Abstract
Aims
The Cockcroft-Gault (CG), the Modification of Diet in Renal Disease (MDRD) and the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formulae are often used to estimate glomerular filtration rate (GFR). The objective was to determine the best method for estimating GFR in older adults.
Methods
A cross-sectional study was conducted at the geriatric wards of two hospitals in The Netherlands. Patients aged 70 years or above with an estimated (e)GFR below 60 ml min−1 1.73 m−2 were included. The CG, CG calculated with ideal bodyweight (IBW), MDRD and CKD-EPI formulae were compared with a criterion standard, sinistrin clearance. Renal function was classified into five stages according to the National Kidney Foundation Disease Outcomes Quality Initiative chronic kidney disease classification, as follows (in ml min−1 1.73 m−2): stage 1, eGFR ≥ 90; stage 2, eGFR of 60–89; stage 3, eGFR of 30–59; stage 4, eGFR of 15–29; and stage 5, eGFR < 15.
Results
Sixteen patients, 50% male, with a mean age of 82 years (range 71–87 years) and mean body mass index 26 kg m−2 (range 18–36 kg m−2), were included. On average, all formulae slightly overestimated GFR, as follows (in ml min−1 1.73 m−2): CG +0.05 [95% confidence interval (CI) −28 to +28]; CG with IBW +0.03 (95% CI −20 to +20); MDRD +9 (95% CI −16 to +34); and CKD-EPI +5 (95% CI −20 to +29). They classified kidney disease correctly in 68.8% (CG), 75% (CG with IBW), 43.8% (MDRD) and 68.8% (CKD-EPI) of the participants, respectively.
Conclusions
The CG, CG with IBW, MDRD and CKD-EPI formulae estimate the mean GFR of a population rather well. In individual cases, all formulae may misclassify kidney disease by one stage.
Keywords: Chronic Kidney Disease Epidemiology Collaboration, Cockcroft-Gault, frail elderly, glomerular filtration rate, Modification of Diet in Renal Disease formula
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
A decrease in glomerular filtration rate (GFR) enhances the risk for accumulation of medications that depend on renal excretion.
Glomerular filtration rate is often estimated by formulae such as the Cockcroft-Gault, Modification of Diet in Renal Disease (MDRD) and CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration).
It is unknown whether these formulae can also be used reliably in a geriatric population.
WHAT THIS STUDY ADDS
Cockcroft-Gault, MDRD and CKD-EPI formulae are to be used with caution in older adults.
These formulae can both underestimate and overestimate GFR.
Hence, these formulae can misclassify National Kidney Foundation Disease Outcomes Quality Initiative renal disease category by one stage.
The Cockcroft-Gault formula, calculated with ideal bodyweight instead of actual bodyweight, performs best.
Introduction
When renal function declines, many drugs or their active metabolites that depend on renal excretion may accumulate, and this necessitates dosage adjustment in order to prevent adverse drug reactions [1]. This is especially important in older people, who are more vulnerable to adverse drug reactions due to an increased prevalence of renal impairment (partly due to structural and functional changes in the kidney as a result of ageing), polypharmacy and frailty [2–4]. On average, glomerular filtration rate (GFR) declines with age by approximately 1 ml min−1 1.73 m−2 year−1 over the age of 40 years. The average GFR declines from ∼130 to 80 ml min−1 1.73 m−2 as people age from 30 to 80 years [2]. Chronic kidney disease is characterized by kidney damage in combination with a decreased GFR. The criterion standard to assess renal function is to measure GFR by determining the clearance of exogenous markers, which are stable, completely filtered by the glomerulus, not secreted or reabsorbed in the renal tubule, and not metabolized [5]. However, these are expensive and cumbersome methods, and consequently, not suitable for daily practice. Physicians need a quick, simple and inexpensive method to provide a valid estimate of GFR. Various methods have been developed for this purpose. The most widely used methods are the Cockcroft-Gault (CG) and Modification of Diet in Renal Disease (MDRD) formulae. The CG formula estimates creatinine clearance and the MDRD formula estimates GFR based on serum creatinine concentrations, age, gender and weight (CG only) or race (MDRD only) [6, 7]. More recently, the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula has been developed, which is based on serum creatinine concentrations and age [8].
