Abstract
BACKGROUND AND PURPOSE
Some existing computational methods are used to infer protein targets of small molecules and can therefore be used to find new targets for existing drugs, with the goals of re-directing the molecule towards a different therapeutic purpose or explaining off-target effects due to multiple targeting. Inherent limitations, however, arise from the fact that chemical analogy is calculated on the basis of common frameworks or scaffolds and also because target information is neglected. The method we present addresses these issues by taking into account 3D information from both the ligand and the target.
EXPERIMENTAL APPROACH
ElectroShape is an established method for ultra-fast comparison of the shapes and charge distributions of ligands that is validated here for prediction of on-target activities, off-target profiles and adverse effects of drugs and drug-like molecules taken from the DrugBank database.
KEY RESULTS
The method is shown to predict polypharmacology profiles and relate targets from two complementary viewpoints (ligand- and target-based networks).
CONCLUSIONS AND IMPLICATIONS
The open-access web tool presented here (http://ub.cbm.uam.es/chemogenomics/) allows interactive navigation in a unified ‘pharmacological space’ from the viewpoints of both ligands and targets. It also enables prediction of pharmacological profiles, including likely side effects, for new compounds. We hope this web interface will help many pharmacologists to become aware of this new paradigm (up to now mostly used in the realm of the so-called ‘chemical biology’) and encourage its use with a view to revealing ‘hidden’ relationships between new and existing compounds and pharmacologically relevant targets.
Keywords: adverse drug reactions, chemical fingerprints, drug targets, polypharmacology, side effects
Introduction
The famous ‘magic bullet’ term coined by Paul Ehrlich more than one hundred years ago in the field of chemotherapy (Ehrlich, 1907; Witkop, 1999) paved the way to the classical one-compound–one-target paradigm that has largely dominated drug discovery for the last 25 years or so. This reductionist concept has led to a limited appraisal of the causes underlying the side effects of commercial drugs, derived from modulation of secondary targets that can nevertheless play a fundamental role in explaining pharmacological profiles. This is particularly true for drugs acting on the CNS, which can bind to many different receptors (‘magic shotgun’; Roth et al., 2004), and for some multi-kinase inhibitors in oncology (Knight et al., 2010). This realization suggests that a more direct approach to polypharmacology should be taken in modern drug discovery from the very early stages of screening and lead identification. A multi-target−multi-compound approach would provide a much more accurate description of the underlying pharmacology but, given the large size of both chemical and biological spaces, it is also harder to understand, hence the need for tailor-made computer programs that can handle and relate the enormous, and still increasing, amounts of bioactivity data available for both compounds and targets.
Network analysis (Hopkins, 2008; Berger and Iyengar, 2009) in systems pharmacology (Van Der Greef and McBurney, 2005) has recently emerged and promises to revolutionize the field of drug discovery. Polypharmacology, drug repurposing, target fishing and adverse effect prediction are some of the major applications made possible by this paradigm shift, which contrasts with the traditional one-ligand–one-target approach that is still in use in most high-throughput experimental and virtual screening campaigns nowadays (Ripphausen et al., 2010). Traditionally, in silico target-fishing methods have been related to reverse docking (Cai et al., 2006) using one or several compounds against multiple putative targets (Simon et al., 2012). In recent years, however, ligand-based methods exploiting either fingerprints containing two- (2D) and three-dimensional (3D) chemical information or 3D shape descriptors (Bender et al., 2007; Keiser et al., 2007; Vidal and Mestres, 2010; Besnard et al., 2012) have been employed to predict activity profiles and target–target relationships. Superpositional methods that either compare the shapes of two molecules by analytically optimizing their volume intersection (Grant et al., 1996), as implemented in the program ROCS (Rapid Overlay of Chemical Structures, OpenEye Scientific Software, 2011; Rush et al., 2005) or use a surface-based morphological similarity function while minimizing the overall molecular volume of the aligned structures, such as Surflex-Sim (Jain, 2000), have been shown to perform well in the task of predicting off-target activities of ligands (Ballester et al., 2009; Yera et al., 2011). These approaches, although successful, rely on direct pairwise comparisons and this can reduce global performance when database size grows above tens of millions of molecules (Wang et al., 2009).
ElectroShape is a non-superpositional method for ultra-fast comparison of ligands that expands the capabilities and improves the performance of the Ultra Shape Recognition methodology (Ballester and Richards, 2007) by incorporating the molecular charge distribution (Armstrong et al., 2010). In brief, ElectroShape uses three spatial dimensions and adds partial charge as a fourth dimension to capture electrostatic information in the form of 15 descriptors that account for the first, second and third moments of the distributions of distances from five distinct points of the molecule (centroids) in a four-dimensional (4D) space. Molecular similarity is then calculated as the Manhattan distance between ElectroShape descriptors belonging to two different molecules. The following facts are key to increasing the speed of calculation and improving performance: (i) the descriptors are very small and can be pre-calculated and stored for each compound from ensembles of low-energy conformers, and (ii) the similarity calculation is non-superpositional and requires only a few mathematical operations. The value of Ultra Shape Recognition in ligand-based virtual screening (LBVS) has already been recognized (Ballester et al., 2010).
