Abstract
A male patient with profound mental retardation, athetosis, nystagmus, and severe congenital hypotonia (Duchenne muscular dystrophy [DMD]) was previously shown to carry a pericentric inversion of the X chromosome, 46,Y,inv(X)(p21.2q22.2). His mother carried this inversion on one X allele. The patient's condition was originally misdiagnosed as cerebral palsy, and only later was it diagnosed as DMD. Because the DMD gene is located at Xp21.2, which is one breakpoint of the inv(X), and because its defects are rarely associated with severe mental retardation, the other clinical features of this patient were deemed likely to be associated with the opposite breakpoint at Xq22. Our precise molecular-cytogenetic characterization of both breakpoints revealed three catastrophic genetic events that had probably influenced neuromuscular and cognitive development: deletion of part of the DMD gene at Xp21.2, duplication of the human proteolipid protein gene (PLP) at Xq22.2, and disruption of a novel gene. The latter sequence, showing a high degree of homology to the Sec4 gene of yeast, encoded a putative small guanine-protein, Ras-like GTPase that we have termed “RLGP.” Immunocytochemistry located RLGP at mitochondria. We speculate that disruption of RLGP was responsible for the patient’s profound mental retardation.
Mental retardation, a common clinical condition, is not well studied, despite its high cumulative prevalence. X-linked mental retardation (XLMR) is the most frequent type of mental deficiency seen in males, with an incidence of ∼1 in 500–600 boys, although it appears to be highly heterogeneous (Herbst and Miller 1980; Stevenson 2000). XLMR conditions are classified as either syndromic XLMR (MRXS) or nonspecific (or nonsyndromic) XLMR (MRX). In MRXS, mental retardation is one clinical manifestation within a complex syndrome, whereas patients with MRX share no distinctive clinical or biochemical manifestations apart from cognitive impairment (Stevenson 2000; Chelly and Mandel 2001).
The latest available data concerning XLMR have been compiled by the European XLMR Study Group and released on the XLMR Genes Update Web site. In all, 209 families with XLMR (140 with MRXS and 69 with MRX) have been entered into the database so far, and 118 loci associated with XLMR have been mapped on the X chromosome. Of those, 34 have yielded specific genes as causes for mental retardation. Seven genes responsible for MRX have been cloned; several among them—PAK3 (p21 activating kinase 3 [MIM 300142]), OPHN1 (oligophenin-1 [MIM 300127]), and ARHGEF6 (a guanine nucleotide exchange factor for Rho GTPases [MIM 00267])—are involved in the Rho-GTPase cycle, which mediates organization of the cytoskeleton, cell shape, and motility in neuronal cells (Tonilo 2000; Chelly and Mandel 2001). In addition, mutations in GDI1, one of the Rab GDP-dissociation inhibitors that control recycling of Rab GTPases, have been revealed in two families with MRX (MRX41 and MRX48) (D’Adamo et al. 1998).
On the other hand, 27 genes responsible for MRXS have been identified. Among them are FGD1 (faciogenital dysplasia protein 1), an activator of Rho GTPase (Cdc42) (Zheng et al. 1996), and DDP (deafness/dystonia peptide), which shows similarity to yeast mitochondrial proteins and plays an important role in the mitochondrial protein-import system (Jin et al. 1996). Moreover, a missense mutation in the RSK2 (ribosomal S6 kinase 2 [MIM 300075]) gene, which was first identified as the element responsible for Coffin-Lowry syndrome, has also been reported in one family (MRX19) (Trivier et al. 1996). The combined evidence indicates that genes participating in various stages of intracellular signaling, especially members of the GTP-binding family, are likely to play critical roles in the development of cognitive functions, and impairment of their activities may result in mental retardation (Tonilo 2000; Chelly and Mandel 2001).
Recently we detected a constitutional chromosomal aberration in a 16-year-old male patient with Duchenne-type muscular dystrophy (DMD [MIM 300377]), an inv(X) (p21.2q22.2). This patient’s DMD symptoms were accompanied by profound mental retardation, athetosis, and nystagmus. Such profound neurological symptoms are quite rare in DMD; however, loci associated with MRXS in 10 families and with MRX in 8 others have been mapped around Xq22-23. We hypothesized that the breakpoints rearranged in this patient’s inv(X)(p21.2q22.2) might have disrupted not only the DMD gene but also additional, unidentified gene(s) that could account for his neurological symptoms.
