Abstract
BACKGROUND AND PURPOSE
Heroin, with low affinity for μ-opioid receptors, has been considered to act as a prodrug. In order to study the pharmacokinetics of heroin and its active metabolites after i.v. administration, we gave a bolus injection of heroin to rats and measured the concentration of heroin and its metabolites in blood and brain extracellular fluid (ECF).
EXPERIMENTAL APPROACH
After an i.v. bolus injection of heroin to freely moving Sprague–Dawley rats, the concentrations of heroin and metabolites in blood samples from the vena jugularis and in microdialysis samples from striatal brain ECF were measured by ultraperformance LC-MS/MS.
KEY RESULTS
Heroin levels decreased very fast, both in blood and brain ECF, and could not be detected after 18 and 10 min respectively. 6-Monoacetylmorphine (6-MAM) increased very rapidly, reaching its maximal concentrations after 2.0 and 4.3 min, respectively, and falling thereafter. Morphine increased very slowly, reaching its maximal levels, which were six times lower than the highest 6-MAM concentrations, after 12.6 and 21.3 min, with a very slow decline during the rest of the experiment and only surpassing 6-MAM levels at least 30 min after injection.
CONCLUSIONS AND IMPLICATIONS
After an i.v. heroin injection, 6-MAM was the predominant opioid present shortly after injection and during the first 30 min, not only in the blood but also in rat brain ECF. 6-MAM might therefore mediate most of the effects observed shortly after heroin intake, and this finding questions the general assumption that morphine is the main and most important metabolite of heroin.
Keywords: microdialysis, pharmacokinetic, prodrug, metabolite kinetic, CNS, distribution, opioid, heroin, morphine, acetylmorphine
Introduction
Heroin is considered to be one of the most addicting drugs of abuse (Anthony et al., 1994). It does however have a low-binding affinity (Inturrisi et al., 1983; Gianutsos et al., 1986) and efficacy (Selley et al., 2001) at the μ-opioid receptors (MORs), and is believed to act mainly as a prodrug (Inturrisi et al., 1983; Gianutsos et al., 1986). Heroin is highly lipophilic and has a high apparent permeability through the blood–brain barrier (BBB) (Oldendorf et al., 1972). Thus an effective delivery of active metabolites to the brain has been used as an explanation for the highly addictive properties of heroin, where morphine customarily has been considered to be the most relevant active compound mediating heroin euphoria and reward. However, other metabolites, like 6-monoacetylmorphine (6-MAM) and morphine-6-glucuronide (M6G), also possess pharmacological activity (Way et al., 1960; Inturrisi et al., 1983; Pasternak et al., 1987; Strandberg et al., 2006) and are therefore likely to contribute to the heroin effects. The pharmacological contribution of 6-MAM after acute or chronic administration of heroin has however only to a limited extent been studied.
Andersen et al. (2009) have shown that 6-MAM reached much higher concentrations than heroin or morphine in both blood and brain after s.c. administration of heroin to mice. Moreover, the acute behavioural effects observed after heroin administration were more closely related to brain concentrations of 6-MAM than to heroin and morphine, indicating that 6-MAM was the compound mainly responsible for these effects. Subsequent pharmacokinetic analysis of these data showed that the transfer rate for heroin from blood to brain was much lower than its conversion rate to 6-MAM in blood. These results would imply that heroin was rapidly metabolized to 6-MAM and only a small fraction of the heroin dose was able to reach the brain, while the high 6-MAM concentrations in brain were merely reflecting transfer of 6-MAM formed in blood (Boix et al., 2013). Together, these observations challenge the view that morphine, formed in the brain from rapidly entering heroin, is the compound mainly responsible for the effects of heroin (Oldendorf et al., 1972; National Institute on Drug Abuse, 2005). Accordingly, they also question the generalization of numerous neurobiological studies using morphine, to also account for mechanisms involved in heroin-related effects.
The above-described observations showing that metabolism of heroin to 6-MAM mainly takes place in blood, before reaching the brain (Andersen et al., 2009; Boix et al., 2013), could yet be particularly related to the s.c. administration route used in those studies. During the passage through the s.c. compartment heroin might be subject to metabolism, influencing the disposition of heroin and its metabolites observed in the blood and, therefore, their pharmacokinetics. Furthermore the absorption from a s.c. depot will give a more gradual presentation of drug to the heroin-metabolizing enzymes in blood, making saturation of their catalytic capacity less likely, allowing more 6-MAM to be formed. These factors would however be reduced by an i.v. bolus administration, also representing the most common, and preferred, route of administration in human abusers. Extravascular routes, like s.c. or i.m., do not seem to provide the quick and powerful rush normally associated with i.v. heroin. The high bioavailability and fast distribution of the drug achieved through i.v. administration will enhance the transfer of drug to its main effect site/compartment, in the brain for the case of heroin and its metabolites. In the present study, we tested if the distribution of the active heroin metabolites in blood and brain were similar after i.v. administration compared with the observations seen after s.c. injections in mice, where 6-MAM achieves higher levels than heroin or morphine. Further, as the opioid receptors activated by opioids in the brain are faced towards the extracellular fluid (ECF), the unbound brain ECF concentrations of opioids are more relevant than the concentrations measured in whole brain samples including both extracellular and intracellular fluid (Hammarlund-Udenaes et al., 2008). For this purpose, we administered an i.v. bolus of heroin and measured heroin and the three metabolites [6-MAM, morphine and morphine-3-glucuronide (M3G)] by ultraperformance LC-MS/MS (UPLC-MS/MS) in repeated samples from blood and brain ECF, obtained from striatum by microdialysis, in freely moving rats. To our knowledge this has not been studied previously. The literature related to heroin pharmacokinetics, including experimental animals, is sparse. Knowledge of the distribution of heroin and, especially, its active metabolites over time in blood and brain ECF, the compartment considered relevant for the target receptors involved in the euphorigenic and rewarding effects, is important for a proper understanding of the acute effects of heroin.
