Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease and results from mutations in PKD1 or PKD2. Cyst initiation and expansion arise from a combination of abnormal cell proliferation, fluid secretion and extracellular matrix defects and results in kidney enlargement and interstitial fibrosis. Since its first description over 200 years ago, ADPKD has been considered an untreatable condition and its management is limited to blood pressure reduction and symptomatic treatment of disease complications. Results of the recently reported TEMPO 3/4 trial thus represent a paradigm shift in demonstrating for the first time that cystic disease and loss of renal function can be slowed in humans. In this paper, we review the major therapeutic strategies currently being explored in ADPKD including a range of novel approaches in preclinical models. It is anticipated that the clinical management of ADPKD will undergo a revolution in the next decade with the translation of new treatments into routine clinical use.
Keywords: ADPKD, calcium, cAMP, mTOR, therapeutics, vasopressin
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease known. It is caused by mutations in PKD1 (85%) or PKD2 (15%) [1]. The prevalence of ADPKD is 1:400 to 1:1000 and thus it affects approximately 12.5 million people worldwide [2]. Unlike most other chronic kidney disease (CKD), polycystic kidneys enlarge over time and total kidney volume (TKV) as measured by magnetic resonance imaging (MRI) can exceed 1500 ml compared with 300–400 ml in healthy controls [3]. Cyst initiation and expansion arise from abnormal cell proliferation, fluid secretion and extracellular matrix defects and results in kidney enlargement and interstitial fibrosis [4–8]. By the age of 60 years, half of all ADPKD patients require dialysis or transplantation. There are currently no effective treatments to slow disease progression [9]. On average, PKD2 patients develop end-stage renal disease (ESRD) at a median age approximately 20 years later than PKD1 [10]. An important extrarenal manifestation of ADPKD is polycystic liver disease (PLD), distinct from ADPLD which occurs in patients with PRKCSH and SEC63 mutations [11, 12]. PLD is characterized by multiple liver cysts that originate from the biliary epithelium. It can be detected in almost 80% of ADPKD patients by age 60 years [13]. In severe cases, it may require surgical treatment (cyst fenestration, liver resection or transplantation) [14]. Other systemic features include intracranial aneurysm rupture, cardiac valve defects and the development of cysts in many other organs [15].
Careful radiological imaging follow-up studies have shown that TKV increases by 4 to 10% per year in ADPKD patients. Glomerular filtration rate (GFR), however, remains stable in the early stages despite cyst expansion [3]. A long lag time (up to 6 to 8 years) has been observed between changes in overall kidney structure (TKV) and function (GFR) [16–18]. Therefore a long follow-up period would be needed to see a beneficial effect on GFR. It has been proposed that TKV is a strong predictor of future GFR decline in ADPKD patients [18]. In one study, baseline height-adjusted TKV (> 600 cm3) predicted the risk of developing stage 3 CKD within 8 years [18]. On the basis of this observation, several clinical trials have used TKV as a primary endpoint or surrogate marker of disease activity in the presence of mildly impaired eGFR (70–90 ml min−1) [19, 20].
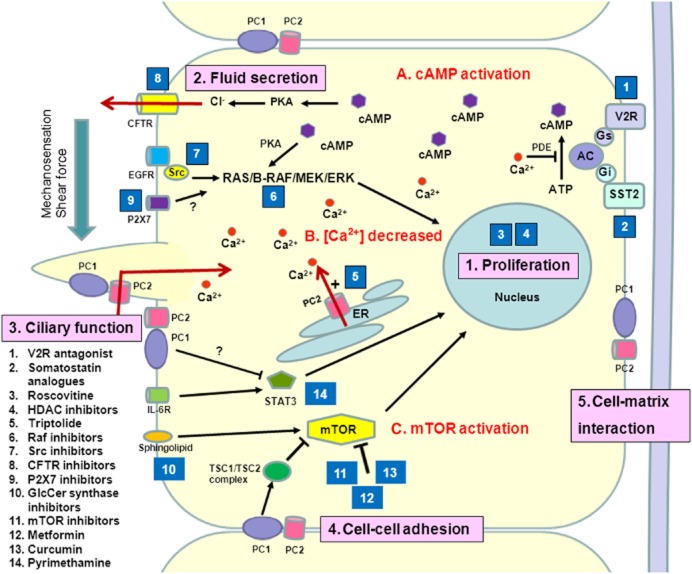
The identification of PKD1 and PKD2 and the discovery of the likely functions of the ADPKD proteins, polycystin-1 (PC-1) and polycystin-2 (PC-2), have revealed new therapeutic targets especially in aberrant downstream signalling pathways (Figure 1) [4]. Nonetheless, the subcellular distribution of both proteins is complex and their functions not fully elucidated. Both proteins have been localized to primary cilia, cell–cell junctions, focal adhesions and the endoplasmic reticulum [21]. PC-1 interacts with PC2 (and probably other proteins) to form a physiological complex which regulates cell proliferation, cell adhesion and Ca2+ signalling [22]. PC-2 is a non-selective calcium channel which belongs to the transient receptor potential (TRP) superfamily [8]. In primary cilia, the polycystin complex functions as a flow-dependent mechanosensor which regulates Ca2+ influx and cAMP levels [23]. The changes in Ca2+ homeostasis are complex with decreases in steady-state calcium, defects in cilia-based Ca2+ influx, store-operated currents and increased leak currents all being reported in polycystin deficient or null cells. This is likely to result in increased cAMP due to the activation of Ca2+ inhibitable adenylate cyclases (V, VI) and the suppression of Ca2+ activated phosphodiesterases (PDE1). Abnormalities in a number of other intracellular signalling pathways not regulated by Ca2+ or cAMP have also been reported. These include Ras/Raf/ERK, mammalian target of rapamycin (mTOR), cystic fibrosis transduction regulator (CFTR), ATP and AMP-kinase (AMPK). These changes could underlie the increases in cell proliferation and fluid secretion observed in ADPKD [4]. Other mechanisms of cyst initiation that have been proposed include abnormalities in planar cell polarity, differentiation, cell adhesion, cilia mechanosensation and centrosome number [24, 25].
Figure 1.
