Abstract
BACKGROUND AND PURPOSE
TAK-875, a selective GPCR40/free fatty acid receptor 1 agonist, improves glycaemic control by increasing glucose-dependent insulin secretion. Metformin is a first-line drug for treatment of type 2 diabetes that improves peripheral insulin resistance. Based on complementary mechanism of action, combining these agents is expected to enhance glycaemic control. Here, we evaluated the chronic effects of TAK-875 monotherapy and combination therapy with metformin in diabetic rats.
EXPERIMENTAL APPROACH
Long-term effects on glycaemic control and β-cell function were evaluated using Zucker diabetic fatty (ZDF) rats, which develop diabetes with hyperlipidaemia and progressive β-cell dysfunction.
KEY RESULTS
Single doses of TAK-875 (3–10 mg·kg−1) and metformin (50–150 mg·kg−1) significantly improved both postprandial and fasting hyperglycaemia, and additive improvements were observed in their combination. Six-week treatment with TAK-875 (10 mg·kg−1, b.i.d.) significantly decreased glycosylated Hb (GHb) by 1.7%, and the effect was additively enhanced by combination with metformin (50 mg·kg−1, q.d.; GHb: −2.4%). This improvement in glycaemic control in the combination group was accompanied by significant 3.2-fold increase in fasting plasma insulin levels. Pancreatic insulin content was maintained at a level comparable to that in normal rats by combination treatment (vehicle: 26, combination: 67.1; normal lean: 69.1 ng·mg−1 pancreas) without affecting pancreatic glucagon content. Immunohistochemical analyses revealed normal morphology, enhanced pancreas duodenum homeobox-1 expression and increased PCNA-positive cells in islets of the combination group.
Conclusion and Implications
Our results indicate that combination therapy with TAK-875 and metformin could be a valuable strategy for glycaemic control and β-cell preservation in type 2 diabetes.
Keywords: GPR40/FFAR1, TAK-875, metformin, Zucker diabetic fatty rats, insulin secretion, glycaemic control, β-cell preservation, hyperlipidaemia
Introduction
Type 2 diabetes mellitus is characterized by elevated plasma glucose levels arising from increased peripheral insulin resistance and the gradual loss of β-cell function (Kahn, 2000). As chronic hyperglycaemia contributes to the complications associated with type 2 diabetes (Giorgino et al., 2005), the primary goal for treatment is to maintain normoglycaemia with fewer adverse effects such as hypoglycaemia. Based on the algorithm for medical management of type 2 diabetes, metformin, a biguanide derivative, is recommended as the initial pharmacological therapy (Inzucchi et al., 2012). Metformin provides glucose-lowering effects via pleiotropic action including decreased hepatic glucose production, increased peripheral glucose disposal and reduced intestinal glucose absorption (Hundal and Inzucchi, 2003). To achieve adequate glycaemic control, combination therapy with multiple drugs that have different mechanisms of action than metformin is often required. However, despite the availability of numerous anti-diabetic agents, many patients have not yet achieved adequate glycaemic control (U.K. Prospective Diabetes Study Group, 1995). Therefore, there remains a need for agents that have improved efficacy and/or a lower risk of adverse effects and can be used in combination treatment, especially with metformin (Charpentier, 2002).
GPCR40 (GPR40)/free fatty acid receptor 1 is a GPCR highly expressed in pancreatic β-cells (Briscoe et al., 2003; Itoh et al., 2003). Long- and medium-chain free fatty acids (FFAs) are endogenous ligands for GPR40, and the activation of GPR40 by FFAs leads to glucose-dependent augmentation of insulin secretion through activation of the Gαq-pathway (Fujiwara et al., 2005; Shapiro et al., 2005), which is a distinct mechanism from other oral insulinotropic drugs, such as sulfonylureas (SU; Rendell, 2004) and dipeptidyl peptidase-4 (DPP-4) inhibitors (Pratley, 2009). Nagasumi et al. (2009) have previously reported that mice overexpressing human GPR40 in pancreatic β-cells exhibit improved glucose excursion and increased insulin secretion during an oral glucose tolerance test (OGTT). This finding suggests that GPR40 could be an attractive drug target to enhance insulin secretion in type 2 diabetes.
TAK-875 is a potent, selective and orally available GPR40 agonist that enhances insulin secretion in a glucose concentration-dependent manner (Negoro et al., 2010; 2012; Tsujihata et al., 2011). Oral administration of TAK-875 markedly improves postprandial hyperglycaemia in diabetic rats, while TAK-875 does not affect normoglycaemia even at a dose higher than the effective dose in fasted normal rats (Tsujihata et al., 2011). In addition, it was demonstrated that TAK-875 significantly improved glycaemic control in type 2 diabetic patients with minimum risk of hypoglycaemia compared with SUs (Araki et al., 2012; Burant et al., 2012).
FFAs acutely stimulate insulin secretion, while chronic exposure to FFAs causes β-cell dysfunction and death, so-called lipotoxicity. Because endogenous ligands of GPR40 are medium- and long-chain FFAs, there remained concern regarding the involvement of GPR40 in lipotoxicity (Steneberg et al., 2005). We have previously shown that chronic exposure to TAK-875 does not cause β-cell dysfunction in rat insulinoma cells, in contrast to FFAs (Tsujihata et al., 2011). However, there are no published reports examining in detail the effects on β-cell function after long-term activation of GPR40 in vivo. Because such analyses in clinical trials are difficult, animal studies are essential for the development of novel anti-diabetic drugs.
Because of the complementary mechanisms of action between GPR40 agonists and metformin, combination therapy with these agents is expected to provide favourable effects on glycaemic control. Recently, combination treatment with a DPP-4 inhibitor and metformin in a diabetic rat model and drug-naive patients with type 2 diabetes has been shown to improve glycaemic control and β-cell function (Han et al., 2011; Williams-Herman et al., 2012). However, the effects of combination treatment with a GPR40 agonist and metformin on development of diabetes and on β-cell dysfunction remain poorly understood.
In the present study, we evaluated the chronic effects of TAK-875 in combination with metformin on glycaemic control in Zucker diabetic fatty (ZDF) rats. The ZDF rat is a severe type 2 diabetic model, which exhibits hyperglycaemia, hyperlipidaemia and insulin resistance. The islets of these rats show disruption of normal islet architecture, β-cell degranulation and increased β-cell death (Lee et al., 1994; Finegood et al., 2001). It has been reported that plasma FFA and triglyceride (TG) content of islets begins to rise progressively prior to the onset of hyperglycaemia (Lee et al., 1994), suggesting the involvement of lipotoxicity in β-cell dysfunction in this model. Therefore, this model is also suitable for evaluating the chronic effects of TAK-875 on β-cell function. In addition, our study was designed to evaluate the chronic effects of combination treatment with TAK-875 and metformin on plasma hormone profiles, pancreatic hormone content and β-cell morphology in these rats.
Methods
Animals
Male ZDF (leprfa/CrlCrlj fatty fa/fa) rats and non-diabetic littermates, male Zucker lean (ZL; leprfa/CrlCrlj lean +/+ or fa/+) rats, were obtained from Charles River Laboratories Japan, Inc. (Yokohama, Japan). All rats were housed in individual metal cages in a room with controlled temperature (23°C), humidity (55%) and lighting, and were allowed free access to powdered standard laboratory chow diet (CE-2, Clea, Japan Inc., Tokyo, Japan) and tap water, unless otherwise indicated. Rats were group housed in single dosing study, while they were single housed in multiple dosing study due to precise measurement of each food intake. All studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010). The care and use of the animals and experimental protocols used in this study were approved by the Experimental Animal Care and Use Committee of Takeda Pharmaceutical Company Limited (Kanagawa, Japan).