The patient was the third child of nonconsanguineous Japanese parents; he was born by spontaneous normal delivery, with a birth weight of 3,000 g. No history of neuromuscular anomalies had been described in his family. As a newborn, he exhibited less-than-normal movement and activity, as well as weakness in suckling. A muscle biopsy at age 4 mo indicated that these features were compatible with muscular dystrophy. His condition was subsequently diagnosed as congenital muscular dystrophy accompanied by mental retardation, congenital nystagmus, and athetosis. He was never able to speak any meaningful words or to sit without support. When he was 15 years old, DMD was diagnosed by use of DNA analysis that indicated deletion of DMD exons 43–60. Subsequent cytogenetic analysis of peripheral lymphocytes revealed karyotypes in the patient and his mother of 46,Y,inv(X)(p21.2q22.2) and 46,X,inv(X)(p21.2q22.2), respectively. By this time (age 16 years), he was severely hypotonic, with extreme weakness of the head and neck muscles, but retained smooth-pursuit eye movements. He presented rigidity of the spine, scoliosis, and increased lordosis as well. His mental retardation was too severe for its actual degree to be assessed. At the age of 18 years, he died of suffocation due to tracheal obstruction by viscid sputum. Before the present study, appropriate informed consent was obtained from the patient’s mother.
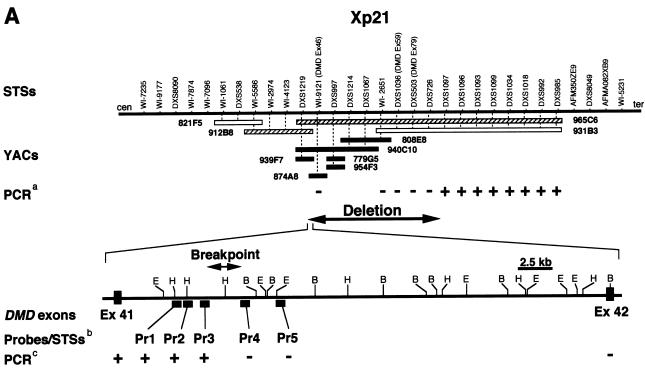
Epstein-Barr virus–transformed lymphoblastoid cell lines (LCLs) were established from peripheral blood lymphocytes of the patient, his mother, and normal volunteers and were employed in the present study. We first performed FISH, as described elsewhere (Ariyama et al. 1995; Saito et al. 1995), to investigate the breakpoints involved in the inv(X). A total of 22 CEPH YACs representing the regions of interest at Xp21-p22 (YACs 931B3, 965C6, 808E8, 874A8, 940C10, 779G5, 954F3, 912B8, 939F7, and 821F5) and Xq21-q22 (YACs 768A10, 756A5, 886H6, 896F12, 874B5, 223D3, 883D1, 768E1, 857G9, 962C9, 821C4, and 940G5), and a BAC clone (RP11-208J19) containing the human PLP gene were used as probes. Among the 22 YACs examined, we observed no specific signals for 6 (808E8, 940C10, 939F7, 779G5, 954F3, and 874A8), and the signals for YACs 912B8 and 965C6 were reduced on inv(X) chromosomes (fig. 1A) from both the patient and his mother. However, strong and specific signals of all eight of those YACs were detected on Xp21 in normal X chromosomes from the mother and from normal volunteers, indicating that submicroscopic regions of DNA involved in the inv(X) were deleted between DXS1219 and WI-2651 and that the distal site of the deletion lay within YAC 965C6. When FISH analysis was performed using a BAC that contained human PLP sequence, an extra-copy signal was detected at Xp21 in addition to a normal copy of PLP on inv(X) in both the patient (fig. 1B) and his mother (data not shown).
Figure 1.
