At the end of the 6 month primary end point, the control patients were offered the denervation procedure and all patients were then followed for a further 6 months. The findings of this analysis [24] were that in the initial denervation group (n = 47) blood pressure lowering was maintained out to 12 months from the procedure with a reduction of 28/10 mmHg compared with baseline. In the crossover group (n = 35) who were evaluated 6 month post-denervation, mean reduction was 24/8 mmHg compared with their 6 month pre-denervation value of +7/1 mmHg vs. baseline.
Symplicity hypertension 3 study
Symplicity HTN-1 and 2 have provided strong safety and efficacy data to support the utility of this procedure in patients with refractory hypertension. However, there are a number of design deficiencies in both of these studies and the United States (US) Food and Drug Administration mandated a definitive US study to overcome some of these design issues. Specifically, Symplicity HTN-3 [25] will require more aggressive achievement of target or at least highest tolerated dose of background antihypertensive medications, qualifying blood pressure will include a requirement for systolic blood pressure of >135 mmHg by ABPM (as well as a subsequent office systolic blood pressure >160 mmHg) to be confirmed following initial screening and most importantly a sham procedure performed in the renal artery of control subjects which includes everything but the actual RF energy application. Because the operator will be aware of which patients do and do not receive the active procedure, a separate group of investigators will perform the end point assessments. As with Symplicity HTN-2, the primary efficacy end point is at 6 months at which time patients in the control group can then receive the procedure if they wish.
Efficacy beyond blood pressure-lowering
Experience with percutaneous renal sympathetic denervation has, in addition to demonstration of substantive blood pressure lowering, also shed light on underlying mechanisms as well as pointing to future therapeutic possibilities beyond hypertension.
A key mechanistic observation was of reduced muscle sympathetic nerve activity following denervation [21, 22]. This finding strongly supports the concept that renal sympathetic afferents (not just efferents) are being disrupted by this procedure. This in turn suggests that sympathetic activity mediated centrally and signalling to various organ systems may also be reduced. This has significant implications for a number of co-morbid diseases which accompany treatment-resistant hypertension.
Diabetes mellitus
A sub-study of Symplicity Hypertension II has demonstrated improvements in fasting glucose and homeostatic model assessment (HOMA) as well as reductions in insulin and C-peptide concentrations at 1 and 3 months compared with controls following renal denervation [26] (Figure 5). While these data need to be confirmed they suggest that renal denervation via disruption of afferent signalling may improve blood flow in the periphery and thus increase uptake of glucose into peripheral skeletal muscle to improve overall glycaemic control [27]. The mechanisms underlying these glycaemic improvements need to be further explored.
Figure 5.
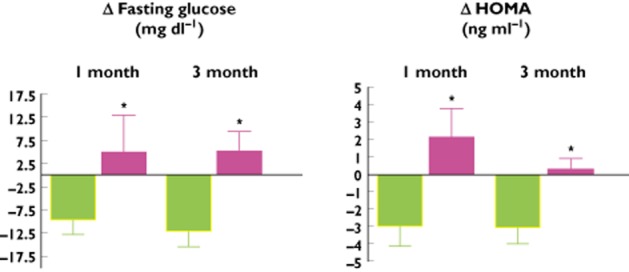
Changes (mean and SD) in fasting glucose (left panel) and homeostatic model assessment (HOMA, right panel) in renal denervation patients and controls in a sub-group from the Symplicity HTN-2 study.  , control (n = 15);
, control (n = 15);  , renal denervation (n = 35), *P < 0.05
, renal denervation (n = 35), *P < 0.05
Obstructive sleep apnoea
Obstructive sleep apnoea (OSA) is a common accompaniment of hypertension, particularly in the setting of obesity. Various overlapping mechanisms drive this condition. In this setting it is of interest that a small sub-study of Symplicity HTN-1 demonstrated improvements in the apnoea/hypopnoea index in the majority of patients with refractory hypertension studied at 6 months (8/10) [28], albeit without a control group for comparison. The mechanism underlying why renal denervation might assist with OSA control requires further exploration. One hypothesis is that laryngeal oedema may be sympathetically mediated (at least in part) and denervation therefore improves that oedema by reducing central sympathetic outflows into the region [29].