Materials
TAK-875 ([(3S)-6-({2′,6′-dimethyl-4′-[3-(methylsulfonyl)propoxy]biphenyl-3-yl}methoxy)-2,3-dihydro-1-benzofuran-3-yl]acetic acid hemihydrate) was synthesized at Chemical Development Laboratories, Takeda Pharmaceutical Company Limited. Metformin hydrochloride was purchased from Wako Pure Chemical Industries Limited (Osaka, Japan). TAK-875 and metformin were suspended in 0.5% methylcellulose solution (Wako Pure Chemical Industries Limited).
OGTT
At the age of 8 weeks, ZDF and ZL rats were fasted overnight. ZDF rats were divided into four groups (n = 6) so that each mean of plasma glucose, TG and body weight had no statistically significant differences among groups. Each group of ZDF rats was orally given vehicle, TAK-875 (3 mg·kg−1), metformin (50 mg·kg−1) or TAK-875 (3 mg·kg−1) in combination with metformin (50 mg·kg−1). ZL rats (n = 5) were orally given vehicle. Plasma parameters measured for the grouping of the animals were used as baseline (pre) data. Sixty minutes after drug administration, all animals received an oral glucose load (1 g·kg−1). Blood samples were collected from the tail vein just before glucose load (time 0), and 10, 30, 60 and 120 min after glucose load for determination of plasma glucose and insulin levels.
Single dosing study in the fasted state
At the age of 19 weeks, non-fasted ZDF rats were divided into four groups (n = 6) so that each mean of glycosylated Hb (GHb), plasma glucose, TG and body weight had no statistically significant differences among groups. Rats were fasted overnight, and each group was orally given vehicle, TAK-875 (10 mg·kg−1), metformin (150 mg·kg−1) or TAK-875 (10 mg·kg−1) in combination with metformin (150 mg·kg−1). Plasma glucose and insulin levels were determined using blood samples collected from the tail vein at time 0 and 0.5, 1, 2, 4 and 6 h after dosing.
Multiple dosing study
ZDF and ZL rats were fed powder normal chow twice a day in the morning and evening (2 h each) for 5 days. At the age of 10 weeks, ZDF rats were divided into four groups (n = 6) so that each mean of GHb, plasma glucose, TG, insulin and body weight had no statistically significant differences among groups. The diet was switched to a high-calorie diet (Quick Fat, Clea, Japan Inc.) under the same time-restricted feeding conditions, and each group was orally given vehicle, TAK-875 (10 mg·kg−1, b.i.d.), metformin (50 mg·kg−1, q.d. in the evening) or TAK-875 (10 mg·kg−1, b.i.d.) in combination with metformin (50 mg·kg−1, q.d. in the evening) 30 min prior to feeding. Five ZL rats were treated with vehicle as a healthy control. After 2, 4 and 6 weeks of treatment, blood samples were collected from the tail vein before feeding in the morning, and GHb, plasma glucose, TG, total cholesterol (TC), non-esterified fatty acids (NEFA), insulin and glucagon were measured. Body weight and food intake were measured once weekly. After 44 days of treatment, all rats were killed, and a portion of the pancreas was cut into two pieces. One piece was used for determination of pancreatic hormone content, and the other was placed in Bouin's fixative solution (Polysciences, Inc., Warrington, PA, USA) for immunohistochemical analysis.
Measurements of plasma and pancreatic parameters
Plasma glucose, TG, TC and NEFA were enzymatically measured with an Autoanalyzer 7080 (Hitachi, Tokyo, Japan). GHb was measured by an HPLC-based automated Analyzer HLC-723 G7 (TOSOH, Tokyo, Japan). Plasma insulin and glucagon were measured using a RIA kit (Millipore, Bedford, MA, USA). A piece of pancreas was homogenized in acid-ethanol (74% ethanol containing 0.15 mol·L−1 HCl), and centrifuged. The insulin and glucagon levels in the supernatant were measured using an RIA kit (Millipore, for insulin and TFB, Tokyo, Japan for glucagon).
Immunohistochemical analysis
After overnight fixation, four pancreata from each group were embedded in paraffin. The deparaffinized sections were exposed to primary antibodies, guinea pig anti-insulin antibody (DAKO A/S, Glostrup, Denmark), rabbit anti-glucagon antibody (DAKO), rabbit anti-pancreas duodenum homeobox-1 [PDX-1, a key transcriptional factor of β-cell function (Melloul, 2004) ] polyclonal antibody (TransGenic, Kumamoto, Japan) or mouse anti-PCNA, a marker of cell proliferation (Rafacho et al., 2008) monoclonal antibody (DAKO) overnight at 4°C. After detection of bound antibody using a polymer-labelled Envision+ system (DAKO), the sections were developed using 3,3′-diaminobenzidine tetrahydrochloride substrate and counterstained with haematoxylin. Digital images were obtained with an inverted microscope IX71 (Olympus, Tokyo, Japan), or with an Aperio ScanScope XT system (Aperio Technologies, Vista, CA, USA).
Statistical analysis
The homeostasis model assessment of β-cell function (HOMA-β) index was calculated using the following formula: [fasting insulin (μU·mL−1) × 360] / [fasting glucose (mg·dL−1) – 63] (Matthews et al., 1985). Statistical differences compared with vehicle were analysed with Dunnett's test or Steel's test. Statistical differences between ZDF rats and ZL rats were analysed with Student's t-test or the Aspin–Welch test. To evaluate if combination treatment had significant additive or synergistic effects, two-way anova was performed (Moritoh et al., 2009). The results were interpreted as follow (i) When a significant interaction effect (P ≤ 0.05) was observed, the effect of combination was synergistic (when the effect of the combination treatment exceeds the sum of the effect of the monotreatment); and (ii) When no significant interaction and significant main effects of both drugs (P ≤ 0.05) were observed, the effect was additive (when the effects of the combination treatment equals the sum of the effect of the monotreatment). All data were presented as mean ± SD.
Results
Acute administration of TAK-875 and metformin in combination additively improves postprandial and fasting hyperglycaemia
Before multiple dosing studies, we examined the acute effects of TAK-875, metformin and their combination on postprandial hyperglycaemia during OGTT in young ZDF rats (8 weeks of age). TAK-875 exhibits high oral bioavailability (76%), Cmax of 5.8 μg mL−1, Tmax of 1 h following a 3 mg kg−1 oral dosing in fasted rats (Negoro et al., 2010). In this study, 3–10 mg·kg−1 of TAK-875 was selected based on the pharmacokinetic profiles: this dose is similar to clinical doses of TAK-875 (Naik et al., 2012). Oral administration of TAK-875 (3 mg·kg−1), metformin (50 mg·kg−1), and their combination 1 h before glucose load significantly decreased plasma glucose AUC by 21.0, 15.4 and 33.8%, respectively, compared with vehicle, and the glucose tolerance in the combination group was even better than that in normal ZL rats (Figure 1A, C). Two-way anova showed additive glucose-lowering effects in the combination (TAK-875, P ≤ 0.01; metformin, P ≤ 0.01; interaction, not significant). TAK-875 significantly enhanced insulin secretion before a glucose load (P ≤ 0.01) and tended to augment it at 10 min after glucose load, while metformin showed a trend towards reduce insulin release at 10 min (Figure 1B, D). TAK-875 and metformin in combination also increased insulin secretion just before glucose challenge compared with vehicle, although the increase was not statistically significant (P = 0.059; Figure 1B, D).
Figure 1.
