Abstract
We have identified five different homozygous recessive mutations in a novel gene, TMIE (transmembrane inner ear expressed gene), in affected members of consanguineous families segregating severe-to-profound prelingual deafness, consistent with linkage to DFNB6. The mutations include an insertion, a deletion, and three missense mutations, and they indicate that loss of function of TMIE causes hearing loss in humans. TMIE encodes a protein with 156 amino acids and exhibits no significant nucleotide or deduced amino acid sequence similarity to any other gene.
We previously reported hereditary deafness segregating in a consanguineous family (5020-22) from southern India and identified an 18-cM interval between markers D3S1619 and D3S1766 that defined the DFNB6 locus (MIM 600971) (Fukushima et al. 1995). With institutional review board approval and written informed consent, additional families with two or more individuals who had severe-to-profound hearing impairment were genotyped for possible linkage to 3p21, and four additional families showing linkage to DFNB6 were found.
Usher syndrome type 2B (USH2B [MIM 276905]), an autosomal recessive disorder comprising moderate hearing loss and retinitis pigmentosa (Hmani et al. 1999), maps to the same chromosomal region as DFNB6. To ascertain whether these families showing linkage to DFNB6 have ocular abnormalities, medical histories were taken with an emphasis on the eye phenotype. There is no history of nyctalopia in these DFNB6 families, and electroretinography was normal in six affected individuals (aged 15–19 years) from families PKSN21 and PKSR22B. Although some of the affected individuals are too young to manifest visual abnormalities, electroretinography can usually diagnose retinitis pigmentosa in presymptomatic children (Young et al. 1996). Deaf members of family I-51 had no clinical evidence of vestibular abnormalities, although affected members of family PKSN21 did have delayed development of independent ambulation, which may reflect an underlying vestibular defect.
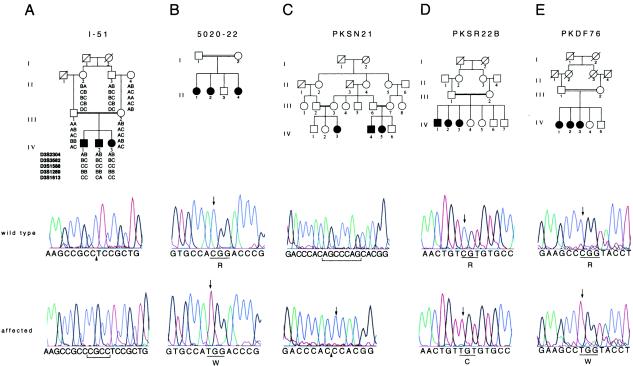
Haplotype analysis in one of the DFNB6 families, I-51, refined the DFNB6 interval to a 3-cM region between markers D3S3582 and D3S1613 (fig. 1A). On the basis of conserved synteny between distal mouse chromosome 9 and human chromosome 3p, the spinner strain of deaf mice was postulated to be the mouse model for nonsyndromic hearing loss linked to DFNB6 (Fukushima et al. 1995). Mice with heritable inner ear abnormalities are models with which to explore the pathophysiology of hearing loss in humans (Steel 1995; Petit et al. 2001). Recently, two independent mutations in the novel gene Tmie were demonstrated to be responsible for hearing loss and vestibular dysfunction in spinner mice (Mitchem et al. 2002). The human ortholog, TMIE, is located in the DFNB6 interval. Therefore, we sequenced the four exons of TMIE from affected individuals in families demonstrating linkage to DFNB6.
Figure 1.
Families showing linkage to DFNB6 and TMIE mutation analyses. A, Family I-51, from India, defined the minimum interval for DFNB6 as being between markers D3S3582 and D3S1613. Sequence chromatograms from a noncarrier (II:4) and a deaf individual (IV:2) show the wild-type sequence, the place of insertion (arrowhead), and the insertion of CGCC (bracketed) in exon 2, respectively. B, 250C→T transition in exon 3 in an affected individual (II:1) from family 5020-22, compared with the wild-type sequence. C, The chromatograms show sequence of the intron 1–exon 2 boundary in PKSN21, from a noncarrier and an affected individual with the deletion of seven bases (bracketed in wild type). The site of the deletion is indicated by an arrowhead, along with an insertion of cytosine (arrow). D, The sequence traces of a wild type and an affected individual (IV:2) in family PKSR22B with the 241C→T transition (arrows). E, Chromatograms from an unaffected control individual and an affected individual (IV:1) from family PKDF76, showing the 274C→T transition (arrows).
