Abstract
Studies of sex differences in the timing of human circadian rhythms have reported conflicting results. This may be because the studies conducted to date have not controlled for the masking effects of the rest-activity cycle on the circadian rhythms being assessed. In the present analysis of data collected under controlled conditions, we examined sex differences in the timing of circadian rhythms while minimizing masking from behavioral and environmental factors using a constant routine (CR) protocol. All participants (28 women and 28 men paired by habitual wake time; age range 18-30) maintained a regular self-selected sleep-wake schedule at home prior to the study. After three baseline days in the laboratory, participants began a CR. Women were found to have a significantly higher melatonin amplitude and lower temperature amplitude than men. While sleep timing was the same between the two groups, the timing of the circadian rhythms of core body temperature and pineal melatonin secretion was earlier relative to sleep time in women as compared to men. Sleep therefore occurred at a later biological time for women than men, despite being at the same clock time. Given that sleep propensity and structure vary with circadian phase and are impacted by circulating melatonin, these findings may have important implications for understanding sex differences in sleep timing and duration, diurnal preference, and the prevalence of sleep disorders such as insomnia.
Keywords: gender differences, circadian phase, entrainment, core body temperature, melatonin, sleep wake cycle
Introduction
The periodic light-dark [LD] cycle entrains (synchronizes) the near-24-hour circadian timing system to the 24-hour environmental day (Pittendrigh and Daan, 1976). Though organisms may share the same LD cycle, the timing of behavioral and physiological rhythms relative to the LD cycle can vary between species and between individuals within a species. In the hamster, sex differences in the timing of behavioral rhythms on an LD cycle have been reported, with females having an earlier activity onset relative to males (Davis et al., 1983). Whether humans display similar sex differences in the timing of biological rhythms remains unresolved.
There are conflicting reports of whether humans show sex differences in the timing of the circadian rhythm of core temperature. In an early study (Mellette et al., 1951), the temperature minimum was found to occur earlier in women, but temperature maximum was found to occur laterer. In contrast, another study (Winget et al., 1977) found that phase angle of entrainment of body temperature (relative to the LD cycle) was similar in women and men studied. It is difficult to interpret this finding, because women and men were exposed to different photoperiods, with men on a 16:8 LD cycle and women on an 18:6 LD cycle. A field study (Kattapong et al., 1995) also found no sex difference in the time of the temperature minimum. Contrary to those results, two field studies have demonstrated significantly earlier temperature rhythms in women (Baehr et al., 2000; Baker et al., 2001), and two studies of older participants have reported significantly earlier core body temperature timing in older women (Campbell et al., 1989; Moe et al., 1991).
Given that both the activity/rest cycle (Waterhouse et al., 1999) and menstrual phase (Coyne et al., 2000; Lee, 1988) can mask the endogenous circadian component of the core body temperature rhythm, using temperature as a phase marker is not ideal for determining whether there are sex differences in the timing of human circadian rhythms. Because melatonin levels are less influenced by activity, posture, sleep, and menstrual phase, the melatonin rhythm has been advocated as a better marker of circadian phase in humans (Klerman et al., 2002). A recent study (Mongrain et al., 2004) reported that young women had a significantly earlier dim light melatonin onset (DLMO) relative to men. In that study, DLMO was determined as the point at which a subject's melatonin levels rose to twice the assay sensitivity. Because there are large inter-individual differences in melatonin levels (Wilson et al., 1977; Zeitzer et al., 1999), using a fixed threshold to determine DLMO cannot always distinguish between an earlier timing of melatonin onset and a higher overall melatonin level. Any sex difference in amplitude of the melatonin rhythm could appear to be a phase difference if the same threshold is used to determine DLMO for all participants.