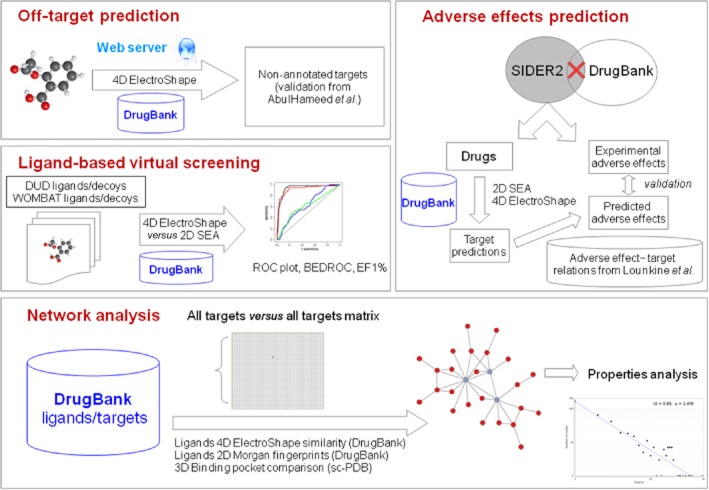
In the following, we show how the ElectroShape method has been validated for off-target prediction (‘target fishing’), LBVS and chemogenomic network analysis (Figure 1). The results obtained have been compared, firstly, to those produced by other well-known 2D techniques that make use of ligand and target annotations from bioactivity databases, and then to structural information from a database of ligand-binding sites in proteins. Implementation on an unsophisticated web server is also presented that can enable pharmacologists and other interested researchers to predict possible adverse effects and secondary targets for a given drug and to explore pharmacological space from the viewpoints of both ligand and target simultaneously.
Figure 1.
Scheme depicting the workflow presented in this article (see main text for details).
Methods
Chemogenomic datasets
To explore the potential use of the ElectroShape approach (Armstrong et al., 2010) for target fishing and prediction of adverse effects, and to compare it with existing 2D methods (Hert et al., 2008), we first built a chemogenomic database using information available from DrugBank (Knox et al., 2011). To this end, target and drug sets were downloaded from http://www.drugbank.ca/ and imported locally into a MySQL relational database. Molecules were then parsed using RDKit (Landrum, 2011) to generate canonical simplified molecular-input line-entry specification (SMILES) strings (Weininger, 1988). Drug–target associations were also imported from DrugBank using Python scripts (http://www.python.org/).
For the 2D analysis, Morgan fingerprints, which are roughly equivalent to the Extended-Connectivity Fingerprints (ECFP4) commonly used in other target-fishing applications (Hert et al., 2008; Rognan and Meslamani, 2011), were generated using RDKit with a radius of two bonds and 2048 bits and inserted in the database. A simple in-house chemistry cartridge was used to allow searching within the database (Cabrera et al., 2011).
To perform the 4D ElectroShape analysis (Cartesian coordinates and charges), the SMILES string representations of the drugs were converted into 3D structures using CORINA (Sadowski et al., 1994; 2003) and point charges were assigned by OpenBabel making use of the Merck Molecular Force Field (MMFF94; Halgren, 1996). Then, a conformational analysis using ALFA (Cabrera et al., 2011), a rule-based conformer generator similar to OMEGA (Hawkins et al., 2010), was undertaken setting the maximum number of conformers to 200 and activating the maximum dissimilarity option to generate the most diverse ensemble possible. By selecting this number we ensure that most of the conformational space is covered for each ligand and avoid the risk of losing alternative similarity matches, a possibility that is almost a certainty when just a single low-energy conformation is taken as a representative 3D structure of ligands with a high number of rotatable bonds. Finally, the molecules were converted to Protein Data Bank (PDB) format (Berman et al., 2007) using OpenBabel (O'Boyle et al., 2011) and 4D descriptors were generated by ElectroShape (Armstrong et al., 2010) for each molecular conformer and inserted into the database to allow simplified queries within chemical space using the cartridge.
To explore the similarity between targets in biological space, we used sc-PDB (Meslamani et al., 2011), an annotated database that contains 3D coordinates for bound ligands and ligand-binding sites, as extracted from the PDB. The sc-PDB complexes were filtered so that only those with a direct match in the DrugBank set were kept.
Similarity calculation and drug–drug and target–target matrices
To calculate target similarity from 2D ligand descriptors, we used the Similarity Ensemble Approach (SEA; Keiser et al., 2007) and Morgan fingerprints (Landrum, 2011) of all molecules, which are annotated to have an effect on every DrugBank target. To this end, an all-drug versus all-drug matrix was built by comparing the fingerprints using the Tanimoto coefficient (Rogers and Tanimoto, 1960). Then, the scores of all the drugs associated with a given target were summed and a final Z-score for each target pair was computed. To obtain the Z-score for the similarity between target A and target B, it is necessary to calculate the expected random average (μ) and standard deviation (σ) of the total score of any two ensembles of n × m drugs (n being the number of drugs that bind to target A and m the number of drugs that bind to target B). Drugs present in DrugBank were randomly grouped in several ensembles ranging in size from 1 to 50 molecules (which is the common range of drugs per target found in DrugBank) and the total score was computed. Then, these scores were fitted to a power law equation and used to calculate the expected values for random targets and the corresponding Z-scores:
In the case of ElectroShape's 4D ligand similarity, we followed an analogous method. Similarities between molecules were computed by calculating the Manhattan distance between each pair of conformer's descriptors for both molecules and the maximum value was kept as the final score between the two ligands. Individual scores between each pair of molecules were summed and the Z-score was calculated using the same procedure as for 2D comparisons: generation of random sets of different sizes followed by calculation of the best power law fit for both average and standard deviation of the total sum score.
