Abstract
The primary purpose of this review is to address the progress towards small molecule modulators of human Transient Receptor Potential Canonical proteins (TRPC1, TRPC3, TRPC4, TRPC5, TRPC6 and TRPC7). These proteins generate channels for calcium and sodium ion entry. They are relevant to many mammalian cell types including acinar gland cells, adipocytes, astrocytes, cardiac myocytes, cochlea hair cells, endothelial cells, epithelial cells, fibroblasts, hepatocytes, keratinocytes, leukocytes, mast cells, mesangial cells, neurones, osteoblasts, osteoclasts, platelets, podocytes, smooth muscle cells, skeletal muscle and tumour cells. There are broad-ranging positive roles of the channels in cell adhesion, migration, proliferation, survival and turning, vascular permeability, hypertrophy, wound-healing, hypo-adiponectinaemia, angiogenesis, neointimal hyperplasia, oedema, thrombosis, muscle endurance, lung hyper-responsiveness, glomerular filtration, gastrointestinal motility, pancreatitis, seizure, innate fear, motor coordination, saliva secretion, mast cell degranulation, cancer cell drug resistance, survival after myocardial infarction, efferocytosis, hypo-matrix metalloproteinase, vasoconstriction and vasodilatation. Known small molecule stimulators of the channels include hyperforin, genistein and rosiglitazone, but there is more progress with inhibitors, some of which have promising potency and selectivity. The inhibitors include 2-aminoethoxydiphenyl borate, 2-aminoquinolines, 2-aminothiazoles, fatty acids, isothiourea derivatives, naphthalene sulfonamides, N-phenylanthranilic acids, phenylethylimidazoles, piperazine/piperidine analogues, polyphenols, pyrazoles and steroids. A few of these agents are starting to be useful as tools for determining the physiological and pathophysiological functions of TRPC channels. We suggest that the pursuit of small molecule modulators for TRPC channels is important but that it requires substantial additional effort and investment before we can reap the rewards of highly potent and selective pharmacological modulators.
Keywords: calcium channel, sodium channel, cationic channel, calcium signalling, ion channel inhibitors
General introduction on Transient Receptor Potential Canonical (TRPC) channels
In mammals, there are 28 genes encoding Transient Receptor Potential (TRP) proteins (Damann et al., 2008; Alexander et al., 2011). These proteins are widely expressed with diverse functions. Assembly of several TRP proteins (monomers) is required for the creation of one ion channel. It is generally considered that the closest structural relatives of TRP proteins are the voltage-gated ion channels such as the voltage-gated KV1.2 potassium channel, which provides the nearest crystal structure. Like KV1.2, each TRP protein is thought to have six membrane-spanning segments and intracellular N- and C-termini. Also like KV1 channels, an individual channel may arise from identical or different members of the family (i.e. there may be homomers or heteromers). Heteromers increase the possibility for more ion channels with different characteristics, conferring advantages and disadvantages for pharmacological discovery and development.
The TRP channels are most commonly non-selective cationic channels with permeability to Ca2+, Na+ and K+. In a few instances, they are Ca2+-selective, Ca2+-impermeable, or permeable also to Mg2+ and other cations. Ion channels of this type are often positive for cell activity because elevated intracellular Ca2+ has diverse positive effects (Clapham, 2007). However, there are exceptions such that TRP channels may have inhibitory functions. The TRP channels generally have weak voltage dependence and are not directly gated by major neurotransmitters. Instead, they enable coupling of relatively slow chemical and physical events to cellular Ca2+ signalling systems, either directly or indirectly. For some TRP channels, there are many modulators, as if the channels are complex integrators of multiple chemical and physical factors.
Based on amino acid sequence alignments, the TRP proteins are categorized into subfamilies that include but are not limited to the TRPC, Vanilloid (TRPV), Melastatin (TRPM), and Polycystin (TRPP) proteins (Damann et al., 2008). In mammals, there are seven TRPC proteins, but one of them (TRPC2) is not expressed in human (Damann et al., 2008; Abramowitz and Birnbaumer, 2009). TRPC1, TRPC4 and TRPC5 are considered to form a subgroup, and TRPC3, TRPC6 and TRPC7 another subgroup.
All of the TRPC channels are non-selective cationic channels, and thus, they are mechanisms for enabling entry of Ca2+ and Na+ into cells (Figure 1). In addition to functional implications of the arising depolarisation and elevated intracellular Ca2+ concentration, the Na+ entry impacts on Na+-dependent mechanisms such as Na+-Ca2+ exchangers. TRPCs may be the most promiscuous TRPs in terms of their tendency to form heteromers. The promiscuity extends outside the TRPC subfamily to TRPV4 and TRPP2 (Tsiokas, 2009; Ma et al., 2010). It has been particularly difficult to determine the compositions of native TRPC channels, although progress has been made for some cell types (Xu et al., 2008; Shi et al., 2010; Sukumar et al., 2012). An issue conferred by heteromers and the similarity of different TRPCs is the possibility of redundancy, where loss or down-regulation of one or several TRPC proteins may be compensated by other TRPCs, other TRPs, or other Ca2+-entry mechanisms such as Orai1 channels (Beech, 2012). It would be wrong, however, to say that one TRPC is equivalent to the next in all regards.
Figure 1.
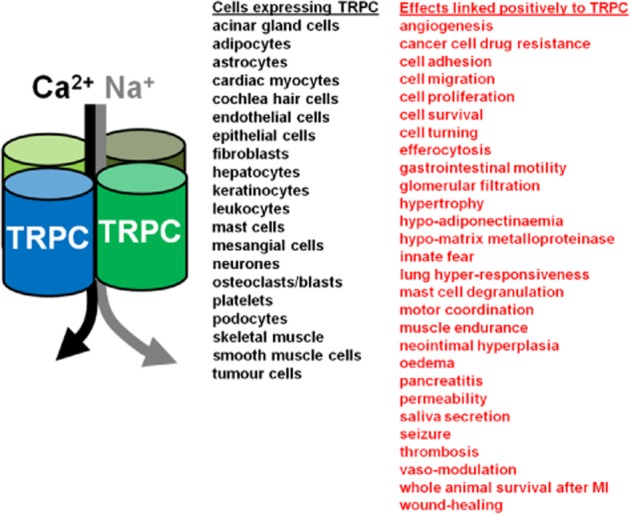
Expression and functions of TRPCs. On the left is a simple schematic diagram of a TRPC heteromer conferring influx of Ca2+ and Na+. In the middle is a list of the cell types that have been suggested to express TRPC mRNA or protein. On the right (in red) is a list of cell or whole tissue/body effects that have been suggested to be driven or potentiated by TRPC activity; in other words, if TRPC channels were to be inhibited, the opposite of the effect is predicted to occur (e.g. less pancreatitis). Example references for cell expression items: acinar gland cells (Liu et al., 2007); adipocytes (Sukumar et al., 2012); astrocytes (Shirakawa et al., 2010); cardiac myocytes (Eder and Molkentin, 2011); cochlea hair cells (Quick et al., 2012); endothelial cells (Ahmmed et al., 2004); epithelial cells (Kim et al., 2011); fibroblasts (Xu et al., 2008); hepatocytes (Rychkov and Barritt, 2011); keratinocytes (Cai et al., 2006); leukocytes (Yildirim et al., 2012); mast cells (Freichel et al., 2012); mesangial cells (Sours et al., 2006); neurones (Bollimuntha et al., 2011); osteoclasts/blasts (Abed et al., 2009); platelets (Ramanathan et al., 2012); podocytes (Dryer and Reiser, 2010); skeletal muscle (Gervasio et al., 2008); smooth muscle cells (Beech et al., 2004); and tumour cells (Thebault et al., 2006). Example references for effect items: angiogenesis (Yu et al., 2010); cancer cell drug resistance (Ma et al., 2012); cell adhesion (Smedlund et al., 2010); cell migration (Xu et al., 2006); cell proliferation (Sweeney et al., 2002); cell survival (Selvaraj et al., 2012); cell turning (Wang and Poo, 2005); efferocytosis (Tano et al., 2011); gastrointestinal motility (Tsvilovskyy et al., 2009); glomerular filtration (Dryer and Reiser, 2010); hypo-adiponectinaemia (Sukumar et al., 2012); hypo-matrix metalloproteinase (Xu et al., 2008); hypertrophy (cardiac) (Eder and Molkentin, 2011); innate fear (Riccio et al., 2009); lung hyper-responsiveness (Yildirim et al., 2012); mast cell degranulation (Ma et al., 2008); motor coordination (Trebak, 2010); muscle endurance (Zanou et al., 2010); neointimal hyperplasia (Kumar et al., 2006); oedema (Weissmann et al., 2012); permeability (Tiruppathi et al., 2002); pancreatitis (Kim et al., 2011); saliva secretion (Liu et al., 2007); seizure (Phelan et al., 2013); survival after MI (myocardial infarction) (Jung et al., 2011); thrombosis (Ramanathan et al., 2012); vaso-modulation (e.g. vasoconstriction) (Weissmann et al., 2006); and wound-healing (Davis et al., 2012).