To assess circadian phase and amplitude in humans while controlling for masking effects induced by day-night changes in posture and behavior, the constant routine (CR) protocol is used (Duffy and Dijk, 2002; Mills et al., 1978). The CR consists of a regimen of semi-recumbent wakefulness in constant dim lighting conditions for at least one circadian cycle (>24 hours), with food and fluid spread evenly across day and night The CR eliminates periodic changes in behavior and holds environmental conditions constant, allowing the endogenous circadian variation in physiologic measures (core temperature, plasma hormone levels) to be assessed with greater precision (Klerman et al., 1999).
For our analysis, we selected data collected in the CR protocol in order to determine whether there are sex differences in the phase angle of entrainment. We hypothesized that women would have an earlier phase than men relative to sleep/wake times for both melatonin and temperature circadian phase markers.
Materials and Methods
Participants
Data from healthy young participants (18-30 years old) were selected for the current analysis. The participants were chosen from a group of 148 (42 women and 106 men) who took part in one of five laboratory protocols carried out in the Intensive Physiological Monitoring Unit of the General Clinical Research Center at the Brigham and Women's Hospital (Duffy et al., 2009; Khalsa et al., 2003; Zeitzer et al., 2000). The distribution of studies was approximately even across seasons for both sexes. These studies each had similar screening procedures, inclusion/exclusion criteria, pre-study sleep-wake schedule requirements, and baseline inpatient study conditions before the CR.
To control for potential sex differences in sleep-wake timing, we matched each woman with a male participant who took part in the same study, based on habitual wake time (± 30 minutes). This was done because women have been reported to be more morning-type than men (Adan and Natale, 2002; Chelminski et al., 1997), and morning-types typically self-select earlier sleep-wake times (Baehr et al., 2000; Duffy et al., 1999). We also considered age when matching the female-male pairs, ensuring the pairs were 5 years or fewer different in age.
Of the 42 women who participated in the five studies, 14 were not used in the current analysis: seven because no man in the same study had a habitual wake time within ± 30 minutes, four due to incomplete or missing data, and three who were using hormonal birth control because of reports that hormonal birth control may raise melatonin levels (Kostoglou-Athanassiou et al., 1998; Wright Jr. and Badia, 1999). The remaining 28 women were paired with 28 men for the current analysis.
All participants were medically and psychologically healthy and were required to have body mass index (BMI) between 20 and 29.9 (Duffy et al., 2009; Khalsa et al., 2003; Zeitzer et al., 2000). They were asked to refrain from using alcohol, nicotine, caffeine, prescription and non-prescription drugs, and dietary supplements both prior to and throughout the course of the study, and compliance was verified using urinary toxicological analysis upon admission to the laboratory. The phase of the menstrual cycle at which the women began the study was not controlled.
Participants had to self-report a habitual sleep duration between seven and nine hours per night. To ensure that each participant was stably entrained to their schedule, no participant who reported recent (within one year) regular night shift work or recent (with three months) travel across more than one time zone, was enrolled. They were also required to maintain a regular sleep-wake schedule for at least one week immediately prior to the study, such that their bedtimes and wake times occurred at the same time (± 30 minutes) each day and were eight hours apart. The timing of the eight hour sleep opportunity was self-selected based on their habitual sleep/wake times. Compliance with the sleep-wake schedule during the week immediately prior to the study was verified by wrist actigraphy. Most participants (21 of the 28 pairs) completed the Horne-Östberg morningness-eveningness questionnaire (Horne and Östberg, 1976) during screening, although this was not used as an inclusion/exclusion criterion.
The studies were approved by the Human Research Committee of the Partners HealthCare System and were conducted in accordance with the Declaration of Helsinki. Each participant gave written informed consent.
Experimental Protocol
All studies began with three 24-hour baseline days consisting of 16 hours of wakefulness and 8 hours of scheduled bed rest in the dark. The sleep/wake schedule for each participant was calculated from bedtimes and wake times reported from the previous week of screening. During baseline wake episodes, the participants were free to move about their study room, but were not allowed to lie down or nap. Sixteen female-male pairs had normal levels of indoor room light [0.23 W/m2 (∼89 lux)] throughout all three baseline days. For the remaining 12 female-male pairs, light levels were dimmed [∼0.0087 W/m2 (∼3.3 lux)] for the last 8 hours of the third baseline day. In all study rooms, the light was produced by overhead fluorescent lamps.
