Abstract
Chronic kidney diseases share common pathogenic mechanisms that, independently from the initial injury, lead to glomerular hyperfiltration, proteinuria, and progressive renal scarring and function loss. Consistent experimental evidence supports the crucial role of proteinuria in accelerating kidney disease progression to end-stage renal failure through multiple pathways, including induction of tubular chemokine expression and complement activation. These events, in turn, lead to inflammatory cell infiltration in the interstitium and sustained fibrogenesis. The extent of proteinuria is widely recognized as a marker of the severity of chronic kidney disease and as a predictor of future decline in glomerular filtration rate. More importantly, a reduction in proteinuria invariably translates into a protection from renal function decline in patients with diabetic and non-diabetic renal disease. Recent evidence also showed the existence of a relatioship between proteinuria levels and cardiovascular risk, which extends to the range of urinary albumin excretion that was previously thought ‘normal’. Thus, proteinuria should be considered a valuable surrogate end point for clinical trials in patients with chronic renal diseases and a target for reno- and cardioprotecive strategies.
Keywords: biomarker, cardiovascular, chronic kidney disease, proteinuria
Introduction
People with chronic kidney disease (CKD) represent 5–7 % of the entire world population and are at higher risk for hospitalization and heart disease than those of corresponding age and gender in the general population. According to a cohort study including almost 1.3 million individuals, the unadjusted mortality rates after myocardial infarction in subjects with CKD were significantly higher than in subjects with a history of cardiovascular disease or diabetes [1]. As a consequence of the few noticeable symptoms of CKD, four out of five affected people are not even aware of their condition and progressively lose renal function over the course of years. Half of CKD patients die from cardiovascular events before reaching end stage renal disease (ESRD) and nearly two-thirds of patients who enter dialysis die within 5 years of initiation of renal replacement therapy, a life expectancy worse than patients with heart failure or most common cancers.
The high burden of CKD and associated costs, related adverse outcomes and decreased productivity make it a significant public health problem worldwide [2]. This issue is even more relevant in emerging nations, where ESRD constitutes a ‘death sentence’, as dialysis or kidney transplantation are often unavailable or unaffordable, leading to the death of nearly 1 million ESRD patients each year.
Improvements in renal disease prevention and management have ameliorated outcomes of patients with CKD over the last decades, but the number of those who still progress to ESRD is unbearably high [3]. New treatment options have arisen from experimental studies over the last decades, but the new molecules with nephroprotective properties that have actually reached the clinical setting is disappointingly low. The chronic, slowly progressing nature of kidney disease makes designing clinical trials with hard endpoints, such as need of replacement therapy or death, extremely challenging. Doubling of serum creatinine concentrations is a Food and Drug Admininistration (FDA) accepted surrogate end point, but is of limited utility in clinical trial design since many years are generally required before this outcome develops.
Slopes of glomerular filtration rate (GFR) have been used as another surrogate endpoint. However, the two most widely used formulae to estimate GFR, namely the ‘Chronic Kidney Disease Epidemiology Collaboration’ (CKD-Epi) and the ‘abbreviated Modification of Diet in Renal Disease’ (aMDRD) have been repeatedly challenged and there is increasing evidence that their use might generate misleading information [4]. According to a recent prospective cohort study in 111 patients with autosomal dominant polycystic kidney disease (ADPKD) patients, estimated GFR (eGFR) values were biased by a significant overestimation with the CKD-Epi and underestimation with the aMDRD formula compared with direct GFR measurements done by iohexol plasma clearance technique [5]. Only direct measurements of the GFR by gold standard techniques could actually assess a treatment effect on GFR decline, but this approach may be hard to implement in large clinical trials.
Thus, novel, surrogate endpoints able to predict easily a clinically relevant end point, possibly long before its occurrence, are needed to test new treatments in CKD and generate evidence for their implementation in patient care [6].