Left ventricular hypertrophy and heart failure with preserved ejection fraction
Left ventricular hypertrophy (LVH) and heart failure with preserved ejection fraction (HFPEF) have a common and frequent basis of hypertension as a major aetiological factor. It is not surprising therefore that measures of LVH such as left ventricular mass index and intra-ventricular septal wall thickness can be successfully abrogated with percutaneous renal denervation in association with lower systemic blood pressure levels [30]. Of interest, LV mass regression was also observed in a small group of patients who did not appear to have a substantial blood pressure response to denervation [30]. This raises the possibility of a ‘blood pressure-independent’ sympathetic reduction-mediated impact on LV mass. Again, this requires further investigation. Furthermore, the implications for HFPEF clearly follow on from this and a number of groups around the world are actively studying this specific patient population where currently no effective treatment exists.
Systolic chronic heart failure
Whilst hypertension is an important aetiological factor in the subsequent development of systolic chronic heart failure (CHF), patients with established systolic CHF generally have normal, and usually low, systemic blood pressure values. They do, however, have a markedly and chronically activated sympathetic nervous system which is strongly linked to progression of underlying disease processes as well as poor clinical outcomes in this setting [31]. The success of β-adrenoceptor blockers in systolic CHF is testament in this regard [32]. Percutaneous renal sympathetic denervation may be particularly beneficial in this setting and indeed complementary to β-adrenoceptor blockade given differing pathways of sympathetic abrogation. This is supported by animal studies which have demonstrated improved cardiac function and reduced pathological fibrosis following denervation [33, 34]. Furthermore, denervation has improved renal blood flow in the (preclinical) heart failure setting and this may have significant implications for the cardiorenal syndrome [35]. A small pilot study of seven patients with chronic mild to moderate systolic heart failure (mean EF 43 ± 15%) did not raise any procedural or safety concerns [36], in particular no major drop in BP despite low baseline levels and no change in renal function, with a mild improvement in the 6 min walk test. However, left ventricular ejection fraction, as well as other cardiac structural and functional changes on echocardiography, were not significantly changed after 6 months. Another proof-of-concept study investigating the safety and efficacy of renal sympathetic denervation in the setting of systolic CHF with impaired renal function is currently ongoing (Symplicity HF) [37].
Chronic kidney disease (CKD)
Due to ethical concerns, patients with low eGFR were excluded from the initial Symplicity HTN studies. However, a separate small series of refractory hypertension patients with stage 3–4 CKD (mean eGFR 31 ml min−1 1.73 m−2) have been denervated [38]. The magnitude of the blood reductions achieved appeared to be similar to that of those patients with relative preservation of renal function. Care needs to be taken with imaging. CO2 angiography was used in this series to delineate the main renal artery.
Conclusion
Hypertension remains a major public health problem, particularly in Western but increasingly in developing countries. Despite effective and largely well tolerated anti-hypertensive pharmacotherapy there exists a population of patients whose blood pressure remains sub-optimally controlled. Provided that appropriate pharmacotherapies and their doses have been adequately explored, new procedures and devices have emerged to assist with blood pressure control in this setting. Renal sympathetic denervation appears thus far to provide significant and durable blood pressure lowering with a highly acceptable peri- and post-procedural adverse event profile. Nevertheless, the number of patients exposed in randomized controlled trials to denervation remains relatively low and further large scale trial data are required before this procedure can reach the point of guideline recommendation as part of a standard algorithm for the management of treatment-resistant hypertension patients.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work.
References
- 1.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008;52:1749–1757. doi: 10.1016/j.jacc.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 3.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A, Ruilope LM. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 5.Garg JP, Elliott WJ, Folker A, Izhar M, Black HR RUSH University Hypertension Service. Resistant hypertension revisited: a comparison of two university-based cohorts. Am J Hypertens. 2005;18:619–626. doi: 10.1016/j.amjhyper.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Václavík J, Sedlák R, Plachy M, Navrátil K, Plásek J, Jarkovsky J, Václavík T, Husár R, Kociánová E, Táborsky M. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double-blind, placebo-controlled trial. Hypertension. 2011;57:1069–1075. doi: 10.1161/HYPERTENSIONAHA.111.169961. [DOI] [PubMed] [Google Scholar]