Schematic illustration of the key mechanisms of ADPKD pathogenesis and targets of potential treatments. Polycystin-1 and polycystin-2 expressed in different subcellular locations and regulate (1) proliferation, (2) fluid secretion, (3) ciliary function, (4) cell-cell adhesion and (5) cell-matrix interaction of renal epithelial cells. Dysfunction of polycystin-1 or polycystin-2 results to aberrant signalling pathways including: (A) activation of cAMP, (B) decreased intracellular calcium concentrtions and (C) activation of mTOR. These changes lead to transformation of normal cells to a ‘cystic phenotype’ and promote cyst formation. The targets of candidate drugs are depicted as blue boxes. Abbreviations: CFTR, cystic fibrosis transmembrane regulator; ER, endoplasmic reticulum; ERK, extracellular-signal regulated kinase; GlcCer, glucosylceramide; HDAC, histone deacetylase; IL-6R, interleukin-6 receptor; MEK, mitogen-activated protein kinase; mTOR, the mammalian target of rapamycin; PC, polycystin; PDE, phosphodiesterase; PKA, protein kinase A; SR, somatostatin receptor; TSC, tuberous sclerosis;V2R, vasopressin V2 receptor
The major treatment strategies attempted in ADPKD have focused on retarding disease progression and reducing cardiovascular risk. To modify disease, the major approaches have been to inhibit cystic cell proliferation and fluid secretion [26–29]. It is worth noting that liver cysts which arise by proliferation of cholangiocytes and dilation of biliary ductules may require different treatments [30]. However, a range of other novel treatment strategies designed to modify disease progression that target ciliary function, membrane glycosphingolipids and extracellular matrix are also currently under investigation [31–33]. Blood pressure control especially through inhibition of the renin-angiotensin-aldosterone system is the main approach to target cardiovascular risk but could be disease-modifying for ADPKD, e.g. the HALT study [20, 34]. In this paper, we review the results of recent clinical trials and the most promising preclinical compounds in development.
Clinical trials in ADPKD
Somatostatin analogs
Somatostatin, a somatotropin release-inhibiting factor, mediates its inhibitory effects through binding to at least five high affinity G-protein-coupled membrane receptors (sstr1–5) [35]. It is known to inhibit the action of all known gastrointestinal tract hormones by reducing intracellular cAMP and Ca2+ concentrations with activation of protein phosphatases [35]. Long acting somatostatin analogues have been developed and are in routine clinical use in the treatment of acromegaly and gastroenteropancreatic neuroendocrine tumours [35].
Octreotide, a long acting somatostatin analogue, has been shown to inhibit hepatic and renal cyst growth by inhibiting cAMP signalling in the PCK rat model [13]. A trial with lanreotide reported a 3% decrease in total liver volume (TLV) and a 1.5% decrease in TKV following 6 months treatment compared with baseline [36] (Table 1). Another study showed that patients treated with long acting repeatable depot® octreotide (OctLAR) for 1 year had a 5% reduction in TLV compared with baseline while TKV remained unchanged [30]. Subjective benefits on quality of life such as pain and physical activity were also significantly improved.
Table 1.
Selected drugs tested in clinical trials for ADPKD
| Drugs | Key mechanism | Dose (patients) | Effects | Main side effects | References |
|---|---|---|---|---|---|
| Sirolimus | mTOR inhibition | 2 mg day−1, 18 months (CKD stage 1–2) | TKV and eGFR: no change | Oral mucositis (82%), diarrhoea (61%), acne (59%), peripheral oedema (16%), amenorrhoea, ovarian cysts | [19, 98] |
| Everolimus | mTOR inhibition | 5 mg day−1, 24 months (CKD stage 2–3) | TKV and eGFR: no change | Oral ulcer (43%), leukopenia (18%), acne (14%), peripheral oedema (21%) | [44] |
| Octreotide | Somatostatin inhibition; cAMP↓ | 40 mg month−1, 1–2 years (CKD stage 1–4) | TLV: decreased, TKV: progression slowed, eGFR: no change | Diarrhoea (61%), tachyphylaxis (2nd year) | [30, 37] |
| Lanreotide | Somatostatin inhibition; cAMP↓ | 120 mg month−1, 6 months (CKD stage 1–4) | TLV: decreased, TKV: decreased, eGFR: no change | Diarrhoea (70%), injection-site nodules (48%) | [36] |
| Tolvaptan | Vasopressin V2 receptor inhibition; cAMP ↓ | 60–120 mg day−1, 36 months (CKD stage 1–2) | TKV and eGFR: progression slowed; fewer ADPKD-specific events (kidney pain, haematuria, and UTI) | Thirst (55%), Polyuria (38%), [Na] > 150 mmol l−1 (4%), ALT > 2.5 × UNL (5%), hyperuricaemia and gout (2.9%) | [54] |
ALT, alanine aminotransferase; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; mTOR, the mammalian target of rapamycin; TKV, total kidney volume; TLV, total liver volume; UNL, upper normal limit; UTI, urinary tract infection.
In a follow-up paper from the octreotide cohort [37], the beneficial effect was sustained into the second year when octreotide was maintained. However, there was no further decline in TLV and TKV after 1 year, suggesting the probability of tachyphylaxis due to downregulation or desensitization of somatostatin receptors [37]. The rate of eGFR decline was unchanged by octeotide treatment in this study.
Somatostain analogues remain attractive long term treatments for ADPKD in view of the convenience of monthly pulse injection and their tolerable side effects (diarrhoea and increased fasting plasma glucose concentration) [37]. Overall, it appears that somatostatin analogues are more effective in reducing liver cyst volume than kidney cyst volume. A clinical trial on the long term effects of somatostatin on renal disease progression using TKV as a primary outcome measure is ongoing (NCT00309283, http://clinicaltrials.gov). Whether more potent somatostatin analogues or a combination of a somatostatin analogue with other growth inhibitors could enhance efficacy warrants further investigation. Pasireotide, a more potent somatostatin analogue with broader receptor specificity, is more effective than octreotide in reducing the growth of liver and kidney cysts in rodent models but has not yet been tested in humans [38]. Another ongoing study is comparing combination therapy with octreotide and everolimus with octreotide monotherapy in PLD (NCT01157858) [39].
mTOR inhibitors
mTOR is a serine/threonine kinase which is a member of the PI3K-related kinase superfamily. Its major functions are to promote cell hypertrophy, cell division and cell survival [40]. Previous studies found that the mTOR pathway is upregulated in ADPKD, possibly due to loss of tonic inhibition on mTOR from a tuberin-polycystin-1 complex [41]. In preclinical studies, mTOR inhibitors (sirolimus, everolimus) were found to inhibit cell proliferation and cyst growth in several rodent models of ADPKD [41–43]. However, two clinical trials using different mTOR inhibitors in ADPKD patients published simultaneously in 2010 (Table 1), reported no significant benefits of either drug on TKV or GFR [19, 44]. In early-stage ADPKD patients (n = 100, eGFR >70 ml min−1 1.73 m−2), 18 months of sirolimus (target dose 2 mg day−1) with achieved steady-state blood concentrations from 4.1–4.9 μg l−1, had no impact on TKV or eGFR compared with the control group [19]. In later stage ADPKD patients (n = 433, eGFR 20–89 ml min−1 1.73 m−2), 2 years of everolimus (2.5 mg twice daily) with a mean trough concentration of 5.3 μg l−1) significantly reduced the annual increase in TKV by 35% in the first year, but the effect was not sustained at the end of the second year [44]. In addition, there was an overall increase in eGFR decline (–5.42 ml min−1) in the treated group compared with the placebo group (–3.22 ml min−1, P = 0.004) during the first year of study.