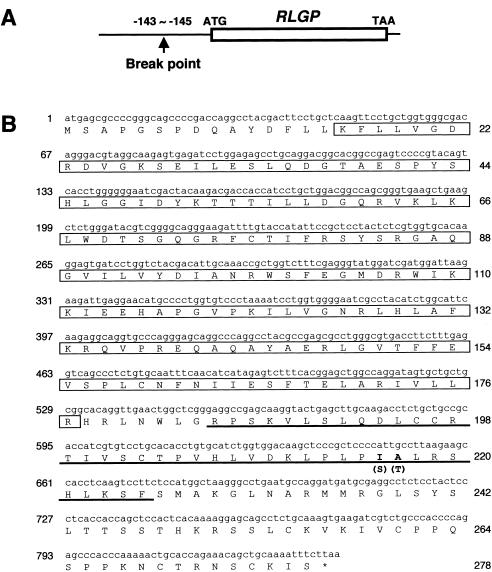
Results of investigations of Xp21. A, Summarized genomic structure at Xp21 around one breakpoint in the patient with inv(X). YACs and STSs between markers WI-7235 and WI-5231 were positioned according to information archived in the Whitehead Institute/MIT Center and Sanger Centre databases. Black, hatched, and white bars indicate YACs that showed no specific signal, reduced signal, or normal signal, respectively. The presence (+) or absence (–) of a PCR product from the STS markers is indicated in the third row of the top panel. The presence (+) or absence (–) of PCR products is indicated in the third row of the bottom panel. E = EcoRI; H = HindIII; B = BglII. B, FISH analysis, using a BAC that contains human PLP sequence. In addition to a normal copy of PLP at Xq22, an extra-copy signal was detected at Xp21 on inv(X) in both the patient and his mother. C, Southern blot analysis, using the Pr3 product as a probe. Lane P, DNA from patient with inv(X)(p21.2q22.2); lane M, patient's mother’s DNA; and lane N, normal DNA. Rearranged fragments, which appeared when the DNA samples of the patient and his mother were digested with HindIII (9.8 kb), HindIII/EcoRI (4.8 kb), or HindIII/BglII (2.6 kb), are indicated by asterisks (*). D, Genomic sequence spanning the inversion breakpoint. Clone c11T3 was isolated from a cosmid library constructed from DNAs of the patient’s LCLs.
PCR amplification was performed with genomic DNA as a template and with primer-pairs designed to amplify the STS markers or the additional seven primer-pairs indicated in figure 1A. (PCR primer sequences and reaction conditions are available on request from the corresponding author.) Amplified products were obtained at loci between DXS1097 and DXS985, as well as in DMD exon 41, whereas no PCR products were generated at loci from DXS726 to WI-9121 or in DMD exon 42 (fig. 1A). These results indicated that the proximal boundary of the Xp21 deletion of inv(X) lay between exons 41 and 42 (intron 41) of the DMD gene and that the telomeric boundary would lie between DXS726 and DXS1097. Additional PCR analyses, using primer pairs Pr1–Pr5 (fig. 1) that were prepared according to the genomic DNA sequence database for this portion of the X chromosome, yielded products only with primers Pr1–Pr3. Therefore, the proximal breakpoint at Xp21 in the inv(X) lies between Pr3 and Pr4 (fig. 1A). We estimated the length of the deletion to be ∼1.3 Mb (Nataraja et al. 1998).
For further analysis of the Xp21 breakpoint, we performed Southern blot analysis. Genomic DNAs extracted from LCLs derived from the patient, his mother, and normal individuals were digested with HindIII, HindIII plus EcoRI, or HindIII plus BglII, as described elsewhere (Imoto et al. 2001). Southern blotting detected rearranged bands only when the PCR product from the Pr3 primer set was used as a probe (fig. 1C), indicating unambiguously that the Xp21 breakpoint lay in the vicinity of the Pr3-amplified sequence. Next, we constructed and performed screening of a cosmid library from genomic DNA derived from the patient, as described elsewhere (Inazawa et al. 1993). Clones containing the DMD (Xp21) breakpoint were screened by colony hybridization, using an [32P]-dCTP–labeled PCR product Pr3 (fig. 1A) as a probe, and we obtained five clones by screening the cosmid library of genomic DNAs derived from the patient with the Pr3 PCR product as the screening probe. When FISH analysis was performed, using these five clones as probes, signals for cos11T3 appeared split into two loci (i.e., Xp21 and Xq22) on the normal chromosome but were seen only at Xp22 on the inv(X) (data not shown). Therefore, cos11T3 was most likely to contain sequence harboring the Xq22 breakpoint of the inv(X). Subsequently, subcloned HindIII/BglII fragments of cos11T3 into pBluescript SK plasmid vector (Stratagene) were sequenced using PRISM 377 ABI autosequencers (Applied Biosystems Japan); sequences were assembled with the Auto-Assembler program (Applied Biosystems) and were characterized using the BLAST program.