Proteinuria is a major risk factor of renal disease progression
Proteinuria is strongly associated with the risk of CKD progression in both non-diabetic and diabetic patients. A mass screening involving 107 192 participants in Okinawa, Japan, identified proteinuria as the most powerful predictor of ESRD risk over 10 years in the general population [7]. In the 274 patients with non-diabetic chronic nephropathies and clinical proteinuria included in the Ramipril Efficacy in Nephropathy (REIN) trial [8], urinary protein excretion was the only baseline variable that correlated with the rate of GFR decline and progression to ESRD. Consistently, when patients were stratified according to baseline proteinuria levels, those in the lowest tertile had the lowest rate of renal disease progression and of ESRD, as compared with patients in the middle and in the highest tertiles. In harmony with these findings, the Modification of Diet in Renal Disease (MDRD) [9] and the African-American Study of Kidney Disease and Hypertension (AASK) [10] studies which included non-diabetic patients with CKD showed that higher baseline proteinuria was associated with faster GFR decline.
The same is true for diabetic patients. Results from the UK Prospective Diabetes Study 74 revealed that increased urinary albumin concentrations within the normal range were independently associated with subsequent development of micro-albuminuria (urine albumin excretion between 30 and 300 mg 24 h−1) or renal impairment in type 2 diabetics with but no albuminuria at baseline [11]. Among patients with diabetic nephropathy, baseline urinary albumin to creatinine ratio was a strong independent predictor of ESRD in the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) study and in the Irbesartan in Diabetic Nephropathy Trial (IDNT) [12, 13].
Thus, proteinuria is a marker of renal risk in the general population and in non-diabetic and diabetic patients with CKD prior to treatment. Furthermore, according to the RENAAL study, baseline albuminuria was the most important independent predictor of ESRD risk in all ethnic groups, including White, Black, Asian and Hispanic [14].
Proteinuria plays a crucialpathogenic role in loss of renal function
Experimental data have provided significant insights on the mechanisms by which CKD progresses. It is now well established that, independent of the underlying cause, chronic proteinuric nephropathies have in common a loss of selectivity of the glomerular barrier to protein filtration. In the experimental model of renal mass reduction by five-sixths nephrectomy, the remnant glomeruli undergo hypertrophy and the tone of afferent arterioles drops by a greater degree than that of efferent ones. These changes increase glomerular capillary hydraulic pressure and lead to more filtrate formed per nephron. These changes, initially helpful to minimize the functional consequences of nephron loss, are ultimately detrimental, causing relentless injury of remaining intact nephrons [15]. Enhancing intraglomerular capillary pressure and perfusion pressure results in stretching of the glomerular capillaries, leading to impaired filter function and loss of larger molecules, such as proteins, in the urine (Figure 1).
Figure 1.
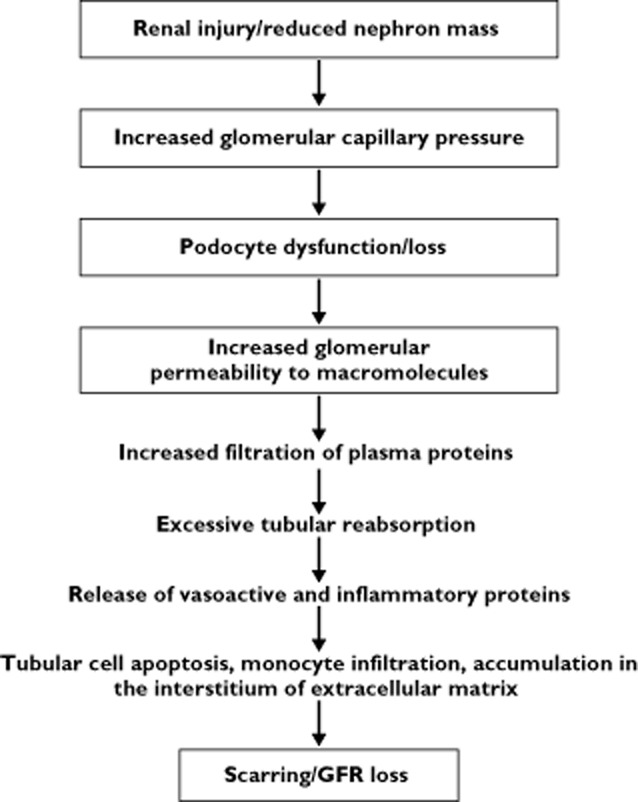
The common pathway in the progression of chronic proteinuric nephropathies. Modified from [52]
Recent data showed the existence of a direct relationship between podocyte detachment, endothelial cell fenestration and albuminuria in patients with type 2 diabetes [16]. Similarly to previous findings in type 1 diabetes [17], these three parameters worsen in parallel with the classic histological features of diabetic kidney disease, providing a structural link between glomerular injury, albuminuria and renal scarring.