Methods
Chemicals and reagents
Heroin hydrochloride, heroin-d3, heroin-d9, 6-MAM, 6-MAM-d3, 6-MAM-d6, morphine-d3, M6G, M3G and M3G-d3 were obtained from Lipomed (Lipomed GmbH, Arlesheim, Switzerland), morphine from NMD (NMD Grossisthandel AS, Oslo, Norway) and morphine-d6 from Toronto Research Chemicals Inc. (Ontario, Canada). Standard compounds were stored according to supplier recommendations. HPLC-grade methanol and acetonitrile were purchased from Labscan Ltd. (POCH SA, Gliwice, Poland), and analytical grade ammonium formate and formic acid from Merck (Whitehouse Station, NJ, USA). All water used was provided by a MilliQ A10 purification system (Merck KGaA, Darmstadt, Germany). Stock and working solutions were prepared as described previously (Gottas et al., 2012).
Animals and conditions
Male Spraque–Dawley rats from Taconic (Borup, Denmark), 7–8-week-old and weighing about 300 g at surgery, were used (n = 14). They were housed in pairs at standard housing conditions (08:00–20:00 h lights on), with free access to food and water. The surgery was performed at least 4 days after arrival. After implantations of catheters and the guide cannula, each rat was placed in a special individual cage to avoid injury of the rat and the implants.
The experimental protocol was approved by the Norwegian National Animal Research Authority and carried out in accordance to Norwegian regulations and international standards.
In vivo experiment
Surgical implantation of the microdialysis guide cannula and the catheters, as well as the microdialysis experimental procedures, have been detailed earlier (Gottas et al., 2012). Briefly, under isoflurane anaesthesia (2.5%), the animals were implanted with a PE-50 catheter fused with silastic tubing in vena jugularis and a PE-50 fused with PE-10 catheter in vena femoralis. A microdialysis brain guide cannula was implanted through a hole in the skull at the following coordinates relative to bregma: anterior + 0.5 mm; lateral ± 3.0 mm; and lowered 4.0 mm ventral relative to bregma, aimed at the striatum (Paxinos and Watson, 1998). At least 2 days after surgery and 18 h before the experiment, a microdialysis probe, with a 4 mm long dialysis membrane, was inserted into the guide cannula (Figure 1). The animal was then coupled to the sampling equipment under light anaesthesia (2.5% isoflurane). The microdialysis probe was perfused with Ringer's solution at 0.2 μL·min−1 and the animal was left for acclimatization overnight. On the following day, the perfusion solution was changed to a Ringer's solution containing deuterated recovery calibrators (heroin-d9, 6-MAM-d3, morphine-d6, M3G-d3) for each compound to be analysed and the flow increased to 2 μL·min−1. The sample collection interval was set to 1 min (2.5 min for one of the animals).
Figure 1.
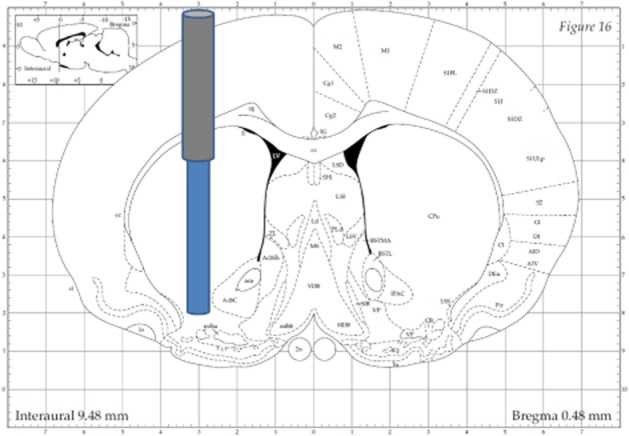
Illustration ofmicrodialysis probe placement in dorsal and ventral striatum. The guide cannula was placed through a hole in the skull at the following coordinates relative to bregma: anterior + 0.5 mm, lateral ± 3.0 mm, and lowered 4.0 mm ventral relative to bregma, microdialysis probe protruding additional 4.0 mm ventral relative to bregma (Paxinos and Watson, 1998).
The rat received an i.v. bolus injection (0.1 mL) of 3 μmol heroin (12.8 mg·mL−1) through the catheter implanted in vena femoralis, followed by flushing with 0.2 mL physiological saline solution. Dialysis samples were collected during a 120 min sampling period. Concurrently, 0.1 mL blood samples were taken through the catheter implanted in vena jugularis around 1, 3, 5, 7, 10, 15, 22, 30, 45, 60 and 120 min after the injection of heroin. The exact time point for each sample was recorded. Blood samples were drawn using a syringe and transferred to microcentrifuge tubes containing 100 μL ammonium format buffer (5 mM, pH 3.1), with 4 mg·mL−1 sodium fluoride and 17.8 IU·mL−1 heparin sodium, handled on ice, and further mixed and immediately frozen in liquid N2 in accordance with a previously established method (Karinen et al., 2009). At the end of the experiment, the rat was killed by an i.v. injection of pentobarbitone administered through the vena jugularis catheter. The brain was immediately removed and dissected for visual control of extensive bleeding or other pathology related to the microdialysis procedure, but no animal had to be excluded on this basis. Some brains were frozen in liquid N2 for later analysis of heroin and metabolites. The injected heroin solution was freshly made at least once a week and had a content of 6-MAM of no more than 1–3% prior to injection.
Chemical analysis
Dialysate sample preparation and analysis were done according to a previously established method (Gottas et al., 2012). In brief, dialysate samples (2 μL) were diluted with internal standard solution (10 μL) present in the vials at collection time and injected into the UPLC system without further sample preparation. The samples were analysed by a Waters Acquity UPLC-MS/MS system (Waters, Milford, MA, USA) equipped with an Acquity HSS T3 column.
Blood and brain tissue samples were prepared for analysis following the method published by Karinen et al. (2009). Briefly, 50 μL internal standard (0.5 μM in water) and 500 μL acetonitrile/methanol (85:15) were added to the collected frozen blood and brain homogenate samples.