Finally, for ligand-binding site similarity, we first used the protein structure alignment algorithm TM-align (Zhang and Skolnick, 2005) to align the binding sites of the targets present in both DrugBank and sc-PDB and then a template modelling score (TM-score) was extracted as a normalized measure of the topological similarity between any two target binding sites.
Web server implementation
The ElectroShape Polypharmacology web server was implemented in Python using the Django Web framework (https://www.djangoproject.com/). It is based on a MySQL database extended with a cartridge previously developed by the authors (Cabrera et al., 2011). The user only has to paste a SMILES string into the search box and then select a method for target fishing. If the traditional 2D SEA method is chosen, the SMILES string is used to generate Morgan fingerprints and perform the query to the database. If an ElectroShape-based method is requested, the server (i) converts this string into a 3D structure, (ii) explores the conformational space using the rule-based program ALFA (Cabrera et al., 2011), (iii) assigns MMFF94 atom point charges using OpenBabel and (iv) calculates the ElectroShape 4D descriptors.
The network representation is based on the Javascript InfoVis toolkit and the information is extracted directly from the database through Django. Finally, 2D images for the small molecules are generated on-the-fly using the RDKit module from Django.
Prediction of side effects and off targets
Reported associations extracted from the literature (Lounkine et al., 2012) between pharmacological effects (including those categorized as ‘adverse effects’) and certain targets were imported into a MySQL relational database. On the other hand, adverse drug reactions (ADR) and corresponding drugs’ SMILES strings were downloaded from the side effect resource SIDER 2 (Kuhn et al., 2010) and a subset comprising 151 drugs not present in DrugBank was created and used for validating our results. For each of these drugs, we calculated the putative binding profile and queried the database for the adverse effects associated to the predicted targets. The goodness of our predictions was assessed by evaluating how many ADR are correctly identified and/or missed out of the total number of 270 ADR contained in the MySQL database. This problem was handled as a group of 270 binary decisions for each drug: 1 = true association; 0 = no association. The true positive and negative rates, as well as the false positive and negative rates, were calculated for each drug and averaged.
For prediction of non-annotated off-targets, the dataset proposed by (AbdulHameed et al., 2012) was used. SMILES strings were extracted from PubChem when possible, or generated manually.
LBVS
To perform an on-target validation, we used the Directory of Useful Decoys (DUD) (Huang et al., 2006) and the ‘WOrld of Molecular BioAcTivity’ (WOMBAT) (Good and Oprea, 2008) subset to account for the analogue bias in the set of active compunds. Ligands and decoys were extracted from the original distributions and processed in an identical way to that followed with the DrugBank molecules in order to allow direct search and comparison because ElectroShape results are dependent on the partial charge method used. Briefly, the original files in multi-molecule structure data format were used to generate directly Morgan fingerprints and SMILES strings using RDKit. The latter were employed to generate initial 3D structures using CORINA (Sadowski et al., 2003) and different conformations for each of them were obtained by using ALFA (Cabrera et al., 2011).
We were able to match every target in the DUD to a corresponding DrugBank target with at least one representative ligand except for β-lactamase AmpC whose only protein-bound ligand was not transformed properly, hence results are presented for only 39 targets. As a measure of the global similarity per ligand, we used the SEA Z-score for 2D and 4D methods, and in the case of ElectroShape 4D, we also used the maximum similarity values among the compounds per target so that comparison with other data fusion techniques could be performed.
Network analysis
The target–target matrix was analysed and the Z-score distribution was fitted to an extreme value distribution (EVD) in order to calculate the expectation values (E-values, e.g. a probability of observing a given Z-score using random data) for the similarity between targets (Hert et al., 2008). Using different cut-off values, we transformed those matrices into threshold networks, adding an edge between two targets or nodes if the E-value or a similar score was above a certain value that depends on the kind of comparison being made (2D ligand chemistry, 3D ligand-binding site or 4D ligand shape and charge distribution). For the ligand-binding sites, we used the TM-score values to build the corresponding threshold network.
The Cytoscape software and network analysis plug-in (Smoot et al., 2011) was employed to study several statistical properties and to compute the union, difference and intersection of 2D ligand, 4D ligand and 3D receptor networks. Finally, to explore the usefulness of the information in the networks, a ‘triviality’ test was performed for each kind of network. In this test, we counted the number of edges due to a name similarity (string matching over 0.6) and percentage of common compounds between targets of more than 60%. These indicators try to measure the quantity of information that could be extracted trivially from the names of the targets or, at plain sight, from the profiles of the ligands.
Results
Chemogenomic datasets
After applying to DrugBank the protocol described above, the database contained a total of 5685 molecules and 3779 targets related by 11 559 annotated activities. In the case of the 3D database for ElectroShape, we ended up with 565 505 different conformers. This number roughly corresponds to an average of 100 conformers per molecule. Only sc-PDB ligand-binding sites belonging to targets present in DrugBank were selected and identified by their UniProt (Wu et al., 2006) identification code. This procedure yielded a total of 1056 targets.
Similarity calculation and drug–drug and target–target matrices
Inspired by the work from Shoichet's lab (Hert et al., 2008) that related biological targets by employing the 2D chemical descriptors of their ligands, we analogously developed a parameterization for the SEA (Keiser et al., 2007) method using random groups of molecules from DrugBank. As can be seen in Table 1, after the fitting procedure, power law values are comparable to the original values obtained by Shoichet et al. (Hert et al., 2008) for the MDL Drug Data Report (MDDR) database (MDDR, 2006). The SEA method was also re-parameterized using ElectroShape descriptors and an equivalent random set of molecules.