The three baseline days were followed by a CR, which began upon awakening after the third baseline night. During the CR, participants remained in a semi-recumbent posture (lying in bed with the head of the bed elevated ∼45°), in dim light [approximately 0.0048 W/m2 (∼1.8 lux)] and were fed equicaloric snacks each hour. Participants were not permitted to change posture throughout the CR, and a staff member remained in the room to help maintain wakefulness. The CRs varied in duration from 27 to 50 hours, depending on the requirements of the particular study the participant took part in. In cases where the CR lasted more than 40 hours, only the first 40 hours of data were used in our analysis.
Core body temperature was recorded at one-minute intervals via disposable rectal thermistor (Measurement Specialties TPG, Dayton, OH).
Blood samples were collected 1-2 times per hour via an indwelling catheter inserted into a forearm vein, and connected to 12-ft tubing passed through a porthole in the wall so that the blood samples (typically 1-3 mL) could be collected from outside the study room. Plasma samples were frozen and sent to Pharmasan, Inc. (Osceola, WI) for radioimmunoassay for melatonin using an assay with a sensitivity of 0.7 pg/ml, an intra-assay coefficient of variation of 9%, and an interassay coefficient of variation of 10%.
Data Analysis
Core body temperature phase was assessed by the maximum likelihood fit of a two-harmonic regression model with first order autoregressive noise (Brown and Czeisler, 1992). The first five hours and the final 30 minutes of temperature data were excluded from analysis to eliminate the masking effects of waking and changing posture at the beginning and end of the CR. The minimum of the fundamental and the first harmonic components of the model fit to the data were averaged. This average was used as the core body temperature minimum (CBTmin) circadian phase reference marker.
Due to the large variability in nocturnal melatonin concentrations between individuals (Wilson et al., 1977; Zeitzer et al., 1999), and potential sex differences in melatonin levels, we chose an individualized method of determining melatonin phase (Benloucif et al., 2008; Klerman et al., 2002). We defined melatonin onset as the time at which plasma melatonin levels rose to 25% of the fitted nightly peak (termed here DLMOn25%). The nightly melatonin peak was determined by fitting a 3-harmonic waveform to the data from the CR (after eliminating the initial 5 hours and last 30 minutes). The amplitude of the fitted waveform (maximum – minimum of fitted waveform) was used to derive the 25% crossover threshhold. We calculated 25% of the amplitude, and interpolated between adjacent samples to determine the minute when the plasma levels rose to 25% of that nightly peak level. Plasma melatonin offset was defined as the time when melatonin levels fell to 25% of their nightly peak (DLMOff25%). This value is used as a circadian reference point and does not represent an estimated offset of melatonin synthesis. Because it is common to use a fixed threshold to determine DLMO (Lewy et al., 1985), we also calculated DLMO as the point at which melatonin levels reached 10 pg/ml. To determine whether the window of melatonin release was different between men and women, the length of time between DLMOn25% and DLMOff25% was calculated. To determine whether there was a sex differences in amount of melatonin release, area under the curve (AUC) between DLMOn25% and DLMOff25% was calculated using the trapezoidal method (Zeitzer et al., 2000).
Phase angles for melatonin and temperature circadian phase markers were calculated for each participant. Phase angle for DLMOn25% was calculated relative to habitual bedtime (bedtime - DLMOn25%), while phase angles for DLMOff25% and CBTmin were calculated relative to habitual wake time (wake time - DLMOff25% or CBTmin).
Two-tailed Student's t-tests were used to compare data from women and men.
Results
Because of our matching criteria, the women and men were not significantly different in age, habitual bedtime (range 22:08-02:08 vs. 21:52-2:13), or habitual wake time (range 06:10-10:07 vs. 05:55-10:14; see Table 1). There was no significant difference between the women and men in morningness-eveniningness score (Horne and Östberg, 1976), and both groups were on average “neither” types. Women and men did not differ in BMI.