These data are consistent with the hypothesis that tubulo-interstitial damage is induced by direct toxicity of filtered proteins. Studies support the possibility that the excessive protein load of podocytes can be a factor underlying progressive injury of these glomerular cells and through their release of transforming growth factor-β, eventually leading to the differentiation of mesangial cells into myofibroblasts [18]. In vitro experiments showed that proximal tubular cells exposed to plasma proteins such as albumin, IgG and transferrin, release profibrotic and pro-inflammatory molecules, including the vasoconstrictor peptide endothelin-1 (ET-1), RANTES and osteopontin [19–22]. Protein overloading of human proximal tubular cells induced the synthesis of fractalkine, which in its membrane-anchored form promotes mononuclear cell adhesion via the CX3CR1 receptor [2]. Gene expression of fractalkine, a CX3C transmembrane chemokine, was increased in kidneys of mice with protein overload proteinuria, and the gene product was detected in tubular epithelial cells mainly in the basal region [2].
Transcription analysis by cDNA microarray of renal tubular cells from patients with proteinuric nephropathies [23] identified more than 160 genes as being regulated differently compared with those of proximal tubular cells from control subjects. The upregulation of cytokines, growth factors and vasoactive substances, results in abnormal accumulation in the interstitium of extracellular matrix collagen, fibronectin and other components that are responsible for interstitial fibrosis [24]. As a consequence of proteinuria, the intrarenal activation of the complement cascade may also promote injury through the formation of membrane attack complex and possibly other biologically active products that interact with specific receptors [25–27]. Thus, experimental data converge to indicate that proteinuria is not just a marker of the entity of renal damage, but it also plays a direct pathogenic role in renal disease progression, promoting loss of kidney function and scarring.
Selectivity has been pointed out as an important feature of proteinuria, modifying its nephrotoxic effect. When proteinuria is highly selective, i.e. when albumin represents its major component, tubulo-interstitial damage is milder than in cases when also larger proteins, together with potentially nephrotoxic components, such as complement factors, growth factors and others, are ultrafiltered in the urinary space.
This evidence, however, should not lead to the underestimation of the injurious effects of albuminuria. Recent experimental data have showed a direct, pro-inflammatory role of urinary albumin. The proteolysis of ultrafiltered albumin by proximal tubular cells provides the substrate to dendritic cells for the generation of antigenic peptides that elicit an inflammatory response [28]. Consistently, patients with persistent selective proteinuria secondary to minimal change disease who do not respond to steroids or immunosuppressive treatments eventually develop renal interstitial inflammation and glomerulosclerosis [15, 29].
Fractional protein clearance has been described as a more accurate indicator of impairment of glomerular permselectivity than total proteinuria in predicting renal outcome of patients with primary glomerulonephritis [30], possibly because it represents a more accurate marker of glomerular permselectivity impairment. According to a recent study in 97 patients with primary glomerulonephritis [31], fractional excretion of albumin and fractional high molecular weight proteinuria were better predictors of developing stage 5 CKD or ESRD than total proteinuria. Intriguingly, the two fractional clearances had a similar predicting power, which further supports the notion that the amount of proteinuria, rather than its quality, represents the major predictor of renal function loss.