Multiple studies have been conducted to compare the CG and MDRD formulae with a criterion standard in the older population, with conflicting results [3, 9–16]. The CKD-EPI formula has recently been the subject of investigation in older adults, in addition to the CG and the MDRD formulae [17]. Flamant et al. [17] concluded that the MDRD or CKD-EPI formulae should be preferred. However, they did not investigate which of these formulae classified renal impairment category best. The aim of this study was to compare the accuracy of the CG, the MDRD and the CKD-EPI formulae in classifying renal impairment in a geriatric population by using a criterion standard.
Methods
Design and setting
A cross-sectional study was conducted at the acute care geriatric wards and outpatient wards of the University Medical Center Utrecht, an academic teaching hospital, and the Jeroen Bosch hospital 's-Hertogenbosch, a nonacademic teaching hospital, in The Netherlands. The Medical Ethics Committee of the University Medical Center Utrecht reviewed and approved this study.
Study population
All patients admitted to these wards or attending the outpatient department from January 2010 until December 2010 were eligible for inclusion if they were aged 70 years or above, in stable medical condition, cognitively able to give informed consent, and with an estimated GFR by the four-variable version of the MDRD of 60 ml min−1 1.73 m−2 or less. All participants gave written informed consent.
Study procedures
The study procedure took 8 h for each included participant. First, information was collected on patient characteristics, including the following: age, sex, weight, height, race, comorbidities and medication use. Body height (in centimetres) and weight (in kilograms) were used to calculate the body mass index [BMI; as follows: weight/(height × height] and body surface area (BSA; as follows: 0.007184 × weight0.425 × height0.725 [18]. Then an infusion tube was inserted into a cubital vein for blood withdrawal and infusion of the sinistrin. The Inutest ‘single shot method’ (Inutest®; 5 g of sinistrin per 20 ml; Fresenius Kabi, Graz, Austria) was used to measure the actual GFR. The active compound of Inutest® is sinistrin, an analogue of inulin with greater water solubility [19].
Serum creatinine concentrations were determined immediately before the 2500 mg sinistrin bolus infusion. The measurements were performed using the kinetic Jaffé method (rate blanked) on an ARCHITECT ci8200sr analyser (2012 Abbott Laboratories, Abbott Park, IL, USA). At 10, 20, 30, 60, 90, 120, 240 and 480 min after the sinistrin infusion, venous blood samples were taken. Sinistrin concentrations were measured in all plasma aliquots, as well as in the plasma aliquot before sinistrin infusion. Given that quantification of sinistrin in serum is limited by its physiological fructose content, which interferes with the natural fructose content of serum, it has to be determined as a serum blank [20]. Sinistrin concentration measurements were performed by colorimetric assessment using a Colorimeter Starrcol (Hoorn, The Netherlands).
Measurement of GFR
To calculate the GFR, the area under the curve of the sinistrin concentration–time curve was determined [21]. According to the manual of Inutest®, the sinistrin concentration-time curves should be analysed individually by a two-compartment model [19]. However, previous research has shown that this may lead to imprecise estimates of the individual pharmacokinetic parameters and that a population analysis estimates individual pharmacokinetic parameters better than individual analysis [22]. A population approach was therefore applied to obtain individual parameter estimates. Modelling was done using the nonlinear mixed-effects modelling software NONMEM VI version 2.0 (Icon Development Solutions, Hanover, MD, USA). The ADVAN 3 and TRANS4 subroutines were used to describe the data with a linear two-compartment model, with parameters CL (clearance), V1 (central volume), Q (intercompartmental clearance) and V2 (peripheral volume). The first-order conditional estimation method was used to obtain the parameter estimates. Additive, proportional and combined residual error models were tested (the residual error comprises the interindividual variability and the intraindividual variability). Interindividual variability on each parameter was modelled assuming a log-normal distribution. The selection of the two-compartment model (instead of one, three or more compartments) was based on the likelihood ratio test, parameter estimates and their relative standard errors, residual error values and goodness-of-fit plots.