Table 1.
Fitting comparison for DrugBank and Shoichet et al. sets in SEA
| Mean exponent | Mean coefficient | Pearson r2 | Standard deviation exponent | Standard deviation coefficient | Pearson r2 | |
|---|---|---|---|---|---|---|
| DrugBank 2D Morgan fingerprints | 1.01 | 5.81 × 10−3 | 0.9991 | 0.534 | 6.03 × 10−2 | 0.9977 |
| DrugBank ElectroShape 4D | 0.99 | 4.76 × 10−2 | 0.9998 | 0.635 | 1.56 × 10−1 | 0.9951 |
| Shoichet et al. (Hert et al., 2008) | 1 | 4.24 × 10−4 | 0.9998 | 0.665 | 4.49 × 10−3 | 0.9882 |
Finally, for the 3D receptor set arising from the intersection of DrugBank and sc-PDB databases, we obtained an all-against-all matrix for the 1056 targets using the TM-score normalized by the average number of residues of the two ligand-binding sites being compared.
Off-target validation results
Using a limited test set from DrugBank (see under Methods) that consisted of recently discovered but not yet annotated secondary targets for a certain number of compounds (Ballester et al., 2009), we tested the ability of the ElectroShape chemogenomic approach to predict these annotations correctly. For three of the four compounds (Table 2), the primary activity was found to be ranked first in the target profile (except for RO-25–6981, in which case it was found in the third place), while all secondary targets were identified within the first seven predicted targets and with very close similarity values to the first scoring target.
Table 2.
Off-target test set from (AbdulHameed et al., 2012)
| Compound | Primary target rank | Secondary target rank |
|---|---|---|
| Dimetholizine | 1 (histamine H1 receptor) | 6 (α1A adrenoceptor) |
| 7 (α1D adrenoceptor) | ||
| 7 (α1B adrenoceptor) | ||
| 7 (5-HT1A receptor) | ||
| 1 (D2 dopamine receptor) | ||
| Denopamine | 1 (β1 adrenoceptor) | 3 (β3 adrenoceptor) |
| Ifenprodil | 1 (NMDA receptor) | 3 (μ opioid receptor) |
| RO-25–6981 (NMDA receptor ligand) | 3 (NMDA receptor) | 4 (D4 dopamine receptor) |
| 1 (noradrenaline transporter) | ||
| 1 (5-HT transporter) |
Prediction of adverse effects
Following on recently published work (Lounkine et al., 2012), we related certain targets belonging to the DrugBank set to 290 probable adverse effects. We then predicted the 2D SEA polypharmacology profiles of the drugs included in the SIDER 2 database but not in DrugBank, and we extracted the adverse effects related to those targets. For every molecule, the specificity and selectivity of the method were calculated and then the values were averaged, yielding 92% for specificity and 16% for selectivity. We also obtained a minimum of 20% of adverse effects predicted for 43% of the compounds. These values appear to indicate that most of the adverse effects that could be predicted are missing but the false positive rate is within a reasonable range. With ElectroShape, we obtained better values, namely, a 95% of specificity and 22% of selectivity, which highlights the improved ability of this method to discover additional targets over the general chemical scaffold comparison merely using 2D fingerprints.
LBVS
Regarding the on-target validation of the dataset (cf. Figure 1), we selected the DUD and a subset of WOMBAT to avoid, to some extent, the effect of analogues in the true binders sets. In the case of the 4D method, we also tested the SEA (4D SEA) and the maximum similarity method (4D MAX).
By-target and average results for AUC of receiver operating characteristic plots are given in Table 3. A perfectly accurate method would have an AUC of 1, while a random method would have an AUC of 0.5. It can be seen that the 2D and 4D MAX methods perform equally and that both outperform the 4D SEA. A closer look at the WOMBAT results (Table 4) reveals that a strong analogue bias is present for all methods but especially for 2D SEA, the performance of which drops more than those of the two 4D methods.
Table 3.