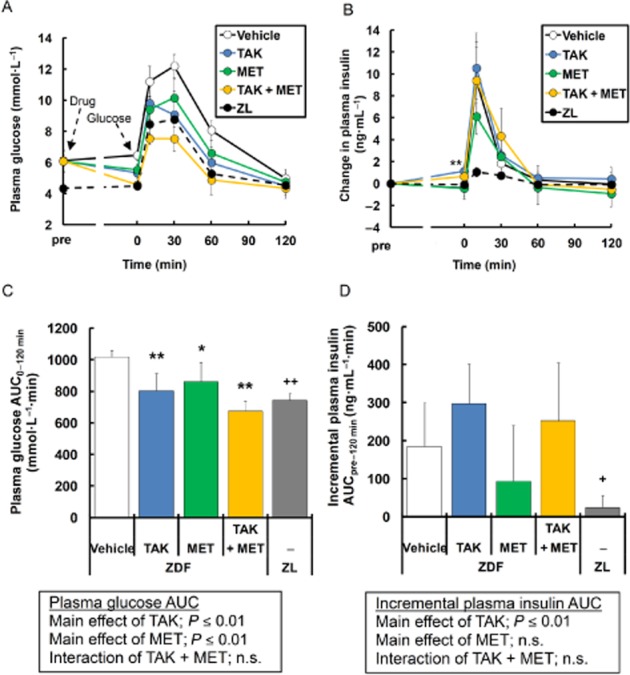
Effects of single dosing of TAK-875 (TAK), metformin (MET) and TAK in combination with MET on glucose excursion and insulin secretion during oral glucose tolerance test in young ZDF rats. (A) and (B) show plasma glucose and change in plasma insulin levels, respectively, during oral glucose tolerance test in ZDF rats treated with vehicle, 3 mg·kg−1 of TAK, 50 mg·kg−1 of MET, or their combination, and in ZL rats treated with vehicle. Data in (C) and (D) represent plasma glucose AUC0-120 min and incremental plasma insulin AUCpre-120 min respectively. *P ≤ 0.05, **P ≤ 0.01 compared to vehicle-treated ZDF rats by Dunnett's test or Steel's test. +P ≤ 0.05, ++P ≤ 0.01 compared to vehicle-treated ZDF rats by Student's t-test or the Aspin–Welch test. The results of two-way anova are indicated in insets. Values are mean ± SD (n = 6 for ZDF rats, n = 5 for ZL rats). n.s., not significant.
Next, effects of these drugs on fasting hyperglycaemia were evaluated in aged ZDF rats. Fasting plasma glucose levels were significantly elevated in 19-week-old ZDF rats compared with age-matched normal rats (13.0 ± 3.0 mmol·L−1 vs. 5.6 ± 0.2 mmol·L−1, P ≤ 0.01). Acute dosing of TAK-875 (10 mg·kg−1) significantly decreased fasting plasma glucose levels with augmentation of insulin secretion in ZDF rats (Figure 2). Metformin (150 mg·kg−1) also improved fasting hyperglycaemia without changes in insulin release (Figure 2). The combination treatment with TAK-875 and metformin additively decreased fasting plasma glucose levels (TAK-875, P ≤ 0.01; metformin, P ≤ 0.01; interaction, not significant), and significantly increased fasting plasma insulin levels compared with vehicle (Figure 2). The amount of the secreted insulin was almost the same as that seen with TAK-875 treatment (Figure 2B, D). These results indicate that combination treatment with TAK-875 and metformin effectively improves postprandial and fasting hyperglycaemia while sparing insulin secretion, compared with each drug alone.
Figure 2.
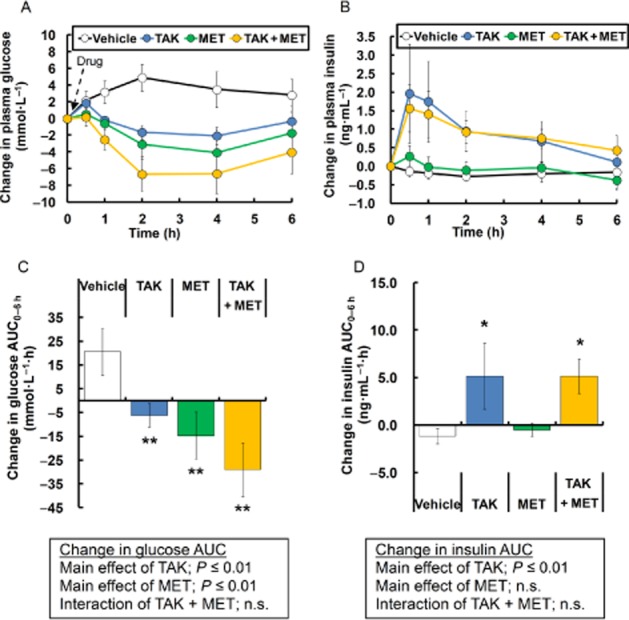
Effects of single dosing of TAK-875 (TAK), metformin (MET) and TAK in combination with MET on fasting hyperglycaemia in aged ZDF rats. (A) and (B) show changes in plasma glucose and insulin levels, respectively, after single treatment with vehicle, 10 mg·kg−1 of TAK, 150 mg·kg−1 of metformin, or their combination. Data in (C) and (D) represent change in glucose AUC0–6 h and change in insulin AUC0–6 h respectively. **P ≤ 0.01 compared to vehicle-treated ZDF rats by Student's t-test or the Aspin–Welch test. The results of two-way anova are indicated in insets. Values are mean ± SD (n = 6). n.s., not significant.
Multiple dosing of TAK-875 slows progression of diabetes, and the effect is additively potentiated by the combination with metformin
To expand the results mentioned earlier after acute dosing to the chronic setting, the effects of multiple dosing of TAK-875 on glycaemic control were examined in ZDF rats. To accurately confirm the effects of insulinotropic drugs taken before meals, rats were subjected to time-restricted feeding, in which a high-calorie diet was fed twice daily for 2 h per feeding. TAK-875 (10 mg·kg−1) or metformin (50 mg·kg−1) was administered 30 min before meal twice daily or once daily in the evening respectively. After 6 weeks of treatment, no significant changes in body weight and cumulative food intake were observed, except for significant increase in body weight in ZDF rats receiving metformin alone (P ≤ 0.01) or the combination of TAK-875 and metformin (P ≤ 0.01; Figure 3). In vehicle-treated ZDF rats, fasting plasma glucose levels began to elevate at week 2, and reached hyperglycaemic levels (26.2 ± 1.2 mmol·L−1) at week 6 (Figure 4A). A 6-week treatment with TAK-875, metformin and their combination showed a trend towards decrease in fasting plasma glucose levels by 22.4, 15.1 and 37.9%, respectively, compared with vehicle (Figure 4A, B). Consistent with fasting plasma glucose levels, GHb levels, which reflect long-term glycaemic control, were significantly decreased by 1.7, 1.8 and 2.4% after 6 weeks of treatment with TAK-875, metformin and their combination, respectively, compared with vehicle (Figure 4C), and an additive GHb-lowering effect was observed in their combination (TAK-875, P ≤ 0.05; metformin, P ≤ 0.01; interaction, not significant).
Figure 3.
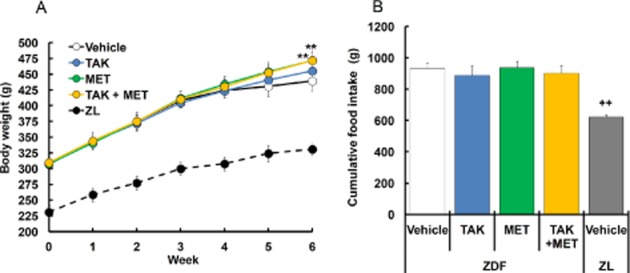
Effects of 6 week treatment with TAK-875 (TAK), metformin (MET) and TAK in combination with MET on body weight and cumulative food intake in ZDF rats. (A) and (B) show body weight and cumulative food intake, respectively, in ZDF rats during 6 weeks of treatment with vehicle, 10 mg·kg−1, b.i.d. of TAK, 50 mg·kg−1, q.d. of MET, or their combination, and in ZL rats during 6 weeks of treatment with vehicle. ** P ≤0.01 compared to vehicle-treated ZDF rats by Dunnett's test or Steel's test. ++P ≤ 0.01 compared to vehicle-treated ZDF rats by Student's t-test or the Aspin–Welch test. Values are mean ± SD (n = 6 for ZDF rats, n = 5 for ZL rats).