An oddity in the TRPC subfamily is TRPC1. It forms ion channels poorly or not at all when expressed alone in vitro in heterologous systems (Xu et al., 2008). Other TRPCs, by contrast, form plasma membrane channels quite readily when expressed alone (i.e. they can form functional homomers). TRPC1 probably functions mostly in heteromers. Although some studies have shown signals when TRPC1 is expressed alone, the signals have generally been small and could be explained by TRPC1 forming heteromers with endogenous TRPs of the expression system.
There are examples of TRPC gene mutations linked to human disease. TRPC6 mutations cause familial focal segmental glomerulosclerosis (Winn et al., 2005), and a single nucleotide polymorphism in TRPC6 has been linked to idiopathic pulmonary hypertension (Yu et al., 2009). Gain-of-function mutation in TRPC4 protects against myocardial infarction in diabetes (Jung et al., 2011). In addition to evidence of genetic linkage to disease, studies from numerous independent laboratories have strongly suggested that TRPC channels have substantial importance in mammalian biology and may be valuable therapeutic drug targets.
Physiological regulation of TRPC channels
TRPCs are promiscuous in their sensitivities to physiological factors and in the mechanisms by which these factors act. Here, physiological modulators are considered only briefly, but it is important to bear them in mind because pharmacological agents may affect TRPC channels indirectly (i.e. via the physiological regulatory systems). More detailed reviews on this aspect are available (Abramowitz and Birnbaumer, 2009; Beech, 2013).
Because of the relatively slow nature of TRPC channels, it is less relevant to think of fast directly-acting physiological modulators than it is for neurotransmitter-gated ion channels, for example. Several of the TRPC channels exhibit constitutive activity (Dietrich et al., 2003; Sukumar et al., 2012), and so increased gene expression or rapid forward-trafficking events, which are a feature of TRPC channels (Bezzerides et al., 2004; Monet et al., 2012), can achieve functional significance in the absence of classical directly-binding activators.
Some modulators are common to the TRPC5 and TRPC6 subgroups, causing similar inhibitory or stimulatory effects. One such common modulator is modest elevation of the intracellular Ca2+ concentration, which enhances channel activity (Hui et al., 2006). Redox factors, such as hydrogen peroxide, are also common stimulants (Graham et al., 2010); as such, relationships of TRPC to mitochondrial function may occur. Another common stimulant is α-subunits of G-proteins, which is why many agonists at G-protein-coupled receptors are stimulators (Abramowitz and Birnbaumer, 2009; Beech, 2013). Agonists at tyrosine kinase receptors may also be common stimulators (Odell et al., 2005). An intermediate in the actions of such agonists is likely to be stimulation of a phospholipase C, leading to inositol 1,4,5-triphosphate (IP3) and diacylglycerol, depletion of phosphatidylinositol 4,5-biphosphate, and elevation of the intracellular Ca2+ concentration, all of which modulate TRPC channels. Cyclic AMP acting via protein kinase A and cGMP acting via protein kinase G are common negative modulators, whereas tyrosine kinases are stimulators. Serine-threonine kinase with-no-lysine 4 is an inhibitor and calmodulin kinase an activator. Protein kinase C mostly inhibits TRPC channels, but there is stimulation when TRPC1 is involved. The latter regulatory effects have been reviewed with more extensive reference to the original works (Beech, 2013).
There are also differential effects of physiological factors, which distinguish the TRPC5 and TRPC6 subgroups. Diacylglycerol directly stimulates TRPC6 but not TRPC5. The effect on TRPC6 is independent of protein kinase C and it is part of the receptor-to-channel coupling mechanism for TRPC6, as well as TRPC3 and TRPC7. The receptor-to-channel coupling mechanism for the TRPC5 subgroup is less well established but may involve direct G-protein action, phosphorylation by protein kinase C, signalling through Group VI PLA2, lysophosphatidylcholine and other factors (Beech, 2013). Arachidonic acid and some of its metabolites are TRPC6 stimulators but seem not to stimulate TRPC5. Conversely, GM1 gangliosides and other lipid factors stimulate TRPC5 but are not known to affect TRPC6. Mild extracellular acidification stimulates TRPC5 whereas it has no effect on TRPC6. There are also differential effects of factors that arise in toxic environments. TRPC5 is stimulated by lanthanum or gadolinium ions whereas TRPC6 is inhibited. There are similar stimulatory effects of lead and mercury, suggesting that the TRPC5 type of channel may have an important role in the detection of toxic substances. References to the original studies of these effects are not provided comprehensively in this article but can be found in a recent review (Beech, 2013).
An intriguing characteristic of TRPC4 and TRPC5 channels is their sensitivity to modulation at the third extracellular (E3) loop (also called the turret). The E3 was found to contain critical amino acid residues for the unusual stimulatory action of lanthanides at TRPC4/5 (Jung et al., 2003) and heteromers with TRPC1 (Xu et al., 2008). A disulfide bridge near to the lanthanide-sensitive residues is susceptible to reduction by the redox protein thioredoxin, leading to channel activation (Xu et al., 2008). The E3 domain may confer special opportunities for pharmacological modulation.
Functions of TRPC channels
TRPC/Trpc gene disruption is not known to confer embryonic lethality or catastrophic phenotype in the adult. Multiple Trpc disruptions (i.e. Trpc1/4/5 or Trpc3/6 in the mouse) (Suresh Babu et al., 2012) and global expression of dominant-negative mutant TRPC5 (Sukumar et al., 2012) also do not cause catastrophic phenotypes. Patients with gain-of-function TRPC6 mutations present only with focal segmental glomerulosclerosis (Winn et al., 2005), and systemic administration of a TRPC channel inhibitor apparently had no major adverse effect (Kim et al., 2011). Nevertheless, changes have been identified in genetically- or pharmacologically-modified mice, suggesting multiple roles (Figure 1). From such findings, it is a working hypothesis that TRPCs have modest importance in passive physiology but comparatively have more importance in active adaptive processes arising in response to chemical or physical insult (Beech, 2013). If this concept is true, it follows that TRPC channels may have value as new therapeutic targets for the treatment of human diseases, albeit with caveats.
Evidence suggesting functional importance of TRPCs is considerable (Figure 1). The functions relate to most systems of the body – both central and peripheral. The primary purpose of this review is not to provide a comprehensive review of this literature but to focus on TRPC pharmacology. Therefore, only a summary is provided (Figure 1). The figure illustrates cell types in which TRPCs are expressed and effects suggested to be potentiated or driven by TRPC activity. The functional data were generated through studies with gene-modified mice and studies of the effects of anti-sense DNA, RNA interference, dominant negative mutants and blocking antibodies on human cells and tissues. For some disease contexts, it is apparent that it could be advantageous to inhibit TRPC activity, whereas in other contexts, it may be disadvantageous or it could be anticipated that specific adverse effects or contraindications would arise. There will be benefit in further validation towards determining the likely consequences of TRPC channel inhibition (or stimulation) in specific human diseases.
A theme arising from TRPC studies is that the channels have positive roles in adaptive processes (Figure 1). These include, for example, positive effects in cell proliferation and migration. Therefore, a case might be made for discovering inhibitors of the channels with a view to developing novel adjuvant anti-cancer agents or drugs to suppress other unwanted hyperplasic or migratory events. Of course there are also beneficial hyperplasic or migratory events to which due attention would need to be paid (e.g. there could be unwanted effects of TRPC inhibitors on wound healing). Whether beneficial effects would outweigh unwanted effects remains to be determined.
Introduction to the pharmacology of TRPC channels
There are few, if any, established small molecules or toxins that are highly selective and potent modulators of TRPC channels, and there are no published crystal structures on which the design of modulators can be based. Therefore, the field is at a stage of seeking high-resolution structural information on the channels, investigating structure-activity relationships (SARs) for known small molecule modulators (to identify molecules with better properties), developing and using screening assays to discover novel modulators from chemical libraries or natural products, or finding alternative approaches for achieving specific TRPC channel effects. One alternative approach is the generation of functional antibodies that modulate TRPC channels. The approach has so far been successful in achieving polyclonal isoform-specific extracellular inhibitors of TRPC1-, TRPC5- and TRPC6-containing channels (Xu et al., 2008; Mohl et al., 2011; Sukumar et al., 2012). Inhibitor antibodies acting via intracellular sites have also been reported (Shi et al., 2010).
Here, we focus on small molecule (chemical) modulators. There are chemicals of this type that affect TRPC channel function. Most of them have inhibitory effects (see succeeding discussion). None of them are especially potent but recent studies have identified chemicals that are effective in the low (and sub-) μM concentration range. It appears that some are relatively selective, even showing a degree of selectivity for one type of TRPC over another. A few appear to modulate the channels through direct binding, but further studies are mostly required to determine if effects are direct or indirect. Because of promiscuity in TRPC modulation by physiological factors, there is particular risk that effects of chemical modulators arise indirectly. A study showed that two quite similar chemicals with similar inhibitory effects on TRPC channels had different mechanisms of action – one direct and the other indirect (see Polyphenols). There is still much work to be done before highly selective, potent, directly-acting modulators are achieved. If such progress is made, the arising pharmacology will importantly aid investigations of the physiological and pathophysiological roles of TRPC channels, facilitate validation of TRPCs as potential therapeutic targets in different disease models and human tissues, and possibly provide foundations for drug discovery and development.