AUC results for the DUD using the three approaches tested
| 4D SEA | 4D MAX | 2D SEA | |
|---|---|---|---|
| ACE | 0.59 | 0.71 | 0.81 |
| AChE | 0.57 | 0.61 | 0.64 |
| ADA | 0.59 | 0.64 | 0.91 |
| ALR2 | 0.64 | 0.82 | 0.58 |
| AmpC | – | – | 0.55 |
| AR | 0.56 | 0.84 | 0.64 |
| CDK2 | 0.55 | 0.78 | 0.72 |
| COMT | 0.54 | 0.63 | 0.80 |
| COX1 | 0.55 | 0.74 | 0.69 |
| COX2 | 0.66 | 0.91 | 0.46 |
| DHFR | 0.77 | 0.78 | 0.99 |
| EGFR | 0.77 | 0.73 | 0.95 |
| ER_agonist | 0.84 | 0.86 | 0.71 |
| ER_antagonist | 0.77 | 0.87 | 0.94 |
| FGFR1 | 0.36 | 0.52 | 0.40 |
| FXa | 0.56 | 0.45 | 0.71 |
| GART | 0.93 | 0.92 | 0.77 |
| GPb | 0.70 | 0.81 | 0.86 |
| GR | 0.44 | 0.60 | 0.75 |
| HIVPr | 0.41 | 0.42 | 0.77 |
| HIVRT | 0.48 | 0.48 | 0.41 |
| HMGA | 0.86 | 0.96 | 0.97 |
| HSP90 | 0.48 | 0.90 | 0.91 |
| InhA | 0.38 | 0.64 | 0.62 |
| MR | 0.69 | 0.84 | 0.88 |
| NA | 0.85 | 0.88 | 0.87 |
| p38 | 0.37 | 0.65 | 0.66 |
| PARP | 0.71 | 0.79 | 0.92 |
| PDE5 | 0.49 | 0.52 | 0.82 |
| PDGFRb | 0.46 | 0.43 | 0.24 |
| PNP | 0.58 | 0.56 | 0.97 |
| PPARγ | 0.80 | 0.79 | 0.93 |
| PR | 0.52 | 0.91 | 0.78 |
| RXRα | 0.87 | 0.82 | 0.97 |
| SAHH | 0.92 | 0.92 | 0.94 |
| Src | 0.70 | 0.66 | 0.41 |
| Thrombin | 0.35 | 0.63 | 0.61 |
| TK | 0.86 | 0.81 | 0.94 |
| Trypsin | 0.39 | 0.77 | 0.82 |
| VEGFR2 | 0.53 | 0.63 | 0.60 |
| Average | 0.62 | 0.73 | 0.75 |
| SD | 0.17 | 0.15 | 0.19 |
ADA, adenosine deaminase; ALR2, aldose reductase; AmpC, AmpC β-lactamase; AR; androgen receptor; CDK2, cyclin-dependent kinase 2; DHFR, dihydrofolate reductase; EGFR, EGF receptor (kinase domain); ER_agonist, oestrogen receptor (agonist-bound conformation); ER_antagonist, oestrogen receptor (antagonist-bound conformation); FGFR1, fibroblast growth factor receptor 1 (kinase domain); FXa, factor Xa; GART, glycinamide ribonucleotide transformylase; GPb, glycogen phosphorylase β; GR, glucocorticoid receptor; HIVPr, HIV protease; HIVRT, HIV reverse transcriptase; HMGR, hydroxymethylglutaryl-CoA reductase; HSP90, human heat shock protein 90; InhA, enoyl-[acyl-carrier-protein] reductase; MR, mineralocorticoid receptor; NA, neuraminidase; p38, p38 MAPK; PDGFRb, PDGF receptor β (kinase domain); PNP, purine nucleoside phosphorylase; PR, progesterone receptor; RXRα, retinoic X receptor α; SAHH, S-adenosyl-homocysteine hydrolase; SRC, tyrosine kinase Src; TK, thymidine kinase; VEGFR2, VEGF receptor 2 (kinase domain).
Table 4.
AUC results for the WOMBAT subset
| 4D SEA | 4D MAX | 2D SEA | |
|---|---|---|---|
| ALR2 | 0.56 | 0.67 | 0.42 |
| AR | 0.32 | 0.39 | 0.52 |
| CDK2 | 0.51 | 0.67 | 0.69 |
| COX2 | 0.58 | 0.81 | 0.43 |
| EGFR | 0.73 | 0.67 | 0.61 |
| ER | 0.65 | 0.73 | 0.66 |
| FXa | 0.50 | 0.43 | 0.77 |
| HIVRT | 0.45 | 0.45 | 0.45 |
| p38 | 0.54 | 0.76 | 0.55 |
| PDE5 | 0.25 | 0.33 | 0.66 |
| PPARγ | 0.83 | 0.81 | 0.72 |
| Average | 0.54 | 0.61 | 0.59 |
| SD | 0.17 | 0.17 | 0.12 |
ALR2, aldose reductase; AR; androgen receptor; CDK2, cyclin-dependent kinase 2; EGF receptor (kinase domain); ER, oestrogen receptor; FXa, factor Xa; HIVRT, HIV reverse transcriptase; p38, p38 MAPK.
It is worth noting that the average performance of our ElectroShape method compares favourably with those obtained through other means. In particular, the 4D MAX approach compared favourably with ROCS max ComboScore (Ballester et al., 2009; reported average of 0.77). Besides, the non-superpositional ElectroShape 4D method is several orders of magnitude faster than ROCS (Grant et al., 1996) because (i) the molecular descriptors are pre-calculated, (ii) a direct comparison requires just a Manhattan distance calculation and (iii) ElectroShape, unlike ROCS, does not require a computationally expensive superposition of one ligand onto another to compute the optimal ligand similarity.
Network analysis
Based on the target–target similarity matrices, we built several threshold networks with different cut-off values and compared several properties. For the case of ligand-based networks, comparison between ligands yielded Z-scores, which are directly transformable into E-values through an EVD fitting procedure. In the case of structure-based networks, the direct comparison between ligand-binding sites yielded TM-score values, which were used as the threshold criteria to trace an edge between two nodes.
To examine other properties of the network, we computed the union, intersection and differences between the three kinds of networks (ligand-based 2D and 4D, and receptor-based 3D) and calculated the triviality score as the percentage of the network edges built from plain-sight knowledge deduced from ligand structures and target names.
According to the network statistical parameters (cluster coefficient, characteristic path length and node degree distribution, which accounts for the distribution of the number of neighbours per node, Figure 2), the ligand-based network could be classified as a broad-scale and small-world network. This is so because it presents a high cluster coefficient, a short characteristic path (e.g. nodes are reachable from others within a few leaps, resulting in the small-world property, Figure 2) and a power law fitting of the node degree distribution (broad scale). Remarkably, the same classification is also present in the 3D target network, whereas the properties of the PSI-BLAST-based (Altschul et al., 1997) network presented by Shoichet et al. (Hert et al., 2008) were not compatible with the categorization of broad-scale and small-world networks.