Estimation of GFR
The primary outcome was to assess which formula estimates the GFR best according to the National Kidney Foundation Disease Outcomes Quality Initiative (NKF KDOQI) chronic kidney disease classification [23]. This classification distinguishes the following five stages: stage 1, kidney damage with estimated glomerular filtration rate (eGFR) ≥ 90 ml min−1 1.73 m−2; stage 2, kidney damage with eGFR of 60–89 ml min−1 1.73 m−2; stage 3, eGFR of 30–59 ml min−1 1.73 m−2; stage 4, eGFR of 15–29 ml min−1 1.73 m−2; and stage 5, eGFR < 15 ml min−1 1.73 m−2. For this study, kidney damage was not assessed.
Glomerular filtration rate was estimated according to the following formulae:
CG (in ml min−1) [7]: {[140 – age (in years)] × bodyweight (in kg)}/[72 × serum creatinine (Scr; in mg dl−1)] (× 0.85 if woman). To give results in μmol l−1, multiply Scr by 88.4.
CG (in ml min−1) with ideal bodyweight (IBW) instead of bodyweight [24]: IBW for men = 50 + 0.9 × [length (in cm) – 152], and minimally equal to or higher than bodyweight; and IBW for women = 45.5 + 0.9 × [length (in cm) – 152], and minimally equal to or higher than bodyweight.
Four-variable version of the MDRD (in ml min−1 1.73 m−2) [6]: 186 × Scr (in mg dl−1)–1.154 × age (in years)–0.203 × 0.742 (women) × 1.2 (dark race).
CKD-EPI (in ml min−1 1.73 m−2) if serum creatinine concentrations are >0.9 mg dl−1 (male) and >0.7 mg dl−1 (female) [8]: men, 141 × [Scr (in mg dl−1)/0.9]−0.411 × (0.993)Age; and women, 144 × [Scr (in mg dl−1)/0.7]−0.329 × (0.993)Age.
In order to compare the estimated and measured GFRs and to classify the results according to the NKF KDOQI classification system, the sinistrin clearance and the CG were normalized per 1.73 m2.
Data analysis
The relationship between different methods of GFR assessment was explored using Bland–Altman plots. The Bland–Altman approach depicts the mean difference and 95% confidence intervals (CIs) of the differences, represented by the limits of agreement (mean difference ± 2SD of differences) [25]. As the GFR measurements are more likely to be closer to the real GFR than the predicted estimates by the formulae, the measured GFR on the x-axes was used instead of the mean of both methods.
Also, the mean absolute difference for each formula was determined by first calculating the absolute difference per patient (|eGFR – mGFR|) and then calculating the average of the absolute differences.
Results
Baseline characteristics
During the study period, 139 patients were approached for participation; 31 patients signed the informed consent form. Eventually, 24 patients completed the study procedures; two patients withdrew consent before the start of the study procedures, and five patients had to be excluded due to practical problems. In the data set consisting of 24 patients, the results of eight patients did not meet the high standards of criterion standard GFR measurements. Two concentration profiles included a very high concentration of 1601 and 2449 μg ml−1 at 10 and 20 min, respectively, resulting in an unreliable estimate of V1. If these two concentrations were excluded, the GFR could not be measured reliably; therefore, these patients were excluded from the analysis. In four profiles, concentrations were zero due to the applied correction in the chemical assay. Given that inclusion or exclusion of these concentrations resulted in significant differences in the estimated GFR, and it was not clear which of these GFRs was most reliable, these patients were excluded from further analysis. In two patients, the estimated values for V1 were close to 1 l, which does not seem physiologically plausible; these patients were excluded from the final analysis.
This left 16 patients for whom all measured plasma concentrations and calculated pharmacokinetic parameters were considered reliable. Three of these patients were included on the last day of hospitalization, while the other 13 patients were recruited from the outpatient department. Table 1 shows that the mean age of the included patients was 82 years (range 71–87 years), eight participants (50%) were male, and mean BMI was 26 kg m−2 (range 18–36 kg m−2).
Table 1.