A third study (n = 55, eGFR 40–80 ml min−1 1.73 m−2) has shown that a higher rapamycin dose (trough concentration ∼6–8 μg l−1) and selection of PKD1 patients was associated with therapeutic benefit with respect to changes in TKV and eGFR [45]. Consistent with these data, a recent study in Pkd1 mice has clearly demonstrated dose-dependent effects of sirolimus on mTOR signalling, suggesting that conventional doses in man (blood concentrations ∼3 μg l−1) are ineffective in slowing cystogenesis [46]. A novel approach to improve direct drug delivery to renal epithelial cells by exploiting folate-conjugated rapamycin has been shown to slow progression in the bpk mouse model [47]. A strategy of combining low dose mTOR inhibitors with non-mTOR based treatments is another approach that could maximize efficacy while minimizing their many potential side effects [39].
Vasopressin V2 receptor antagonists
Tolvaptan is an orally active small molecule vasopressin V2 receptor antagonist which is effective in the treatment of hypervolaemic or euvolaemic hyponatraemia and congestive heart failure [48]. The rationale for its use in ADPKD comes from work showing that arginine vasopressin is a major stimulus for cAMP production in the collecting ducts [4]. Preclinical experiments demonstrated that vasopressin V2 receptor antagonists (OPC-31260 and tolvaptan) consistently inhibited cystogenesis by reducing renal cAMP levels in pcy mice, PCK rats and Pkd2WS25/– mice [49–51]. These encouraging findings have translated to clinical trials under the Tolvaptan Efficacy and Safety in Management of PKD and Outcomes (TEMPO) programme [52]. In the tolvaptan phase 2 open label study (TEMPO 2/4) in 63 ADPKD patients, 3 years of tolvaptan treatment ameliorated cyst growth (annual ΔTKV: +1.7% for tolvaptan vs. + 5.8% for control, P < 0.001) and more importantly, improved the annual decline of GFR compared with a historical cohort (annual eGFR declined: - 0.71 vs. −2.1 ml min−1 1.73 m−2, P = 0.01) [53]. Common adverse effects in these patients included thirst, polyuria, hyperuricaemia and increased creatinine concentrations which accounted for a drop-out rate of 9.5% [53]. The Tolvaptan phase 3 study (TEMPO 3/4), a 3 year multicentre randomized placebo controlled trial (n = 1445), has just reported in 2012. Its results demonstrated that tolvaptan (60–120 mg day−1, twice daily) slowed the rate of TKV increase by almost 50% compared with placebo (2.8% vs. 5.5% per year, P < 0.001) (Table 1). The decline of kidney function was also ameliorated by one-fourth in the tolvaptan group compared with the placebo group (annual eGFR declined: −2.72 vs. −3.70 ml min−1 1.73 m−2, P < 0.001) [54]. Aquaresis-related adverse events led to the discontinuation of tolvaptan in approximately 8% of participants, mostly within the first month. The results are encouraging as this is the first drug proven to have both structural and functional benefits in ADPKD. However, the efficacy, tolerability and adverse effects of long term use will need to be carefully evaluated in non-selected patients in routine clinical practice. An alternative approach to reducing vasopressin-stimulated cAMP production may be simply to increase daily fluid intake (3 l day−1). A pilot study has shown that plasma arginine vasopressin concentrations can be suppressed and urine osmolality decreased to under 300 mOsm l−1 by increased water intake [55].
Angiotensin converting enzyme inhibitors and angiotensin receptor blockers
Blood pressure control remains important in ADPKD to prevent the development of complications such as left ventricular hypertrophy, ischaemic heart disease and stroke [56]. Unlike other forms of CKD, the effect of rigorous blood pressure control and the potential benefit of ACE inhibition on the progression of ADPKD have remained areas of controversy for many years but are being evaluated in the ongoing HALT PKD study (NCT00283686) with a large, well-characterized cohort [34]. HALT will compare rigorous (<= 110/75 mmHg) vs. standard (<= 130/80 mmHg) BP control by using combination therapy (lisinopril and telmisartan) vs. monotherapy (lisinopril) in both early stage and late stage ADPKD patients [20].
Preclinical studies and early clinical trials
Src inhibitors
The Src family of non-receptor tyrosine kinases have been shown to be involved in the pathogenesis of numerous human cancers and may contribute to cell proliferation in ADPKD [57]. Bosutinib (SKI-606), a Src/Abl tyrosine kinase inhibitor, was found to suppress kidney cyst formation in the bpk and PCK rodent models by inhibiting EGF receptor activation and downstream B-Raf/ERK signalling [58]. In addition, SKI-606 reduced cell proliferation and extracellular matrix adhesion in vitro in ADPKD cyst lining cells and retarded cystic phenotype of Pkd1 +/- mice in vivo [32]. A phase 2 trial (NCT01233869) is being conducted to test the safety and efficacy of Src inhibition in ADPKD patients. Table 2 summarizes the drug candidates tested in preclinical studies.
Table 2.