Figure 4.
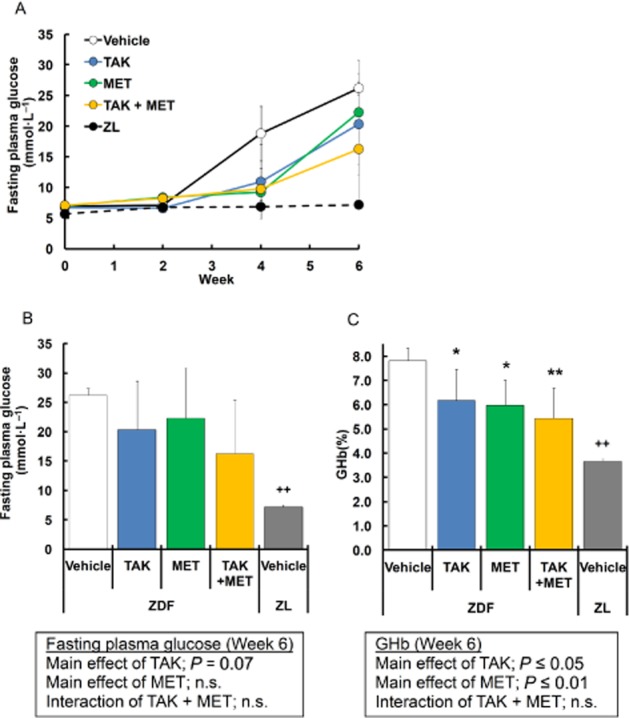
Effects of 6 week treatment with TAK-875 (TAK), metformin (MET) and TAK in combination with MET on glycaemic parameters in ZDF rats. (A) shows fasting plasma glucose levels in ZDF rats during 6 weeks of treatment with vehicle, 10 mg·kg−1, b.i.d. of TAK, 50 mg·kg−1, q.d. of MET, or their combination, and in ZL rats during 6 weeks of treatment with vehicle. Data in (B) and (C) represent fasting plasma glucose and GHb levels after 6 weeks of treatment, respectively. *P ≤ 0.05, **P ≤ 0.01 compared to vehicle-treated ZDF rats by Dunnett's test or Steel's test. ++P ≤ 0.01 compared to vehicle-treated ZDF rats by Student's t-test or the Aspin–Welch test. The results of two-way anova are indicated in insets. Values are mean ± SD (n = 6 for ZDF rats, n = 5 for ZL rats). n.s., not significant.
Multiple dosing of TAK-875 alone or in combination with metformin prevents β-cell dysfunction
To evaluate in vivo effects on pancreatic β-cell function, fasting plasma insulin levels were examined in ZDF rats after multiple dosing. In vehicle-treated ZDF rats, plasma insulin levels were gradually elevated until 4 weeks of treatment, followed by a rapid reduction (Figure 5A), indicating that progressive β-cell dysfunction began around week 4. Plasma insulin levels at week 6 remained 2.2- and 2.4-fold higher in the TAK-875 and metformin groups, respectively, compared with the vehicle group, although the increase was not statistically significant (Figure 5A, B). In combination-treated ZDF rats, plasma insulin levels continued to increase through the study period with a maximal 3.2-fold increase compared with vehicle-treated rats (Figure 5A, B), and the additive increase was observed at week 6 (TAK-875, P ≤ 0.05; metformin, P ≤ 0.01; interaction, not significant). Furthermore, as assessed by HOMA-β after 6 weeks of treatment, β-cell function was increased 5.0-, 4.7- and 9.6-fold in the TAK-875, the metformin and the combination groups, respectively, and significant increases were observed in the metformin and the combination group compared with vehicle (Figure 5C). As often observed in type 2 diabetic patients, ZDF rats exhibited elevated plasma glucagon levels compared with normal rats (Figure 5D). In contrast to plasma insulin levels, plasma glucagon levels were not significantly changed by any of the treatments (Figure 5D). At week 6, vehicle-treated ZDF rats showed significant increases in fasting plasma TG and TC levels, but not NEFA levels, compared with normal rats (Table 1). Plasma TG, TC and NEFA levels were not significantly changed by 6 weeks of treatment with TAK-875 (Table 1). In metformin- and the combination-treated rats, a significant increase in plasma TG was observed compared with vehicle-treated rats (Table 1). Altogether, these results indicate that long-term activation of GPR40 by TAK-875 prevents progressive β-cell dysfunction even in the presence of hyperlipidaemia, and the effects are potentiated by the combination with metformin.
Figure 5.
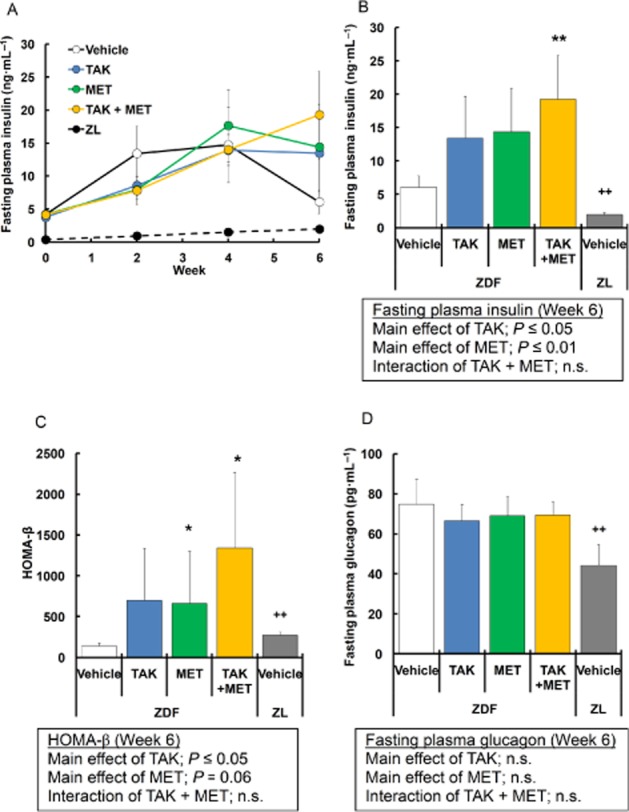
Effects of 6 week treatment with TAK-875 (TAK), metformin (MET) and TAK in combination with MET on plasma insulin and glucagon levels in ZDF rats. (A) shows fasting plasma insulin in ZDF rats during 6 weeks of treatment with vehicle, 10 mg·kg−1, b.i.d. of TAK, 50 mg·kg−1, q.d. of MET, or their combination, and in ZL rats during 6 weeks of treatment with vehicle. Data in (B), (C) and (D) represent fasting plasma insulin levels, homeostasis model assessment of β-cell function (HOMA-β) index calculated from fasting plasma glucose and insulin levels and fasting plasma glucagon levels after 6 weeks of treatment, respectively. *P ≤ 0.05, ** P ≤ 0.01 compared to vehicle-treated ZDF rats by Dunnett's test or Steel's test. ++P ≤ 0.01 compared to vehicle-treated ZDF rats by Student's t-test or the Aspin–Welch test. The results of two-way anova are indicated in insets. Values are mean ± SD (n = 6 for ZDF rats, n = 5 for ZL rats). n.s., not significant.
Table 1.