Baseline characteristics
| Characteristic | n = 16 |
|---|---|
| Age [years; mean (range)] | 82 (71–87) |
| Male gender [n (%)] | 8 (50) |
| Hospitalized [n (%)] | 3 (19%) |
| Caucasian [n (%)] | 16 (100) |
| Body mass index [kg m−2; mean (range)] | 26.4 (18–36) |
| Height [m; mean (range)] | 1.64 (1.48–1.75) |
| Weight [kg; mean (range)] | 71 (47–103) |
| Serum creatinine concentration [μmol l−1; mean (range)] | 128 (80–292) |
| Number of medications [mean (range)] | 9 (3–15) |
| Number of comorbidities [mean (range)] | 5 (2–8) |
| Patients with hypertension [n (%)] | 10 (63) |
| Patients with diabetes mellitus [n (%)] | 4 (25) |
Glomerular filtration rate
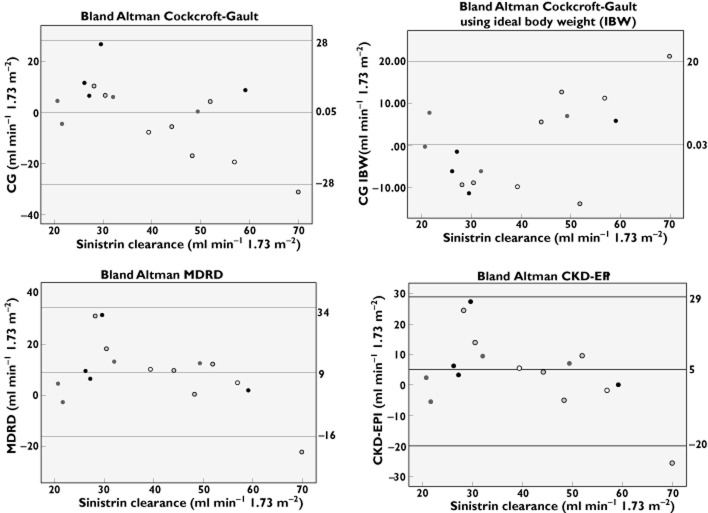
Table 2 shows the measured and estimated GFRs per patient. Figure 1 shows that on average, all formulae slightly overestimated GFR, as follows: CG +0.05 (95% CI −28 to +28) ml min−1 1.73 m−2 [+7.2% (95% CI −62.6 to +77.1%)]; MDRD +9 (95% CI −16 to +34) ml min−1 1.73 m−2 [+29.1% (95% CI −45.6 to +103.9%)]; and CKD-EPI +5 (95% CI −20 to +29) ml min−1 1.73 m−2 [17.8% (95% CI −51.3 to +86.9%)]. When using the IBW instead of actual bodyweights, the CG has a smaller 95% CI, i.e. +0.3 (95% CI −20 to +20) ml min−1 1.73 m−2.
Table 2.
Measured and calculated values of glomerular filtration rate per patient
| Patient number | Sinistrin clearance (ml min−1 1.73 m−2) | CG (ml min−1 1.73 m−2) | CG (ml min−1 1.73 m−2) based on IBW | MDRD (ml min−1 1.73 m−2) | CKD-EPI (ml min−1 1.73 m−2) |
|---|---|---|---|---|---|
| 1 | 30 | 56 | 41 | 61 | 57 |
| 2 | 26 | 38 | 32 | 36 | 32 |
| 3 | 52 | 56 | 66 | 64 | 61 |
| 4 | 70 | 39 | 49 | 48 | 44 |
| 5 | 27 | 34 | 29 | 34 | 30 |
| 6 | 59 | 68 | 53 | 61 | 59 |
| 7 | 44 | 38 | 38 | 54 | 48 |
| 8 | 21 | 25 | 21 | 25 | 23 |
| 9 | 48 | 31 | 36 | 49 | 43 |
| 10 | 22 | 17 | 14 | 19 | 16 |
| 11 | 28 | 39 | 37 | 59 | 53 |
| 12 | 49 | 50 | 42 | 62 | 56 |
| 13 | 57 | 37 | 46 | 62 | 55 |
| 14 | 30 | 37 | 39 | 49 | 44 |
| 15 | 39 | 32 | 49 | 49 | 45 |
| 16 | 32 | 38 | 38 | 45 | 41 |
Abbreviations: CG, Cockcroft-Gault; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; IBW, ideal bodyweight; and MDRD, Modification of Diet in Renal Disease.
Figure 1.