Table 1.
Mean, standard deviation, p value, and number of pairs tested for sex comparisons. Two-tailed t-tests were used for all comparisons.
| Women | Men | p value | pairs | |
|---|---|---|---|---|
| Age | 21.6 yrs ±3.2 | 21.8 yrs ± 3.4 | 0.7802 | 28 |
| Bed time | 08:10 ± 10:4h | 00:13 ± 1:07h | 0.8713 | 28 |
| Wake time | 08:13 ±1:05h | 08:22 ± 1:04h | 0.6300 | 28 |
| M/E Score | 53.2 ± 6.8 | 48.4 ± 10.5 | 0.2996 | 21 |
| BMI | 23.21 ± 2.6 | 24.47 ±2.9 | 0.0992 | 26 |
| CBT amplitude | 0.43°C ± 0.13 | 0.55°C ±0.16 | 0.0031 | 28 |
| CBTmin | 04:46 ± 1:56h | 06:11 ±1:19h | 0.0025 | 28 |
| Melatonin amplitude | 43.58 pg/ml ± 19.28 | 28.63 pg/ml ± 15.32 | 0.0025 | 28 |
| Melatonin AUC | 652.5 pg/ml ± 209.9 | 455.4 pg/ml ± 309.9 | 0.0174 | 28 |
| DLMO25% | 22:49 ± 1:27h | 23:28 ± 1:16h | 0.0839 | 28 |
| DLMO 10pg/ml | 22:27 ± 1:37h | 23:16 ± 1:16h | 0.0238 | 26 |
| DLMOff25% | 08:45 ± 1:30h | 09:46 ± 1:24h | 0.0116 | 28 |
| CBTmin phase angle | 3.45 h ± 1:57h | 1.99 h ±1.10h | 0.0002 | 28 |
| DLMO25% phase angle | 1.34 h ±0.96h | 0.75 h ±0.83h | 0.0169 | 28 |
| DLMOff25% phase angle | −0.53 h ± 0.73h | −1.49 h ± 1.41h | 0.0024 | 28 |
Women had a significantly lower core body temperature amplitude, but a significantly higher melatonin amplitude than men (see Table 1 and Figure 1). As a result of the higher melatonin amplitude, women had a higher 25% DLMO threshold relative to men (21.79 ± 9.64 pg/ml vs. 14.32 ± 7.91 pg/ml; p=0.003). The higher melatonin amplitude in women was not the result of a narrowing of the window of melatonin release in women, because the time between DLMOn25% and DLMOff25% was not significantly different between women and men (9.93 ± 0.80 h vs. 10.30 ± 1.17 h; p=0.17). Average area under the curve between DLMOn25% and DLMOff25% was significantly greater in women than men (see Table 1), indicating greater total nighttime melatonin secretion in women. BMI was not associated with melatonin levels for either women (r=-0.17, p=0.43; n=28) or men (r=-0.02, p=0.94; n=25). Similarly, weight was not associated with melatonin levels for either women (r=-0.29, p=0.13; n=28) or men (r=0.004, p=0.99; n=25).
Figure 1.