A common method for identifying modulators involves measurements from TRPC over-expressing cells loaded with a Ca2+-reactive fluorescent dye such as fura-2. Stimulation of the TRPC channels leads to a rise in the intracellular Ca2+ concentration as the Ca2+ enters through the TRPC channels and binds fura-2. Such methods are often suitable and powerful, but they are also prone to false positives and other artefacts. In addition, as in most cases only one TRPC channel is over-expressed, the largest proportion of the measured Ca2+ flux usually results from the activity of homomeric TRPC channels. Therefore, compounds identified in such an assay should be further evaluated using whole-cell, and preferably also single channel, patch-clamp recordings in voltage-clamp mode, and, where possible, in native channels. In the succeeding sections, we will describe pharmacological TRPC modulators. We include a brief section on TRPC stimulators, but the focus is on inhibitors because there is most information about inhibitors, and current thinking suggests that inhibitors might have most use either for understanding TRPC channel biology or as potential therapeutic agents.
Pharmacology I: TRPC stimulators
A series of TRPC6 stimulators is formed by hyperforin (1) and the diacylated phloroglucinols Hyp1 (2), Hyp5 (3), Hyp7 (4), Hyp8 (5) and Hyp9 (6) (Figure 2). Hyperforin is the main active ingredient of St. John's wort. With an EC50 of about 1 μM, hyperforin causes elevation of the intracellular Ca2+ concentrations and it was subsequently found to be a transient stimulator of TRPC6 without effect on TRPC3, TRPC4 or TRPC5 (Leuner et al., 2007). Diacylated phloroglucinol derivatives 2–6 are also TRPC6 stimulators and structurally less complex than hyperforin (Leuner et al., 2010), which can be regarded as a highly functionalized phloroglucinol derivative. The compounds do not activate TRPC3 and TRPC7. In these studies, it was suggested that hyperforin and the Hyp compounds are structurally related to diacylglycerol, but the chemical basis for such a relationship is unclear in our opinion.
Figure 2.
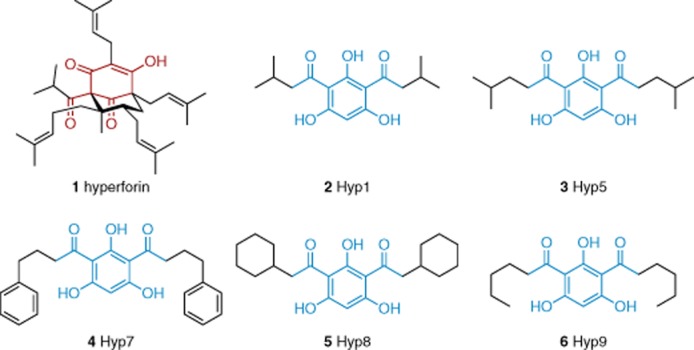
Hyperforin and diacylphloroglucinol-based TRPC6 stimulators. The functionalized acylphloroglucinol core of hyperforin has been highlighted in red to indicate the structural similarities between hyperforin and the Hyp compounds. The diacylated phloroglucinol cores of the Hyp compounds are highlighted in blue. See the main text for details and references.
Genistein (7, Figure 3) was found to stimulate TRPC5 with an EC50 of 93 μM (Wong et al., 2010). Daidzein (8), a genistein analogue that is inactive as a tyrosine kinase inhibitor, acted similarly. The thiazolidinedione rosiglitazone (9) was also found to stimulate TRPC5 channels with an EC50 of 31 μM (Majeed et al., 2011b). The effect of rosiglitazone occurred relatively rapidly and was reversible on washout. The chemically related thiazolidinediones pioglitazone (10) and troglitazone (11) had no effect, suggesting dissociation of the effect of rosiglitazone from its well-recognized target of PPAR-γ. Over-expressed TRPM3 channels were inhibited by rosiglitazone, pioglitazone or troglitazone. Endogenous TRPC1/TRPC5 heteromeric channels were stimulated by rosiglitazone and to a lesser extent by troglitazone, whereas pioglitazone was inactive (Sukumar et al., 2012). TRPC inhibitors may also have stimulatory effects on TRPC channels (see succeeding discussion). The data establish principles for pharmacological stimulation of TRPC5 channels, but the presently identified agents act only in the medium-to-high μM concentration range.
Figure 3.
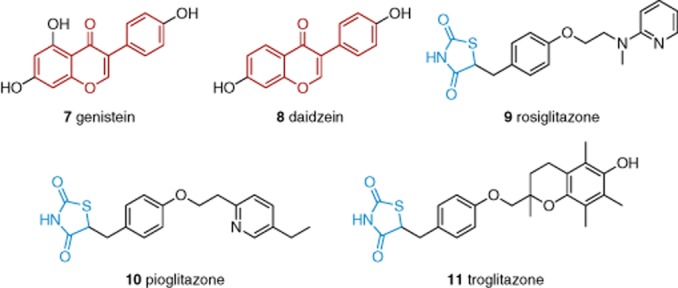
Structures of isoflavone- and thiazolidinedione-based TRPC stimulators. Red indicates the isoflavone core. Blue indicates the thiazolidinedione core. See the main text for details and references.
Pharmacology II: TRPC inhibitors
Inhibitors are classified here according to chemical structure.
Pyrazoles
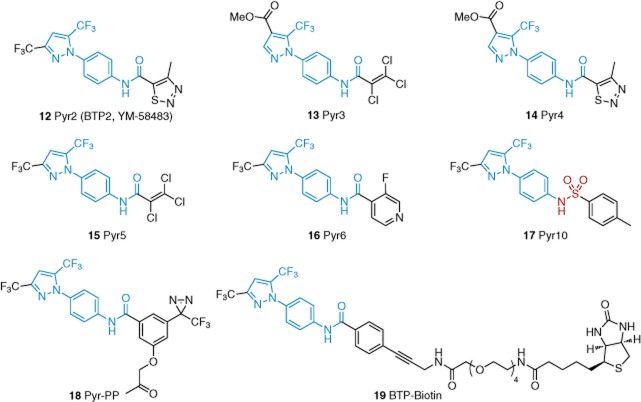
A series of 3,5-bis(trifluoromethyl)pyrazole (BTP) derivatives was discovered as immune suppressants that act by inhibiting NFAT and interleukin-2 production (Djuric et al., 2000). Pyr2 in this series (12 in Figure 4) is now recognized as an inhibitor of Ca2+ entry induced by store depletion and it may be this effect that leads to suppression of NFAT (Zitt et al., 2004; He et al., 2005). The effect on Ca2+ entry in store-depleted cells may arise through an effect on Orai1 channels, which are structurally distinct from TRPC channels (Hou et al., 2012). Nevertheless, Pyr2 also inhibits store-independent activity of TRPC3 and TRPC5 (Table 1).
Figure 4.
Structures of pyrazole-based TRPC inhibitors 12–17 and target identification probes 18 and 19. Blue indicates the similarities between the Pyr cores. Red indicates the distinguishing sulfonamide moiety of 17. See the main text for details and references.
Table 1.
Inhibition by pyrazole-based compounds 12–17 (Kiyonaka et al., 2009)
| Figure 3 compounds | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| TRPC1 | TRPC3 | TRPC4 | TRPC5 | TRPC6 | TRPC7 | |
| 12 (Pyr2) | n.d. | 4.2 | n.d. | 0.1–1 | 1–10 | 1–10 |
| 13 (Pyr3) | n.d. | 0.5 | n.d. | >10 | >10 | >10 |
| 14 (Pyr4) | n.d. | 0.3–3 | n.d. | >10 | 1–10 | 1–10 |
| 15 (Pyr5) | n.d. | 0.1–1 | n.d. | >10 | >10 | >10 |
| 16 (Pyr6) | n.d. | 18.5 | >10 | >10 | >10 | n.d. |
| 17 (Pyr10) | n.d. | 0.7 | >10 | >10 | >10 | n.d. |
n.d., not determined; TRPC, Transient Receptor Potential Canonical.