Selected compounds as potential therapies for ADPKD in preclinical studies
| Compounds | Classification | Key mechanism (target/pathway) | Animal models | Main effects | Ongoing clinical trials | References |
|---|---|---|---|---|---|---|
| Bosutinib (SKI-606) | Src kinase inhibitors | Src tyrosine kinase/EGFR | BPK mice, PCK rat, Pkd1+/− mice | Reduce renal cyst number and volume; decrease BUN | Yes | [32, 58] |
| Triptolide | Diterpenoids | PC2 (agonist) | Pkd1 cKO mice | Reduce renal cyst number and BUN | Yes | [61–63] |
| Statins | HMG-CoA reductase inhibitors | Farnesyl pyrophosphate | Han: SPRD rat | Reduce renal cystic index and BUN | Yes | [99–101] |
| Sorafinib, Plx5568 | Raf inhibitors | B-Raf/MEK/ERK | Pkd2 cKO mice, Han:SPRD rat | Paradoxically increase liver cysts and augment renal and liver fibrosis | No | [14, 68] |
| R-roscovitine, S-CR8 | Cyclin-dependent kinase inhibitors | Cyclin-dependent kinases | cpk mice, jck mice, Pkd1 cKO mice | Reduce renal and hepatic cystic indexes; decrease BUN | No | [26, 69] |
| TSA,VPA | HDAC inhibitors | Modify chromatin and gene expression (epigenetic control) | Pkd1 & Pkd2 cKO mice, pkd2 zebrafish morphants | Reduce renal cystic index and BUN | No | [71, 72] |
| Tetrazolo-CFTR(inh)-172,Ph-GlyH-101 | CFTR inhibitors | CFTR chloride secretion | Pkd1 cKO mice | Decrease renal cyst number and BUN | No | [28] |
| Metformin | Biguanides | mTOR/CFTR (AMPK agonist) | Pkd1 cKO mice | Decrease renal cystic index | No | [79] |
| Genz-123346 | Glucosylceramide inhibitors | Akt/mTOR | pcy mice, jck mice, Pkd1 cKO mice | Decrease renal cystic volume, fibrosis, and BUN | No | [31] |
| Pioglitazone, Rosiglitazone | Thiazolidinediones | PPAR-γ receptor/ CFTR | PCK rat, Han: SPRD rat | Decrease liver and renal cystic index | No | [68, 82] |
| Curcumin | Curcuminoids | mTOR, WNT, STAT3 | Pkd1 cKO mice | Reduce renal cystic index and BUN | No | [24] |
| Ginkgolide B | Terpenoids | Unknown | Pkd1 cKO mice | Reduce renal cystic index but not hepatic cystic index | No | [88] |
| R-568 | Calcimimetics | CaSR/intracellular Ca2+ | Han: SPRD rat | Reduce renal cystic index, fibrosis and BUN | No | [90] |
| A-438079 | P2X7 receptor antagonists | Purinergic pathway/ ERK | pkd2 zebrafish morphants | Reduce pronephric cyst formation | No | [94] |
| Pyrimethamine, S3I-201 | Dihydrofolate reductase inhibitors | STAT3 | Pkd1 cKO mice | Reduce renal cystic index and creatinine | No | [95] |
| Leflunomide | Pyrimidine synthesis inhibitors | STAT6 | Bpk mice | Reduce renal cystic kidney and BUN | No | [97] |
BUN, blood urea nitrogen; CaSR, Calcium-sensing receptor; CFTR, cystic fibrosis transmembrane conductance regulator; cKO, conditional knockout; HMG-CoA, 3-hydroxy-3-methylglutaryl–coenzyme A; PPAR-γ, Peroxisome proliferator-activated receptor γ; STAT, signal transducer and activator of transcription; TSA, Trichostatin A; VPA, Valproic acid.
Triptolide
Triptolide is a natural compound derived from the traditional Chinese medicine Tripterygium wilfordii [59]. It has been used for treating rheumatoid arthritis and systemic lupus erythematosus due to its potent anti-inflammatory effects [60]. It was found to restore cytosolic Ca2+ release in Pkd1 null cells by acting as a PC2 agonist [61]. In vivo studies have shown its consistent effects on cystogenesis in several Pkd1 mouse models [61–63]. However, triptolide has a narrow therapeutic window and toxic side effects such as infertility and immunosuppression have largely limited its clinical use [60]. The potential application of triptolide in ADPKD is being examined in an ongoing clinical trial (NCT00801268).
Sorafenib
Sorafenib is a small molecule non-selective Raf inhibitor that decreases ERK activity and inhibits the proliferation of various human cancer cell lines [64]. Sorafenib could be an effective treatment in ADPKD since B-Raf plays a central role in cAMP-dependent activation of ERK in cystic epithelia [65–67], Surprisingly, paradoxical activation of Raf/MEK/ERK signalling and increased liver cysts were observed in Pkd2 deficient mice treated with sorafenib [14], possibly due to compensatory activation of Raf-1. In the same study, when sorafenib was given in combination with octreotide, the paradoxical increase of a liver cyst growth could be rescued through simultaneous blockade of the cAMP/PKA pathway [14]. Another group however reported increased renal and liver fibrosis in the Han:SPRD rat model with a different small molecule inhibitor (PLX5568); there was attenuation of cyst enlargement without an improvement in kidney function [68].
Roscovitine
R-roscovitine is a cyclin dependent kinase (CDK) inhibitor that has been shown to inhibit cell cycle progression, proliferation and apoptosis in the jck and cpk mouse models of PKD [69]. A recent study using S-CR8, a more potent second-generation analog of roscovitine, has confirmed its effectiveness in an orthologous Pkd1 mice model [26]. S-CR8 is approximately 80-fold more potent than R-roscovitine and suppressed both kidney and liver cyst formation [26].
HDAC inhibitors
Histone acetylation provides epigenetic control on gene expression through the post-translational modification of protein transcription complexes associated with active chromatin [70]. Histone deacetylases (HDACs) inhibitors induce cell cycle arrest and apoptosis in tumour cells and have been developed as a new class of anti-cancer agents currently in clinical trials [70]. Trichostatin A (TSA), a pan-HDAC inhibitor, was identified from a chemical library screen, to suppress pronephric cyst formation in a pkd2 zebrafish model [71]. Consistent with this, selective knockdown of HDAC1 or administration of a class I HDAC inhibitor, valproic acid (VPA), had identical effects as TSA [71]. These effects have been confirmed in both Pkd1 and Pkd2 mutant mice [71–73].
CFTR inhibitors
The CFTR channel is thought to play a major role in the increased chloride and fluid secretion into cyst lumen in ADPKD. An attenuated cystic phenotype has been observed in patients who carry both CFTR and PKD mutations [74]. The rapid development of CFTR inhibitors by high through-put screening has led to the discovery of many small-molecule inhibitors targeted at different domains of the CFTR protein [75]. Tetrazolo-172 and Phenyl-GlyH-101 represent the thiazolidinone and glycine hydrazide classes of CFTR inhibitors respectively. Both classes of compounds were found to inhibit cyst formation and enlargement in MDCK cyst models and in Pkd1 mice [28]. More potent and stable CFTR inhibitors are being developed and have been tested in embryonic kidney explant PKD models [76]. Nevertheless, their efficacy and safety in human ADPKD requires future clinical trials.
KCa3.1 channels mediate K+ efflux and maintain a negative intracellular membrane potential which indirectly enhances apical Cl− secretion by the CFTR [77]. A specific KCa3.1 channel inhibitor, TRAM-34, has been shown to inhibit Cl− secretion and cyst formation by MDCK cells [77]. This suggests that KCa3.1 channel inhibitors could be alternative or complimentary to CFTR inhibitors as anti-secretory drugs. However, animal studies and clinical trials to support its use for ADPKD treatment are still lacking.
Metformin
Metformin, a biguanide derivate, is a safe and well-tolerated drug in general clinical use for the treatment of hyperglycemia in type 2 diabetic mellitus [78]. Its therapeutic potential in other conditions such as polycystic ovary disease and the prevention or treatment of cancer has also been studied [78]. Recently, metformin was also shown to inhibit renal cystogenesis by activating AMPK and suppressing mTOR and CFTR in Pkd1 mice [79]. However, the effective dosage in mice was 300 mg kg−1 day−1, ∼15 times higher than that used in treating type 2 diabetes mellitus in humans. It may exert anticancer effects via AMPK-medicated mTOR complex 1 inhibition [40]. Further studies could consider combining metformin with mTOR or mTOR kinase inhibitors (TOR-KIs) for synergistic effects. The potential risk of metformin to cause lactic acidosis in advanced CKD will limit its use to early stage ADPKD. The use of metformin in ADPKD patients has not been investigated in clinical trials.