Effects of 6-week treatment with TAK-875, MET and TAK-875 in combination with MET on plasma lipid profile
| TG | TC | NEFA | ||
|---|---|---|---|---|
| Animal | Compound | (mmol·L−1) | (mmol·L−1) | (mEq·L−1) |
| ZDF | Vehicle | 12.4 ± 2.9 | 4.8 ± 0.5 | 0.44 ± 0.05 |
| ZDF | TAK | 12.9 ± 3.9 | 4.5 ± 0.6 | 0.49 ± 0.11 |
| ZDF | MET | 18.3 ± 3.7a | 5.1 ± 0.4 | 0.48 ± 0.09 |
| ZDF | TAK + MET | 18.0 ± 3.3a | 4.9 ± 0.4 | 0.53 ± 0.1 |
| ZL | Vehicle | 2.1 ± 0.5b | 2.1 ± 0.1b | 0.48 ± 0.05 |
Data show plasma TG, TC and NEFA levels in ZDF rats after 6 weeks of treatment with vehicle, 10 mg·kg−1, b.i.d. of TAK-875 (TAK), 50 mg·kg−1, q.d. of MET, or their combination, and in ZL rats during 6 weeks of treatment with vehicle.
P ≤ 0.05 compared with vehicle-treated ZDF rats by Dunnett's test or Steel's test.
P ≤ 0.01 compared with vehicle-treated ZDF rats by Student's t-test or the Aspin–Welch test. Values are mean ± SD (n = 6 for ZDF rats, n = 5 for ZL rats). MET, metformin.
Pancreatic insulin content is maintained at normal levels in rats given TAK-875 and metformin in combination
Pancreatic hormone contents were measured at the end of study period. In vehicle-treated ZDF rats, pancreatic insulin content was significantly decreased to 37.7% of that in normal rats (Figure 6A). After 44 days of treatment with TAK-875 and metformin, pancreatic insulin contents were 1.6- and 2.2-fold higher, respectively, than in the vehicle group, although the differences did not reach statistical significance (Figure 6A). The combination of TAK-875 and metformin maintained a significantly higher level of pancreatic insulin content, which was almost equivalent to that in normal rats (vehicle: 26, combination: 67.1; normal lean: 69.1 ng·mg−1 pancreas; Figure 6A). On the other hand, pancreatic glucagon contents were not significantly different among the ZDF groups (Figure 6B).
Figure 6.
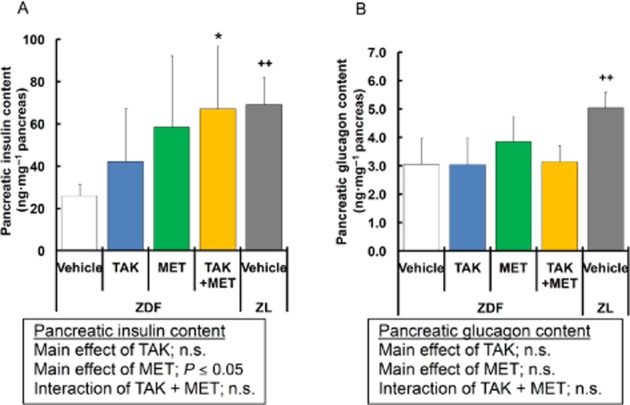
Effects of 44 day treatment with TAK-875 (TAK), metformin (MET) and TAK in combination with metformin on pancreatic insulin and glucagon content. Data in (A) and (B) represent pancreatic insulin and glucagon content, respectively, in ZDF rats after 44 days of treatment with vehicle, 10 mg·kg−1, b.i.d. of TAK, 50 mg·kg−1, q.d. of MET, or their combination, and in ZL rats after 44 days of treatment with vehicle. *P ≤ 0.05 compared to vehicle-treated ZDF rats by Dunnett's test or Steel's test. ++P ≤ 0.01 compared to vehicle-treated ZDF rats by Student's t-test or the Aspin–Welch test. The results of two-way anova are indicated in insets. Values are mean ± SD (n = 6 for ZDF rats, n = 5 for ZL rats). n.s., not significant.
Structural abnormality and altered protein expression necessary for β-cell function and proliferation are improved by TAK-875 and metformin in combination
Pancreata isolated from rats were analysed by immunohistochemistry using anti-insulin, anti-glucagon, anti-PDX-1 and anti-PCNA antibodies at the end of the study period. Immunostaining for insulin revealed that islets in vehicle-treated ZDF rats were enlarged and disorganized with extensions into the surrounding exocrine tissue compared with those in normal rats (Figure 7A, Q). Moreover, widespread distribution of glucagon-positive α-cells and reduced PDX-1 expression throughout the islets were observed in vehicle-treated ZDF rats (Figure 7B, C, R, S). Multiple dosing of TAK-875 and metformin did not produce detectable changes in islet morphology or in the expression of insulin, glucagon and PDX-1 in ZDF rats (Figure 7A-C, E–G, I–K). In contrast, in ZDF rats receiving TAK-875 and metformin in combination, many insulin-positive cells displayed relatively normal round and dense islet architecture compared with those in vehicle-treated ZDF rats (Figure 7A, M). The distributions of glucagon-positive cells in islets were not different between the combination and vehicle groups (Figure 7B, N). Interestingly, high levels of PDX-1 expression were maintained in nucleus and cytosol of islets from combination-treated ZDF rats, as observed in those from ZL rats (Figure 7C, O, S). Moreover, immunostaining of PCNA showed increased PCNA-positive cell numbers in islets from the combination group compared with the vehicle group (arrows in Figure 7P). The double staining revealed the co-localizations of PCNA-positive nuclei with insulin staining in these islets (Supporting Information Figure S1). Taken together, these results indicate that multiple dosing of TAK-875 and metformin in combination could exert beneficial effects on pancreatic β-cell function and mass in ZDF rats.
Figure 7.
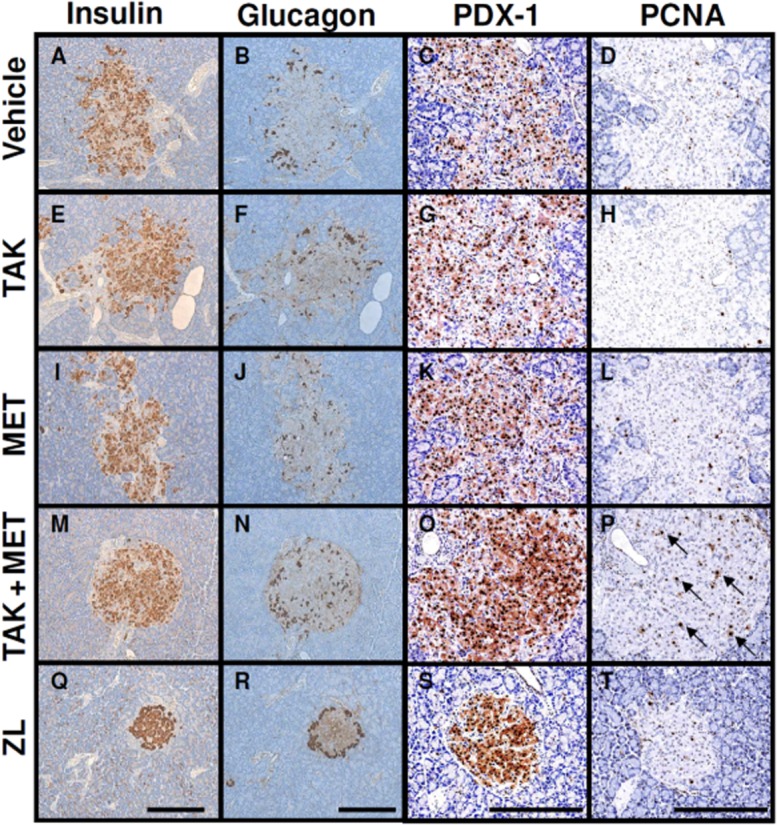
Effects of 44 day treatment with TAK-875 (TAK), metformin (MET) and TAK in combination with MET on insulin staining, glucagon staining, PDX-1 expression and PCNA-positive cell numbers in pancreatic islets from ZDF rats. ZDF rats were administered vehicle (A–D), 10 mg·kg−1, b.i.d. of TAK (E–H), 50 mg·kg−1, q.d. of MET (I–L), or their combination (M–P) for 44 days, and ZL rats were administered vehicle (Q–T). The pancreata were isolated and immunostained with anti-insulin (A, E, I, M, Q), anti-glucagon (B, F, J, N, R), anti-PDX-1 (C, G, K, O, S) and anti-PCNA (D, H, L, P, T) antibodies. Representative images for each group are shown. Arrows indicate PCNA-positive cells. Scale bar = 250 μm.