Figure 2.

Node degree distribution for ElectroShape threshold network (A) and 3D binding site network (B).
Ligand-based networks appear to be populated by very close clusters, which are themselves mostly unconnected and represent pharmacological families such as GPCR, kinases, metabolic enzymes, proteases and nuclear hormone receptors (NHR). This trend can also be observed from the 3D target network where some clusters become even clearer, that is, the NHR family because their members share very similar ligand-binding sites (Figure 3).
Figure 3.

Small-world, broad-scale 3D receptor network with some pharmacologically relevant clusters highlighted (NHRs; kinases and dehydrogenases) and coloured by ‘betweenness’.
Interestingly, the intersection of, and the difference between, the 3D target- and 4D ligand-based networks revealed that only a minimal fraction of the network is shared between them (from 0.36 to 6.80% in highly or minimally restrictive cut-off networks respectively). Low percentages may indicate that both methods yield complementary results: while the direct comparison of target ligand-binding sites could give valuable information in order to achieve some kind of target specificity, ligand-based networks could contribute with information about unexpected interactions for adverse effect prediction and polypharmacology profile optimization.
Regarding the presence of trivial information in the network, some dependence on the cut-off is observed. When the cut-off used to build the ligand-based network is low, the percentage of the network that could be considered trivial tends to increase, the score first rising due to very similar names and then, after a certain cut-off value, changing the regime to a shared-compound triviality (more than 60% of compounds in common with the other target).
In the case of 3D target networks, decreasing the cut-off has the opposite effect as it decreases the percentage of trivial edges in the network that are mostly related to a certain name similarity and independent from the number of shared compounds. This is in agreement with the origin of the network since no ligand comparisons were used to build it. In addition, the similarity in the name could be explained by the tendency of systematically naming targets that belong to the same families with similar names, which also tend to have similar functions and hence similar active (or ligand-binding) sites.
Discussion
Comparison with other methods
To our knowledge, only three other web tools are available for performing similar tasks. TarFisDock (Li et al., 2006) applies the ‘reverse docking’ method, which consists of systematically docking a ligand into hundreds of putative pharmacological target sites. This approach has the advantages of not requiring previous knowledge about true binders and not depending on functional information about the target. However, it needs a 3D model of the protein, which may be unknown or not available straightforwardly, and also suffers from the traditional problems associated with the docking methodology, namely, limited flexibility of the receptor, low speed, inaccurate scoring functions and excessive influence of the target conformation on the results. The SEA tool circumvents the docking problems by relating protein targets on the basis of the set-wise chemical similarity among all compounds that are known to interact with those targets (Keiser et al., 2007). Thus, the problem is transferred to chemical space but at the expense of limiting the kind of compounds that can be predicted in a reliable manner. This method can also be used to search large chemical libraries rapidly and to build cross-target similarity maps. The third tool is STITCH 3 (Kuhn et al., 2012), which allows the exploration of interactions between chemicals and targets on the basis of evidence from scientific documents. This resource currently contains interactions among 300 000 small molecules and 2.6 million proteins from 1133 organisms, and allows the visual display of interactive networks relating protein targets to which the same chemical binds.
Methodological advantages and limitations
The web tool presented here (http://ub.cbm.uam.es/chemogenomics/) implements the well-known SEA approach for target comparison using 2D ligand descriptors and also an ElectroShape comparison module that allows the estimation of 4D molecular similarities faster than other 3D methods. This is due to the pre-computation of the shape and partial charge distribution of the molecules in a format that can be stored in a database for further use, unlike the methods that need to perform n comparisons for every query. Our server also has a network explorer that allows the user to navigate the chemical (2D and 4D ligand) and the biological (target sites) regions of pharmacological space (Figure 4).
Figure 4.
Screenshot of the ElectroShape Polypharmacology web server after submitting the SMILES string for acetylsalicylic acid as the query.
This methodology is obviously limited by the extent that pharmacological space is covered in current databases in terms of both compounds and targets. Therefore, only currently available information can be used to predict new targets or possible adverse effects for candidate molecules. It is also evident that chemical space has not been uniformly explored so that some parts of it (due to synthetic accessibility or other causes) can be better represented than others (or even not be represented at all).
Conclusions
In conclusion, we have presented here a new target-fishing approach that makes use of the ultra-fast LBVS ElectroShape methodology and is able to predict drug adverse effects, build polypharmacology profiles and relate targets from two complementary viewpoints (ligand- and target-based networks). The DUD and WOMBAT sets were employed for on-target validation and the results were directly comparable to those obtained using other state-of-the-art target-fishing approaches. Off-target validation was performed using a limited set of non-annotated secondary targets for already known drugs. Finally, comparison of the predicted adverse effects with data contained in the SIDER 2 database showed good specificity and reasonable selectivity. All of these features are freely available from an undemanding and user-friendly web interface that (i) can be queried for both polypharmacology profiles and adverse effects, (ii) hyperlinks related targets in the three networks (2D, 4D ligand and 3D receptor) and (iii) displays the 2D structure of already annotated drugs.