By sequencing, we identified two clones (pc1105 and pc1117) that contained parts of DMD intron 41 and also a sequence from cosmid U237H1 (Xq22; GenBank accession number Z95624) that we identified as a novel ras-like GTPase gene and designated “RLGP.” On the basis of the genomic database of the U.S. National Center for Biotechnology Information, physical distance between PLP and RLGP genes is ∼836 kb (GenBank accession number NT_027217). Complete sequence analyses of these two plasmids revealed that the breakpoint at Xq22 lay 143–145 bp upstream from the putative start codon of RLGP (figs. 1D and 2). The region surrounding this breakpoint contained sequence (−250 to −120 bp from the putative start codon) that a computer search (PROSCAN) predicted as a promoter. The BLAST program predicted that the amino acid sequence of RLGP protein would contain a GTP-binding domain similar to RAS small GTPase, as well as an SOCS (suppressors of cytokine signaling) domain. The putative gene product showed 99%, 88%, and 72% identities, respectively, to RAR2A (Xq22.1), RAR (17q25.3), and RAR-like protein (16p13.3), all of which are human homologues of Saccharomyces cerevisiae SEC4 proteins (data not shown).
Figure 2.
Results of investigations of RLGP. A, Genomic structure of the RLGP gene in relation to the breakpoint located in its 5′ region. B, The nucleotide and deduced amino acid sequences of the RLGP-encoding cDNA. The nucleotide sequence is numbered on the left, and the deduced amino acid sequence is numbered on the right. The ras-like GTPase domain is boxed, and an SOCS domain is underlined. The amino acids that are not identical to those in RAR2A are indicated in boldface type, and the amino acids in RAR2A are shown in parentheses.
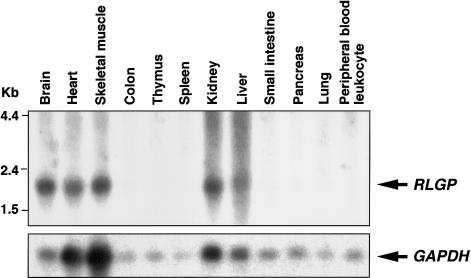
To gain insight into the distribution of RLGP mRNA, we performed northern blots containing RNA from different human tissues (human 12-lane MTN blots) (Clontech). As shown in figure 3, a single RLGP transcript was detected in brain, heart, skeletal muscle, kidney, and liver but was undetectable in seven other tissues, including peripheral blood leukocytes. Adjustment of the signal to that of GAPDH mRNA indicated that the brain had the highest level of expression among the 12 tissues examined. In addition, we confirmed RLGP-specific RT-PCR product, containing a putative coding region and 5′ as well as 3′ UTR of this gene, could be detected in human brain tissue (data not shown). However, because RNA from the patient or his mother was available only from LCLs established from peripheral lymphocytes and because RLGP was not expressed in these materials (data not shown), we were unable to evaluate expression of this gene in the patient.
Figure 3.
Expression of RLGP mRNA in normal human tissues. The same blot was rehybridized with a GAPDH probe, as a control, for RNA loading and transfer.
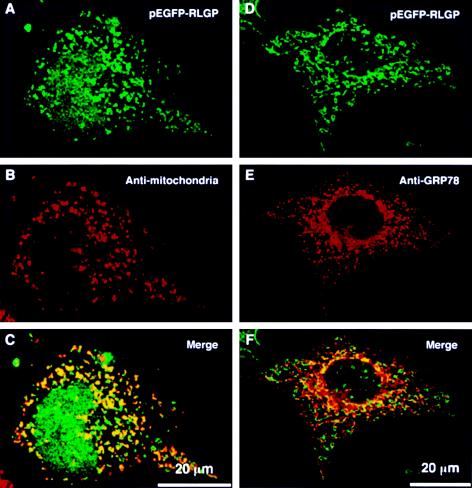
To further determine the subcellular localization of RLGP, we performed indirect fluorescent immunocytochemistry using a pEGFP-RLGP (green) construct (fig. 4). Expression constructs of RLGP were assembled by cloning the coding sequence of this gene in-frame with green fluorescent protein (GFP) in the pEGFP-C1 or N1 vector (Clontech), and transfected into COS7 cells, using Lipofectamine Plus (Invitrogen). Cells fixed with 2% paraformaldehyde were incubated with mouse monoclonal anti–human mitochondria (1:100 dilution; Chemicon International) or anti-Grp78 (1:200 dilution; Stressgen Biotechnologies) antibodies. Binding was detected by rhodamine-conjugated anti–mouse IgG (1:1,000 dilution; Molecular Probes). Fluorescence was observed by means of a confocal laser-scanning microscope (Personal Confocal System, Olympus). The stained RLGP overlapped with antimitochondrial staining but showed no specific accumulation in the endoplasmic-reticulum compartment labeled by anti-Grp78. The staining patterns of both N- and C-terminal–tagged RLGP constructs were similar. Nuclear accumulation of green fluorescence was occasionally observed in cells transfected with pEGFP-RLGP (fig. 4). However, since this color was detected predominantly in the nucleus of cells transfected with pEGFP mock-vector only (data not shown), the apparent nuclear accumulation may have been nonspecific background due to the proteolytic cleavage of the GFP-RLGP fusion protein.