Discussion and Conclusions
TAK-875, a potent, selective and orally available GPR40 agonist, provides glucose-dependent insulin secretion through activation of the Gαq-signalling pathway and amplification of intracellular Ca2+ (Tsujihata et al., 2011; Yashiro et al., 2012), which is a mechanism distinct from those of other clinically available oral insulinotropic drugs, including SUs and DPP-4 inhibitors (Winzell and Ahren, 2007). We previously showed that acute administration of TAK-875 improved postprandial hyperglycaemia due to augmentation of insulin secretion in non-obese type 2 diabetic rats (Tsujihata et al., 2011). In addition, it has been demonstrated that TAK-875 significantly improved glycaemic control in type 2 diabetic patients with minimum risk of hypoglycaemia compared with SUs (Araki et al., 2012; Burant et al., 2012). Given that TAK-875 could become a new class of anti-hyperglycaemic agent, there is a need to evaluate its potential use in combination treatment with existing anti-hyperglycaemic agents. In the present study, we examined the effects of combination treatment with TAK-875 and metformin, which is a first-line drug for treatment of type 2 diabetes, on glycaemic control, pancreatic β-cell function and islet morphology in male ZDF rats. This model shows insulin resistance at a young age, after which severe hyperglycaemia and progressive β-cell dysfunction develop, due at least in part to insufficient compensation for insulin resistance. It is therefore suitable for evaluation of combination effects of insulin secretagogues and insulin sensitizers (Lee et al., 1994; Finegood et al., 2001).
In this study, 6-week multiple dosing of TAK-875 significantly decreased GHb levels by 1.7%, and the efficacy was almost comparable with that of metformin. Several non-clinical studies with GPR40 agonists other than TAK-875 have been described in recent years. These results show that glucose-lowering and insulinotropic effects during an intraperitoneal glucose tolerance test are well maintained in obese rodents after multiple dosing (Tan et al., 2008; Lin et al., 2011), indicating a lack of tachyphylaxis of GPR40 agonists. However, distinct anti-diabetic effects by long-term activation of GPR40 have not been demonstrated. In this study, we demonstrated for the first time that administration of the GPR40 agonist TAK-875 not only acutely improved both postprandial and fasting hyperglycaemia, but also chronically delayed the progression of diabetes in a rodent model. Our findings strongly support clinical results (Araki et al., 2012; Burant et al., 2012).
While FFAs acutely stimulate insulin secretion, chronic exposure to them is reported to cause β-cell dysfunction and cell death. Although GPR40 has been considered to be possibly involved in lipotoxicity (Steneberg et al., 2005), a number of experimental observations do not support a central role for GPR40 in lipotoxicity (Latour et al., 2007; Kebede et al., 2008; Nagasumi et al., 2009). In our previous study, prolonged exposure to TAK-875 did not cause β-cell dysfunction in vitro, in contrast to FFA (Tsujihata et al., 2011). In ZDF rats, hyperlipidaemia precedes the rise in plasma glucose, and these lipotoxic effects on β-cells are thought to contribute to gradual progression of β-cell dysfunction (Lee et al., 1994). Actually, in the present study, hyperlipidaemia was observed in ZDF rats compared with normal rats. Despite the high plasma lipid levels, TAK-875 achieved the improvement in glycaemic control with a trend towards increase in fasting plasma insulin levels and pancreatic insulin content, and no changes in islet morphology. These results indicate that long-term activation of GPR40 by TAK-875 could have beneficial effects on pancreatic β-cell function even in the presence of hyperlipidaemia. Our results support a previous in vitro study showing that GPR40 is not involved in the chronic toxic effects of FFAs (Tsujihata et al., 2011).
Metformin provides glucose-lowering effects via pleiotropic mechanisms including decreased hepatic glucose production, increased peripheral glucose disposal and reduced intestinal glucose absorption (Hundal and Inzucchi, 2003). As expected from their complementary mechanisms of action, acute administration of TAK-875 and metformin in combination resulted in a greater decrease in postprandial and fasting hyperglycaemia compared with each drug alone, despite the fact that insulin secretion was almost the same as that in rats treated with TAK-875 alone. This insulin-sparing action of the combination could lead to the alleviation of β-cell exhaustion. In fact, chronic dosing of TAK-875 and metformin in combination significantly decreased GHb levels by 2.4% and prevented the progressive decline in plasma insulin levels, both of which effects were greater than those seen with either drug alone. Furthermore, the combination treatment showed a significant increase in HOMA-β and pancreatic insulin content, and the insulin content was comparable with that in normal rats. As assessed by immunohistochemistry, normal rounded islet architecture, elevated PDX-1 expression and increased PCNA-positive cell numbers were observed in the combination group. Considering that the balance of β-cell proliferation and apoptosis can control pancreatic β-cell mass, thereby forming islet architecture (Butler et al., 2003), relatively normal islet architecture in the combination group may be maintained due to increased β-cell proliferation. Altogether, these results suggest that this combination strategy is effective for preserving β-cell function in ZDF rats.
The mechanism of the strong protective effects on β-cells by the combination of TAK-875 and metformin is not fully understood. Previous reports have demonstrated that prevention of progressive hyperglycaemia per se preserves insulin and PDX-1 gene expression by relieving glucotoxicity on β-cells in ZDF rats (Harmon et al., 1999). Thus, improvement in β-cell function in the combination group observed in the present study may be mediated, at least in part, by improved glycaemic control. On the other hand, Janssen et al. (2003) have reported that glycaemic control by treatment with phlorizin, a renal sodium-dependent glucose transport inhibitor, prevents the decrease in plasma insulin, but not the progressive histochemical changes in islets in these rats, implying the involvement of direct trophic effects of TAK-875 and metformin on β-cell survival in our studies. It has been very recently shown that chronic activation of designer Gq-coupled receptor in β-cells leads to the sequential activation of ERK1/2 and IRS-2 signalling, thus triggering a series of events that greatly improve β-cell function (Jain et al., 2013). Because GPR40 is one of the Gq-coupled receptors highly expressed in β-cells, there is a possibility that TAK-875 may directly activate ERK1/2 and IRS-2 cascade in β-cells, leading to improved β-cell function. Indeed, it has been reported that activation of GPR40 by endogenous ligand FFA causes increased ERK1/2 phosphorylation in mouse insulinoma MIN6 cells (Itoh et al., 2003). While metformin suppresses hepatic glucose production in liver, the direct effects in pancreatic β-cells have also been reported. The therapeutic concentration of metformin has been also shown to cause β-cell protection against glucolipotoxicity (Lupi et al., 1999; 2002; Marchetti et al., 2004), although there is an ongoing debate whether activation of AMPK has positive or negative impact on pancreatic β-cell function (Fu et al., 2013). These direct protective effects of TAK-875 and metformin in β-cells might be particularly potentiated in their combination treatment, by which the additive glycaemic control has been achieved. Further studies are required to clarify how TAK-875 directly affects β-cell growth or how TAK-875 and metformin can interact with each other in β-cells. Alternatively, recent clinical observation indicates that metformin increases glucagon-like peptide-1 (GLP-1) concentration (Mannucci et al., 2001). GPR40 is also expressed in enteroendocrine cells, and mediates FFA-induced GLP-1 secretion (Edfalk et al., 2008). It is well known that GLP-1 has protective effects on β-cells (Perfetti and Hui, 2004). Although plasma GLP-1 levels were not assessed in this study, the potential role of GLP-1 in the combination effects should be assessed in a future study.