Reducing proteinuria retards renal disease progression
Clinical trials consistently showed the renoprotective effect of proteinuria reduction [15]. Results of the MDRD study established that a reduction of proteinuria by antihypertensive treatment was associated with a decrease in the rate of GFR loss, and that protection of renal function achieved by lowering blood pressure was dependent on the extent of initial proteinuria [9]. The REIN study was designed to assess the hypothesis that, through a specific antiproteinuric effect, angiotensin converting enzyme (ACE) inhibitors could be superior to other antihypertensive drugs in limiting the GFR decline and preventing ESRD in patients with chronic nephropathies. In this study, patients were randomly assigned to receive ramipril or conventional antihypertensive therapy to maintain diastolic blood pressure at 90 mmHg or less. The study showed that despite similar blood pressure control in the two treatment groups, ACE inhibitor therapy decreased proteinuria significantly more than conventional antihypertensive therapy and this translated into a 50% reduced progression to ESRD [32]. Patients who had more proteinuria to start with benefited more from treatment than those who had less proteinuria. Of note, independent of the initial level of proteinuria, the extent of short term proteinuria reduction significantly correlated with the reduction in the rate of GFR decline and progression to ESRD in the long term [33]. Similar findings were observed in diabetic kidney disease. Bjorck et al. [34] found that in type 1 diabetics with overt nephropathy, enalapril reduced the rate of GFR decline more than did treatment with a β-adrenoceptor blocker, despite comparable blood pressure control. Another trial of 409 type 1 diabetics [35] showed less progression to the combined end point of doubling serum creatinine, ESRD or death while on captopril compared with placebo. In both trials, blood pressure was comparable between treatment groups and slower progression was associated with more proteinuria reduction in patients on ACE inhibitor therapy. Similar data are available for type 2 diabetics. Two large trials of patients with overt nephropathy showed that proteinuria reduction by angiotensin receptor blocker treatment was associated with a lower incidence of serum creatinine doubling and risk of ESRD [12, 13]. Actually, all post hoc analyses of randomized clinical trials aimed at evaluating the relationships between changes in proteinuria and disease outcome consistently found that short term proteinuria reduction was invariably associated with slower GFR decline and progression to ESRD in the long term.
A meta-analysis of 1860 patients with diabetic or non-diabetic chronic nephropathies showed that proteinuria was the most important modifiable risk factor to slow progression and that reduction of urine protein excretion was the main goal for treatment [36]. Consistently, a pooled analysis of 2387 patients included in 11 randomized clinical trials, found that, irrespective of treatment adopted, short term changes were strongly consistent with long term renal outcome. Reduction of proteinuria was invariably associated with improved outcome, whereas no effect on proteinuria predicted no long term benefit. A worsening of proteinuria was never associated with improvement [37].
A recent trial in 227 diabetic patients with eGFR <45 ml min−1 1.73 m−2 showed that bardoxolone methyl, an anti-oxidant and anti-inflammatory molecule, increased eGFR compared with placebo in a dose-dependent fashion, an effect that was associated with increased blood pressure and albuminuria [38]. This phenomenon, raised concerns whether the renal effect of increasing eGFR was actually due to hyperfiltration, a major determinant of accelerated glomerular damage. Consistent with this hypothesis, treatment with an analogue of bardoxolone methyl in Zucker diabetic fatty (ZDF) rats with overt type 2 diabetes was associated with increased proteinuria and glomerulosclerosis [39]. Therefore, data from the bardoxolone trial actually confirm that hyperfiltration-induced proteinuria is associated with increased glomerulosclerosis in rats and may lead to accelerated renal function loss in patients. Unfortunately, a long term randomized trial designed to understand the impact of bardoxolone on long term renal survival in stage IV–V CKD patients with diabetes has been recently halted due to increased mortality in the treatment group (ClinicalTrials.gov identifier: NCT01351675).