Pyr3 (13, Figure 3) is suggested to inhibit TRPC3 relatively selectively (Table 1). It incorporates a trichloroethylene moiety that appears to be crucial for selectivity (Kiyonaka et al., 2009). In addition to showing selectivity within the TRPC subgroup (Table 1), Pyr3 fails to inhibit TRPM2, TRPM4 or TRPM7 channels (Kiyonaka et al., 2009). Whole-cell patch-clamp studies indicated that the effect of Pyr3 on TRPC3 was direct, extracellular and partly (and slowly) reversible on washout. Photo cross-linking experiments with probe Pyr-PP (18), in combination with competition studies with Pyr3, supported the idea that Pyr3 can directly bind to TRPC3. Structural similarities of Pyr3 and Pyr-PP to the other five Pyr compounds in Figure 4, in combination with inhibition data, strongly suggest that these compounds may also be direct TRPC3 binders. Pyr3 was also used in vivo to suppress cardiac hypertrophy in mice (Kiyonaka et al., 2009), which was linked previously to TRPC channel activity (Figure 1). Also reported are Pyr6 (16) and Pyr10 (17, which contains a sulfonamide linkage instead of an amide linkage). Pyr6 showed preference for inhibition of Orai1 channels and Pyr10 for TRPC3 (Schleifer et al., 2012). Pyr3 was also found to inhibit Orai1 channels, and so, the compound may not be as specific for TRPC3 as is widely suggested. Pyr6 and Pyr10 were found to be selective for TRPC3 over TRPC4, TRPC5 and TRPC6. NFAT translocation was sensitive to Pyr6 but not Pyr10. It should be noted that unbiased analysis of Jurkat T cell lysates with probe BTP-biotin (19) suggested binding to the actin reorganizing protein drebrin and follow-up studies revealed an important role for drebrin in supporting Ca2+ entry (Mercer et al., 2010). Therefore, Pyr/BTP compounds may have effects on Ca2+ entry channels via an intermediate protein.
2-Aminoquinolines
Identification of 2-aminoquinolines as selective TRPC4/TRPC5 inhibitors has been reported (Miller et al., 2011) (Figure 5). This class of inhibitor was found during a screen for modulators of agonist-evoked TRPC4 responses in HEK 293 cells. Based on SAR studies around the 2-aminoquinoline scaffold, ML204 (20) was selected for detailed study. Table 2, however, shows activities of several of the most potent and selective compounds in the class (20–31). SAR data showed that five-, six- and seven-membered ring 2-amino substituents were tolerated but that acyclic or aryl substituents decreased activity. The four-methyl group proved optimal. A number of 6-substituents were tolerated but 8-substituents were not. Activities of the compounds against TRPC4 were confirmed by patch-clamp recording, showing that inhibition did not depend on receptor activation and was reversible. A direct inhibitory effect was suggested. There was no inhibition of TRPA1, TRPM8, TRPV1 and TRPV3 channels or voltage-gated Na+, K+ or Ca2+ channels. ML204 was further tested for binding of a panel of 68 receptors, ion channels and transporters; at 10 μM, it showed moderate binding in seven cases. Our own experiments have confirmed that ML204 inhibits TRPC5-dependent Ca2+ entry (D. J. Beech and Y. Majeed, unpubl. data). Therefore, ML204 seems to be a useful chemical tool for modulating TRPC4 or TRPC5 channels.
Figure 5.
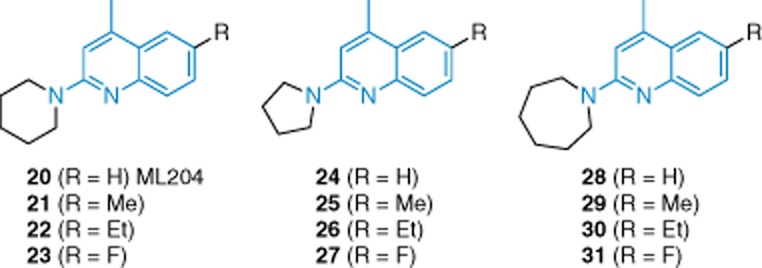
Structures of 2-aminoquinoline-based TRPC inhibitors 20–31. Blue indicates the 2-aminoquinoline scaffold the inhibitors have in common. See the main text for details and references.
Table 2.
Structures and inhibition profiles of the most potent and selective 2-aminoquinazoline-based TRPC4/C5 inhibitors (Miller et al., 2011)
| Figure 4 compounds | IC50 TRPC4 (μM) | IC50 TRPC6 (μM) | |
|---|---|---|---|
| Ca2+ assay | QPatch assay | FLIPR assay | |
| 20 (ML204) | 0.96 | 2.6 | 18.4 |
| 21 | 5.82 | 3.93 | 8.58 |
| 22 | (45.8%)a | 3.73 | 14.99 |
| 23 | 10.50 | 6.38 | 20.21 |
| 24 | 2.75 | 8.02 | 14.93 |
| 25 | 1.5 | 3.91 | 2.03 |
| 26 | 0.58 | 2.06 | 4.24 |
| 27 | 1.05 | 3.94 | 7.15 |
| 28 | 1.50 | 1.96 | 9.74 |
| 29 | 1.30 | 0.9 | 12.83 |
| 30 | 7.37 | 3.3 | (42.7%)a |
| 31 | 3.99 | 1.63 | (10.3%)a |
Percentage inhibition at 20 μM compound.
TRPC, Transient Receptor Potential Canonical.
Steroids
Investigation of neuroactive steroids led to identification of inhibitory effects against TRPC5 (Figure 6) (Majeed et al., 2011a). The most potent inhibitor was progesterone (35), with an IC50 of 5 μM. In Ca2+ assays, dihydrotestosterone (37) caused only weak inhibition at 10 μM, but it had stronger effects in patch-clamp recordings. The effects of progesterone and dihydrotestosterone were strong and reversible in outside-out patch recordings, suggesting relatively direct mechanisms of action. Pregnenolone sulfate (33), a so-called ‘fountain-of-youth’ steroid, inhibited Gd3+-induced TRPC5 activity with an IC50 of 19 μM. Pregnenolone sulfate did not inhibit TRPM2. Pregnanolone (32), pregnanolone sulfate (33) and pregnenolone sulfate (34) inhibited TRPC5 but pregnenolone and allopregnanolone did not. Norgestimate (36) was also found to inhibit TRPC channels (Miehe et al., 2012). Inhibition of TRPC3, TRPC4, TRPC5 and TRPC6 occurred with IC50 values of 12, 6, 12 and 18 μM. These studies suggest direct inhibitory effects of steroids acting with stereo-specificity but at relatively high concentrations that are only likely to have significance if exogenous steroids are administered.
Figure 6.
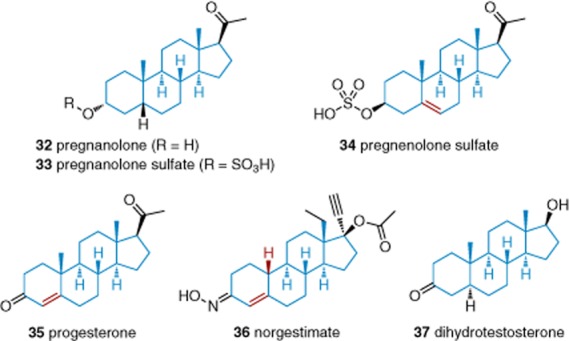
Structures of steroid-based TRPC inhibitors 32–37. Blue indicates the common steroid cores, whereas red indicates minor deviations from this core such as alternative bond oxidation states and substituents. See the main text for details and references.
Phenylethylimidazoles
Phenylethylimidazoles are widely used as non-specific inhibitors of Ca2+ channels. SKF96365 (38), the antifungal econazole (39) and its analogue calmidazolium chloride (40, also known as a calmodulin antagonist) (Figure 7) are inhibitors of Ca2+ entry in store-depleted cells (Sweeney et al., 2009). The compounds inhibit TRPC3 and TRPC6 channels at relatively high μM concentrations (Harteneck and Gollasch, 2011). Voltage-gated Ca2+ channels are more potently inhibited (Singh et al., 2010). Therefore, these compounds are TRPC channel inhibitors, but they are not potent or selective.
Figure 7.
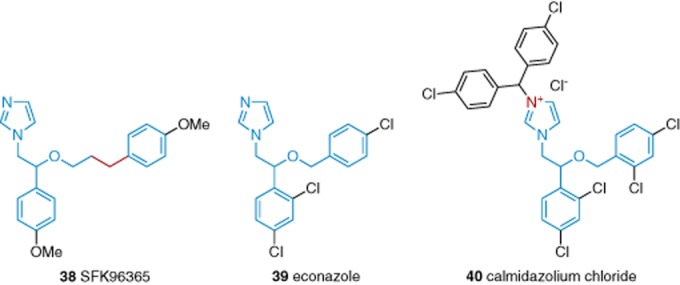
Structures of phenylethylimidazole-based TRPC inhibitors 38–40. Blue indicates the structural elements that the scaffolds of the compounds have in common, whereas red indicates deviations from this scaffold. See the main text for details and references.