The combination of SU drugs and metformin is one of the most frequently used combination therapies, but increases the risk of hypoglycaemia (Blonde et al., 2002). Our study showed that plasma glucose levels just before glucose load (time 0) in ZDF rats receiving a combination of TAK-875 and metformin were similar to those of normal rats. These results suggest that the combination of TAK-875 and metformin may pose a low risk of hypoglycaemia due to the glucose-dependent insulin secretion seen with TAK-875 (Tsujihata et al., 2011). Combination therapy with metformin and DPP-4 inhibitors, which augments insulin secretion via elevation of active forms of incretins, is currently attracting attention because of the additive improvement in glycaemic control with a minimum risk of hypoglycaemia (Goldstein et al., 2007). The recent in vitro report has shown that high concentration of glucose or metformin regulates expressions of GLP-1 and glucose-dependent insulinotropic polypeptide receptor, but not GPR40 in β-cells (Pan et al., 2009). In future studies, it will be of interest to determine whether expression of GPR40 can be regulated by diabetic condition and combination with other anti-diabetic drugs in vivo.
In diabetic patients, hyperglucagonemia has been reported (Sloop et al., 2005) and is involved in hyperglycaemia by increasing hepatic glucose production. In the present study, ZDF rats showed elevated plasma glucagon levels compared with normal rats. It has been previously reported that GPR40 mediates FFA-induced glucagon secretion via GPR40 in α-cells (Flodgren et al., 2007). In our study, TAK-875 did not affect plasma glucagon levels, pancreatic glucagon content or distribution of glucagon-positive cells in ZDF rats. These results are consistent with the previously reported in vitro study using human pancreatic islets and in clinical data on diabetic patients (Araki et al., 2012; Yashiro et al., 2012).
In our study, increases in body weight were observed in the metformin and the combination groups. Consistent with these observations, the DPP-4 inhibitor alogliptin and pioglitazone in combination increased body weight compared with pioglitazone alone in db/db mice, a diabetic model with progressive β-cell dysfunction like ZDF rats (Moritoh et al., 2009). This may be associated with anabolic effects of elevated plasma insulin. Moreover, significant increases in fasting plasma TG levels in the metformin and combination groups were observed at week 6 in this study. Because hyperinsulinaemia promotes hepatic lipogenesis via activation of lipogenic transcriptional factor sterol regulatory element-binding protein-1c (Yecies et al., 2011), thereby causing an increase in plasma TG levels, plasma TG levels in the vehicle-treated ZDF rats may have been decreased by the reduction of plasma insulin levels at week 6.
In conclusion, the present study is the first to demonstrate that chronic activation of GPR40 by TAK-875 has the potential to slow the progression of diabetes without accelerating the progression of β-cell dysfunction in a diabetic animal model. Our findings also suggest that combination therapy with TAK-875 and metformin may be a valuable strategy for glycaemic control and β-cell preservation in patients with type 2 diabetes.
Acknowledgments
We are very grateful to Drs. Seigo Izumo, Yukio Yamada and Masakuni Noda for valuable discussions and helpful suggestions. Also, thanks are due to Dr. Yusuke Moritoh for helpful support, Shoichi Asano for technical assistance with animal testing, and Kazuko Hirai for technical assistance with immunohistochemistry. Parts of these studies were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, CA, 24–28 June 2011.
Glossary
- DPP-4
dipeptidyl peptidase-4
- FFAs
free fatty acids
- GHb
glycosylated Hb
- GLP-1
glucagon-like peptide-1
- GPR40
GPCR40
- HOMA-β
homeostasis model assessment of β-cell function
- NEFA
non-esterified fatty acids
- OGTT
oral glucose tolerance test
- PDX-1
pancreas duodenum homeobox-1
- SU
sulfonylureas
- TC
total cholesterol
- TG
triglyceride
- ZDF
Zucker diabetic fatty
- ZL
Zucker lean
Conflicts of interest
All authors are current employees of Takeda Pharmaceutical Company Limited, Kanagawa, Japan.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Effects of 44-day treatment with TAK-875, metformin and TAK-875 in combination with metformin on insulin- and PCNA-double positive cell numbers in pancreatic islets from ZDF rats. ZDF rats were administered the combination 10 mg·kg−1, b.i.d. of TAK-875 and 50 mg·kg−1, q.d. of metformin for 44 days. The pancreata were isolated and immunostained with anti-insulin (red colour) and anti-PCNA (brown colour) antibodies. Arrows indicate the representative image of the co-localization of PCNA-positive nuclei with insulin staining. Scale bar = 250 μm.
References
- Araki T, Hirayama M, Hiroi S, Kaku K. GPR40-induced insulin secretion by the novel agonist TAK-875: first clinical findings in patients with type 2 diabetes. Diabetes Obes Metab. 2012;14:271–278. doi: 10.1111/j.1463-1326.2011.01525.x. [DOI] [PubMed] [Google Scholar]
- Blonde L, Rosenstock J, Mooradian AD, Piper BA, Henry D. Glyburide/metformin combination product is safe and efficacious in patients with type 2 diabetes failing sulphonylurea therapy. Diabetes Obes Metab. 2002;4:368–375. doi: 10.1046/j.1463-1326.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- Burant CF, Viswanathan P, Marcinak J, Cao C, Vakilynejad M, Xie B, et al. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:1403–1411. doi: 10.1016/S0140-6736(11)61879-5. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Charpentier G. Oral combination therapy for type 2 diabetes. Diabetes Metab Res Rev. 2002;18(Suppl. 3):S70–S76. doi: 10.1002/dmrr.278. [DOI] [PubMed] [Google Scholar]
- Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, et al. Beta-cell mass dynamics in Zucker diabetic fatty rats: rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- Flodgren E, Olde B, Meidute-Abaraviciene S, Winzell MS, Ahren B, Salehi A. GPR40 is expressed in glucagon producing cells and affects glucagon secretion. Biochem Biophys Res Commun. 2007;354:240–245. doi: 10.1016/j.bbrc.2006.12.193. [DOI] [PubMed] [Google Scholar]
- Fu A, Eberhard CE, Screaton RA. Role of AMPK in pancreatic beta cell function. Mol Cell Endocrinol. 2013;366:127–134. doi: 10.1016/j.mce.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Maekawa F, Yada T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab. 2005;289:E670–E677. doi: 10.1152/ajpendo.00035.2005. [DOI] [PubMed] [Google Scholar]
- Giorgino F, Laviola L, Leonardini A. Pathophysiology of type 2 diabetes: rationale for different oral antidiabetic treatment strategies. Diabetes Res Clin Pract. 2005;68(Suppl. 1):S22–S29. doi: 10.1016/j.diabres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–1987. doi: 10.2337/dc07-0627. [DOI] [PubMed] [Google Scholar]
- Han SJ, Choi SE, Kang Y, Jung JG, Yi SA, Kim HJ, et al. Effect of sitagliptin plus metformin on beta-cell function, islet integrity and islet gene expression in Zucker diabetic fatty rats. Diabetes Res Clin Pract. 2011;92:213–222. doi: 10.1016/j.diabres.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Harmon JS, Gleason CE, Tanaka Y, Oseid EA, Hunter-Berger KK, Robertson RP. In vivo prevention of hyperglycemia also prevents glucotoxic effects on PDX-1 and insulin gene expression. Diabetes. 1999;48:1995–2000. doi: 10.2337/diabetes.48.10.1995. [DOI] [PubMed] [Google Scholar]