Proteinuria is a cardiovascular risk factor
Beyond its association with renal disease progression, proteinuria increases risk for cardiovascular events and mortality in patients with and without diabetes. Patients with proteinuria have augmented risk for cardiovascular events, cardiovascular mortality and all-cause mortality [40]. In addition, proteinuria has been implicated in myocardial disease of the left ventricle and increased risk for incident stroke. Finally, proteinuria is also associated with increased risk for atherosclerotic events in the peripheral vasculature [40].
In the RENAAL Study, which randomized 1513 patients with type 2 diabetes to losartan vs. placebo, for each 50% reduction in albuminuria, there was an 18% and 27% reduction in cardiovascular and heart failure risk, respectively [41]. Similar results were noted in the LIFE trial [42], in which time varying albuminuria was related to the primary composite cardiovascular endpoint such that the risk decreased with lower levels of albuminuria in follow-up. Larger proteinuria reduction predicted slower renal disease progression and even less cardiovascular events [43]. Similar data were reported for type 2 diabetes patients with overt nephropathy included in the IDNT trial [44]. Thus, evidence that interventions that reduce urinary albumin or total protein excretion are invariably reno- and cardioprotective confirms that albuminuria and proteinuria are sensitive and both reliable surrogate end points to monitor and predict treatment effect in clinics and research.
Proteinuria-associated cardiovascular risk extends to any degree of measurable albuminuria
Patients with type 2 diabetes and micro- or macro-albuminuria have a significantly higher risk for cardiovascular death than that observed in patients with less albuminuria. In a pooled analysis of 11 cohort studies including 2138 patients with type 2 diabetes followed up for a mean of 6.4 years, micro-albuminuria was found to be associated with an adjusted overall odds ratio for all-cause mortality of 2.4 and for cardiovascular morbidity and mortality of 2.0 [45]. Even higher cardiovascular risk has been reported for patients with macro-albuminuria [46]. This largely accounts for the excess cardiovascular mortality observed in patients with diabetes compared with age-matched subjects without the disease.
In contrast with diabetic patients with micro- or macro-albuminuric, those with urinary albumin excretion <20 μg min−1 (the upper limit of what is generally considered the normal range) were initially supposed to have a cardiovascular risk close to that of the general population. However, this concept has been recently challenged. In the Third Copenhagen City Heart Study, individuals with >4.8 μg min−1 levels of albuminuria had increased risk of coronary artery disease and all-cause mortality as compared with those below this level [47]. Similarly, 1568 non-hypertensive, non-diabetic Framingham Offspring Study participants free of cardiovascular disease, those with urinary albumin/creatinine greater than the gender-specific median (≥3.9 μg mg−1 for men and 7.5 μg mg−1 for women) had a three-fold increased risk of cardiovascular disease as compared with those below this level [48].
More strikingly, according to a recent analysis of 1208 hypertensive, normo-albuminuric patients with type 2 diabetes from the BErgamo NEphrologic Diabetes Complication Trial (BENEDICT), any degree of measurable albumin excretion bore significant heart risks [49]. For each 1 μg min−1 in albumin excretion at the start of the study, there was a progressive incremental risk of experiencing heart problems during follow-up. Second degree polynomial multivariable analysis showed a continuous non-linear relationship between albuminuria and events without thresholds. Even albuminuria of 1–2 μg min−1 was significantly associated with increased risk compared with albuminuria <1 μg min−1 (Figure 2).
Figure 2.