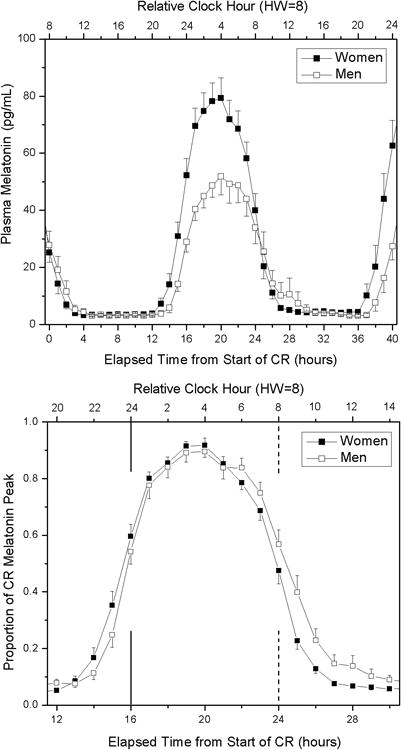
Melatonin waveforms for women and men on CR. Upper panel: Average (± standard error) melatonin waveforms for women (black boxes) and men (open boxes). Plasma melatonin values were averaged per hour beginning at wake time on the CR for each participant. Data for all participants within each group were averaged per hour across the CR. Only those hourly bins in which at least 14 participants remained on CR (hours 0-40) were included. Lower panel: Average proportion of fitted peak melatonin values for women and men. As the normalized peak occurred at different times for each participant, the average waveform for each group has a peak that is less than 1.Vertical lines represents the habitual sleep time (solid line) and habitual wake time (dashed line). HW= Habitual Wake time.
The timing of the circadian phase markers was earlier in the women than in men, despite their similar sleep times. While the timing of DLMOn25% in women was earlier (see Table 1), this difference did not reach statistical significance. The timing of DLMOff25%, DLMO (10 pg/ml threshold), and CBTmin were all significantly ealrier in the women (see Table 1 and Figure 2).
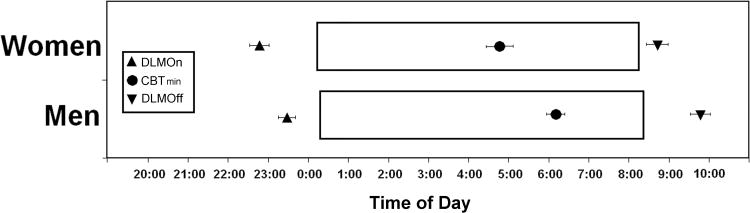
Figure 2.
Relative timing of the circadian phase markers with respect to sleep timing in women and men. Open boxes indicate the average timing of the habitual sleep episode in women (upper bar) and men (lower bar). Upward triangle indicates average (± standard error) dim light melatonin onset (DLMOn25%). Downward triangle indicates average (± standard error) dim light melatonin offset (DLMOff25%). Circle indicates average (± standard error) core body temperature minimum (CBTmin).
We examined the phase angle (relative timing) between melatonin or core body temperature circadian phase markers and habitual bed or wake time. In all cases, we found significant differences between the women and men. Women had a significantly longer interval between DLMOn25% and bedtime, a significantly shorter interval between DLMOff25% and wake time, and a significantly longer interval between CBTmin and wake time than men (see Table 1 and Figure 2).
Discussion
When measured in controlled environmental and behavioral conditions, we found that the timing of the circadian phases of the melatonin and core body temperature rhythms were earlier, relative to self-selected, habitual sleep-wake time, in women compared to men. This advanced circadian timing in women occurred despite the two groups having habitual bedtimes and wake times that were nearly identical. This resulted in a sex difference in the relative timing of circadian rhythms with respect to usual sleep-wake times, such that the women were sleeping and waking at the same clock time, but a later biological time, than the men.
Previous studies on sex differences in the timing of the body temperature rhythm have reported inconsistent findings. Two common methodological weaknesses in those studies may have contributed to the varying results. First, body temperature was measured in ambulatory participants who were allowed to sleep. If women and men had similar sleep-wake timing, the influence of activity and sleep on the observed temperature likely masked any underlying difference in body temperature between the sexes (Klerman et al., 1999). Second, in those studies, women and men were not matched for habitual sleep-wake times. Given that women tend to be more morning-type than men (Adan and Natale, 2002; Chelminski et al., 1997), it is likely that the women on average had earlier sleep-wake times, and this in turn could have resulted in earlier temperature rhythm timing in the women. In fact, one of the studies that reported an earlier temperature timing in women also reported that the sleep timing of women was significantly earlier (Campbell et al., 1989). Thus, the difference in the timing of the body temperature rhythm in the women may have reflected a sex difference in self-selected sleep-wake/light-dark timing, not a difference in actual biological timing relative to the sleep-wake/light-dark timing. In the present experiment, we minimized the effect of masking on rhythms of temperature and melatonin by using the CR protocol, and we controlled for differences in sleep-wake/light-dark timing by matching the women and men in our analysis by habitual wake times and then keeping them in strictly-controlled sleep-wake/light-dark conditions for three days prior to assessing the timing of their circadian rhythms. Under these highly controlled conditions, we found that women had a core body temperature phase that was nearly an hour and a half earlier than men.