Piperazine/piperidine analogues
Known relationships between the Sigma-1 receptor and Ca2+ signalling led to investigation of the effects of Sigma-1 receptor ligands (Figure 8) on Ca2+ responses of endothelial cells (Amer et al., 2013). Sigma-1 receptor antagonists BD1063 (41) and BD1047 (43) and the agonist 4-IBP (42) inhibited the sustained but not the initial transient Ca2+ response evoked by histamine or vascular endothelial growth factor. These data indicated specific inhibitory effects on Ca2+ entry mechanisms, and so, it was investigated if TRP channels are sensitive to Sigma-1 receptor ligands. BD1063, BD1047 and 4-IBP inhibited TRPC5. They also inhibited TRPM3 but not TRPM2. TRPC5 inhibition was independent of the type of stimulant used to initially evoke TRPC5 activity. The effect was confirmed in patch-clamp recordings. At a concentration of 10 μM, BD1047 inhibited TRPC5 by about 60%. The effect was rapid in onset and reversible upon washout, suggesting a direct inhibitory extracellular action. Despite these compounds being Sigma-1 receptor ligands, their effects on the channels did not depend on the Sigma-1 receptor, and no relationship between the channels and Sigma-1 receptor was detected.
Figure 8.
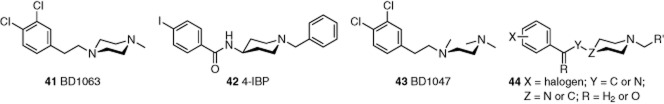
Structures of the Sigma-1-receptor ligands and TRPC5 inhibitors 41–43, and proposed pharmacophore 44. Lowest energy conformations of six-membered rings were drawn to emphasize structural similarities. Compounds 41 and 43 were used as their commercially available di-HBr salts. See the main text for details and references.
There are chemical similarities between BD1047, BD1063 and 4-IBP. Whereas BD1047 can be considered a ring-opened analogue of BD1063, 4-IBP also contains a halogenated aryl ring attached, via a two-atom linker, to a six-membered ring containing at least one basic nitrogen. Based on these findings, we suggested a new TRPC pharmacophore 44 (Figure 8).
Naphthalene sulfonamides
The myosin light chain kinase (MLCK) inhibitors ML-7 (45) and ML-9 (46), applied extra- or intra-cellularly, inhibited TRPC6 (Figure 9) (Shi et al., 2007). ML-9 displayed an IC50 of 7.8 μM. Wortmannin and peptide-based MLCK inhibitors did not affect TRPC6 or the inhibitory action of ML-9, suggesting inhibition independent of MLCK. W-7 (47), another MLCK inhibitor and calmodulin antagonist, is an analogue of ML-9 and ML-7 with an open chain diamine. W-7, several of its analogues, and its isomer 48 inhibit hyperforin-induced TRPC6 activity (Harteneck and Gollasch, 2011). W-7 inhibited TRPC6, TRPM2, TRPM3 and TRPV4 with IC50 values of 28, 26, 15 and 65 μM. These findings suggest that naphthalene sulfonamides are μM non-specific TRP channel inhibitors.
Figure 9.
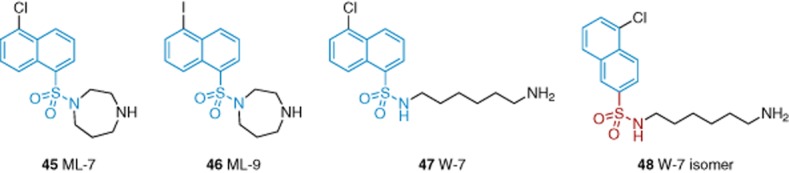
Structures of naphthalene sulfonamide-based TRPC inhibitors 45–48. Blue indicates the common naphthalene sulfonamide core, whereas red indicates the alternative position for naphthalene substitution in 48. See the main text for details and references.
N-phenylanthranilic acids and analogues
Flufenamic acid (FFA, 49), N-(p-amylcinnamoyl)anthranilic acid (ACA, 53) and other N-phenylanthranilic acids (or fenamates, Figure 10) stimulate TRPC6 (Foster et al., 2009) and inhibit other TRPC (and TRPM) channels (Kraft and Harteneck, 2005; Harteneck et al., 2007; Ilatovskaya et al., 2011). FFA, mefenamic acid (MFA) (50), niflumic acid (51), diclofenac sodium (52) and several FFA analogues (54) have been investigated in some detail against TRPC4 and TRPC5 (Jiang et al., 2012). All of these non-steroidal anti-inflammatory drugs are inhibitors of TRPC4 or TRPC5 with IC50s of 37–170 μM. Changes of the phenyl ring substituents resulted in analogues that were less potent than FFA and MFA.
Figure 10.
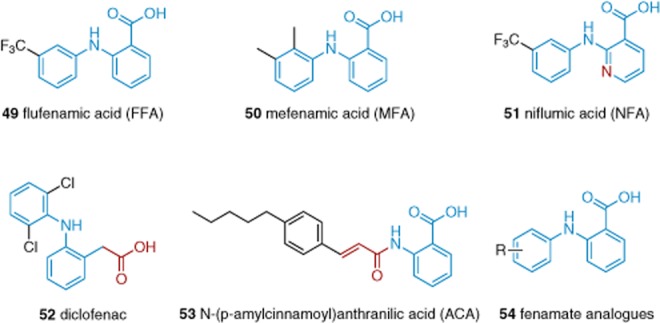
Structures of N-phenylanthranilic acids and analogues 49–54. Blue indicates the N-phenylanthranilic acid scaffold, whereas red indicates deviations from this scaffold. See the main text for details and references.
Fatty acids
Upon adipocyte maturation, there is up-regulation of constitutively active TRPC1 and TRPC5 heteromeric channels (Sukumar et al., 2012). Because adipocytes are closely involved with fatty acid metabolism and storage, a library of 66 fatty acids was screened for activity against TRPC5 channels; 19 were found to be inhibitors, whereas the others had no effect. Further investigation was carried out on three dietary ω-3 fatty acids, α-linolenic acid (55), eicosapentaenoic acid (56) and docosahexaenoic acid (57) (Figure 11), which occur in plants or oily fish. There was inhibition of TRPC5 and TRPC1/TRPC5 heteromers. The IC50 of α-linolenic acid against TRPC5 was 22 μM, which is in the concentration range achieved by ingestion of this fatty acid. Strikingly, α-linolenic acid induced the production of adiponectin (a key anti-inflammatory adipokine) in adipose tissue of wild-type mice but not adipose tissue of mice expressing mutant TRPC5 that disabled the TRPC1/TRPC5 heteromer.
Figure 11.
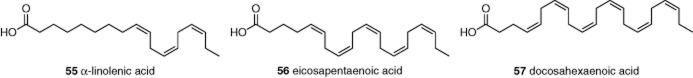
Structures of ω-3 fatty acid inhibitors of TRPC channels, 55–57. See the main text for details and references.
Polyphenols
Based on the idea that TRPC channels are promiscuous sensors of a variety of dietary factors, further dietary factors including antioxidants were investigated. The antioxidant gallic acid (58, Figure 12) (a component of green tea) inhibited hydrogen peroxide- but not lysophosphatidylcholine-induced TRPC5 activity (Naylor et al., 2011). Resveratrol (59) (a component of red wine) inhibited TRPC5 activity that was evoked not only by hydrogen peroxide but also lysophosphatidylcholine. Whole-cell patch-clamp recordings confirmed the inhibitory effect with an IC50 ranging from 4 to 30 μM depending on voltage. Inhibition by resveratrol was relatively slow and the effect could not be readily reversed on washout. These findings, in combination with the lack of significant effect on TRPC5 in excised membrane patches, indicated that resveratrol was an indirect inhibitor of TRPC5. The oestrogen receptor modulator diethylstilbestrol (60) inhibited TRPC5 with similar potency (IC50 3–9 μM depending on the recording technique) but its effect occurred rapidly, was readily reversible on washout and occurred in excised outside-out membrane patch recordings, indicating a direct inhibitory effect. One of the main differences between the two trans-stilbenes (59, 60) is the presence of two ethyl groups on 60, which causes a difference in preferred conformation of the aromatic rings of 59 (in the same plane as the double bond) and 60 (twisted out of the plane of the double bond).
Figure 12.
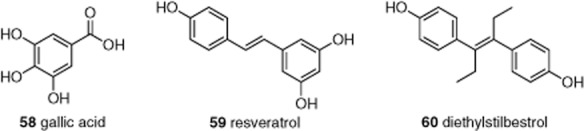
Structures of the anti-oxidant gallic acid and trans-stilbene-based TRPC5 inhibitors, 58–60. See the main text for details and references.
2-Aminothiazoles
Pharmaceutical company GlaxoSmithKline (GSK) patented compounds with general structure 61 as TRPC3 and/or TRPC6 inhibitors (Figure 13) (Dodson et al., 2012). The compounds are based on a 2-aminothiazole core, and most of the 49 reported examples also have a tetrahydroisoquinoline-based substituent (e.g. 63–65). Compounds listed in ‘example 3’ (62) and ‘example 42’ (65) are reported to display IC50s against TRPC3 of 0.01–0.1 μM, and 62 to inhibit TRPC6 with an IC50 of 0.01–0.1 μM. Compounds listed in ‘examples 3, 9, 11 and 42’ (62–65) were also reported to inhibit the expression of phenylephrine-induced atrial naturietic factor (ANF) in neonatal rat ventricular myocytes.