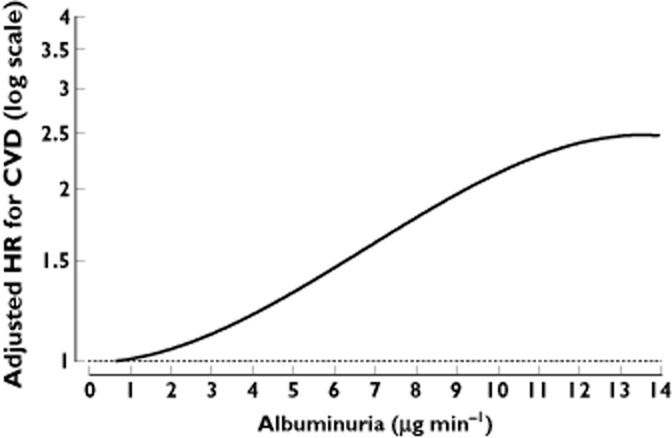
Adjusted hazard ratios (HR) for major cardiovascular events according to baseline albuminuria in 1208 hypertensive, normo-albuminuric patients with type 2 diabetes from the BErgamo NEphrologic Diabetes Complication Trial (BENEDICT) [49]
Thus, in type 2 diabetes patients, any degree of measurable urinary protein excretion, even in what is considered the normal range, increases their risk of experiencing heart problems. This is a major health issue, since patients with normo-albuminuria account for at least 90% of the diabetic population.
Conclusion
Surrogate endpoints of renal failure are instrumental to test new treatments in patients with CKD, whose natural history is characterized by a slow, asymptomatic, decline of function. Proteinuria plays a direct pathogenic role in renal disease progression and its extent is widely recognized as a marker of the severity of glomerulopathy. Population-based studies and controlled trials have identified proteinuria as a predictor of future decline in GFR and the development of ESRD. Reduction in proteinuria invariably translates into protection from renal function decline in non-diabetic and diabetic renal disease with overt proteinuria. Even more importantly, proteinuria reduction and preservation of renal function translates into improved cardiovascular outcomes. Altogether, proteinuria should be considered a valuable surrogate endpoint for clinical trials in proteinuric renal diseases and a target for cardiovascular protection.
Though proteinuria is still not recognized by the FDA or the European Medicines Agency (EMA) as a surrogate endpoint for trials of CKD interventions, the aforementioned evidence prompted a lively discussion between leading nephrologists and FDA representatives on the qualification of proteinuria as a surrogate marker [50, 51].
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, James MT, Hemmelgarn BR. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380:807–814. doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 2.Donadelli R, Zanchi C, Morigi M, Buelli S, Batani C, Tomasoni S, Corna D, Rottoli D, Benigni A, Abbate M, Remuzzi G, Zoja C. Protein overload induces fractalkine upregulation in proximal tubular cells through nuclear factor kappaB- and p38 mitogen-activated protein kinase-dependent pathways. J Am Soc Nephrol. 2003;14:2436–2446. doi: 10.1097/01.asn.0000089564.55411.7f. [DOI] [PubMed] [Google Scholar]
- 3.Cravedi P, Ruggenenti P, Remuzzi G. Kidney failure stabilizes after an increase over 2 decades. J Ren Care. 2007;33:100–104. doi: 10.1111/j.1755-6686.2007.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 4.Poggio ED, Nef PC, Wang X, Greene T, Van Lente F, Dennis VW, Hall PM. Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill hospitalized patients. Am J Kidney Dis. 2005;46:242–252. doi: 10.1053/j.ajkd.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Ruggenenti P, Gaspari F, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Prandini S, Ene-Iordache B, Diadei O, Perico N, Ondei P, Pisani A, Buongiorno E, Messa P, Dugo M, Remuzzi G. Measuring and estimating GFR and treatment effect in ADPKD patients: results and implications of a longitudinal cohort study. PLoS ONE. 2012;7:e32533. doi: 10.1371/journal.pone.0032533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cravedi P, Ruggenenti P, Remuzzi G. Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol. 2012;8:301–306. doi: 10.1038/nrneph.2012.42. [DOI] [PubMed] [Google Scholar]
- 7.Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int. 1996;49:800–805. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 8.Ruggenenti P, Perna A, Mosconi L, Matalone M, Pisoni R, Gaspari F, Remuzzi G. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The ‘Gruppo Italiano di Studi Epidemiologici in Nefrologia’ (GISEN) Kidney Int Suppl. 1997;63:S54–57. [PubMed] [Google Scholar]