Bland–Altman plots for Cockcroft-Gault (CG), CG with ideal bodyweight, Modification of Diet in Renal Disease (MDRD) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formulae. Key:  , body mass index (BMI) > 30 kg m−2;
, body mass index (BMI) > 30 kg m−2;  , BMI 26–30 kg m−2;
, BMI 26–30 kg m−2;  , BMI 19–25 kg m−2; and ◯, BMI < 19 kg m−2
, BMI 19–25 kg m−2; and ◯, BMI < 19 kg m−2
The formulae predict GFR to be similar in both male and female participants (results not shown). Furthermore, Figure 1 suggests that CG tends to overestimate GFR in patients with morbid obesity (BMI > 30 kg m−2) and to underestimate GFR in patients who are underweight (BMI < 19 kg m−2). When the CG is calculated with IBW, this over- and underestimation is no longer present.
The mean absolute difference was highest for the MDRD formula, i.e. 12.1 ml min−1 1.73 m−2, compared with 10.8 ml min−1 1.73 m−2 for CG, 8.6 ml min−1 1.73 m−2 for CG using IBW and 9.4 ml min−1 1.73 m−2 for CKD-EPI.
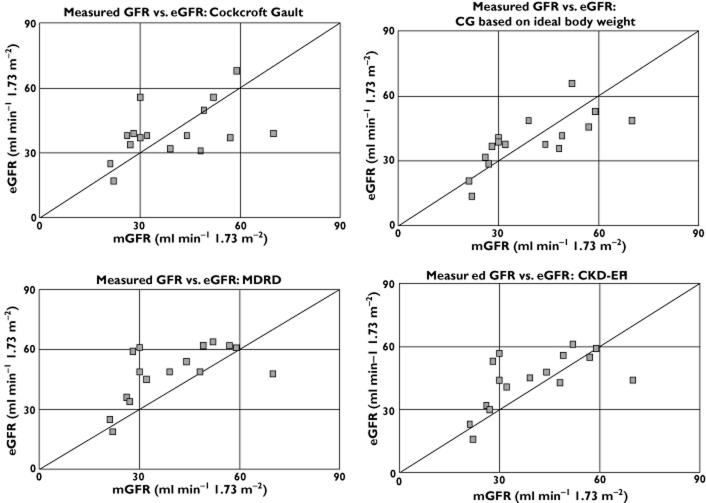
Figure 2 shows that the participants had kidney disease varying from stage 2 to stage 4. The CG formula classified the kidney disease stage correctly in 11 participants (68.8%), the CG with IBW in 12 participants (75.0%), the MDRD formula made a correct classification in seven participants (43.8%), and the CKD-EPI in 11 participants (68.8%). All incorrect classifications differed by one stage (CG, one higher and four lower; CG with IBW, three higher and one lower; MDRD, eight higher and one lower; and CKD-EPI, four higher and one lower) from the actual disease stage. This could result in incorrect classification of a patient as having an impaired renal function, or vice versa. Combining the estimates of the three formulae did not improve classification (results not shown).
Figure 2.
Measured glomerular filtration rate (GFR) vs. estimatate GFR (eGFR)
Discussion
The results of the present study indicate that the CG, MDRD and CKD-EPI formulae estimate the mean GFR rather well in a geriatric population. However, in individual cases all formulae may misestimate the GFR by up to 31 ml min−1 1.73 m−2 (103%), thereby misclassifying kidney disease by one stage in >30% of the participants. Both underestimation and overestimation occurred with all formulae. The CG formula, calculated with IBW, classified the renal impairment category best. The mean absolute difference was also smallest for the CG formula, calculated with IBW.
This study compared the CG, CG with IBW, MDRD and the CKD-EPI formulae with a criterion standard in a geriatric population. Other studies have generated conflicting results. Van den Noortgate et al. found similar results to the present study in a comparable patient population [14]. Burkhardt et al. concluded that both CG and MDRD formulae underestimated GFR by 20 and 40 ml min−1, respectively [12]. However, in their study, only patients without signs of reduced renal function were included. Péquignot et al. stated that the CG formula performed in a superior manner to the MDRD formula, because the MDRD formula strongly overestimated the GFR [15]. In their study, however, only hospitalized patients with an indwelling urinary catheter were included, which may imply a more frail population than ours, in an unstable medical condition. The finding that the CG formula performs less well in patients at the extremes of BMI is in concordance with the results from previous studies [26, 27]. When IBW is applied instead of actual bodyweight, the CG formula performs much better. The results of the present study also confirm that MDRD and CKD-EPI formulae perform in a similar manner in individuals, although MDRD tends slightly to overestimate GFR compared with CKD-EPI, resulting in more misclassifications than CKD-EPI [28]. Thus, sound advice on which formula to use to estimate renal function in the geriatric population cannot yet be offered, although the CG formula with IBW classified renal impairment category best, with the smallest mean absolute difference. It is possible that the reliability of the different formulae is influenced by more variables than age, weight and sex, such as the medical condition of the patient, medication use or comorbidities, or that bodyweight may correlate less or in a different manner with muscle mass in older people than in younger adults.