Glucosylceramide inhibitors
Sphingolipids are ‘bioactive lipids’ that are not only components of cell membranes but also play signalling roles in the regulation of cell proliferation, apoptosis, adhesion, and inflammation [80]. Alterations of glycosphingolipid metabolism with elevated glucosylceramide (GlcCer) in cystic epithelial cells may have a major role in driving cyst growth [31]. A seminal study has shown that Genz-123346, a GlcCer synthase inhibitor, blocked cell cycle progression and proliferation through inhibition of the Akt-mTOR pathway in mouse models for ADPKD and nephronopthisis [31]. A more recent study identified another glycosphingolipid, ganglioside GM3, as a potential therapeutic target [81]. Crossing jck mice with mice carrying a mutation in the GM3 synthase (St3gal5) gene led to a milder cystic phenotype [81].
Thiazolidinediones
Agonists of peroxisome proliferator activated receptor-gamma (PPARγ) receptors have been shown to have modest anti-cystogenic properties in PKD animal models [82–85]. Pioglitazone feeding inhibits renal and hepatic bile duct cyst growth possibly through reducing CFTR expression in the PCK rat [82]. In another study, rosiglitazone delayed the onset of renal failure but was associated with cardiac enlargement due to excessive renal sodium reabsorption in the Han:SPRD rat [85]. Nevertheless, the efficacy of these drugs has not yet been tested in ADPKD patients and their potential side effects (cardiac failure, fluid retention) could be major safety concerns [86].
Curcumin
Curcumin (diferuloylmethane) is a yellow spice derived from the rhizome of the plant Curcuma longa [24, 87]. It is a polyphenol natural product which is known to modulate several pathways (mTOR, WNT, STAT3) altered in ADPKD [24]. A recent study showed that curcumin reduced cystogenesis and postponed renal failure in Pkd1 mice [24]. In MDCK cells, curcumin could inhibit forskolin-promoted cyst formation [87]. The involvement of multiple pathways, rather than a single pathway, may be an advantage in terms of efficacy. However, clinical trials have not yet been done to support its clinical use. The bioavailability of oral curcumin in the renal epithelium also needs further investigation.
Ginkgolide B
A recent study proposed that ginkgolide B, a natural compound isolated from the leaves of Ginkgo biloba, inhibits renal cyst development in Pkd1 knockout mice [88]. The exact mechanism of ginkgolide B on cyst formation and progression remains unclear and requires confirmation.
Calcimimetics
Calcimimetics are allosteric modulators of the calcium-sensing receptor developed to treat secondary hyperparathyroidism. Calcium sensing receptors are expressed in all nephron segments except the glomerulus. The major site for plasma membrane localization however differs between the apical membrane in the proximal tubule and the basolateral membrane in the thick ascending limb and the distal convoluted tubule [89]. Interestingly, the calcimimetic R-568 was found to halt late-stage progression of renal cysts in the Han:SPRD rat through a direct modulation of intracellular calcium [90] and the effect was stronger than with calcium supplementation alone. However, R-568 had no significant effects on cyst formation in two other models, the Pkd2WS25/− mouse and the PCK rat [91]. Whether calcimimetics have additional effects on cyst progression other than correction of secondary hyperparathyroidism warrants further investigation.
Purinergic receptor inhibitors
Activation of purinergic receptors by ATP has been hypothesized to modulate fluid secretion, cell proliferation, apoptosis and ciliary function in ADPKD [92, 93]. Blockade of the P2X7 receptor by morpholino knockdown or a selective inhibitor (A438079) reduced cyst formation in a pkd2 zebrafish model [94], suggesting that P2X7 antagonists could have therapeutic potential in ADPKD.
Pyrimethamine
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway plays an important role in kidney development and mediates tubular cell proliferation after ischaemic injury [95, 96]. A chemical library screen for STAT3 inhibitors identified a number of compounds including pyrimethamine, an antiparasitic drug in clinical use. Further testing showed that pyrimethamine could effectively inhibit cyst growth in Pkd1 mice [95]. A STAT3 specific inhibitor (S3I-201) confirmed these beneficial effects [95]. How PC1 regulates STAT3 activity is still not completely understood [96].
Leflunomide
In addition to STAT3, overexpression of STAT6 in cyst-lining epithelium has been found in two mouse models of ADPKD [97]. Leflunomide, an FDA-approved disease modifying anti-rheumatic drug, significantly reduced cystic disease and preserved kidney function in bpk mice through suppression of the IL4/IL13/STAT6 pathway [97]. Specific STAT6 inhibitors could prove more effective in future studies.
Concluding remarks and future directions
The identification of PKD1 and PKD2 has stimulated rapid progress in the study of the molecular mechanisms underlying ADPKD pathogenesis and the development of mechanism-based therapeutics. Translation of preclinical data to clinical practice is fast becoming a reality with the recent results of the TEMPO 3/4 study. Indeed, these results represent a paradigm shift in the management of ADPKD patients. Nonetheless, it is anticipated that further drug development will be needed as it is unlikely that a single agent, even if safe and well tolerated, will work in all patients. Careful risk stratification (genotyping, phenotyping, imaging, biomarkers) will also be necessary to identify the patients who need treatment, what agents to use and when to initiate treatment. Since the majority of patients are asymptomatic, any treatments approved for clinical use will need to have an excellent safety record and tolerability in view of their likely life long duration. In this respect, combination therapies may be advantageous by maximizing efficacy while minimizing side effects. It will be crucial to address important clinical issues such as the duration of treatment, the effect of pulse therapies and the consequence of stopping treatment on subsequent disease progression. Finally, effective drugs to treat cystic liver disease will be needed for the proportion of patients with significant PLD.
Competing Interest
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare ACMO has participated in the TEMPO 3/4 trial as a centre investigator, has served on advisory boards for Otsuka and as a consultant for Pfizer as a member of the external data monitoring committee for the Bosunitib study.