The method validation and analysis for the blood and brain tissue samples were performed with a Waters Quattro Premier XE MS/MS using the same instrumental parameters as for dialysate (Gottas et al., 2012), with some modifications. The blood UPLC gradient profile is shown in Table 1. Data were processed with the TargetLynx program (Waters, Milford, MA, USA), using peak area for quantification. Calibration curves with five to seven points were applied for the analytes. The lowest level of quantification (LLOQ) and lowest level of detection (LOD) are presented in Table 2. The within-day and between-day precision and accuracy (Table 3) showed a good precision and accuracy for the analytical method. No endogenous interferences were found in blank rat blood samples.
Table 1.
UPLC gradient profile
| Time (min) | Methanol mobile phase (%) | Ammonium format mobile phase (%) |
|---|---|---|
| 0.00 | 0 | 100 |
| 0.50 | 10 | 90 |
| 2.60 | 50 | 50 |
| 3.00 | 90 | 10 |
| 4.50 | 10 | 90 |
| 5.10 | 0 | 100 |
| 5.20 | 100 | 0 |
Table 2.
Molecular weight, limit of detection and lower limit of quantitation with precision and bias
| LOD | LLOQ | ||||||
|---|---|---|---|---|---|---|---|
| Substance | Molecular weight | nmol·mL−1 | ng·mL−1 | nmol·mL−1 | ng·mL−1 | CV (%) | Bias (%) |
| Heroin | 369.4 | 0.0002 | 0.07 | 0.008 | 3.0 | 14 | 4 |
| 6-MAM | 327.4 | 0.0011 | 0.36 | 0.006 | 2.0 | 10 | −1 |
| Morphine | 285.3 | 0.0009 | 0.26 | 0.006 | 1.7 | 18 | −6 |
| M3G | 461.5 | 0.0005 | 0.23 | 0.02 | 2.2 | 14 | −5 |
Table 3.
Added concentrations, within-day precision and accuracy (bias) and between-day precision and accuracy (bias)
| Added concentration | Within day | Between day | ||||
|---|---|---|---|---|---|---|
| Substance | nmol·mL−1 | ng·mL−1 | CV (%) | Bias (%) | CV (%) | Bias (%) |
| Heroin | ||||||
| QC (high) | 5 | 1851 | 3 | −9 | 3 | −6 |
| QC (medium) | 0.5 | 185 | 4 | −11 | 5 | −5 |
| QC (low) | 0.05 | 18 | 4 | −9 | 5 | −4 |
| 6-MAM | ||||||
| QC (high) | 5 | 1643 | 3 | −1 | 5 | −1 |
| QC (medium) | 0.5 | 164 | 2 | 4 | 5 | 0.5 |
| QC (low) | 0.05 | 16 | 4 | 10 | 5 | 3 |
| Morphine | ||||||
| QC (high) | 5 | 1427 | 5 | −2 | 7 | 0 |
| QC (medium) | 0.5 | 143 | 6 | 14 | 8 | 3 |
| QC (low) | 0.05 | 14 | 6 | 17 | 12 | 8 |
| M3G | ||||||
| QC (high) | 4.97 | 2293 | 4 | −5 | 12 | −6 |
| QC (medium) | 0.5 | 229 | 8 | 0 | 10 | −1 |
| QC (low) | 0.014 | 6 | 11 | 7 | 18 | −2 |
Microdialysis probe recovery
Recovery for each microdialysis probe and the analytes were calculated by retrodialysis using isotope analogues as described by Gottas et al. (2012). This procedure was used for calculating the concentration of unbound analyte (Cu) in brain ECF.
The average recovery values (±SD) for the deuterated recovery calibrators were 28 ± 12% for heroin-d9, 22 ± 9% for 6-MAM-d3, 21 ± 10% for morphine-d6 and 19 ± 9% for M3G-d3 (n = 7, n = 3 for M3G-d3). For samples without recovery estimation, the average recovery was used to calculate Cu.
Data analysis
From a total of 14 animals, and due to technical problems with the sampling equipment, it was possible to collect both blood and brain ECF data from five animals: two animals provided only brain ECF data and three animals only blood data. Both sampling methods failed after various time periods in four animals that could supply only full-tissue (homogenate) brain data.
To compensate for samples taken at different time intervals, and the fact that not all animals gave complete data sets, blood and microdialysis sample data were fitted by a population method using the program Kinetica 5.1 (Thermo Fisher Scientific Inc., Waltham, MA, USA). All results were fitted by an extravascular model, except for heroin in blood collected after i.v. administration, which was fitted by an i.v. bolus model. The number of compartments in the model did not have any noteworthy influence on the results, and the model with the lowest Akaike information criteria was selected in order to assure the best fitting for each analyte (Ludden et al., 1994; Glatting et al., 2007).
The calculated fitted results were further used in a non-compartmental analysis to calculate the area under the concentration–time curve from time zero to last sample time (AUCLast), the estimated maximum concentration (Cmax) and the time to reach Cmax (Tmax) for all the different substances. The t1/2 were calculated for both heroin and 6-MAM in blood and brain ECF after taking fitted log concentration versus time plots into account. Additionally, clearance (Cl) and volume of distribution during the terminal phase (Vd) were calculated based on heroin levels measured in blood. Because virtually all heroin is metabolized to 6-MAM without loss into urine (Rook et al., 2006b), the virtual dose of 6-MAM can be considered similar to the actual heroin dose, allowing the calculation of Cl and Vd for 6-MAM based on blood concentrations as well. The AUC calculations were based on a mixed log linear model implemented in the Kinetica software. For heroin in blood an i.v. bolus model was used, whereas an extravascular model was used for the metabolites in blood and all the compounds in brain ECF. The log-transformed concentration versus time plots were used to assess the distribution (α) and elimination phase. All data below LLOQ were discarded from the data set, except for M3G in dialysate, where LOD was used as limit for further analysis to avoid too few data points for analysis in Kinetica.
Results
Physiological observations
The heroin dose (1.3 mg) chosen in this study was relatively high to ensure measurable concentrations of heroin in the brain ECF compartment. Immediately after injection the animals showed a complete sedation, with severe muscle rigor and reduced respiration rate. Indication of cyanosis, with change in blood colour and thickening of blood, was observed and made blood sampling challenging.