Figure 13.
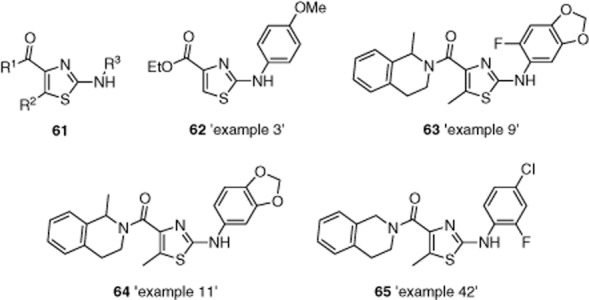
General structure of putative TRPC3 and/or TRPC6 inhibitors in a GSK patent (61) and specific examples of potent inhibitors mentioned in the patent 62–65. See the main text for details and reference.
Miscellaneous compounds
The isothiourea derivative KB-R7943 (66, Figure 14) is an inhibitor of Na+-Ca2+ exchange, but it was also found to inhibit TRPC3, TRPC5 and TRPC6 with relatively low IC50s of 0.46, 1.38 and 0.71 μM (Kraft, 2007). The inhibitory effects of KB-R7943 were slow, and recovery on washout was weak. Indirect effects via Na+-Ca2+ exchanger are not ruled out.
Figure 14.
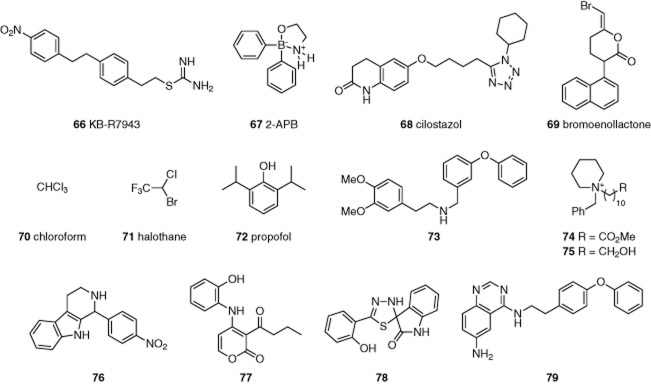
Structures of miscellaneous TRPC inhibitors, 66–79. See the main text for details and references.
The organoborane 2-aminoethoxydiphenyl borate (2-APB, 67, Figure 14) is an inhibitor of IP3 receptors and Ca2+ entry in store-depleted cells. 2-APB also inhibits TRPC and TRPM channels. It stimulates TRPV channels (Hu et al., 2004). Studies of TRPC5 suggested an IC50 of about 20 μM and an effect that occurs relatively rapidly and reversibly (Xu et al., 2005). The effect appears to arise through a direct extracellular action.
The anti-platelet drug cilostazol (68, Figure 13) inhibited TRPC3, TRPC6 and TRPC7 by enabling PK A-dependent phosphorylation of the channels (Nishioka et al., 2011). It does not appear to be a directly acting TRPC channel inhibitor. Similarly, bromoenollactone (69, Figure 14) inhibited TRPC5 activity evoked by sphingosine-1-phosphate (AL-Shawaf et al., 2011) and the effect was suggested to occur indirectly via an effect on Group VI PLA2. The latter hypothesis was supported by knock-down studies of the PLA2. The lack of effect of 69 on Gd3+-evoked activity also argued against a direct inhibitory effect on TRPC5 channels.
Chemical requirements for activation of TRPC5 channels by lysophospholipids are similar to those of the TREK-1 K+ channel which is suggested to be a target of general anaesthetics through modulation of biophysical properties of the lipid bilayer. Anaesthetics were tested for effects on TRPC5. The classical inhalation general anaesthetics chloroform (70) and halothane (71) and the commonly used i.v. anaesthetic propofol (72) (Figure 14) were inhibitors of TRPC5 (Bahnasi et al., 2008). Effects occurred at concentrations that may be relevant to clinical anaesthesia in some situations. It is unlikely that any of these agents has specific binding sites on TRPC5 channels.
Screening of the Chembionet library for effects against TRPC6 has been reported (Urban et al., 2012). Follow-up studies with nine of the identified 20 inhibitors led to identification of seven confirmed hits with IC50s in the low-to-medium μM range. Because one compound was toxic to cells, selectivity studies were performed only on six of the compounds using TRPC6, TRPC3, TRPC7, TRPA1, TRPM2 and TRPV1. Compounds 2910-0498 (73), 6228-0353 (74), 6228-0473 (75), 8009-5364 (76) and 8016–8488 (77) (Figure 14) displayed selectivity for the TRPC3/6/7 subgroup, with some evidence for selectivity within this subgroup. The most selective TRPC6 inhibitor was 76 (2.5-fold with respect to TRPC3 and >10-fold with respect to TRPC7), and the most selective TRPC3 inhibitor was 73 (fourfold with respect to TRPC6 and sixfold with respect to TRPC7). Compound 5408-0428 (78) was selective for TRPC6 with respect to the other members of the TRPC3/6/7 subfamily but also inhibited TRPA1, TRPM2 (partially) and TRPV1 with similar potencies. Inhibition of TRPC6 and TRPC3 by 73, 75 and 76 was further studied using whole-cell patch-clamp, confirming activity. In these experiments, only 76 displayed the selectivity for TRPC6 seen in Ca2+ recordings. Some of these new compounds may provide interesting starting points for further optimisation towards selective tool compounds.
Screening of a quinazoline-derived library in a Drosophila huntingtin gene assay identified compounds that inhibit TRPC1-dependent cationic current evoked by store depletion, such as 79 (Figure 14) (Wu et al., 2011). Ionic currents induced by over-expression of TRPC1 were not affected however, suggesting an effect that was indirect.
Conclusions
In conclusion, important progress has been made in recent years towards the identification of selective and potent small molecule modulators of TRPC channels. The published data suggest that it is possible to achieve such pharmacology and that there will be high quality and widely available modulators within the next 5–10 years. Presently, although some agents have quite good potencies [i.e. IC50s in the low (and sub-) μM range], we ideally need about 100-fold improvement in nearly all cases. More information is required on specificity, especially in relation to channels with overlapping or similar functions (e.g. Orai1 channels). More detailed information is needed on direct binding and on the precise mechanisms of action. Ideally, specific binding pockets for key modulators should be identified and characterized. Furthermore, modulators should be generated with properties that make them suitable for in vivo use. It would be ideal to show that in vivo effects are lost in mice lacking expression of the target TRPC. Once such modulators are determined, it will be timely to progress to in vivo studies of human disease models in large animals with a view to delivering therapeutic agents.
Pyr3 is one of the encouraging but also intriguing modulators. It is often specified in the literature as a TRPC3-specific inhibitor, especially as it became commercially available. However, we would advise caution at this stage because it is not clear that Pyr3 distinguishes TRPC3 channels from Orai1 channels (Schleifer et al., 2012). Notably, Pyr3 is similar in chemical structure to BTP2 and Synta66, which are widely used as Orai1 channel [calcium release-activated channel (CRAC)] inhibitors. BTP2 is the same compound as Pyr2, which is closely related to Pyr3 and has also been described as TRPC5-specific. We have found that Pyr3 and Synta66 similarly inhibit a common Ca2+ entry pathway in endothelial cells, consistent with them both inhibiting the Orai1 (CRAC) channel (P. Turner & D. J. Beech, unpubl. data).
ML204 is promising as a selective TRPC4/TRPC5 inhibitor, but there should again be caution until there has been wider use by independent groups, further description of its specificity and evaluation of its use in vivo. Similarly, some hit compounds from the Chembionet library look promising, but further work is needed regarding their optimisation and profiling. Piperazines/piperidines have some good properties, but substantial research is needed if improved potency and specificity are to be achieved. Identification of natural TRPC modulators is intriguing in terms of the biology of TRPC channels, suggesting that the channels may serve as points for integration with or sensing of external chemical environment. It is unclear, however, if such modulators will serve well as templates for highly potent TRPC modulators. The 2-aminothiazole-based TRPC3/6 inhibitors may constitute the most potent TRPC inhibitors so far, but further details and clarification are needed.
We hope that the promising progress outlined in this review will encourage multidisciplinary teams to focus on the important and worthwhile challenge of developing highly potent, specific, readily usable and widely available TRPC pharmacology. In our opinion, it is more likely to be a pot of gold than a mirage.
Acknowledgments
The research is supported by the Wellcome Trust and the British Heart Foundation.
Glossary
- ACA
N-(p-amylcinnamoyl)anthranilic acid
- ANF
atrial naturietic factor
- BTP
3,5-bis(trifluoromethyl)pyrazole
- CRAC
calcium release-activated channel
- E3
third extracellular
- FFA
Flufenamic acid
- IP3
inositol 1,4,5-triphosphate
- MFA
mefenamic acid
- MLCK
myosin light chain kinase
- NFAT
Nuclear factor of activated T cells
- PLA2
phospholipase A2
- SAR
Structure-activity relationship
- TRP
Transient Receptor Potential
- TRPC
Transient Receptor Potential Canonical
- TRPM
Melastatin
- TRPP
Polycystin
- TRPV
Vanilloid
- 2-APB
2-aminoethoxydiphenyl borate
Conflict of interest
None.