References
- 1.Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2010;6:197–206. doi: 10.1038/nrneph.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leuenroth SJ, Crews CM. Targeting cyst initiation in ADPKD. JASN. 2009;20:1–3. doi: 10.1681/ASN.2008101118. [DOI] [PubMed] [Google Scholar]
- 3.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 4.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang MY, Ong AC. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and treatment. Nephron Physiol. 2008;108:1–7. doi: 10.1159/000112495. [DOI] [PubMed] [Google Scholar]
- 6.Terryn S, Ho A, Beauwens R, Devuyst O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta. 2011;1812:1314–1321. doi: 10.1016/j.bbadis.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Drummond IA. Polycystins, focal adhesions and extracellular matrix interactions. Biochim Biophys Acta. 2011;1812:1322–1326. doi: 10.1016/j.bbadis.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng S, Rodat-Despoix L, Delmas P, Ong AC. A single amino acid residue constitutes the third dimerization domain essential for the assembly and function of the tetrameric polycystin-2 (TRPP2) channel. J Biol Chem. 2011;286:18994–19000. doi: 10.1074/jbc.M110.192286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–1485. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- 10.Barua M, Cil O, Paterson AD, Wang K, He N, Dicks E, Parfrey P, Pei Y. Family history of renal disease severity predicts the mutated gene in ADPKD. JASN. 2009;20:1833–1838. doi: 10.1681/ASN.2009020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li A, Davila S, Furu L, Qian Q, Tian X, Kamath PS, King BF, Torres VE, Somlo S. Mutations in PRKCSH cause isolated autosomal dominant polycystic liver disease. Am J Hum Genet. 2003;72:691–703. doi: 10.1086/368295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davila S, Furu L, Gharavi AG, Tian X, Onoe T, Qian Q, Li A, Cai Y, Kamath PS, King BF, Azurmendi PJ, Tahvanainen P, Kaariainen H, Hockerstedt K, Devuyst O, Pirson Y, Martin RS, Lifton RP, Tahvanainen E, Torres VE, Somlo S. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat Genet. 2004;36:575–577. doi: 10.1038/ng1357. [DOI] [PubMed] [Google Scholar]
- 13.Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3',5'-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Spirli C, Morell CM, Locatelli L, Okolicsanyi S, Ferrero C, Kim AK, Fabris L, Fiorotto R, Strazzabosco M. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology. 2012;56:2363–2374. doi: 10.1002/hep.25872. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 15.Irazabal MV, Huston J, 3rd, Kubly V, Rossetti S, Sundsbak JL, Hogan MC, Harris PC, Brown RD, Jr, Torres VE. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. CJASN. 2011;6:1274–1285. doi: 10.2215/CJN.09731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres VE, King BF, Chapman AB, Brummer ME, Bae KT, Glockner JF, Arya K, Risk D, Felmlee JP, Grantham JJ, Guay-Woodford LM, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP. Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. CJASN. 2007;2:112–120. doi: 10.2215/CJN.00910306. [DOI] [PubMed] [Google Scholar]
- 17.Torres VE, Grantham JJ, Chapman AB, Mrug M, Bae KT, King BF, Jr, Wetzel LH, Martin D, Lockhart ME, Bennett WM, Moxey-Mims M, Abebe KZ, Lin Y, Bost JE. Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. CJASN. 2011;6:640–647. doi: 10.2215/CJN.03250410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. CJASN. 2012;7:479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wuthrich RP. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 20.Chapman AB, Torres VE, Perrone RD, Steinman TI, Bae KT, Miller JP, Miskulin DC, Rahbari Oskoui F, Masoumi A, Hogan MC, Winklhofer FT, Braun W, Thompson PA, Meyers CM, Kelleher C, Schrier RW. The HALT polycystic kidney disease trials: design and implementation. CJASN. 2010;5:102–109. doi: 10.2215/CJN.04310709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong AC, Harris PC. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int. 2005;67:1234–1247. doi: 10.1111/j.1523-1755.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 22.Streets AJ, Wagner BE, Harris PC, Ward CJ, Ong AC. Homophilic and heterophilic polycystin 1 interactions regulate E-cadherin recruitment and junction assembly in MDCK cells. J Cell Sci. 2009;122(Pt 9):1410–1417. doi: 10.1242/jcs.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 24.Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, de Heer E, Peters DJ. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol. 2011;300:F1193–1202. doi: 10.1152/ajprenal.00419.2010. [DOI] [PubMed] [Google Scholar]
- 25.Luyten A, Su X, Gondela S, Chen Y, Rompani S, Takakura A, Zhou J. Aberrant regulation of planar cell polarity in polycystic kidney disease. JASN. 2010;21:1521–1532. doi: 10.1681/ASN.2010010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bukanov NO, Moreno SE, Natoli TA, Rogers KA, Smith LA, Ledbetter SR, Oumata N, Galons H, Meijer L, Ibraghimov-Beskrovnaya O. CDK inhibitors R-roscovitine and S-CR8 effectively block renal and hepatic cystogenesis in an orthologous model of ADPKD. Cell Cycle. 2012;11:4040–4046. doi: 10.4161/cc.22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvet JP. Strategies to inhibit cyst formation in ADPKD. CJASN. 2008;3:1205–1211. doi: 10.2215/CJN.05651207. [DOI] [PubMed] [Google Scholar]
- 28.Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. JASN. 2008;19:1300–1310. doi: 10.1681/ASN.2007070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang MY, Ong AC. Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clin Pract. 2012;120:c25–34. doi: 10.1159/000334166. discussion c35. [DOI] [PubMed] [Google Scholar]
- 30.Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, Kim B, King BF, Glockner J, Holmes DR, 3rd, Rossetti S, Harris PC, LaRusso NF, Torres VE. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. JASN. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natoli TA, Smith LA, Rogers KA, Wang B, Komarnitsky S, Budman Y, Belenky A, Bukanov NO, Dackowski WR, Husson H, Russo RJ, Shayman JA, Ledbetter SR, Leonard JP, Ibraghimov-Beskrovnaya O. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med. 2010;16:788–792. doi: 10.1038/nm.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott J, Zheleznova NN, Wilson PD. c-Src inactivation reduces renal epithelial cell-matrix adhesion, proliferation, and cyst formation. Am J Physiol Cell Physiol. 2011;301:C522–529. doi: 10.1152/ajpcell.00163.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X. Epigenetics and autosomal dominant polycystic kidney disease. Biochim Biophys Acta. 2011;1812:1213–1218. doi: 10.1016/j.bbadis.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres VE, Chapman AB, Perrone RD, Bae KT, Abebe KZ, Bost JE, Miskulin DC, Steinman TI, Braun WE, Winklhofer FT, Hogan MC, Oskoui FR, Kelleher C, Masoumi A, Glockner J, Halin NJ, Martin DR, Remer E, Patel N, Pedrosa I, Wetzel LH, Thompson PA, Miller JP, Meyers CM, Schrier RW. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int. 2012;81:577–585. doi: 10.1038/ki.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modlin IM, Pavel M, Kidd M, Gustafsson BI. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther. 2010;31:169–188. doi: 10.1111/j.1365-2036.2009.04174.x. [DOI] [PubMed] [Google Scholar]