Blood pharmacokinetics
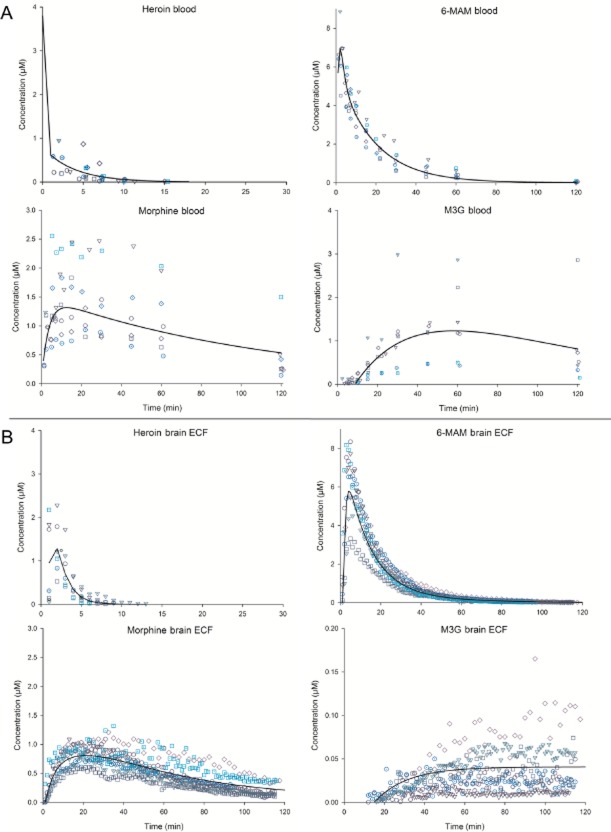
The concentration versus time profiles are shown in Figure 2A, and the calculated pharmacokinetic values are listed in Table 4. The heroin level in blood declined below LLOQ already after about 18 min, with a biphasic concentration versus time curve. The Cmax for heroin was 3.8 μM as calculated by Kinetica. This value must however be considered as extremely uncertain due to few data points within the distribution phase (α phase). The initial α phase (ultra-rapid distribution, between 0 and 1 min) had an apparent t1/2 of at least 0.3 min and the terminal elimination phase (between 2 and 18 min) a t1/2 of 2.7 min. However, the highest measured concentration of heroin from an animal was 0.95 μM, obtained in the first sample taken after 2 min. The corresponding concentration of 6-MAM in the same sample was 8.86 μM, about nine times higher than the heroin concentration. In general, however, the 6-MAM concentrations were about 18 times higher than the heroin concentration in the first blood samples measured. Further, based on the population-fitted results, 6-MAM reached blood concentrations about five times higher than morphine, with a Cmax at 7 μM and Tmax after 2 min. The decline in 6-MAM blood concentration curve was also biphasic, with an initial α phase (between 3 and 5 min) with an apparent t1/2 of 5.8 min, and a terminal elimination phase (between 18 and 120 min) with a t1/2 of 12.8 min. In the α phase, heroin was still present and could therefore influence the concentration curve for 6-MAM, whereas virtually all heroin was cleared from the blood during the terminal elimination phase. The morphine concentrations increased gradually, with a Cmax of 1.3 μM after 12.6 min (Tmax). The blood level of M3G increased even slower than morphine, with a Cmax of 1.2 μM after 58 min (Tmax). The Cl for heroin from blood was estimated to be 979 mL·min−1, and 27 mL·min−1 for 6-MAM. Vd for heroin was calculated to be 3787 mL, and 493 mL for 6-MAM, by Kinetica.
Figure 2.
Blood and brain ECF concentrations of heroin, 6-MAM, morphine and M3G after i.v. administration of 3 μmol (1.3 mg) heroin. Points represent the observed concentrations (the different symbols representing the different animals), lines show the fitted values calculated in Kinetica software. (A) Observed and fitted values for each separate compound in blood (n = 8, M3G n = 7). (B) Observed Cu and fitted values for each separate compound in brain ECF (n = 7, M3G n = 6).
Table 4.
Pharmacokinetic parameters after i.v. bolus injection. (All parameters are based on population-fitted data in Kinetica.)
| Drug | Parameter | Blood | Brain ECF |
|---|---|---|---|
| Heroin | AUCLast (μM·min) | 3.1 | 5.9 |
| Cmax (μM) | 3.8* | 1.5 | |
| Tmax (min) | 0.0 | 1.5 | |
| t1/2 α phase (min) | 0.3 | – | |
| t1/2 elimination phase (min) | 2.7 | 0.9 | |
| Cl (mL·min) | 979 | – | |
| Vd (mL) | 3787 | – | |
| 6-MAM | AUCLast (μM·min) | 112.3 | 102.7 |
| Cmax (μM) | 7.0 | 5.8 | |
| Tmax (min) | 2.0 | 4.3 | |
| t1/2 α phase (min) | 5.8 | 9.6 | |
| t1/2 elimination phase (min) | 12.8 | 23.3 | |
| Cl (mL·min) | 27 | – | |
| Vd (mL) | 493 | – | |
| Morphine | AUCLast (μM·min) | 107.6 | 59.6 |
| Cmax (μM) | 1.3 | 0.8 | |
| Tmax (min) | 12.6 | 21.3 | |
| M3G | AUCLast (μM·min) | 109.3 | 3.6 |
| Cmax (μM) | 1.2 | 0.04 | |
| Tmax (min) | 58.2 | 120** |
Cmax calculated in Kinetica must be considered as uncertain.
Tmax observed used for M3G in brain ECF.
Brain ECF pharmacokinetics
The concentration versus time profiles are shown in Figure 2, panel B, and the calculated pharmacokinetic values are listed in Table 4. The calculated Cmax for heroin was 1.5 μM, with Tmax after 1.5 min. Heroin levels declined below LLOQ after about 10 min, with an apparent t1/2 of 0.9 min. 6-MAM reached concentrations approximately four and seven times higher than heroin and morphine, respectively, with a Cmax of 5.8 μM after 4.3 min (Tmax). The decline in 6-MAM brain ECF concentration curve was biphasic, with an initial α phase (between 6 and 26 min) that showed an apparent t1/2 of 9.6 min, and a following terminal phase (between 80 and 120 min) with a t1/2 of 23.3 min. Morphine levels increased during a longer period and reached Cmax of 0.8 μM after 21.3 min (Tmax). The brain ECF levels of M3G increased even slower than morphine, with an apparent Cmax of 0.04 μM. The M3G level was still increasing towards the end of experiment, after 120 min.