Acknowledgments
A. C. C. gratefully acknowledges the FPU 2009-0203 grant and its foreign internship programme from the Spanish Ministry of Education. A. M. acknowledges financial support from Comunidad de Madrid through Fundación Severo Ochoa's AMAROUTO program. This research has been funded in part by the Spanish Comisión Interministerial de Ciencia y Tecnología (SAF2009-13914-C02-02) and Comunidad de Madrid (S2010-BMD-2457). The authors are grateful to Professor W. Graham Richards for encouragement and Dr Sree Vadlamudi for fruitful discussions during the elaboration of this work. We also would like to thank the three anonymous reviewers for their knowledgeable and insightful comments that helped to improve the quality of the original paper.
Glossary
- ADR
adverse drug reactions
- DUD
Directory of Useful Decoys
- EVD
extreme value distribution
- LBVS
ligand-based virtual screening
- MDDR
MDL Drug Data Report
- MMFF94
Merck Molecular Force Field
- NHR
nuclear hormone receptors
- PDB
Protein Data Bank
- ROCS
Rapid Overlay of Chemical Structures
- SEA
Similarity Ensemble Approach
- SMILES
simplified molecular-input line-entry specification
- WOMBAT
WOrld of Molecular BioAcTivity
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1 Compounds used for off-target validation.
Table S2 Enrichment analysis of LBVS. BEDROC analysis (α = 20, 80% weight for top 8%).
Appendix S1 Details on parameterization of the networks.
References
- AbdulHameed MDM, Chaudhury S, Singh N, Sun H, Wallqvist A, Tawa G. Exploring polypharmacology using a ROCS-based target fishing approach. J Chem Inf Model. 2012;52:492–505. doi: 10.1021/ci2003544. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MS, Morris GM, Finn PW, Sharma R, Moretti L, Cooper RI, et al. ElectroShape: fast molecular similarity calculations incorporating shape, chirality and electrostatics. J Comput Aided Mol Des. 2010;24:789–801. doi: 10.1007/s10822-010-9374-0. [DOI] [PubMed] [Google Scholar]
- Ballester PJ, Richards WG. Ultrafast shape recognition to search compound databases for similar molecular shapes. J Comput Chem. 2007;28:1711–1723. doi: 10.1002/jcc.20681. [DOI] [PubMed] [Google Scholar]
- Ballester PJ, Finn PW, Richards WG. Ultrafast shape recognition: evaluating a new ligand-based virtual screening technology. J Mol Graph Model. 2009;27:836–845. doi: 10.1016/j.jmgm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Ballester PJ, Westwood I, Laurieri N, Sim E, Richards WG. Prospective virtual screening with Ultrafast Shape Recognition: the identification of novel inhibitors of arylamine N-acetyltransferases. J R Soc Interface. 2010;7:335–342. doi: 10.1098/rsif.2009.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Scheiber J, Glick M, Davies JW, Azzaoui K, Hamon J, et al. Analysis of pharmacology data and the prediction of adverse drug reactions and off-target effects from chemical structure. ChemMedChem. 2007;2:861–873. doi: 10.1002/cmdc.200700026. [DOI] [PubMed] [Google Scholar]
- Berger SI, Iyengar R. Network analyses in systems pharmacology. Bioinformatics. 2009;25:2466–2472. doi: 10.1093/bioinformatics/btp465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H, Henrick K, Nakamura H, Markley JL. The worldwide Protein Data Bank (wwPDB): ensuring a single, uniform archive of PDB data. Nucleic Acids Res. 2007;35(Suppl 1):D301–D303. doi: 10.1093/nar/gkl971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang X-P, et al. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera AC, Gil-Redondo R, Perona A, Gago F, Morreale A. VSDMIP 1.5: an automated structure-and ligand-based virtual screening platform with a PyMOL graphical user interface. J Comput Aided Mol Des. 2011;25:813–824. doi: 10.1007/s10822-011-9465-6. [DOI] [PubMed] [Google Scholar]
- Cai J, Han C, Hu T, Zhang J, Wu D, Wang F, et al. Peptide deformylase is a potential target for anti-Helicobacter pylori drugs: reverse docking, enzymatic assay, and X-ray crystallography validation. Protein Sci. 2006;15:2071–2081. doi: 10.1110/ps.062238406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. On immunity with special reference to the relationship between distribution and action of antigens. J R Inst Public Health. 1907;15:321–340. [Google Scholar]
- Good AC, Oprea TI. Optimization of CAMD techniques 3. Virtual screening enrichment studies: a help or hindrance in tool selection? J Comput Aided Mol Des. 2008;22:169–178. doi: 10.1007/s10822-007-9167-2. [DOI] [PubMed] [Google Scholar]
- Grant JA, Gallardo M, Pickup B. A fast method of molecular shape comparison: a simple application of a Gaussian description of molecular shape. J Comput Chem. 1996;17:1653–1666. [Google Scholar]
- Halgren TA. Merck molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions. J Comput Chem. 1996;17:520–552. [Google Scholar]
- Hawkins PCD, Skillman AG, Warren GL, Ellingson BA, Stahl MT. Conformer generation with OMEGA: algorithm and validation using high quality structures from the Protein Databank and Cambridge Structural Database. J Chem Inf Model. 2010;50:572–584. doi: 10.1021/ci100031x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hert J, Keiser MJ, Irwin JJ, Oprea TI, Shoichet BK. Quantifying the relationships among drug classes. J Chem Inf Model. 2008;48:755–765. doi: 10.1021/ci8000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Huang N, Shoichet BK, Irwin JJ. Benchmarking sets for molecular docking. J Med Chem. 2006;49:6789–6801. doi: 10.1021/jm0608356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AN. Morphological similarity: a 3D molecular similarity method correlated with protein-ligand recognition. J Comput Aided Mol Des. 2000;14:199–213. doi: 10.1023/a:1008100132405. [DOI] [PubMed] [Google Scholar]
- Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, Shoichet BK. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25:197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- Knight ZA, Lin H, Shokat KM. Targeting the cancer kinome through polypharmacology. Nat Rev Cancer. 2010;10:130–137. doi: 10.1038/nrc2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, et al. DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res. 2011;39(Suppl 1):D1035–D1041. doi: 10.1093/nar/gkq1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M, Campillos M, Letunic I, Jensen LJ, Bork P. A side effect resource to capture phenotypic effects of drugs. Mol Syst Biol. 2010;6:343. doi: 10.1038/msb.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M, Szklarczyk D, Franceschini A, von Mering C, Jensen LJ, Bork P. STITCH 3: zooming in on protein-chemical interactions. Nucleic Acids Res. 2012;40:D876–D880. doi: 10.1093/nar/gkr1011. (database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrum G. Rdkit: Open-Source Cheminformatics. Basel: Novartis Institutes for BioMedical Research; 2011. ( http://www.rdkit.org/) [Google Scholar]
- Li H, Gao Z, Kang L, Zhang H, Yang K, Yu K, et al. TarFisDock: a web server for identifying drug targets with docking approach. Nucleic Acids Res. 2006;34:W219–W224. doi: 10.1093/nar/gkl114. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounkine E, Keiser MJ, Whitebread S, Mikhailov D, Hamon J, Jenkins JL, et al. Large-scale prediction and testing of drug activity on side-effect targets. Nature. 2012;486:361–367. doi: 10.1038/nature11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MDDR. MDL Drug Data Report. San Leandro, CA: MDL; 2006. [Google Scholar]
- Meslamani J, Rognan D, Kellenberger E. sc-PDB: a database for identifying variations and multiplicity of ‘druggable’ binding sites in proteins. Bioinformatics. 2011;27:1324–1326. doi: 10.1093/bioinformatics/btr120. [DOI] [PubMed] [Google Scholar]
- O'Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OpenEye Scientific Software. 2011. ROCS. OpenEye Scientific Software: Santa Fe, NM.
- Ripphausen P, Nisius B, Peltason L, Bajorath J. Quo vadis, virtual screening? A comprehensive survey of prospective applications. J Med Chem. 2010;53:8461–8467. doi: 10.1021/jm101020z. [DOI] [PubMed] [Google Scholar]
- Rogers DJ, Tanimoto TT. A computer program for classifying plants. Science. 1960;132:1115–1118. doi: 10.1126/science.132.3434.1115. [DOI] [PubMed] [Google Scholar]
- Rognan D, Meslamani J. Enhancing the accuracy of chemogenomic models with a three-dimensional binding site kernel. J Chem Inf Model. 2011;51:1593–1603. doi: 10.1021/ci200166t. [DOI] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Rush TS, 3rd, Grant JA, Mosyak L, Nicholls A. A shape-based 3-D scaffold hopping method and its application to a bacterial protein-protein interaction. J Med Chem. 2005;48:1489–1495. doi: 10.1021/jm040163o. [DOI] [PubMed] [Google Scholar]
- Sadowski J, Gasteiger J, Klebe G. Comparison of automatic three-dimensional model builders using 639 X-ray structures. J Chem Inf Comput Sci. 1994;34:1000–1008. [Google Scholar]
- Sadowski J, Schwab C, Gasteiger J. 2003. CORINA, 3D Structure Generator: version 3.1, Molecular Networks GmbH, Erlangen, Germany.
- Simon Z, Peragovics A, Vigh-Smeller M, Csukly G, Tombor L, Yang Z, et al. Drug effect prediction by polypharmacology-based interaction profiling. J Chem Inf Model. 2012;52:134–145. doi: 10.1021/ci2002022. [DOI] [PubMed] [Google Scholar]
- Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Greef J, McBurney RN. Rescuing drug discovery: in vivo systems pathology and systems pharmacology. Nat Rev Drug Discov. 2005;4:961–967. doi: 10.1038/nrd1904. [DOI] [PubMed] [Google Scholar]
- Vidal D, Mestres J. In silico receptorome screening of antipsychotic drugs. Mol. Inf. 2010;29:543–551. doi: 10.1002/minf.201000055. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xiao J, Suzek TO, Zhang J, Wang J, Bryant SH. PubChem: a public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009;37(Suppl 2):W623–W633. doi: 10.1093/nar/gkp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weininger D. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J Chem Inf Comput Sci. 1988;28:31–36. [Google Scholar]
- Witkop B. Paul Ehrlich and his magic bullets – revisited. Proc Am Philos Soc. 1999;143:540–557. [PubMed] [Google Scholar]
- Wu CH, Apweiler R, Bairoch A, Natale DA, Barker WC, Boeckmann B, et al. The Universal Protein Resource (UniProt): an expanding universe of protein information. Nucleic Acids Res. 2006;34(suppl 1):D187–D191. doi: 10.1093/nar/gkj161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yera ER, Cleves AE, Jain AN. Chemical structural novelty: on-targets and off-targets. J Med Chem. 2011;54:6771–6785. doi: 10.1021/jm200666a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Skolnick J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005;33:2302–2309. doi: 10.1093/nar/gki524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.