Figure 4.
Subcellular localization of RLGP. COS7 cells were transiently transfected with pEGFP-RLGP (A and D), fixed, and immunocytochemically stained using mouse monoclonal anti-human mitochondria (B) or anti-Grp78 (E) antibodies. Note that the localization of RLGP overlapped with antimitochondrial staining (C) but showed no specific accumulation in the endoplasmic-reticulum compartment labeled by anti-Grp78 (F).
Mild mental retardation is a clinical feature in about one-third of patients with DMD; studies of large cohorts of patients with DMD have revealed a mean IQ ∼1 SD below the normal mean (Worden and Vignos 1962; Dubowitz 1965). The relationship between mental retardation and specific deletions of the DMD gene has been studied, with a significant correlation now recognized between intellectual impairment and macrodeletions of DNA in the distal part of DMD (Bushby 1995; Bardoni et al. 2000; Felisari et al. 2000). However, those studies were conducted only on patients with DMD who had mild mental retardation. Although very severe mental retardation is quite rare as a clinical complication of DMD, a few patients do have severe mental retardation; this group seems to represent a different entity (i.e., mental retardation–complicated DMD) (Bandoh et al. 1987; Bushby et al. 1995). So far as we know, very severe mental retardation has been reported in one patient with a complete deletion of DMD, including its 5′ and 3′ noncoding regions (Alimsardjono et al. 1995), and in two unrelated families in which DMD exon 66 was skipped in transcription (Wibawa et al. 2000). However, the patient presented here merely showed a common type of DMD deletion, involving exons 41–79.
Renier et al. (1981) recognized three types of Pelizaeus-Merzbacher disease (PMD [MIM 312080]), a mental retardation syndrome. The classic type, with onset in infancy and death in late adolescence or young adulthood, is the most common form and is characterized by initial signs of nystagmus and extrapyramidal effects, including rolling head movements or head tremor. The second, “connatal” type shows rapid progression and is fatal in infancy or childhood; the third, a transitional form, is intermediate. Duplication of the PLP gene is detected in 60%–70% of patients with PMD (Sistermans et al. 1998; Inoue et al. 1999), and such patients invariably show the classic form with a milder phenotype (weaker demyelination) than that of patients who carry PLP point mutations (Sistermans et al. 1998; Inoue et al. 1999). Patients with PLP duplications commonly maintain head control and can speak in sentences, and some are able to walk with assistance. FISH analysis in the present patient unambiguously revealed duplication of PLP in the inv(X); some of his clinical features, such as nystagmus, extrapyramidal athetosis, and relatively long course, met the criteria for the classic type of PMD. Moreover, pathological findings in brain sections, stained with Kluver-Barrera and Holzer stains after his death, showed weak demyelination in the cerebral white matter, especially in both temporal lobes (data not shown), an observation that was compatible with the classic type of PMD involving PLP duplication (Sistermans et al. 1998; Inoue et al. 1999). Among our patient’s clinicopathological features, only the degree of mental retardation was discordant with the classic type of PMD. We inferred that additional molecular mechanisms might be implicated in the pathogenesis of his very severe mental retardation.