Relation between drug concentrations in blood and brain ECF
Figure 3 shows the fitted results for the concentrations of heroin and metabolites in blood and brain ECF. The concentration of heroin in brain ECF increased until reaching a maximum 1.5 min after the bolus injection, indicating that the absorption into this compartment lasted for at least this period of time. The concentration of 6-MAM in brain ECF reached a Cmax approximately 2.3 min after the Tmax for 6-MAM in blood. After Cmax was reached in brain ECF, the concentration of both heroin and 6-MAM declined in parallel with the respective concentrations in blood, with an initial time lag of a few minutes. The concentration of morphine and M3G in brain ECF reached Cmax approximately 10 min and more than 60 min after the respective Tmax values in the blood.
Figure 3.
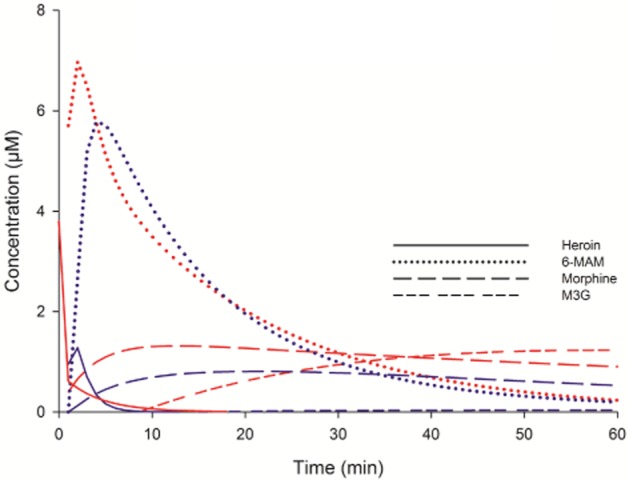
Blood and brain ECF concentrations of heroin, 6-MAM, morphine and M3G after i.v. administration of 3 μmol (1.3 mg) heroin. Lines show the fitted values calculated in Kinetica software. Red lines: fitted blood values time 0–60 min (n = 8, M3G n = 7). Blue lines: fitted brain ECF values from time 0 to 60 min (n = 7, M3G n = 6).
Opioid concentrations in brain tissue
Due to technical problems with the sampling set-up, some rats (n = 5) were killed during the experimental period of 120 min at 2, 35, 70, 71 and 80 min and their brains removed immediately for measurement of drug content. Brains from other animals (n = 5) killed at the end of the experimental period were also analysed. The concentration of heroin in brain tissue from the rat killed after 2 min was 1.25 nmol·g−1, from the rat killed after 35 min, the concentration was 0.018 nmol·g−1, whereas heroin levels below LLOQ were found in the brains collected at later time points. The concentrations of 6-MAM in the brain tissue were 18.36, 1.70, 0.16, 0.06 and 0.07 nmol·g−1 for each of the time points indicated above respectively. In brains collected after 120 min, the mean concentration of 6-MAM was 0.08 nmol·g−1 ±0.03 SEM (n = 5). The concentrations of morphine were 1.16, 1.33, 0.03, 0.77 and 1.34 nmol·g−1 for each respective time point. In brains collected after 120 min the mean concentration was 0.57 nmol·g−1 ±0.02 SEM (n = 5).
Discussion
In the present study we investigated the distribution of heroin and its metabolites in blood and brain after an i.v. bolus injection of heroin to freely moving rats. When concentration measurements started, from 1 min onwards, the levels of heroin were relatively low in blood and brain ECF, and the levels of 6-MAM increased rapidly in both compartments and amply exceeded that of heroin already after 1 min in blood and after 2 min in brain ECF. The results showed that 6-MAM was the predominating compound present in blood and brain during the first 30 min after heroin administration. In brain ECF, the compartment where binding to opioid receptors involved in euphoria and reward takes place (Hammarlund-Udenaes, 2010; Pan and Pasternak, 2011), the maximum 6-MAM concentration was about four and seven times higher compared with heroin and morphine respectively. Moreover, the AUC for 6-MAM in the brain ECF was about twice as high as for morphine, and approximately 17 times higher compared with heroin. The AUC ratios between brain ECF and blood was 1.9, 0.9, 0.6 and 0.03 for heroin, 6-MAM, morphine and M3G, respectively, indicating that transfer of heroin and 6-MAM were mainly mediated by passive diffusion, whereas both morphine and M3G have a restricted transport over the BBB. However, the AUC ratio for heroin was probably deviating from its true value and was more likely closer to 1, as the extrapolation to Cmax for heroin at time zero was difficult to perform due to the rapid metabolism and distribution. The true concentration at time zero was more likely around 145 μM, calculated by dividing the administrated dose with blood volume of 6.9 mL/100 g rat (Probst et al., 2006), resulting in an AUC in blood of about 8 (μM·min) and a brain ECF : blood concentration ratio of about 0.7. Our present results were in line with previous findings from our laboratory showing that 6-MAM reached much higher Cmax and AUCs than heroin and morphine after s.c. injections of heroin in mice (Andersen et al., 2009), as measured in both whole blood and brain tissue samples. Thus, the high level of 6-MAM compared with heroin and morphine found shortly after heroin administration in the previous study (Andersen et al., 2009) was not a consequence of the s.c. route of administration, as similar relationships between 6-MAM and heroin or morphine also were observed after i.v. bolus administration in the present study. In particular it should be noted that even though the dose used in this study was relatively high, there was no indication of saturation of the metabolic capacity in blood, as high concentration of 6-MAM was obtained rapidly.