References
- Abed E, Labelle D, Martineau C, Loghin A, Moreau R. Expression of transient receptor potential (TRP) channels in human and murine osteoblast-like cells. Mol Membr Biol. 2009;26:146–158. doi: 10.1080/09687680802612721. [DOI] [PubMed] [Google Scholar]
- Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, et al. Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Shawaf E, Tumova S, Naylor J, Majeed Y, Li J, Beech DJ. GVI phospholipase A2 role in the stimulatory effect of sphingosine-1-phosphate on TRPC5 cationic channels. Cell Calcium. 2011;50:343–350. doi: 10.1016/j.ceca.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer MS, McKeown L, Tumova S, Liu R, Seymour VA, Wilson LA, et al. Inhibition of endothelial cell Ca2+ entry and transient receptor potential channels by Sigma-1 receptor ligands. Br J Pharmacol. 2013;168:1445–1455. doi: 10.1111/bph.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahnasi YM, Wright HM, Milligan CJ, Dedman AM, Zeng F, Hopkins PM, et al. Modulation of TRPC5 cation channels by halothane, chloroform and propofol. Br J Pharmacol. 2008;153:1505–1512. doi: 10.1038/sj.bjp.0707689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ. Orai1 calcium channels in the vasculature. Pflugers Arch. 2012;463:635–647. doi: 10.1007/s00424-012-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ. Characteristics of Transient Receptor Potential Canonical calcium-permeable channels and their relevance to vascular physiology and disease. Circ J. 2013;77:570–579. doi: 10.1253/circj.cj-13-0154. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol. 2004;559(Pt 3):685–706. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Bollimuntha S, Selvaraj S, Singh BB. Emerging roles of canonical TRP channels in neuronal function. Adv Exp Med Biol. 2011;704:573–593. doi: 10.1007/978-94-007-0265-3_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Fatherazi S, Presland RB, Belton CM, Roberts FA, Goodwin PC, et al. Evidence that TRPC1 contributes to calcium-induced differentiation of human keratinocytes. Pflugers Arch. 2006;452:43–52. doi: 10.1007/s00424-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol. 2008;18:R880–R889. doi: 10.1016/j.cub.2008.07.063. [DOI] [PubMed] [Google Scholar]
- Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23:705–715. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Mederos y Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J Biol Chem. 2003;278:47842–47852. doi: 10.1074/jbc.M302983200. [DOI] [PubMed] [Google Scholar]
- Djuric SW, BaMaung NY, Basha A, Liu H, Luly JR, Madar DJ, et al. 3,5-Bis(trifluoromethyl)pyrazoles: a novel class of NFAT transcription factor regulator. J Med Chem. 2000;43:2975–2981. doi: 10.1021/jm990615a. [DOI] [PubMed] [Google Scholar]
- Dodson JW, Marino JP, McAtee JJ, Terrell LM, Washburn DG. 2012. Compounds. Patent WO 2012/037349 A2.
- Dryer SE, Reiser J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am J Physiol. 2010;299:F689–F701. doi: 10.1152/ajprenal.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder P, Molkentin JD. TRPC channels as effectors of cardiac hypertrophy. Circ Res. 2011;108:265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- Foster RR, Zadeh MA, Welsh GI, Satchell SC, Ye Y, Mathieson PW, et al. Flufenamic acid is a tool for investigating TRPC6-mediated calcium signalling in human conditionally immortalised podocytes and HEK293 cells. Cell Calcium. 2009;45:384–390. doi: 10.1016/j.ceca.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Freichel M, Almering J, Tsvilovskyy V. The role of TRP proteins in mast cells. Front Immunol. 2012;3:1–15. doi: 10.3389/fimmu.2012.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasio OL, Whitehead NP, Yeung EW, Phillips WD, Allen DG. TRPC1 binds to caveolin-3 and is regulated by Src kinase – role in Duchenne muscular dystrophy. J Cell Sci. 2008;121(Pt 13):2246–2255. doi: 10.1242/jcs.032003. [DOI] [PubMed] [Google Scholar]
- Graham S, Ding M, Ding Y, Sours-Brothers S, Luchowski R, Gryczynski Z, et al. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem. 2010;285:23466–23476. doi: 10.1074/jbc.M109.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteneck C, Gollasch M. Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels. Curr Pharm Biotechnol. 2011;12:35–41. doi: 10.2174/138920111793937943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteneck C, Frenzel H, Kraft R. N-(p-amylcinnamoyl)anthranilic acid (ACA): a phospholipase A2 inhibitor and TRP channel blocker. Cardiovasc Drug Rev. 2007;25:61–75. doi: 10.1111/j.1527-3466.2007.00005.x. [DOI] [PubMed] [Google Scholar]
- He LP, Hewavitharana T, Soboloff J, Spassova MA, Gill DL. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J Biol Chem. 2005;280:10997–11006. doi: 10.1074/jbc.M411797200. [DOI] [PubMed] [Google Scholar]
- Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338:1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, et al. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- Hui H, McHugh D, Hannan M, Zeng F, Xu SZ, Khan SU, et al. Calcium-sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth. J Physiol. 2006;572(Pt 1):165–172. doi: 10.1113/jphysiol.2005.102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilatovskaya DV, Levchenko V, Ryan RP, Cowley AW, Jr, Staruschenko A. NSAIDs acutely inhibit TRPC channels in freshly isolated rat glomeruli. Biochem Biophys Res Commun. 2011;408:242–247. doi: 10.1016/j.bbrc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Zeng B, Chen GL, Bot D, Eastmond S, Elsenussi SE, et al. Effect of non-steroidal anti-inflammatory drugs and new fenamate analogues on TRPC4 and TRPC5 channels. Biochem Pharmacol. 2012;83:923–931. doi: 10.1016/j.bcp.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Jung C, Gene GG, Tomas M, Plata C, Selent J, Pastor M, et al. A gain-of-function SNP in TRPC4 cation channel protects against myocardial infarction. Cardiovasc Res. 2011;91:465–471. doi: 10.1093/cvr/cvr083. [DOI] [PubMed] [Google Scholar]
- Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, et al. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011;140:2107–2115. doi: 10.1053/j.gastro.2011.02.052. 2115 e2101-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, et al. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci U S A. 2009;106:5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R. The Na+/Ca2+ exchange inhibitor KB-R7943 potently blocks TRPC channels. Biochem Biophys Res Commun. 2007;361:230–236. doi: 10.1016/j.bbrc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kraft R, Harteneck C. The mammalian melastatin-related transient receptor potential cation channels: an overview. Pflugers Arch. 2005;451:204–211. doi: 10.1007/s00424-005-1428-0. [DOI] [PubMed] [Google Scholar]
- Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, et al. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–563. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K, Kazanski V, Muller M, Essin K, Henke B, Gollasch M, et al. Hyperforin – a key constituent of St. John's wort specifically activates TRPC6 channels. FASEB J. 2007;21:4101–4111. doi: 10.1096/fj.07-8110com. [DOI] [PubMed] [Google Scholar]
- Leuner K, Heiser JH, Derksen S, Mladenov MI, Fehske CJ, Schubert R, et al. Simple 2,4-diacylphloroglucinols as classic transient receptor potential-6 activators–identification of a novel pharmacophore. Mol Pharmacol. 2010;77:368–377. doi: 10.1124/mol.109.057513. [DOI] [PubMed] [Google Scholar]
- Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(-/-) mice. Proc Natl Acad Sci U S A. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180:2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cao J, Luo J, Nilius B, Huang Y, Ambudkar IS, et al. Depletion of intracellular Ca2+ stores stimulates the translocation of vanilloid transient receptor potential 4-C1 heteromeric channels to the plasma membrane. Arterioscler Thromb Vasc Biol. 2010;30:2249–2255. doi: 10.1161/ATVBAHA.110.212084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cai Y, He D, Zou C, Zhang P, Lo CY, et al. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc Natl Acad Sci U S A. 2012;109:16282–16287. doi: 10.1073/pnas.1202989109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed Y, Amer MS, Agarwal AK, McKeown L, Porter KE, O'Regan DJ, et al. Stereo-selective inhibition of transient receptor potential TRPC5 cation channels by neuroactive steroids. Br J Pharmacol. 2011a;162:1509–1520. doi: 10.1111/j.1476-5381.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed Y, Bahnasi Y, Seymour VA, Wilson LA, Milligan CJ, Agarwal AK, et al. Rapid and contrasting effects of rosiglitazone on transient receptor potential TRPM3 and TRPC5 channels. Mol Pharmacol. 2011b;79:1023–1030. doi: 10.1124/mol.110.069922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JC, Qi Q, Mottram LF, Law M, Bruce D, Iyer A, et al. Chemico-genetic identification of drebrin as a regulator of calcium responses. Int J Biochem Cell Biol. 2010;42:337–345. doi: 10.1016/j.biocel.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miehe S, Crause P, Schmidt T, Lohn M, Kleemann HW, Licher T, et al. Inhibition of diacylglycerol-sensitive TRPC channels by synthetic and natural steroids. PLoS ONE. 2012;7:e35393. doi: 10.1371/journal.pone.0035393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, et al. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J Biol Chem. 2011;286:33436–33446. doi: 10.1074/jbc.M111.274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohl MC, Iismaa SE, Xiao XH, Friedrich O, Wagner S, Nikolova-Krstevski V, et al. Regulation of murine cardiac contractility by activation of alpha(1A)-adrenergic receptor-operated Ca2+ entry. Cardiovasc Res. 2011;91:310–319. doi: 10.1093/cvr/cvr081. [DOI] [PubMed] [Google Scholar]