We successfully isolated a novel gene, RLGP, from the inv(X) breakpoint at Xq22.2 in this patient and showed that the breakpoint disrupted its putative promoter region. So far, only seven genes have been identified with mutations in families with MRX, and three of the genes were isolated from breakpoints of X;autosome translocations (Billuart et al. 1998; Kutsche et al. 2000; Zemni et al. 2000). Of these seven genes, six (GDI1, OPHN1, PAK3, RPS6KA3, IL1RAPL, and TM4SF2) have been shown to participate in various stages of intracellular signaling (Tonilo 2000; Chelly and Mandel 2001). For example, GDI1 and OPHN1 regulate Rab and Rho GTPases involved in vesicle cycling, neurotransmitter release, cell migration, and neurite outgrowth (Chelly and Mandel 2001). PAK3 is a downstream effector of Rho GTPases (Rac and Cdc42), carrying signals to the actin cytoskeleton (Sells et al. 1997) and mediating MAP kinase cascades, including JNK1 and p38 (Bagrodia et al. 1995). These circumstances strongly support our view that interruption of RLGP expression might have affected cognitive development in the subject of the present study.
Immunostaining of RLGP protein in cells, by use of expression constructs tagged with GFP, clearly demonstrated colocalization of RLGP with mitochondria. A high-affinity GDP/GTP-binding site is present on mitochondrial ribosomes, which may mediate protein synthesis by regulating the initiation complex (Denslow et al. 1991). Moreover, several GTP-binding proteins, mainly small G proteins, have been identified at points of contact between the inner and outer mitochondrial membranes (Thomson et al. 1998; Sleer and Hall 2000). RLGP protein may work in a similar manner. Moreover, many diseases resulting from defects in mitochondrial DNA involve neurological symptoms. Nuclear genetic disorders such as Friedreich ataxia (FRDA [MIM 229300]), Wilson disease (WND [MIM 277900]), and autosomal hereditary spastic paraplegia (SPG7 [ MIM 602783]) also involve mitochondrial dysfunction as a direct or indirect cause of their neurological degenerative phenotypes (Chinnery et al. 1999). Taking these observations together, we consider it possible that RLGP may play a critical role in the development or function of the CNS, by transducing signals or transporting molecules across mitochondrial membranes.
In keeping with the marked progression of the Human Genome Project, numerous genes associated with MRX and/or MRXS have been identified to date. Here, we have shown that a Ras-like GTPase gene, RLGP, was disrupted by the inv(X)(Xp21.2;q22.2) in a patient with very severe mental retardation, although the actual function of this gene remains unclear. To understand the role that RLGP might have played in intellectual development of the patient, as well as in his other clinical features, we acknowledge a need to conduct further clinicopathological characterizations of RLGP, including mutational screening of this gene in a large group of patients with XLMR. It should be borne in mind that genes associated with at least six MRXSs and eight MRXs have been mapped on Xq22-23 but remain unidentified.
Acknowledgments
We thank Dr. T. Toda, for valuable advice about the clinical features of the patient, and Dr. A. Iwaki, for technical advice about FISH experiments using BACs as probes. This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan (J.I. and I.I.) and by the Special Program of Integrated Genomics and Advanced Medical Frontier Research (J.I.), and the Research Grant 13B-1 for Nervous and Mental Disorders from the Ministry of Health, Labor and Welfare of Japan (F.S.-O.).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- BLAST, http://www.ncbi.nlm.nih.gov/BLAST/
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for accession numbers Z95624 and NT_027217)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for DMD [MIM 300377], FRDA [MIM 229300], OPHN1 [MIM 300127], PAK3 [MIM 300142], PLP and PMD [MIM 312080], RPS6KA3 [MIM 300075], SPG7 [MIM 602783], and WND [MIM 277900])