Affected individuals in family 5020-22 have a missense mutation (250C→T) in exon 3 of TMIE, changing an arginine residue to a tryptophan (R84W) within the putative cytoplasmic domain of TMIE (fig. 1B). We identified mutations in TMIE in four additional families showing linkage to DFNB6 (one Indian and three Pakistani). In family I-51, we found a 4-bp insertion, 125-126insCGCC, in exon 2, segregating with hearing loss (fig. 1A). The insertion is predicted to result in a frameshift substituting 72 incorrect amino acids followed by premature truncation of the protein. Sequence analysis of affected individuals in family PKSN21 showed a deletion/insertion IVS1-2_98delAGCCCAGinsC (fig. 1C). Since the mutation removes the predicted acceptor splice site preceding exon 2, this exon may be skipped, resulting in a frameshift. Alternatively, a Genscan-predicted cryptic splice site in exon 2 could be utilized, resulting in the deletion of 21 nucleotides and removal of seven amino acids (PSTAPPK) from the putative extracellular domain of TMIE. In family PKSR22B, we found a C→T transition (241C→T) in exon 3, causing a substitution of arginine for cysteine, R81C, in the predicted cytoplasmic domain (fig. 1D). In the fifth family (PKDF76), there is a missense mutation, 274C→T, substituting tryptophan for arginine, R92W (fig. 1E). Homozygosity for these mutations cosegregated with the deafness phenotype in all five families, and none of these mutations was detected in 150 Pakistani and 104 Indian individuals with normal hearing.
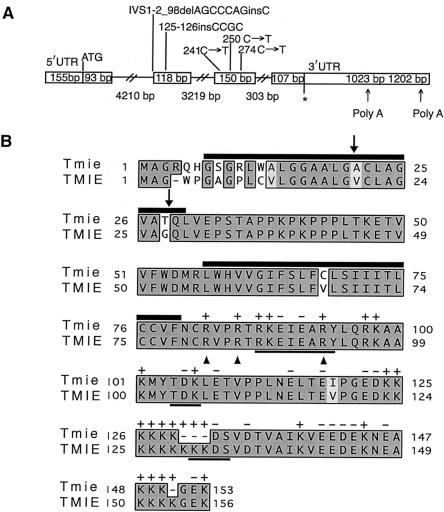
Northern-blot analysis demonstrated that TMIE is expressed in many human tissues and encodes a transcript of ∼2.5 kb (data not shown). Repeated RACE experiments on cDNA from human retina and various other tissues yielded a ∼1.7-kb transcript; no other TMIE transcripts were identified (fig. 2A). The predicted translation initiation codon of TMIE is present in an adequate Kozak context (Kozak 1996), with an in-frame upstream stop codon. The TMIE transcript has an ORF of 468 bp (156 amino acids), and the encoded protein shares 92% identity with mouse Tmie (fig. 2B). TMIE exhibits no significant similarity to any proteins in the databases—except that residues 47–109 of TMIE have 27% identity and 51% similarity to a hypothetical protein from Drosophila melanogaster (AAF53893; Adams et al. 2000). Analysis of TMIE with TMHMM2 (Krogh et al. 2001) predicts an intracellular amino terminus, two transmembrane regions separated by an extracellular loop, and an intracellular carboxy terminus. The cleavage of a signal peptide (amino acids 1–24 or 1–27 predicted by SigCleave) would result in an extracellular amino terminus and a single transmembrane domain in TMIE. The carboxy terminus of TMIE is rich in positively charged amino acids interspersed with negatively charged residues and has three potential protein kinase phosphorylation sites (fig. 2B). Arginine residues seem to be critical for the function of this protein, since three missense mutations in families showing linkage to DFNB6 affect three different arginine residues clustered closely in the cytoplasmic domain (fig. 2B).
Figure 2.