Consistent with our present results, Mongrain et al. (Mongrain et al., 2004) reported an earlier timing of melatonin onset (DLMO) in women than men. In that study, DLMO was defined as the point that melatonin levels reached twice the minimum detectable concentration, and was reported to be 96 min earlier in women. When we assessed melatonin onset by adjusting for each individual's melatonin levels (DLMOn25%), we found an difference of less than half the magnitude of that reported by Mongrain et al., and it did not reach statistical significance. However, when we defined melatonin onset using a fixed threshold, our results were closer to the magnitude reported by Mongrain et al. By using the same fixed threshold for women and men, the sex difference in biological timing was exaggerated due to higher melatonin levels in women. Because of their higher melatonin amplitude, women will tend to reach a fixed threshold value before men, even if the actual circadian timing is identical. This finding reinforces the usefulness of collecting melatonin across an entire circadian cycle, rather than assessing only the onset of secretion. Furthermore, it highlights the utility of using an individualized method of determining DLMO when comparing groups that may differ in melatonin levels.
Our finding of earlier melatonin timing in women is in contrast to a recent study by Burgess & Fogg (2008). In that study, 170 participants were sampled for salivary melatonin for at least 20 hours under dim light. No sex difference was reported in the timing of DLMOn or DLMOff. One possible reasons for the discrepancy between that study and our present findings concerns the method of sampling. Salivary melatonin is approximately a third of the levels obtained in plasma (Benloucif et al., 2008), and those lower levels would tend to magnify the influence of assay variability on phase estimates. As both our study and the Burgess & Fogg study used the same assay, the assay variance in the Burgess & Fogg study is greater relative to the melatonin levels reported. Second, in the Burgess & Fogg study, the bed times and wake times for the women and men were not controlled, while in our study, we used female-male pairs who were matched by habitual sleep-wake times in order to control for potential sex differences in sleep-wake timing and associated light-dark exposure. It is possible that males in the Burgess and Fogg study had earlier bed times or wake times (a sex difference was not reported), which would tend to obscure the ability to observe a relatively advanced circadian timing in women.
We found that women had lower core body temperature amplitude but higher melatonin amplitude than the men. Several studies have reported a lower temperature amplitude in women in the luteal phase compared with the follicular phase of the menstrual cycle (Coyne et al., 2000; Lee, 1988), and no difference in temperature amplitude between follicular phase women and men, but a significantly lower temperature amplitude between luteal phase women and men (Baker et al., 2001). Our finding of a lower temperature amplitude in the women suggests that more of the women may have been in the luteal phase than the follicular phase of the menstrual cycle, although we did not assess this at the time of study. Prior reports have suggested that melatonin levels in the luteal phase are increased (Brun et al., 1987; Webley and Leidenberger, 1986; Wetterberg et al., 1976), decreased (Shibui et al., 2000) or are unchanged (Berga and Yen, 1990; Brzezinski et al., 1988; Delfs et al., 1994; Ito et al., 1993; Parry et al., 1997; Wright Jr. and Badia, 1999) when compared to the follicular phase. It has been suggested that differences in lighting conditions and masking effects in those studies may have contributed to the varying results (Baker and Driver, 2007). Controlling for lighting conditions and masking effects using a CR, Wright & Badia (Wright Jr. and Badia, 1999) found no effect of menstrual phase on melatonin levels; therefore, menstrual phase is unlikely to account for the difference in melatonin amplitude between women and men found in our study. It is also unlikely that menstrual phase is responsible for the advanced biological timing of women in our study, as the menstrual cycle has not been found to affect circadian phase in women (Berga and Yen, 1990; Parry et al., 1997).