- 9.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL Group ftMoDiRDMS. Blood pressure control, proteinuria, and the progression of renal disease. The modification of diet in renal disease study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 12.Keane WF, Zhang Z, Lyle PA, Cooper ME, de Zeeuw D, Grunfeld JP, Lash JP, McGill JB, Mitch WE, Remuzzi G, Shahinfar S, Snapinn SM, Toto R, Brenner BM. Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: the RENAAL study. Clin J Am Soc Nephrol. 2006;1:761–767. doi: 10.2215/CJN.01381005. [DOI] [PubMed] [Google Scholar]
- 13.Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45:281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 14.de Zeeuw D, Ramjit D, Zhang Z, Ribeiro AB, Kurokawa K, Lash JP, Chan J, Remuzzi G, Brenner BM, Shahinfar S. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int. 2006;69:1675–1682. doi: 10.1038/sj.ki.5000326. [DOI] [PubMed] [Google Scholar]
- 15.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weil EJ, Lemley KV, Mason CC, Yee B, Jones LI, Blouch K, Lovato T, Richardson M, Myers BD, Nelson RG. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012;82:1010–1017. doi: 10.1038/ki.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 18.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79:944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoja C, Morigi M, Figliuzzi M, Bruzzi I, Oldroyd S, Benigni A, Ronco P, Remuzzi G. Proximal tubular cell synthesis and secretion of endothelin-1 on challenge with albumin and other proteins. Am J Kidney Dis. 1995;26:934–941. doi: 10.1016/0272-6386(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 20.Drumm K, Bauer B, Freudinger R, Gekle M. Albumin induces NF-kappaB expression in human proximal tubule-derived cells (IHKE-1) Cell Physiol Biochem. 2002;12:187–196. doi: 10.1159/000066278. [DOI] [PubMed] [Google Scholar]
- 21.Tang S, Leung JC, Abe K, Chan KW, Chan LY, Chan TM, Lai KN. Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest. 2003;111:515–527. doi: 10.1172/JCI16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 23.Rudnicki M, Eder S, Perco P, Enrich J, Scheiber K, Koppelstatter C, Schratzberger G, Mayer B, Oberbauer R, Meyer TW, Mayer G. Gene expression profiles of human proximal tubular epithelial cells in proteinuric nephropathies. Kidney Int. 2007;71:325–335. doi: 10.1038/sj.ki.5002043. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DW, Saunders HJ, Baxter RC, Field MJ, Pollock CA. Paracrine stimulation of human renal fibroblasts by proximal tubule cells. Kidney Int. 1998;54:747–757. doi: 10.1046/j.1523-1755.1998.00048.x. [DOI] [PubMed] [Google Scholar]
- 25.Biancone L, David S, Della Pietra V, Montrucchio G, Cambi V, Camussi G. Alternative pathway activation of complement by cultured human proximal tubular epithelial cells. Kidney Int. 1994;45:451–460. doi: 10.1038/ki.1994.59. [DOI] [PubMed] [Google Scholar]
- 26.Nangaku M, Pippin J, Couser WG. Complement membrane attack complex (C5b-9) mediates interstitial disease in experimental nephrotic syndrome. J Am Soc Nephrol. 1999;10:2323–2331. doi: 10.1681/ASN.V10112323. [DOI] [PubMed] [Google Scholar]
- 27.Rangan GK, Pippin JW, Coombes JD, Couser WG. C5b-9 does not mediate chronic tubulointerstitial disease in the absence of proteinuria. Kidney Int. 2005;67:492–503. doi: 10.1111/j.1523-1755.2005.67106.x. [DOI] [PubMed] [Google Scholar]
- 28.Macconi D, Chiabrando C, Schiarea S, Aiello S, Cassis L, Gagliardini E, Noris M, Buelli S, Zoja C, Corna D, Mele C, Fanelli R, Remuzzi G, Benigni A. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J Am Soc Nephrol. 2009;20:123–130. doi: 10.1681/ASN.2007111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 30.Bazzi C, Petrini C, Rizza V, Arrigo G, Beltrame A, Pisano L, D'Amico G. Urinary excretion of IgG and alpha(1)-microglobulin predicts clinical course better than extent of proteinuria in membranous nephropathy. Am J Kidney Dis. 2001;38:240–248. doi: 10.1053/ajkd.2001.26080. [DOI] [PubMed] [Google Scholar]