Diagrammatic representations of TMIE and TMIE. A, The boxed regions depict exons of TMIE. Slashed lines represent introns. The length of each exon and intron is given in base pairs (bp), within boxes (for exons) and below slashed lines (for introns). “ATG” denotes the initiation codon, and the asterisk (*) marks the stop codon. Mutations found in the five families showing linkage to DFNB6 are shown above the respective exons. We obtained a 1023-bp 3′ UTR followed by a poly A tail. BLAST analysis of the TMIE cDNA sequence identified ESTs corresponding to a 1202-bp 3′ UTR because of utilization of a different polyadenylation signal. B, Clustal W alignment of deduced amino acid sequences of Tmie and TMIE. The shared amino acid identity between Tmie (mouse) and TMIE (human) is indicated by shaded boxes: dark gray for absolute identity and light gray for conservative changes. Dashes (—) show gaps in the alignment. TMIE has two potential sites of signal peptide cleavage (arrows), and two predicted transmembrane regions (black bars on top of amino acid residues). Cleavage of the signal peptide would result in a protein with an extracellular amino terminus, one transmembrane segment, and an intracellular carboxy terminus. The C-terminus has many positively charged amino acids interspersed with negatively charged residues (charge indicated on top of each residue) and three potential protein kinase phosphorylation sites (bold underlines). Arrowheads indicate arginine residues in TMIE mutated in affected individuals in three families linked to DFNB6.
Given that allelism has been demonstrated for several other colocalizing Usher and nonsyndromic recessive deafness loci (Weil et al. 1996, 1997; Liu et al. 1997a, 1997b; Bork et al. 2001; Ahmed et al. 2002), a mutant allele of TMIE may be the basis for USH2B, even though predicted null alleles of TMIE are associated with nonsyndromic deafness. It will be interesting to determine whether TMIE is a significant cause of recessively inherited deafness in different populations, since the relative contribution of any one gene to the etiology of deafness in different parts of the world varies dramatically (Connexins and Deafness Homepage).
In summary, mutations in 15 different genes, including TMIE, have been shown to cause nonsyndromic, recessively inherited hearing loss in humans (Petit et al. 2001; Hereditary Hearing Loss Homepage). The primary sequence of TMIE does not suggest an obvious biochemical function for the predicted protein. However, the identification of loss-of-function mutations causing deafness in mice and humans indicates that Tmie/TMIE has a conserved, critical role in the auditory system. The inner ear pathology in affected spinner mice suggests that Tmie is required for normal postnatal maturation of sensory hair cells in the cochlea, including correct development of stereocilia bundles (Mitchem et al. 2002). Determination of the cellular-expression pattern of Tmie in cochlea, its development timing, and the subcellular localization of the Tmie protein will provide insight into the protein function. Further characterization of the morphological and molecular pathology in spinner mice will assist in the understanding of the interaction of Tmie with other important developmental and homeostatic pathways in the inner ear.
Acknowledgments
We thank the families for their participation. This data was generated through the use of Celera Discovery System and the associated databases. The research was supported in part by National Institute on Deafness and other Communication Disorders (NIDCD) grants R01-DC02842 (to R.J.H.S.) and R01-DC04410 (to D.C.K.) and by intramural funds from NIDCD (1 Z01 DC00035-04, 1 Z01 DC 00039-04, and 1 Z01 DC000064-01) to E.R.W., T.B.F., and A.J.G.