MORs mediate the rewarding and reinforcing effects of heroin and morphine (Johnson and North, 1992) (for reviews see Koob and Bloom, 1988; Feltenstein and See, 2008). Brain striatum, which has a high abundance of κ-opioid receptors and MORs (Kling et al., 2000), plays an important role regarding the effects seen after use of drugs of abuse (Everitt and Robbins, 2005). Further, in order to measure opiate concentrations in brain ECF for 1 min sampling periods and to get adequate recovery measurements, it was essential to use a 4 mm microdialysis membrane. The rat striatum allows the use of such long dialysis probe in a relative homogenous area. Thus, in this study, opiate concentrations were sampled from both ventral and dorsal striatum. 6-MAM binds to MORs with an affinity similar to morphine (Inturrisi et al., 1983), but with a greater efficacy (Selley et al., 2001). Because the binding to the MORs takes place on the extracellular surface of the neurons, our measurements of 6-MAM in striatal brain ECF indicated that 6-MAM should be important for the acute effects seen shortly after heroin intake, as is observed in the study of Andersen et al. (2009). The higher and faster increase of 6-MAM compared with morphine in brain ECF further substantiates the potentially important role of 6-MAM in mediating the acute effects seen after i.v. administration of heroin, as the rate of increase in concentration has been shown to be important for determining the potency of a drug of abuse (Volkow et al., 2004; Samaha and Robinson, 2005). Whether this applies to all effects observed after heroin administration, for example, analgesia is however open for discussion. There are, for example, evidence for several MOR subtypes with selective binding affinities for opioid agonists present in varying concentrations throughout different brain areas (Bare et al., 1994; Zimprich et al., 1995; Pan et al., 1999; 2000; 2001; 2009; Abbadie et al., 2000; Xu et al., 2009). These receptor subtypes mediate different downstream signal pathways, resulting in different analgesic and behaviour effects (Rossi et al., 1995a,b; 1997; Pan et al., 1999; Schuller et al., 1999) (for review see Pasternak, 2010).
As can be observed in Figure 3, heroin entered the brain ECF quickly and attained a Cmax already 1.5 min after the i.v. administration. At this point in time, the brain ECF 6-MAM concentration was already of the same order of magnitude and continued to rise thereafter, reaching the Cmax 4.3 min after the heroin injection when almost no heroin was found in the brain ECF. Thus, the brain ECF 6-MAM concentrations appeared to reflect the 6-MAM concentrations in the blood, both with respect to time course and concentrations, and not the brain ECF concentrations of heroin. The rats became rigorous and got respiratory depression immediately after heroin administration, when both heroin and 6-MAM levels were present in blood and brain ECF, but such observations were not systematically protocolled during the experiment, because this study focused mainly on pharmacokinetics. Even though morphine was present shortly after heroin injection, its levels in blood and brain were much lower than for heroin and 6-MAM in this initial stage, and the contribution of morphine to the observed effects was probably limited. This is supported by ongoing studies, where equimolar doses of morphine do not result in rigor and only mild respiratory depression and sedation are observed.
Our results revealed a rapid elimination of heroin in blood, with an ultra-rapid distribution phase with a t1/2 of at least 0.3 min and a terminal elimination phase with t1/2 of 2.7 min. To our knowledge, there are no previous reports on in vivo heroin t1/2 in rats, but these findings are in accordance with earlier findings of t1/2 of 2.5 min in mice (Way et al., 1960) and 1.3–7.8 min in humans (Rook et al., 2006a). A traditional explanation for the fast disappearance of heroin from blood has been its putative fast transfer to the brain, in addition to its rapid hydrolysis (Oldendorf et al., 1972; Umans and Inturrisi, 1982). The distribution of drugs from the blood stream across the BBB to the brain depends largely on their lipophilicity, the existence of active transport systems and the extent of protein binding. The partition coefficients in octanol/water have been calculated to be 0.85, 0.61, −0.07 and −1.12, for heroin, 6-MAM, morphine and M3G, respectively (Avdeef et al., 1996), indicating a high lipophilicity for heroin and 6-MAM. It is well known that highly lipophilic drugs easily pass the BBB by passive diffusion and are redistributed to the brain (Oldendorf et al., 1972; Umans and Inturrisi, 1981). Therefore, a fast transfer of heroin to the brain based on its lipophilicity could explain in part the low concentration of heroin in the blood, but would also imply that heroin should rapidly reach a relative high initial concentration in brain ECF. The Cmax for heroin in brain ECF was however relatively low compared with 6-MAM, and the same appeared to AUC for heroin compared with AUC for 6-MAM. The relatively low concentration of heroin in brain ECF could however have been due to heroin distributing more rapidly than 6-MAM to other brain tissue compartments than ECF. It has been shown that drug concentrations measured in ECF may not correlate with the concentrations measured in total brain homogenate (Hammarlund-Udenaes, 2010). In the present study, heroin concentration in brain tissue from a rat killed after 2 min was of 1.29 nmol·g−1 compared with 18.90 nmol·g−1 for 6-MAM, indicating that heroin did not have a higher distribution to brain tissue than 6-MAM. A similar relationship between the concentrations of heroin and 6-MAM was also seen for the rat killed after 35 min. Further, in vitro studies in mice (Boix et al., 2013) showed a much slower metabolism of heroin to 6-MAM in brain tissue compared with blood, observations that also corroborate with ongoing in vitro experiments in our laboratory in rats (to be published). We found a very large Vd for heroin compared with 6-MAM based on blood measurements. The present study did not investigate to which organs heroin distributed, but fat tissue might be a likely candidate.