- Hundal RS, Inzucchi SE. Metformin: new understandings, new uses. Drugs. 2003;63:1879–1894. doi: 10.2165/00003495-200363180-00001. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Jain S, de Azua IR, Lu H, White MF, Guettier JM, Wess J. Chronic activation of a designer Gq-coupled receptor improves beta cell function. J Clin Invest. 2013;123:1750–1762. doi: 10.1172/JCI66432. doi: 10.1172/JCI66432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen SW, Martens GJ, Sweep CG, Span PN, Verhofstad AA, Hermus AR. Phlorizin treatment prevents the decrease in plasma insulin levels but not the progressive histopathological changes in the pancreatic islets during aging of Zucker diabetic fatty rats. J Endocrinol Invest. 2003;26:508–515. doi: 10.1007/BF03345212. [DOI] [PubMed] [Google Scholar]
- Kahn SE. The importance of the beta-cell in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000;108(Suppl. 6a):2S–8S. doi: 10.1016/s0002-9343(00)00336-3. [DOI] [PubMed] [Google Scholar]
- Kebede M, Alquier T, Latour MG, Semache M, Tremblay C, Poitout V. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes. 2008;57:2432–2437. doi: 10.2337/db08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56:1087–1094. doi: 10.2337/db06-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DC, Zhang J, Zhuang R, Li F, Nguyen K, Chen M, et al. AMG 837: a novel GPR40/FFA1 agonist that enhances insulin secretion and lowers glucose levels in rodents. PLoS ONE. 2011;6:e27270. doi: 10.1371/journal.pone.0027270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi R, Del Guerra S, Tellini C, Giannarelli R, Coppelli A, Lorenzetti M, et al. The biguanide compound metformin prevents desensitization of human pancreatic islets induced by high glucose. Eur J Pharmacol. 1999;364:205–209. doi: 10.1016/s0014-2999(98)00807-3. [DOI] [PubMed] [Google Scholar]
- Lupi R, Del Guerra S, Fierabracci V, Marselli L, Novelli M, Patanè G, et al. Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes. 2002;51(Suppl. 1):S134–S137. doi: 10.2337/diabetes.51.2007.s134. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, et al. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care. 2001;24:489–494. doi: 10.2337/diacare.24.3.489. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, et al. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab. 2004;89:5535–5541. doi: 10.1210/jc.2004-0150. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Melloul D. Transcription factors in islet development and physiology: role of PDX-1 in beta-cell function. Ann N Y Acad Sci. 2004;1014:28–37. doi: 10.1196/annals.1294.003. [DOI] [PubMed] [Google Scholar]
- Moritoh Y, Takeuchi K, Asakawa T, Kataoka O, Odaka H. Combining a dipeptidyl peptidase-4 inhibitor, alogliptin, with pioglitazone improves glycaemic control, lipid profiles and beta-cell function in db/db mice. Br J Pharmacol. 2009;157:415–426. doi: 10.1111/j.1476-5381.2009.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasumi K, Esaki R, Iwachidow K, Yasuhara Y, Ogi K, Tanaka H, et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes. 2009;58:1067–1076. doi: 10.2337/db08-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik H, Vakilynejad M, Wu J, Viswanathan P, Dote N, Higuchi T, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamic properties of the GPR40 agonist TAK-875: results from a double-blind, placebo-controlled single oral dose rising study in healthy volunteers. J Clin Pharmacol. 2012;52:1007–1016. doi: 10.1177/0091270011409230. [DOI] [PubMed] [Google Scholar]
- Negoro N, Sasaki S, Mikami S, Ito M, Suzuki M, Tsujihata Y, et al. Discovery of TAK-875: a potent, selective, and orally bioavailable GPR40 agonist. ACS Medicinal. Chem Lett. 2010;1:290–294. doi: 10.1021/ml1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoro N, Sasaki S, Mikami S, Ito M, Tsujihata Y, Ito R, et al. Optimization of (2,3-dihydro-1-benzofuran-3-yl)acetic acids: discovery of a non-free fatty acid-like, highly bioavailable G-protein coupled receptor 40/free fatty acid receptor 1 agonist as a glucose-dependent insulinotropic agent. J Med Chem. 2012;55:3960–3974. doi: 10.1021/jm300170m. [DOI] [PubMed] [Google Scholar]
- Pan QR, Li WH, Wang H, Sun Q, Xiao XH, Brock B, et al. Glucose, metformin, and AICAR regulate the expression of G protein-coupled receptor members in INS-1 beta cell. Horm Metab Res. 2009;41:799–804. doi: 10.1055/s-0029-1234043. [DOI] [PubMed] [Google Scholar]
- Perfetti R, Hui H. The role of GLP-1 in the life and death of pancreatic beta cells. Horm Metab Res. 2004;36:804–810. doi: 10.1055/s-2004-826167. [DOI] [PubMed] [Google Scholar]
- Pratley RE. Alogliptin: a new, highly selective dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2009;10:503–512. doi: 10.1517/14656560802694713. [DOI] [PubMed] [Google Scholar]
- Rafacho A, Ribeiro DL, Boschero AC, Taboga SR, Bosqueiro JR. Increased pancreatic islet mass is accompanied by activation of the insulin receptor substrate-2/serine-threonine kinase pathway and augmented cyclin D2 protein levels in insulin-resistant rats. Int J Exp Pathol. 2008;89:264–275. doi: 10.1111/j.1365-2613.2008.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs. 2004;64:1339–1358. doi: 10.2165/00003495-200464120-00006. [DOI] [PubMed] [Google Scholar]
- Shapiro H, Shachar S, Sekler I, Hershfinkel M, Walker MD. Role of GPR40 in fatty acid action on the beta cell line INS-1E. Biochem Biophys Res Commun. 2005;335:97–104. doi: 10.1016/j.bbrc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Sloop KW, Michael MD, Moyers JS. Glucagon as a target for the treatment of type 2 diabetes. Expert Opin Ther Targets. 2005;9:593–600. doi: 10.1517/14728222.9.3.593. [DOI] [PubMed] [Google Scholar]
- Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Tan CP, Feng Y, Zhou YP, Eiermann GJ, Petrov A, Zhou C, et al. Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice. Diabetes. 2008;57:2211–2219. doi: 10.2337/db08-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujihata Y, Ito R, Suzuki M, Harada A, Negoro N, Yasuma T, et al. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J Pharmacol Exp Ther. 2011;339:228–237. doi: 10.1124/jpet.111.183772. [DOI] [PubMed] [Google Scholar]
- U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16: overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- Williams-Herman D, Xu L, Teng R, Golm GT, Johnson J, Davies MJ, et al. Effect of initial combination therapy with sitagliptin and metformin on beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2012;14:67–76. doi: 10.1111/j.1463-1326.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- Winzell MS, Ahren B. G-protein-coupled receptors and islet function – implications for treatment of type 2 diabetes. Pharmacol Ther. 2007;116:437–448. doi: 10.1016/j.pharmthera.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Yashiro H, Tsujihata Y, Takeuchi K, Hazama M, Johnson PR, Rorsman P. The effects of TAK-875, a selective GPR40/FFA1 agonist, on insulin and glucagon in isolated rat and human islets. J Pharmacol Exp Ther. 2012;340:483–489. doi: 10.1124/jpet.111.187708. [DOI] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