The sample size of the study population was, however, small, which has a negative influence on the generalizability of the study results. At the time when this study was undergoing preparation, there were only limited data available on renal function assessment in frail older people; therefore, we could not make a proper sample size calculation and chose a sample size of 30, based on the study by Burkhardt et al. [12]. Unfortunately, during the time available for inclusion, we were able to include only 24 participants. Furthermore, a significant number of the participants had to be excluded. These exclusions were related to the chosen method for measuring the sinistrin clearance, the Inutest® single-shot method. The advantage of the single-shot method is that only one bolus infusion of sinistrin is necessary and that additional urinary collections are not required. The normal Inutest® single-shot measurements take 4 h. Given that reduced renal function was expected in this population, we prolonged these measurements to 8 h. Unfortunately, in four cases this prolongation turned out to be insufficient, and reliable GFR measurements could not be made. Furthermore, we suffered more than expected from interference with the natural fructose content of serum, which hindered accurate measurement of serum sinistrin, which could not be sufficiently counteracted by subtracting the serum blank concentration from the sinistrin measurements. In two cases, we detected an improbably high sinistrin concentration at 10 and 20 min, respectively. Whether these measured concentrations were caused by a flaw in the analysis procedure or due to insufficient flushing after the sinistrin infusion is unknown.
For future research, it will be of great importance to collect all variables that might influence the reliability of the formulae and may explain the large interindividual variability found in this and previous studies.
Conclusion
The results of the present study indicate that the CG, MDRD and CKD-EPI formulae estimate the mean GFR rather well in a geriatric population. However, in individual cases all formulae may misestimate the GFR by up to 31 ml min−1 1.73 m−2 (103%). The formulae predict kidney disease stage correctly in 50% of patients. The CG formula calculated with IBW performed better than the CG calculated with bodyweight, MDRD and CKD-EPI formulae. Furthermore, the Inutest® single-shot method should not be the preferred method to assess renal function in older patients.
Acknowledgments
Funding was provided by the Netherlands Organisation for Health Research and Development.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: ACDvM, PAFJ, RJvM and TCGE had support from The Netherlands Organisation for Health Research for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Falconnier AD, Haefeli WE, Schoenenberger RA, Surber C, Martin-Facklam M. Drug dosage in patients with renal failure optimized by immediate concurrent feedback. J Gen Intern Med. 2001;16:369–375. doi: 10.1046/j.1525-1497.2001.016006369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamb EJ, O'Riordan SE, Delaney MP. Kidney function in older people: pathology, assessment and management. Clin Chim Acta. 2003;334:25–40. doi: 10.1016/s0009-8981(03)00246-8. [DOI] [PubMed] [Google Scholar]
- 3.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 4.Markota NP, Markota I, Tomic M, Zelenika A. Inappropriate drug dosage adjustments in patients with renal impairment. J Nephrol. 2009;22:497–501. [PubMed] [Google Scholar]
- 5.Price CP, Finney H. Developments in the assessment of glomerular filtration rate. Clin Chim Acta. 2000;297:55–66. doi: 10.1016/s0009-8981(00)00233-3. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van LF, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamb EJ, Webb MC, O'Riordan SE. Using the modification of diet in renal disease (MDRD) and Cockcroft and Gault equations to estimate glomerular filtration rate (GFR) in older people. Age Ageing. 2007;36:689–692. doi: 10.1093/ageing/afm121. [DOI] [PubMed] [Google Scholar]