- Bioinformatics and Molecular Analysis, http://bimas.dcrt.nih.gov/molbio/proscan/ (for promoter sequence analysis by PROSCAN) [Google Scholar]
- Sanger Centre, http://www.sanger.ac.uk/
- Whitehead Institute/MIT Center database, http://www-genome.wi.mit.edu/ (for position of YACS and STSs)
- XLMR Genes Update Web site, http://xlmr.interfree.it/home.htm
References
- Alimsardjono H, Patria SY, Nishio H, Honke K, Matsuo M (1995) The largest intra-gene deletion of the dystrophin gene identified in a DMD case with full clinical spectrum of dystrophin deficiency. Am J Hum Genet 57:A335 [Google Scholar]
- Ariyama T, Inazawa J, Uemura Y, Kakazu N, Maekawa T, Urase F, Irimajiri K, Horiuchi A, Nakamura Y, Abe T (1995) Clonal origin of Philadelphia chromosome negative cells with trisomy 8 appearing during the course of alpha-interferon therapy for Ph positive chronic myelocytic leukemia. Cancer Genet Cytogenet 81:20–23 [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Derijard B, Davis RJ, Cerione RA (1995) Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem 270:27995–27998 [DOI] [PubMed] [Google Scholar]
- Bandoh T, Kawai H, Adachi K, Ii K (1987) Clinical and pathological studies on intellectual impairment in Duchenne muscular dystrophy, with special reference to patients with severe intellectual impairment [in Japanese]. Rinsyo Shinkeigaku 27:692–701 [PubMed] [Google Scholar]
- Bardoni A, Felisari G, Sironi M, Comi G, Lai M, Robotti M, Bresolin N (2000) Loss of Dp140 regulatory sequences is associated with cognitive impairment in dystrophinopathies. Neuromuscul Disord 10:194–199 [DOI] [PubMed] [Google Scholar]
- Billuart P, Bienvenu T, Ronce N, des Portes V, Vinet MC, Zemni R, Roest Crollius H, Carrie A, Fauchereau F, Cherry M, Briault S, Hamel B, Fryns JP, Beldjord C, Kahn A, Moraine C, Chelly J (1998) Oligophrenin-1 encodes a rho GAP protein involved in X-linked mental retardation. Nature 392:923–926 [DOI] [PubMed] [Google Scholar]
- Bushby KM, Appleton R, Anderson LV, Welch JL, Kelly P, Gardner-Medwin D (1995) Deletion status and intellectual impairment in Duchenne muscular dystrophy. Dev Med Child Neurol 37:260–269 [DOI] [PubMed] [Google Scholar]
- Chelly J, Mandel JL (2001) Monogenic causes of X-linked mental retardation. Nat Rev Genet 2:669–680 [DOI] [PubMed] [Google Scholar]
- Chinnery P, Howell N, Andrews RM, Turnbull DM (1999) Clinical mitochondrial genetics. J Med Genet 36:425–436 [PMC free article] [PubMed] [Google Scholar]
- D'Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanni F, Bienvenu T, Gedeon AK, Oostra B, Wu SK, Tandon A, Valtorta F, Balch WE, Chelly J, Toniolo D (1998) Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat Genet 19:134-139 [DOI] [PubMed] [Google Scholar]
- Denslow ND, Anders JC, O'Brien TW (1991) Bovine mitochondrial ribosomes possess a high-affinity binding site for guanine nucleotides. J Biol Chem 266:9586–9590 [PubMed] [Google Scholar]
- Dubowitz V (1965) Intellectual impairment in muscular dystrophy. Arch Dis Child 40:296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felisari G, Martinelli Boneschi F, Bardoni A, Sironi M, Comi GP, Robotti M, Turconi AC, Lai M, Corrao G, Bresolin N (2000) Loss of Dp140 dystrophin isoform and intellectual impairment in Duchenne dystrophy. Neurology 55:559–564 [DOI] [PubMed] [Google Scholar]
- Herbst DS, Miller JR (1980) Nonspecific X-linked mental retardation II: the frequency in British Columbia. Am J Med Genet 7:461–469 [DOI] [PubMed] [Google Scholar]
- Imoto I, Yang ZQ, Pimkhaokham A, Tsuda H, Shimada Y, Imamura M, Ohki M, Inazawa J (2001) Identification of cIAP1 as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomas. Cancer Res 61:6629–6634 [PubMed] [Google Scholar]
- Inazawa J, Saito H, Ariyama T, Abe T, Nakamura Y (1993) High-resolution cytogenetic mapping of 342 new cosmid markers including 43 RFLP markers on human chromosome 17 by fluorescence in situ hybridization. Genomics 17:153–162 [DOI] [PubMed] [Google Scholar]