In summary, the present study of i.v. bolus administration of heroin to rats indicated a rapid metabolism of heroin before it reached the brain, suggesting that 6-MAM was the main substance transported over the BBB shortly after i.v. injection of heroin. These results extended previous observations in our laboratory, in experiments where heroin was given s.c. (Andersen et al., 2009; Boix et al., 2013), showing that 6-MAM is the dominating compound in blood and brain also after rapid i.v. injection. We were further able to show that this also applied to 6-MAM concentrations in brain ECF, the relevant compartment where opioids interact with their receptors to attain their main effects. This rapid increase of 6-MAM concentration in the striatal brain area can explain the differences in relation to the acute effects and the drug addiction potential of heroin as compared with morphine. These findings are important as results from studies with morphine are frequently generalized to heroin. 6-MAM was the predominant substance present in brain ECF after heroin administration during the first 30 min and is likely to be responsible for the effects of heroin registered during this period. 6-MAM also reached concentrations several fold higher than those ever reached by morphine. This further emphasizes the importance of 6-MAM as the main metabolite of heroin rather than/in addition to morphine, and calls for including 6-MAM in future studies. Our results might also indicate new possibilities to manipulate acute heroin effects by interfering with the levels of the metabolite, 6-MAM, in blood, for example, by binding with antibodies, where some interesting studies have been performed the recent years (Stowe et al., 2011; Pravetoni et al., 2012; Schlosburg et al., 2013) (for reviews see Anton et al., 2009; Kinsey et al., 2009).
Acknowledgments
We would like to thank Bjørg S. Pettersen for her assistance in sample preparation and chemical analysis. This study has been partly supported by the grant 196621/V50 from the Norwegian Research Council.
Glossary
- 6-MAM
6-monoacetylmorphine
- AUCLast
area under the concentration–time curve from time zero to last sample time
- Cmax
maximum concentration;
- BBB
blood–brain barrier
- Cl
clearance
- Cu
concentration of unbound analyte in brain ECF
- ECF
extracellular fluid
- LLOQ
lowest level of quantification
- LOD
lowest level of detection
- M3G
morphine-3-glucuronide
- M6G
morphine-6-glucuronide
- μM
concentration (μmol·L); μmol, molar units
- Tmax
time to reach Cmax
- UPLC-MS/MS
ultraperformance LC-MS/MS
- Vd
volume of distribution during the terminal phase
Conflict of interest
The authors state no conflict of interest.
References
- Abbadie C, Pan YX, Pasternak GW. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol. 2000;419:244–256. doi: 10.1002/(sici)1096-9861(20000403)419:2<244::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Andersen JM, Ripel A, Boix F, Normann PT, Morland J. Increased locomotor activity induced by heroin in mice: pharmacokinetic demonstration of heroin acting as a pro-drug for the mediator, 6-monoacetylmorphine, in vivo. J Pharmacol Exp Ther. 2009;331:153–161. doi: 10.1124/jpet.109.152462. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Anton B, Salazar A, Flores A, Matus M, Marin R, Hernandez JA, et al. Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccin. 2009;5:214–229. doi: 10.4161/hv.5.4.7556. [DOI] [PubMed] [Google Scholar]
- Avdeef A, Barrett DA, Shaw PN, Knaggs RD, Davis SS. Octanol-, chloroform-, and propylene glycol dipelargonat-water partitioning of morphine-6-glucuronide and other related opiates. J Med Chem. 1996;39:4377–4381. doi: 10.1021/jm960073m. [DOI] [PubMed] [Google Scholar]
- Bare LA, Mansson E, Yang DM. Expression of 2 variants of the human mu- opioid receptor messenger-Rna in Sk-N-Sh cells and human brain. FEBS Lett. 1994;354:213–216. doi: 10.1016/0014-5793(94)01129-x. [DOI] [PubMed] [Google Scholar]
- Boix F, Andersen JM, Mørland J. Pharmacokinetic modeling of subcutaneous heroin and its metabolites in blood and brain of mice. Addict Biol. 2013;18:1–7. doi: 10.1111/j.1369-1600.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianutsos G, Cohen SD, Carlson G, Heyman R, Salva P, Morrow G, et al. Alteration of in vivo and in vitro effects of heroin by esterase inhibition. Toxicol Appl Pharmacol. 1986;82:14–18. doi: 10.1016/0041-008x(86)90432-1. [DOI] [PubMed] [Google Scholar]
- Glatting G, Kletting P, Reske SN, Hohl K, Ring C. Choosing the optimal fit function: comparison of the Akaike information criterion and the F-test. Med Phys. 2007;34:4285–4292. doi: 10.1118/1.2794176. [DOI] [PubMed] [Google Scholar]
- Gottas A, Oiestad EL, Boix F, Ripel A, Thaulow CH, Pettersen BS, et al. Simultaneous measurement of heroin and its metabolites in brain extracellular fluid by microdialysis and ultra performance liquid chromatography tandem mass spectrometry. J Pharmacol Toxicol Methods. 2012;66:14–21. doi: 10.1016/j.vascn.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Hammarlund-Udenaes M. Active-site concentrations of chemicals – are they a better predictor of effect than plasma/organ/tissue concentrations? Basic Clin Pharmacol Toxicol. 2010;106:215–220. doi: 10.1111/j.1742-7843.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- Hammarlund-Udenaes M, Friden M, Syvanen S, Gupta A. On the rate and extent of drug delivery to the brain. Pharm Res. 2008;25:1737–1750. doi: 10.1007/s11095-007-9502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci. 1983;33(Suppl. 1):773–776. doi: 10.1016/0024-3205(83)90616-1. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karinen R, Andersen JM, Ripel A, Hasvold I, Hopen AB, Morland J, et al. Determination of heroin and its main metabolites in small sample volumes of whole blood and brain tissue by reversed-phase liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2009;33:345–350. doi: 10.1093/jat/33.7.345. [DOI] [PubMed] [Google Scholar]
- Kinsey BM, Jackson DC, Orson FM. Anti-drug vaccines to treat substance abuse. Immunol Cell Biol. 2009;87:309–314. doi: 10.1038/icb.2009.17. [DOI] [PubMed] [Google Scholar]