- 36.van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, de Man RA, Drenth JP. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137:1661–1668. doi: 10.1053/j.gastro.2009.07.052. e1661-1662. [DOI] [PubMed] [Google Scholar]
- 37.Hogan MC, Masyuk TV, Page L, Holmes DR, 3rd, Li X, Bergstralh EJ, Irazabal MV, Kim B, King BF, Glockner JF, Larusso NF, Torres VE. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant. 2012;27:3532–3539. doi: 10.1093/ndt/gfs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masyuk TV, Radtke BN, Stroope AJ, Banales JM, Gradilone SA, Huang B, Masyuk AI, Hogan MC, Torres VE, Larusso NF. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology. 2012 doi: 10.1002/hep.26140. doi: 10.1002/hep.26140 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chrispijn M, Drenth JP. Everolimus and long acting octreotide as a volume reducing treatment of polycystic livers (ELATE): study protocol for a randomized controlled trial. Trials. 2011;12:246. doi: 10.1186/1745-6215-12-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 43.Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. JASN. 2010;21:489–497. doi: 10.1681/ASN.2009040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N, Arns W, Pavenstadt H, Gaedeke J, Buchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 45.Stallone G, Infante B, Grandaliano G, Bristogiannis C, Macarini L, Mezzopane D, Bruno F, Montemurno E, Schirinzi A, Sabbatini M, Pisani A, Tataranni T, Schena FP, Gesualdo L. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD-study): a randomized, controlled study. Nephrol Dial Transplant. 2012;27:3560–3567. doi: 10.1093/ndt/gfs264. [DOI] [PubMed] [Google Scholar]
- 46.Novalic Z, van der Wal AM, Leonhard WN, Koehl G, Breuning MH, Geissler EK, de Heer E, Peters DJ. Dose-dependent effects of sirolimus on mTOR signaling and polycystic kidney disease. JASN. 2012;23:842–853. doi: 10.1681/ASN.2011040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shillingford JM, Leamon CP, Vlahov IR, Weimbs T. Folate-conjugated rapamycin slows progression of polycystic kidney disease. JASN. 2012;23:1674–1681. doi: 10.1681/ASN.2012040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irazabal MV, Torres VE, Hogan MC, Glockner J, King BF, Ofstie TG, Krasa HB, Ouyang J, Czerwiec FS. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80:295–301. doi: 10.1038/ki.2011.119. [DOI] [PubMed] [Google Scholar]
- 49.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH., 2nd Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:102–108. doi: 10.1681/ASN.2007060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 52.Torres VE. Role of vasopressin antagonists. CJASN. 2008;3:1212–1218. doi: 10.2215/CJN.05281107. [DOI] [PubMed] [Google Scholar]
- 53.Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, Horie S, Nutahara K, Ouyang J, Krasa HB, Czerwiec FS. Tolvaptan in autosomal dominant polycystic kidney disease: three years' experience. CJASN. 2011;6:2499–2507. doi: 10.2215/CJN.03530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barash I, Ponda MP, Goldfarb DS, Skolnik EY. A pilot clinical study to evaluate changes in urine osmolality and urine cAMP in response to acute and chronic water loading in autosomal dominant polycystic kidney disease. CJASN. 2010;5:693–697. doi: 10.2215/CJN.04180609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang MY, Kuok CM, Chen YC, Ryu SJ, Tian YC, Wu-Chou YH, Tseng FJ, Yang CW. Comparison of intracerebral hemorrhage and subarachnoid hemorrhage in patients with autosomal-dominant polycystic kidney disease. Nephron Clin Pract. 2010;114:c158–164. doi: 10.1159/000256568. [DOI] [PubMed] [Google Scholar]
- 57.Puls LN, Eadens M, Messersmith W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist. 2011;16:566–578. doi: 10.1634/theoncologist.2010-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sweeney WE, Jr, von Vigier RO, Frost P, Avner ED. Src inhibition ameliorates polycystic kidney disease. JASN. 2008;19:1331–1341. doi: 10.1681/ASN.2007060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Man S, Gao W, Wei C, Liu C. Anticancer drugs from traditional toxic Chinese medicines. PTR. 2012;26:1449–1465. doi: 10.1002/ptr.4609. [DOI] [PubMed] [Google Scholar]
- 60.Liu L, Jiang Z, Liu J, Huang X, Wang T, Zhang Y, Zhou Z, Guo J, Yang L, Chen Y, Zhang L. Sex differences in subacute toxicity and hepatic microsomal metabolism of triptolide in rats. Toxicology. 2010;271:57–63. doi: 10.1016/j.tox.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, Crews CM. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci U S A. 2007;104:4389–4394. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leuenroth SJ, Bencivenga N, Igarashi P, Somlo S, Crews CM. Triptolide reduces cystogenesis in a model of ADPKD. JASN. 2008;19:1659–1662. doi: 10.1681/ASN.2008030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leuenroth SJ, Bencivenga N, Chahboune H, Hyder F, Crews CM. Triptolide reduces cyst formation in a neonatal to adult transition Pkd1 model of ADPKD. Nephrol Dial Transplant. 2010;25:2187–2194. doi: 10.1093/ndt/gfp777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamaguchi T, Reif GA, Calvet JP, Wallace DP. Sorafenib inhibits cAMP-dependent ERK activation, cell proliferation, and in vitro cyst growth of human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2010;299:F944–951. doi: 10.1152/ajprenal.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parker E, Newby LJ, Sharpe CC, Rossetti S, Streets AJ, Harris PC, O'Hare MJ, Ong AC. Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int. 2007;72:157–165. doi: 10.1038/sj.ki.5002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kugita M, Nishii K, Morita M, Yoshihara D, Kowa-Sugiyama H, Yamada K, Yamaguchi T, Wallace DP, Calvet JP, Kurahashi H, Nagao S. Global gene expression profiling in early-stage polycystic kidney disease in the Han:SPRD Cy rat identifies a role for RXR signaling. Am J Physiol Renal Physiol. 2011;300:F177–188. doi: 10.1152/ajprenal.00470.2010. [DOI] [PubMed] [Google Scholar]