- 10.Fabre EE, Raynaud-Simon A, Golmard JL, Gourgouillon N, Beaudeux JL, Nivet-Antoine V. Interest and limits of glomerular filtration rate (GFR) estimation with formulae using creatinine or cystatin C in the malnourished elderly population. Arch Gerontol Geriatr. 2009;50:55–58. doi: 10.1016/j.archger.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Fliser D. Assessment of renal function in elderly patients. Curr Opin Nephrol Hypertens. 2008;17:604–608. doi: 10.1097/MNH.0b013e32830f454e. [DOI] [PubMed] [Google Scholar]
- 12.Burkhardt H, Bojarsky G, Gretz N, Gladisch R. Creatinine clearance, Cockcroft-Gault formula and cystatin C: estimators of true glomerular filtration rate in the elderly? Gerontology. 2002;48:140–146. doi: 10.1159/000052832. [DOI] [PubMed] [Google Scholar]
- 13.Van den Noortgate NJ, Janssens WH, Afschrift MB, Lameire NH. Renal function in the oldest-old on an acute geriatric ward. Int Urol Nephrol. 2001;32:531–537. doi: 10.1023/a:1014454031451. [DOI] [PubMed] [Google Scholar]
- 14.Van den Noortgate NJ, Janssens WH, Delanghe JR, Afschrift MB, Lameire NH. Serum cystatin C concentration compared with other markers of glomerular filtration rate in the old old. J Am Geriatr Soc. 2002;50:1278–1282. doi: 10.1046/j.1532-5415.2002.50317.x. [DOI] [PubMed] [Google Scholar]
- 15.Péquignot R, Belmin J, Chauvelier S, Gaubert JY, Konrat C, Duron E, Hanon O. Renal function in older hospital patients is more accurately estimated using the cockcroft-gault formula than the modification diet in renal disease formula. J Am Geriatr Soc. 2009;57:1638–1643. doi: 10.1111/j.1532-5415.2009.02385.x. [DOI] [PubMed] [Google Scholar]
- 16.Roberts GW, Ibsen PM, Schioler CT. Modified diet in renal disease method overestimates renal function in selected elderly patients. Age Ageing. 2009;38:698–703. doi: 10.1093/ageing/afp168. [DOI] [PubMed] [Google Scholar]
- 17.Flamant M, Haymann JP, Vidal-Petiot E, Letavernier E, Clerici C, Boffa JJ, Vrtovsnik F. GFR estimation using the cockcroft-gault, MDRD study, and CKD-EPI equations in the elderly. Am J Kidney Dis. 2012;60:847–849. doi: 10.1053/j.ajkd.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–311. discussion 312–3. [PubMed] [Google Scholar]
- 19.Summary of product characteristics Inutest. 2012.
- 20.Oettl K, Payerl D, Zitta S, Muller T, Estelberger W, Reibnegger G. Quantitative analysis of sinistrin in serum with high-performance liquid chromatography for renal function testing. Anal Biochem. 2004;331:183–188. doi: 10.1016/j.ab.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Buclin T, Pechere-Bertschi A, Sechaud R, Decosterd LA, Munafo A, Burnier M, Biollaz J. Sinistrin clearance for determination of glomerular filtration rate: a reappraisal of various approaches using a new analytical method. J Clin Pharmacol. 1997;37:679–692. doi: 10.1002/j.1552-4604.1997.tb04355.x. [DOI] [PubMed] [Google Scholar]
- 22.Proost JH, Eleveld DJ. Performance of an iterative two-stage bayesian technique for population pharmacokinetic analysis of rich data sets. Pharm Res. 2006;23:2748–2759. doi: 10.1007/s11095-006-9116-0. [DOI] [PubMed] [Google Scholar]
- 23.KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: evaluation, classification, and stratification. 2002. p. 2012. [PubMed]
- 24.Chennavasin P, Brater DC. Aminoglycoside dosage adjustment in renal failure: a hand-held calculator program. Eur J Clin Pharmacol. 1982;22:91–94. doi: 10.1007/BF00606431. [DOI] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 26.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5:1003–1009. doi: 10.2215/CJN.06870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 28.Corsonello A, Pedone C, Lattanzio F, Semeraro R, D'Andria F, Gigante M, Coppola A, Cadeddu G, Laino I, Incalzi RA. Agreement between equations estimating glomerular filtration rate in elderly nursing home residents and in hospitalised patients: implications for drug dosing. Age Ageing. 2011;40:583–589. doi: 10.1093/ageing/afr011. [DOI] [PubMed] [Google Scholar]