- Kling MA, Carson RE, Borg L, Zametkin A, Matochik JA, Schluger J, et al. Opioid receptor imaging with positron emission tomography and [18F]cyclofoxy in long-term, methadone-treated former heroin addicts. J Pharmacol Exp Ther. 2000;295:1070–1076. [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug-dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike information criterion, the Schwarz criterion and the F-test as guides to model selection. J Pharmacokinet Biopharm. 1994;22:431–445. doi: 10.1007/BF02353864. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. 2005. Heroin abuse and addiction. National Institutes of Health. Available at: http://www.drugabuse.gov/publications/research-reports/heroin-abuse-addiction (accessed 7/28/2013)
- Oldendorf WH, Hyman S, Braun L, Oldendorf SZ. Blood-brain barrier: penetration of morphine, codeine, heroin, and methadone after carotid injection. Science. 1972;178:984–986. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- Pan YX, Pasternak GW. Molecular biology of mu opioid receptors. In: Pasternak GW, editor. The Opiate Receptors. 2nd edn. New York: Springer; 2011. pp. 121–160. [Google Scholar]
- Pan YX, Xu J, Bolan E, Abbadie C, Chang A, Zuckerman A, et al. Identification and characterization of three new alternatively spliced mu-opioid receptor isoforms. Mol Pharmacol. 1999;56:396–403. doi: 10.1124/mol.56.2.396. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Chang A, Mahurter L, Rossi G, et al. Isolation and expression of a novel alternatively spliced mu opioid receptor isoform, MOR-1F. FEBS Lett. 2000;466:337–340. doi: 10.1016/s0014-5793(00)01095-4. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu A, Mahurter L, Bolan E, Xu MM, Pasternak GW. Generation of the mu opioid receptor (MOR-1) protein by three new splice variants of the Oprm gene. Proc Natl Acad Sci U S A. 2001;98:14084–14089. doi: 10.1073/pnas.241296098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YX, Xu J, Xu MM, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci U S A. 2009;106:4917–4922. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW. Molecular insights into mu opioid pharmacology from the clinic to the bench. Clin J Pain. 2010;26:S3–S9. doi: 10.1097/AJP.0b013e3181c49d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW, Bodnar RJ, Clark JA, Inturrisi CE. Morphine-6-glucuronide, a potent mu agonist. Life Sci. 1987;41:2845–2849. doi: 10.1016/0024-3205(87)90431-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Pravetoni M, Raleigh MD, Le Naour M, Tucker AM, Harmon TM, Jones JM, et al. Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine. 2012;30:4617–4624. doi: 10.1016/j.vaccine.2012.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst RJ, Lim JM, Bird DN, Pole GL, Sato AK, Claybaugh JR. Gender differences in the blood volume of conscious Sprague-Dawley rats. J Am Assoc Lab Anim Sci. 2006;45:49–52. [PMC free article] [PubMed] [Google Scholar]
- Rook EJ, Huitema AD, van den Brink W, Van Ree JM, Beijnen JH. Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: review of the literature. Curr Clin Pharmacol. 2006a;1:109–118. doi: 10.2174/157488406775268219. [DOI] [PubMed] [Google Scholar]
- Rook EJ, Van Ree JM, van den Brink W, Hillebrand MJX, Huitema ADR, Hendriks VM, et al. Pharmacokinetics and pharmacodynamics of high doses of pharmaceutically prepared heroin, by intravenous or by inhalation route in opioid-dependent patients. Basic Clin Pharmacol Toxicol. 2006b;98:86–96. doi: 10.1111/j.1742-7843.2006.pto_233.x. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pan YX, Brown GP, Pasternak GW. Antisense mapping the Mor-1 opioid receptor – evidence for alternative splicing and a novel morphine-6-beta-glucuronide receptor. FEBS Lett. 1995a;369:192–196. doi: 10.1016/0014-5793(95)00757-z. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Standifer KM, Pasternak GW. Differential blockade of morphine and morphine-6-beta-glucuronide analgesia by antisense oligodeoxynucleotides directed against Mor-1 and G-protein alpha-subunits in rats. Neurosci Lett. 1995b;198:99–102. doi: 10.1016/0304-3940(95)11977-5. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Leventhal L, Pan YX, Cole J, Su W, Bodnar RJ, et al. Antisense mapping of MOR-1 in rats: distinguishing between morphine and morphine-6 beta-glucuronide antinociception. J Pharmacol Exp Ther. 1997;281:109–114. [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AA, et al. Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci U S A. 2013;110:9036–9041. doi: 10.1073/pnas.1219159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, et al. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- Selley DE, Cao CC, Sexton T, Schwegel JA, Martin TJ, Childers SR. [mu] Opioid receptor-mediated G-protein activation by heroin metabolites: evidence for greater efficacy of 6-monoacetylmorphine compared with morphine. Biochem Pharmacol. 2001;62:447–455. doi: 10.1016/s0006-2952(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Stowe GN, Schlosburg JE, Vendruscolo LF, Edwards S, Misra KK, Schulteis G, et al. Developing a vaccine against multiple psychoactive targets: a case study of heroin. CNS Neurol Disord Drug Targets. 2011;10:865–875. doi: 10.2174/187152711799219316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg JJ, Kugelberg FC, Alkass K, Gustavsson A, Zahlsen K, Spigset O, et al. Toxicological analysis in rats subjected to heroin and morphine overdose. Toxicol Lett. 2006;166:11–18. doi: 10.1016/j.toxlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Umans JG, Inturrisi CE. Pharmacodynamics of subcutaneously administered diacetylmorphine, 6-acetylmorphine and morphine in mice. J Pharmacol Exp Ther. 1981;218:409–415. [PubMed] [Google Scholar]
- Umans JG, Inturrisi CE. Heroin: analgesia, toxicity and disposition in the mouse. Eur J Pharmacol. 1982;85:317–323. doi: 10.1016/0014-2999(82)90218-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Way EL, Kemp JW, Young JM, Grassetti DR. The pharmacologic effects of heroin in relationship to its rate of biotransformation. J Pharmacol Exp Ther. 1960;129:144–154. [PubMed] [Google Scholar]
- Xu J, Xu MM, Hurd YL, Pasternak GW, Pan YX. Isolation and characterization of new exon 11-associated N-terminal splice variants of the human mu opioid receptor gene. J Neurochem. 2009;108:962–972. doi: 10.1111/j.1471-4159.2008.05833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Simon T, Hollt V. Cloning and expression of an isoform of the rat mu-opioid receptor (Rmor1B) which differs in agonist-induced desensitization from Rmor1. FEBS Lett. 1995;359:142–146. doi: 10.1016/0014-5793(95)00028-8. [DOI] [PubMed] [Google Scholar]