- 67.Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, Helmkamp GM, Jr, Grantham JJ. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 2004;66:964–973. doi: 10.1111/j.1523-1755.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- 68.Buchholz B, Klanke B, Schley G, Bollag G, Tsai J, Kroening S, Yoshihara D, Wallace DP, Kraenzlin B, Gretz N, Hirth P, Eckardt KU, Bernhardt WM. The Raf kinase inhibitor PLX5568 slows cyst proliferation in rat polycystic kidney disease but promotes renal and hepatic fibrosis. Nephrol Dial Transplant. 2011;26:3458–3465. doi: 10.1093/ndt/gfr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 70.Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- 71.Cao Y, Semanchik N, Lee SH, Somlo S, Barbano PE, Coifman R, Sun Z. Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci U S A. 2009;106:21819–21824. doi: 10.1073/pnas.0911987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xia S, Li X, Johnson T, Seidel C, Wallace DP, Li R. Polycystin-dependent fluid flow sensing targets histone deacetylase 5 to prevent the development of renal cysts. Development. 2010;137:1075–1084. doi: 10.1242/dev.049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fan LX, Li X, Magenheimer B, Calvet JP. Inhibition of histone deacetylases targets the transcription regulator Id2 to attenuate cystic epithelial cell proliferation. Kidney Int. 2012;81:76–85. doi: 10.1038/ki.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu N, Glockner JF, Rossetti S, Babovich-Vuksanovic D, Harris PC, Torres VE. Autosomal dominant polycystic kidney disease coexisting with cystic fibrosis. J Nephrol. 2006;19:529–534. [PubMed] [Google Scholar]
- 75.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snyder DS, Tradtrantip L, Yao C, Kurth MJ, Verkman AS. Potent, metabolically stable benzopyrimido-pyrrolo-oxazine-dione (BPO) CFTR inhibitors for polycystic kidney disease. J Med Chem. 2011;54:5468–5477. doi: 10.1021/jm200505e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Albaqumi M, Srivastava S, Li Z, Zhdnova O, Wulff H, Itani O, Wallace DP, Skolnik EY. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;74:740–749. doi: 10.1038/ki.2008.246. [DOI] [PubMed] [Google Scholar]
- 78.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takiar V, Nishio S, Seo-Mayer P, King JD, Jr, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci U S A. 2011;108:2462–2467. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 81.Natoli TA, Husson H, Rogers KA, Smith LA, Wang B, Budman Y, Bukanov NO, Ledbetter SR, Klinger KW, Leonard JP, Ibraghimov-Beskrovnaya O. Loss of GM3 synthase gene, but not sphingosine kinase 1, is protective against murine nephronophthisis-related polycystic kidney disease. Hum Mol Genet. 2012;21:3397–3407. doi: 10.1093/hmg/dds172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blazer-Yost BL, Haydon J, Eggleston-Gulyas T, Chen JH, Wang X, Gattone V, Torres VE. Pioglitazone attenuates cystic burden in the PCK rodent model of polycystic kidney disease. PPAR Res. 2010;2010:274376. doi: 10.1155/2010/274376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshihara D, Kurahashi H, Morita M, Kugita M, Hiki Y, Aukema HM, Yamaguchi T, Calvet JP, Wallace DP, Nagao S. PPAR-{gamma} agonist ameliorates kidney and liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. Am J Physiol Renal Physiol. 2011;300:F465–474. doi: 10.1152/ajprenal.00460.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muto S, Aiba A, Saito Y, Nakao K, Nakamura K, Tomita K, Kitamura T, Kurabayashi M, Nagai R, Higashihara E, Harris PC, Katsuki M, Horie S. Pioglitazone improves the phenotype and molecular defects of a targeted Pkd1 mutant. Hum Mol Genet. 2002;11:1731–1742. doi: 10.1093/hmg/11.15.1731. [DOI] [PubMed] [Google Scholar]
- 85.Dai B, Liu Y, Mei C, Fu L, Xiong X, Zhang Y, Shen X, Hua Z. Rosiglitazone attenuates development of polycystic kidney disease and prolongs survival in Han:SPRD rats. Clin Sci (Lond) 2010;119:323–333. doi: 10.1042/CS20100113. [DOI] [PubMed] [Google Scholar]
- 86.Mao Z, Ong AC. Peroxisome proliferator-activated receptor gamma agonists in kidney disease – future promise, present fears. Nephron Clinical Practice. 2009;112:c230–241. doi: 10.1159/000224789. [DOI] [PubMed] [Google Scholar]
- 87.Gao J, Zhou H, Lei T, Zhou L, Li W, Li X, Yang B. Curcumin inhibits renal cyst formation and enlargement in vitro by regulating intracellular signaling pathways. Eur J Pharmacol. 2011;654:92–99. doi: 10.1016/j.ejphar.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 88.Zhou H, Gao J, Zhou L, Li X, Li W, Xia Y, Yang B. Ginkgolide B inhibits renal cyst development in in vitro and in vivo cyst models. Am J Physiol Renal Physiol. 2012;302:F1234–1242. doi: 10.1152/ajprenal.00356.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang C, Miller RT. Regulation of renal ion transport by the calcium-sensing receptor: an update. Curr Opin Nephrol Hypertens. 2007;16:437–443. doi: 10.1097/MNH.0b013e3282b974a6. [DOI] [PubMed] [Google Scholar]
- 90.Gattone VH, 2nd, Chen NX, Sinders RM, Seifert MF, Duan D, Martin D, Henley C, Moe SM. Calcimimetic inhibits late-stage cyst growth in ADPKD. JASN. 2009;20:1527–1532. doi: 10.1681/ASN.2008090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X, Harris PC, Somlo S, Batlle D, Torres VE. Effect of calcium-sensing receptor activation in models of autosomal recessive or dominant polycystic kidney disease. Nephrol Dial Transplant. 2009;24:526–534. doi: 10.1093/ndt/gfn527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Turner CM, Ramesh B, Srai SK, Burnstock G, Unwin RJ. Altered ATP-sensitive P2 receptor subtype expression in the Han:SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cells Tissues Organs. 2004;178:168–179. doi: 10.1159/000082247. [DOI] [PubMed] [Google Scholar]
- 93.Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2009;296:F1464–1476. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang MY, Lu JK, Tian YC, Chen YC, Hung CC, Huang YH, Chen YH, Wu MS, Yang CW, Cheng YC. Inhibition of the P2X7 receptor reduces cystogenesis in PKD. JASN. 2011;22:1696–1706. doi: 10.1681/ASN.2010070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, Zhou J. Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet. 2011;20:4143–4154. doi: 10.1093/hmg/ddr338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT, Watnick T, Weimbs T. Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci U S A. 2011;108:7985–7990. doi: 10.1073/pnas.1103816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, Todorov G, Song X, Pei Y, Weimbs T. Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proc Natl Acad Sci U S A. 2011;108:18067–18072. doi: 10.1073/pnas.1111966108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Braun M, Young J, Reiner CS, Poster D, Wuthrich RP, Serra AL. Ovarian toxicity from sirolimus. New Engl J Med. 2012;366:1062–1064. doi: 10.1056/NEJMc1113145. [DOI] [PubMed] [Google Scholar]
- 99.Fassett RG, Coombes JS, Packham D, Fairley KF, Kincaid-Smith P. Effect of pravastatin on kidney function and urinary protein excretion in autosomal dominant polycystic kidney disease. Scand J Urol Nephrol. 2010;44:56–61. doi: 10.3109/00365590903359908. [DOI] [PubMed] [Google Scholar]
- 100.Cadnapaphornchai MA, George DM, Masoumi A, McFann K, Strain JD, Schrier RW. Effect of statin therapy on disease progression in pediatric ADPKD: design and baseline characteristics of participants. Contemp Clin Trials. 2011;32:437–445. doi: 10.1016/j.cct.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gile RD, Cowley BD, Jr, Gattone VH, 2nd, O'Donnell MP, Swan SK, Grantham JJ. Effect of lovastatin on the development of polycystic kidney disease in the Han:SPRD rat. Am J Kidney Dis. 1995;26:501–507. doi: 10.1016/0272-6386(95)90497-2. [DOI] [PubMed] [Google Scholar]