Our finding that women have a longer interval between circadian phase (e.g., core body temperature nadir, melatonin offset) and usual wake time may be related to the tendency for women be earlier chronotypes than men (Adan and Natale, 2002; Chelminski et al., 1997). Morning-types have been reported to have a longer interval between the timing of their circadian rhythms and their usual wake time than evening-types (Baehr et al., 2000; Duffy et al., 1999), as we found in the women in our study. This biological timing relative to the LD cycle may predispose women to be more morning-type. However, in the present study, there was no significant sex difference in morningness-eveningness score, with women having an average morningness-eveningness score that was 3.8 points (from a 86-point scale) different from men. While this difference in morningness-eveningness score was not statistically significant, it is similar in magnitude to the significant sex difference observed in a larger study of over 2,000 participants (Adan and Natale, 2002).
Under a given light-dark (wake-sleep) cycle, the relationship between circadian phase and the light-dark cycle is determined by two factors: circadian period and the sensitivity of the circadian system to the phase-shifting effects of light (Pittendrigh, 1979; Pittendrigh and Daan, 1976). The women and men in the present study were selected for their similar sleep and wake times, and therefore would have been exposed to similar light-dark cycles before entering the study. Once in the laboratory, women and men experienced the same light-dark cycles for three baseline days prior to the circadian phase assessment procedure. Because of this, a sex difference in light-dark exposure in our study is an unlikely explanation of our finding of a difference in phase angle of entrainment. The more likely explanations, therefore, are that the women and men in our study differed in circadian period, differed in sensitivity to the phase shifting effects of light (either reduced sensitivity to phase delaying light or greater sensitivity to phase advancing light), or both.
Studies in animals have found that females are less sensitive to the phase shifting effects of light than males. In the hamster, Davis et al. (1983) reported that phase shifts to discrete light pulses were smaller in females than males. Female Octodon degus (Goel and Lee, 1995) and hamsters (Davis et al., 1983) have been found to take longer to adjust to shifted LD cycles than male animals, suggesting a difference in light sensitivity. If our present findings are the result of a sex difference in the circadian sensitivity to light, it may be due to a reduced sensitivity to phase delaying light in women, an increased sensitivity to phase advancing light in women, or both.
Studies in non-human animals have demonstrated that circadian period is shorter in females (Davis et al., 1983; Schull et al., 1989) and that the shorter period may be related to estrogen levels (Morin et al., 1977). In humans, Wever (1984) reported that women had a shorter period than men when studied in free-running conditions. However, we now understand that the free-running circadian period estimates derived from those experiments were systematically confounded by the influence of the participants' self-selected light exposure (Czeisler, 1995), so whether women indeed have shorter circadian periods is still an open question requiring additional studies.
The sex difference we observed in the relative timing between circadian rhythms and sleep in our study may have implications for understanding the development of sleep problems in women. Under conditions where the timing of sleep has been experimentally separated from the timing of the underlying circadian system, high sleep efficiency and the ability to consolidate sleep for an eight hour episode occurrs in young men when sleep is initiated approximately six hours before the circadian phase of the core body temperature minimum (Dijk and Czeisler, 1994; Dijk and Czeisler, 1995). In the present study, the biological time of sleep in the men was nearly ideal for the maintenance of high sleep efficiency. In contrast, the women in the present study slept at a later biological time. Why these healthy women had self-selected sleep times that may have been less than ideal is not understood, but if generalized, this delayed biological time of sleep may be a contributing factor for the higher incidence of insomnia in women than men (Mellinger et al., 1985; Partinen and Hublin, 2005).