- Inoue K, Osaka H, Imaizumi K, Nezu A, Takanashi J, Arii J, Murayama K, Ono J, Kikawa Y, Mito T, Shaffer LG, Lupski JR (1999) PLP duplication causing Pelizaeus-Merzbacher disease: phenotype manifestation and molecular mechanism. Ann Neurol 45:624–632 [PubMed] [Google Scholar]
- Jin H, May M, Tranebjaerg L, Kendall E, Fontan G, Jackson J, Subramony SH, Arena F, Lubs H, Smith S, Stevenson R, Schwartz C, Vetrie D (1996) A novel X-linked gene, DDP, shows mutations in families with deafness (DFN-1), dystonia, mental deficiency and blindness. Nat Genet 14:177–180 [DOI] [PubMed] [Google Scholar]
- Kutsche K, Yntema H, Brandt A, Jantke I, Nothwang HG, Orth U, Boavida MG, David D, Chelly J, Fryns JP, Moraine C, Ropers HH, Hamel BC, van Bokhoven H, Gal A (2000) Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for Rho GTPases, in patients with X-linked mental retardation. Nat Genet 26:247–250 [DOI] [PubMed] [Google Scholar]
- Nataraja R, Jermak C, Trusgnich M, Yoon J, MacMillan S, McCauley B, Brownstein B, Schlessinger D (1998) YAC/STS map of 15 Mb of Xp21.3-p11.3, at 100 kb resolution, with refined comparisons of genetic distances and DMD structure. Gene 215:259–267 [DOI] [PubMed] [Google Scholar]
- Renier WO, Gabreels FJ, Hustinx TW, Jaspar HH, Geelen JA, Van Haelst UJ, Lommen EJ, Ter Haar BG (1981) Connatal Pelizaeus-Merzbacher disease with congenital stridor in two maternal cousins. Acta Neuropathol 54:11–17 [DOI] [PubMed] [Google Scholar]
- Saito F, Sasaki S, Chepelinsky AB, Fushimi K, Marumo F, Ikeuchi T (1995) Human AQP2 and MIP genes, two members of the MIP family, map within chromosome band 12q13 on the basis of two-color FISH. Cytogenet Cell Genet 68:45–48 [DOI] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J (1997) Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol 7:202–210 [DOI] [PubMed] [Google Scholar]
- Sistermans EA, de Coo RFM, De Wijs IJ, Van Oost BA (1998) Duplication of the proteolipid protein gene is the major cause of Pelizaeus-Merzbacher disease. Neurology 50:1749–1754 [DOI] [PubMed] [Google Scholar]
- Sleer LS, Hall PF (2000) Partial characterization of mitochondrial G proteins in adrenal cells. Biochim Biophys Acta 1463:99–106 [DOI] [PubMed] [Google Scholar]
- Stevenson RE (2000) Splitting and lumping in the nosology of XLMR. Am J Med Genet 97: 174–182 [DOI] [PubMed] [Google Scholar]
- Thomson M, Lim G, Hall PF, Kuyznierewicz I (1998) Overlay blot identification of GTP-binding proteins in mitochondria from human placenta. Placenta 19:209–215 [DOI] [PubMed] [Google Scholar]
- Toniolo D (2000) In search of the MRX genes. Am J Med Genet 97:221–227 [DOI] [PubMed] [Google Scholar]
- Trivier E, De Cesare D, Jacquot S, Pannetier S, Zackai E, Young I, Mandel JL, Sassone-Corsi P, Hanauer A (1996) Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature 384:567–570 [DOI] [PubMed] [Google Scholar]
- Wibawa T, Takeshima Y, Mitsuyoshi I, Wada H, Surono A, Nakamura H, Matsuo M (2000) Complete skipping of exon 66 due to novel mutations of the dystrophin gene was identified in two Japanese families of Duchenne muscular dystrophy with severe mental retardation. Brain Dev 22:107–112 [DOI] [PubMed] [Google Scholar]
- Worden DK, Vignos PJ (1962) Intellectual function in childhood progressive muscular dystrophy. Pediatrics 29:968–977 [PubMed] [Google Scholar]
- Zemni R, Bienvenu T, Vinet MC, Sefiani A, Carrie A, Billuart P, McDonell N, et al (2000) A new gene involved in X-linked mental retardation identified by analysis of an X;2 balanced translocation. Nat Genet 24:167–170 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Fischer DJ, Santos MF, Tigyi G, Pasteris NG, Gorski JL, Xu Y (1996) The faciogenital dysplasia gene product FGD1 functions as a Cdc42Hs-specific guanine-nucleotide exchange factor. J Biol Chem 271:33169–33172 [DOI] [PubMed] [Google Scholar]