Footnotes
Nucleotide sequence data reported herein are available in the DDBJ/EMBL/GenBank databases; for details, see the Electronic-Database Information section of this article.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Connexins and Deafness Homepage, http://www.crg.es/deafness/ (for population differences in carrier rates and deafness due to CX26 mutations)
- GenBank Overview, http://www.ncbi.nlm.nih.gov/Genbank/GenbankOverview.html (for human TMIE, [accession number AY081842] and D. melanogaster hypothetical protein [CG15130; accession number AAF53893.1])
- Genscan, http://genes.mit.edu/GENSCAN.html (for cryptic splice-site prediction)
- Hereditary Hearing Loss Homepage, http://dnalab-www.uia.ac.be/dnalab/hhh/ (for a list of all known genes causing hearing loss)
- NCBI BLAST home page, http://www.ncbi.nlm.nih.gov/BLAST/ (for TMIE homologs)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for DFNB6 [MIM 600971] and USH2B [MIM 276905])
- Sigcleave, http://web.umassmed.edu/ (for signal peptide prediction)
- TMHMM2, http://www.cbs.dtu.dk/services/TMHMM-2.0/ (for prediction of transmembrane regions)
References
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, et al (2000) The genome sequence of Drosophila melanogaster. Science 287:2185–2195 [DOI] [PubMed] [Google Scholar]
- Ahmed ZM, Smith TN, Riazuddin S, Makishima T, Ghosh M, Bokhari S, Menon SNP, Deshmukh D, Griffith AJ, Riazuddin S, Friedman TB, Wilcox ER (2002) Nonsyndromic recessive deafness DFNB18 and Usher syndrome type IC are allelic mutations of USH1C. Hum Genet 110:527–531 [DOI] [PubMed] [Google Scholar]
- Bork JM, Peters LM, Riazuddin S, Bernstein SL, Ahmed ZM, Ness SL, Polomeno R, et al (2001) Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet 68:26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K, Ramesh A, Srisailapathy CR, Ni L, Wayne S, O'Neill ME, Van Camp G, Coucke P, Jain P, Wilcox ER, Smith SD, Kenyon JB, Zbar RIS, Smith RJH (1995) An autosomal recessive nonsyndromic form of sensorineural hearing loss maps to 3p-DFNB6. Genome Res 5:305–308 [DOI] [PubMed] [Google Scholar]
- Hmani M, Ghorbel A, Boulila-Elgaied A, Ben Zina Z, Kammoun W, Drira M, Chaabouni M, Petit C, Ayadi H (1999) A novel locus for Usher syndrome type II, USH2B, maps to chromosome 3 at p23-24.2. Eur J Hum Genet 7:363–367 [DOI] [PubMed] [Google Scholar]
- Kozak M (1996) Interpreting cDNA sequences: some insights from studies on translation. Mamm Genome 7:563–574 [DOI] [PubMed] [Google Scholar]
- Krogh A, Larson B, von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580 [DOI] [PubMed] [Google Scholar]
- Liu XZ, Walsh J, Mburu P, Kendrick-Jones J, Cope MJ, Steel KP, Brown SD (1997a) Mutations in the myosin VIIA gene cause non-syndromic recessive deafness. Nat Genet 16:188–190 [DOI] [PubMed] [Google Scholar]
- Liu XZ, Walsh J, Tamagawa Y, Kitamura K, Nishizawa M, Steel KP, Brown SD (1997b) Autosomal dominant non-syndromic deafness caused by a mutation in the myosin VIIA gene. Nat Genet 17:268–269 [DOI] [PubMed] [Google Scholar]
- Mitchem KL, Hibbard E, Beyer LA, Bosom K, Dootz GA, Dolan DF, Johnson KR, Raphael Y, Kohrman DC (2002) Mutation of Tmie, a gene encoding a predicted integral membrane protein, results in stereocilia defects in the mouse deafness mutant spinner. Hum Mol Genet 11:1887–1898 [DOI] [PubMed] [Google Scholar]
- Petit C, Levilliers J, Hardelin JP (2001) Molecular genetics of hearing loss. Annu Rev Genet 35:589–646 [DOI] [PubMed] [Google Scholar]
- Steel KP (1995) Inherited hearing defects in mice. Annu Rev Genet 29:675–701 [DOI] [PubMed] [Google Scholar]
- Weil D, Kussel P, Blanchard S, Levy G, Levi-Acobas F, Drira M, Ayadi H, Petit C (1997) The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat Genet 16:191–193 [DOI] [PubMed] [Google Scholar]
- Weil D, Levy G, Sahly I, Levi-Acobas F, Blanchard S, El-Amraoui A, Crozet F, Philippe H, Abitbol M, Petit C (1996) Human myosin VIIA responsible for the Usher 1B syndrome: a predicted membrane-associated motor protein expressed in developing sensory epithelia. Proc Natl Acad Sci USA 93:3232–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NM, Mets MB, Hain TC (1996) Early diagnosis of Usher syndrome in infants and children. Am J Otol 17:30–34 [PubMed] [Google Scholar]