- 7.DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol. 2010;298:R245–253. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- 8.Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens. 2004;17:217–222. doi: 10.1016/j.amjhyper.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Esler M, Jennings G, Korner P, Blombery P, Burke F, Willett I, Leonard P. Total, and organ-specific, noradrenaline plasma kinetics in essential hypertension. Clin Exp Hypertens A. 1984;6:507–521. doi: 10.3109/10641968409062580. [DOI] [PubMed] [Google Scholar]
- 10.Krum H, Sobotka P, Mahfoud F, Böhm M, Esler M, Schlaich M. Device-based antihypertensive therapy: therapeutic modulation of the autonomic nervous system. Circulation. 2011;123:209–215. doi: 10.1161/CIRCULATIONAHA.110.971580. [DOI] [PubMed] [Google Scholar]
- 11.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 12.DiBona GF. Sympathetic nervous system influences on the kidney. Role in hypertension. Am J Hypertens. 1989;2:119S–124. doi: 10.1093/ajh/2.3.119s. [DOI] [PubMed] [Google Scholar]
- 13.Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol. 1983;245:F1–14. doi: 10.1152/ajprenal.1983.245.1.F1. [DOI] [PubMed] [Google Scholar]
- 14.DiBona GF. Physiology in perspective: the wisdom of the body: neural control of the kidney. Am J Physiol. 2005;289:R633–641. doi: 10.1152/ajpregu.00258.2005. [DOI] [PubMed] [Google Scholar]
- 15.Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995;25:878–882. doi: 10.1161/01.hyp.25.4.878. [DOI] [PubMed] [Google Scholar]
- 16.Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, Dietl KH, Rahn KH. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106:1974–1979. doi: 10.1161/01.cir.0000034043.16664.96. [DOI] [PubMed] [Google Scholar]
- 17.Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1266 cases. J Am Med Assoc. 1953;152:1501–1504. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 18.Allen TR. Current status of lumbar sympathectomy. Am Surg. 1976;42:89–91. [PubMed] [Google Scholar]
- 19.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 20.Symplicity HTN-2 Investigators. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 21.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 22.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert G, Esler M, Schlaich MP. Substantial reduction in single sympathetic nerve fring after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 23.Sobotka PA, Esler MD, Schlaich M, Schmieder RE, Böhm M, Krum H. Symplicity HTN-1: long-term follow-up of catheter-based renal sympathetic denervation for resistant hypertension confirms durable blood pressure reduction. Presented at ACC 2012, Chicago, IL.
- 24.Esler MD, Krum H, Schlaich M, Schmieder RE, Böhm M, Sobotka PA. Renal sympathetic denervation for treatment of drug-resistant hypertension: one year results from the Symplicity HTN-2 randomized controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 25.Kandzari DE, Bhatt DL, Sobotka PA, O'Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR, Bakris G. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol. 2012;35:528–535. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Böhm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 27.Grassi G, Dell'Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, Gamba PL, Mancia G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005;48:1359–1365. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- 28.Witkowski A, Prejbisz A, Florczak E, Kądziela J, Śliwiński P, Bieleń P, Michałowska I, Kabat M, Warchoł E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewicz A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 29.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease. A bidirectional relationship. Circulation. 2012;126:1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 30.Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Böhm M, Hoppe UC. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–909. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 31.Floras JS. Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. J Am Coll Cardiol. 1993;22(4 Suppl. A):72A–84A. doi: 10.1016/0735-1097(93)90466-e. [DOI] [PubMed] [Google Scholar]
- 32.Krum H. Sympathetic activation and the role of beta-blockers in chronic heart failure. Aust N Z J Med. 1999;29:418–427. doi: 10.1111/j.1445-5994.1999.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 33.Sobotka PA, Krum H, Böhm M, Francis DP, Schlaich MP. The role of renal denervation in the treatment of heart failure. Curr Cardiol Rep. 2012;14:285–292. doi: 10.1007/s11886-012-0258-x. [DOI] [PubMed] [Google Scholar]
- 34.Nozawa T, Igawa A, Fujii N, Kato B, Yoshida N, Asanoi H, Inoue H. Effects of long-term renal sympathetic denervation on heart failure after myocardial infarction in rats. Heart Vessels. 2002;16:51–56. doi: 10.1007/s380-002-8317-8. [DOI] [PubMed] [Google Scholar]
- 35.Villarreal D, Freeman RH, Johnson RA, Simmons JC. Effects of renal denervation on postprandial sodium excretion in experimental heart failure. Am J Physiol. 1994;266:R1599–1604. doi: 10.1152/ajpregu.1994.266.5.R1599. [DOI] [PubMed] [Google Scholar]
- 36.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, Francis DP. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162:189–192. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 37. Symplicity HF trial. Available at http://clinicaltrials.gov/ct2/show/study/NCT01392196?term=krum&rank=10 (last accessed 19 September 2012)
- 38.Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA, Sobotka PA, Böhm M, Cremers B, Esler MD, Schlaich MP. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–1257. doi: 10.1681/ASN.2011111062. [DOI] [PMC free article] [PubMed] [Google Scholar]