- Monet M, Francoeur N, Boulay G. Involvement of phosphoinositide 3-kinase and PTEN protein in mechanism of activation of TRPC6 protein in vascular smooth muscle cells. J Biol Chem. 2012;287:17672–17681. doi: 10.1074/jbc.M112.341354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor J, Al-Shawaf E, McKeown L, Manna PT, Porter KE, O'Regan D, et al. TRPC5 channel sensitivities to antioxidants and hydroxylated stilbenes. J Biol Chem. 2011;286:5078–5086. doi: 10.1074/jbc.M110.196956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K, Nishida M, Ariyoshi M, Jian Z, Saiki S, Hirano M, et al. Cilostazol suppresses angiotensin II-induced vasoconstriction via protein kinase A-mediated phosphorylation of the transient receptor potential canonical 6 channel. Arterioscler Thromb Vasc Biol. 2011;31:2278–2286. doi: 10.1161/ATVBAHA.110.221010. [DOI] [PubMed] [Google Scholar]
- Odell AF, Scott JL, Van Helden DF. Epidermal growth factor induces tyrosine phosphorylation, membrane insertion, and activation of transient receptor potential channel 4. J Biol Chem. 2005;280:37974–37987. doi: 10.1074/jbc.M503646200. [DOI] [PubMed] [Google Scholar]
- Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, et al. Canonical Transient Receptor Channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol. 2013;83:429–438. doi: 10.1124/mol.112.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick K, Zhao J, Eijkelkamp N, Linley JE, Rugiero F, Cox JJ, et al. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol. 2012;2:1–14. doi: 10.1098/rsob.120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan G, Gupta S, Thielmann I, Pleines I, Varga-Szabo D, May F, et al. Defective diacylglycerol-induced Ca2+ entry but normal agonist-induced activation responses in TRPC6-deficient mouse platelets. J Thromb Haemost. 2012;10:419–429. doi: 10.1111/j.1538-7836.2011.04596.x. [DOI] [PubMed] [Google Scholar]
- Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychkov GY, Barritt GJ. Expression and function of TRP channels in liver cells. Adv Exp Med Biol. 2011;704:667–686. doi: 10.1007/978-94-007-0265-3_35. [DOI] [PubMed] [Google Scholar]
- Schleifer H, Doleschal B, Lichtenegger M, Oppenrieder R, Derler I, Frischauf I, et al. Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca2+ entry pathways. Br J Pharmacol. 2012;167:1712–1722. doi: 10.1111/j.1476-5381.2012.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, et al. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest. 2012;122:1354–1367. doi: 10.1172/JCI61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Takahashi S, Jin XH, Li YQ, Ito Y, Mori Y, et al. Myosin light chain kinase-independent inhibition by ML-9 of murine TRPC6 channels expressed in HEK293 cells. Br J Pharmacol. 2007;152:122–131. doi: 10.1038/sj.bjp.0707368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Ju M, Saleh SN, Albert AP, Large WA. TRPC6 channels stimulated by angiotensin II are inhibited by TRPC1/C5 channel activity through a Ca2+- and PKC-dependent mechanism in native vascular myocytes. J Physiol. 2010;588(Pt 19):3671–3682. doi: 10.1113/jphysiol.2010.194621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H, Sakimoto S, Nakao K, Sugishita A, Konno M, Iida S, et al. Transient receptor potential canonical 3 (TRPC3) mediates thrombin-induced astrocyte activation and upregulates its own expression in cortical astrocytes. J Neurosci. 2010;30:13116–13129. doi: 10.1523/JNEUROSCI.1890-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Hildebrand ME, Garcia E, Snutch TP. The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br J Pharmacol. 2010;160:1464–1475. doi: 10.1111/j.1476-5381.2010.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedlund K, Tano JY, Vazquez G. The constitutive function of native TRPC3 channels modulates vascular cell adhesion molecule-1 expression in coronary endothelial cells through nuclear factor kappaB signaling. Circ Res. 2010;106:1479–1488. doi: 10.1161/CIRCRESAHA.109.213314. [DOI] [PubMed] [Google Scholar]
- Sours S, Du J, Chu S, Ding M, Zhou XJ, Ma R. Expression of canonical transient receptor potential (TRPC) proteins in human glomerular mesangial cells. Am J Physiol. 2006;290:F1507–F1515. doi: 10.1152/ajprenal.00268.2005. [DOI] [PubMed] [Google Scholar]
- Sukumar P, Sedo A, Li J, Wilson LA, O'Regan D, Lippiat JD, et al. Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ Res. 2012;111:191–200. doi: 10.1161/CIRCRESAHA.112.270751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh Babu S, Wojtowicz A, Freichel M, Birnbaumer L, Hecker M, Cattaruzza M. Mechanism of stretch-induced activation of the mechanotransducer zyxin in vascular cells. Sci Signal. 2012;5:ra91. doi: 10.1126/scisignal.2003173. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- Sweeney ZK, Minatti A, Button DC, Patrick S. Small-molecule inhibitors of store-operated calcium entry. ChemMedChem. 2009;4:706–718. doi: 10.1002/cmdc.200800452. [DOI] [PubMed] [Google Scholar]
- Tano JY, Smedlund K, Lee R, Abramowitz J, Birnbaumer L, Vazquez G. Impairment of survival signaling and efferocytosis in TRPC3-deficient macrophages. Biochem Biophys Res Commun. 2011;410:643–647. doi: 10.1016/j.bbrc.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Thebault S, Flourakis M, Vanoverberghe K, Vandermoere F, Roudbaraki M, Lehen'kyi V, et al. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006;66:2038–2047. doi: 10.1158/0008-5472.CAN-05-0376. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, et al. Impairment of store-operated Ca2+ entry in TRPC4(-/-) mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- Trebak M. The puzzling role of TRPC3 channels in motor coordination. Pflugers Arch. 2010;459:369–375. doi: 10.1007/s00424-009-0740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokas L. Function and regulation of TRPP2 at the plasma membrane. Am J Physiol. 2009;297:F1–F9. doi: 10.1152/ajprenal.90277.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, et al. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137:1415–1424. doi: 10.1053/j.gastro.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban N, Hill K, Wang L, Kuebler WM, Schaefer M. Novel pharmacological TRPC inhibitors block hypoxia-induced vasoconstriction. Cell Calcium. 2012;51:194–206. doi: 10.1016/j.ceca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A. 2006;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos y Schnitzler M, et al. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun. 2012;3:1–10. doi: 10.1038/ncomms1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- Wong CO, Huang Y, Yao X. Genistein potentiates activity of the cation channel TRPC5 independently of tyrosine kinases. Br J Pharmacol. 2010;159:1486–1496. doi: 10.1111/j.1476-5381.2010.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Shih HP, Vigont V, Hrdlicka L, Diggins L, Singh C, et al. Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington's disease treatment. Chem Biol. 2011;18:777–793. doi: 10.1016/j.chembiol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, et al. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, et al. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim E, Carey MA, Card JW, Dietrich A, Flake GP, Zhang Y, et al. Severely blunted allergen-induced pulmonary Th2 cell response and lung hyperresponsiveness in type 1 transient receptor potential channel-deficient mice. Am J Physiol. 2012;303:L539–L549. doi: 10.1152/ajplung.00389.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PC, Gu SY, Bu JW, Du JL. TRPC1 is essential for in vivo angiogenesis in zebrafish. Circ Res. 2010;106:1221–1232. doi: 10.1161/CIRCRESAHA.109.207670. [DOI] [PubMed] [Google Scholar]
- Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, et al. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation. 2009;119:2313–2322. doi: 10.1161/CIRCULATIONAHA.108.782458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanou N, Shapovalov G, Louis M, Tajeddine N, Gallo C, Van Schoor M, et al. Role of TRPC1 channel in skeletal muscle function. Am J Physiol. 2010;298:C149–C162. doi: 10.1152/ajpcell.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitt C, Strauss B, Schwarz EC, Spaeth N, Rast G, Hatzelmann A, et al. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem. 2004;279:12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]