- 31.McQuarrie EP, Shakerdi L, Jardine AG, Fox JG, Mackinnon B. Fractional excretions of albumin and IgG are the best predictors of progression in primary glomerulonephritis. Nephrol Dial Transplant. 2011;26:1563–1569. doi: 10.1093/ndt/gfq605. [DOI] [PubMed] [Google Scholar]
- 32.Group TG. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 33.Ruggenenti P, Perna A, Remuzzi G. Retarding progression of chronic renal disease: the neglected issue of residual proteinuria. Kidney Int. 2003;63:2254–2261. doi: 10.1046/j.1523-1755.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 34.Bjorck S, Mulec H, Johnsen SA, Norden G, Aurell M. Renal protective effect of enalapril in diabetic nephropathy. BMJ. 1992;304:339–343. doi: 10.1136/bmj.304.6823.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 36.Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 37.Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal diseases. Lancet. 2001;357:1601–1608. doi: 10.1016/S0140-6736(00)04728-0. [DOI] [PubMed] [Google Scholar]
- 38.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 39.Zoja C, Corna D, Nava V, Locatelli M, Abbate M, Gaspari F, Carrara F, Sangalli F, Remuzzi G, Benigni A. Analogues of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am J Physiol Renal Physiol. 2013;304:f808–819. doi: 10.1152/ajprenal.00376.2012. [DOI] [PubMed] [Google Scholar]
- 40.Agrawal V, Marinescu V, Agarwal M, McCullough PA. Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat Rev Cardiol. 2009;6:301–311. doi: 10.1038/nrcardio.2009.11. [DOI] [PubMed] [Google Scholar]
- 41.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110:921–927. doi: 10.1161/01.CIR.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- 42.Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlof B, Devereux RB, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wan Y. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a. [DOI] [PubMed] [Google Scholar]
- 43.Holtkamp FA, de Zeeuw D, de Graeff PA, Laverman GD, Berl T, Remuzzi G, Packham D, Lewis JB, Parving HH, Lambers, Heerspink HJ. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32:1493–1499. doi: 10.1093/eurheartj/ehr017. [DOI] [PubMed] [Google Scholar]
- 44.Hunsicker LG, Atkins RC, Lewis JB, Braden G, de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ. Impact of irbesartan, blood pressure control, and proteinuria on renal outcomes in the Irbesartan Diabetic Nephropathy Trial. Kidney Int Suppl. 2004;66:S99–101. doi: 10.1111/j.1523-1755.2004.09223.x. [DOI] [PubMed] [Google Scholar]
- 45.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 46.Sasso FC, Chiodini P, Carbonara O, De Nicola L, Conte G, Salvatore T, Nasti R, Marfella R, Gallo C, Signoriello S, Torella R, Minutolo R. High cardiovascular risk in patients with type 2 diabetic nephropathy: the predictive role of albuminuria and glomerular filtration rate. The NID-2 Prospective Cohort Study. Nephrol Dial Transplant. 2012;27:2269–2274. doi: 10.1093/ndt/gfr644. [DOI] [PubMed] [Google Scholar]
- 47.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, Appleyard M, Jensen JS. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 48.Arnlov J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D′Agostino RB, Vasan RS. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 49.Ruggenenti P, Porrini E, Motterlini N, Perna A, Ilieva AP, Iliev IP, Dodesini AR, Trevisan R, Bossi A, Sampietro G, Capitoni E, Gaspari F, Rubis N, Ene-Iordache B, Remuzzi G. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol. 2012;23:1717–1724. doi: 10.1681/ASN.2012030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, Kasiske B, Hostetter T. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 51.Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol. 2012;23:1917–1928. doi: 10.1681/ASN.2012040390. [DOI] [PubMed] [Google Scholar]