Limitations
The present study of sex differences in entrained circadian phase and melatonin amplitude was a retrospective analysis of data from a series of studies that did not monitor the menstrual phase in the women at the time of study. While prior studies have suggested that menstrual phase does not impact circadian timing or the amplitude of melatonin secretion, future prospective studies that control for and the menstrual phase of subjects and document hormonal status in those subjects should be conducted to further explore the role of menstrual phase on phase angle of entrainment and melatonin levels.
Acknowledgments
We thank the participants; the technical, nursing, and dietary staff of the Brigham and Women's Hospital General Clinical Research Center for their assistance; the technical staff of the Division of Sleep Medicine for data collection and participant monitoring during the CRs; and J.M. Ronda for technical support with the study execution, data management, and data analysis. This research was supported in part by grants from the NIA (P01 AG09975) and NIMH (R01 MH45130) awarded to CAC, and was conducted in the Brigham and Women's Hospital General Clinical Research Center supported by NIH grant M01 RR02635. Support was also provided by grants from the NIA (R01 AG06072) and NHLBI (R01 HL090978) awarded to JFD, an NSBRI grant awarded to CAC, and an NIH Senior NRSA grant (F33 HL09588) awarded to SBK. SWC was supported in part by a fellowship from the Natural Sciences and Engineering Research Council of Canada.
References
- Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiology International. 2002;19:709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: With an emphasis on morningness-eveningness. Journal of Sleep Research. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Medicine. 2007;3 doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Baker FC, Waner JI, Vieira F, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. Journal of Physiology (London) 2001;530:565–574. doi: 10.1111/j.1469-7793.2001.0565k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–69. [PMC free article] [PubMed] [Google Scholar]
- Berga SL, Yen SSC. Circadian pattern of plasma melatonin concentrations during four phases of the human menstrual cycle. Neuroendocrinology. 1990;51:606–612. doi: 10.1159/000125398. [DOI] [PubMed] [Google Scholar]
- Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. Journal of Biological Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- Brun J, Claustrat B, David M. Urinary melatonin, LH, oestradiol, progesterone excretion during the menstrual cycle or in women taking oral contraceptives. Acta Endocrinologica (Cophenhagen) 1987;116:145–149. doi: 10.1530/acta.0.1160145. [DOI] [PubMed] [Google Scholar]
- Brzezinski A, Lynch HJ, Seibel MM, Deng MH, Nader TM, Wurtman RJ. The circadian rhythm of plasma melatonin during the normal menstrual cycle and in amenorrheic women. Journal of Clinical Endocrinology and Metabolism. 1988;66:891–895. doi: 10.1210/jcem-66-5-891. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS ONE. 2008;3:e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SS, Gillin JC, Kripke DF, Erikson P, Clopton P. Gender differences in the circadian temperature rhythms of healthy elderly subjects: Relationships to sleep quality. Sleep. 1989;12:529–536. [PubMed] [Google Scholar]
- Chelminski I, Ferraro FR, Petros T, Plaud JJ. Horne and Ostberg Questionnaire: a score distribution in a large sample of young adults. Pers Individ Differ. 1997;23:647–652. [Google Scholar]
- Coyne MD, Kesick CM, Doherty TJ, Kolka MA, Stephenson LA. Circadian rhythm changes in core temperature over the menstrual cycle: method for noninvasive monitoring. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2000;279:R1316–R1320. doi: 10.1152/ajpregu.2000.279.4.R1316. [DOI] [PubMed] [Google Scholar]
- Czeisler CA. The effect of light on the human circadian pacemaker. In: Waterhouse JM, editor. Circadian Clocks and Their Adjustment. John Wiley and Sons, Inc.; Chichester: pp. 254–302. Ciba Found. Symp. 183. [DOI] [PubMed] [Google Scholar]
- Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. American Journal of Physiology. 1983;244:R93–R105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- Delfs TM, Baars S, Fock C, Schumacher M, Olcese J, Zimmermann RC. Sex steroids do not alter melatonin secretion in the human. Human Reproduction. 1994;9:49–54. doi: 10.1093/oxfordjournals.humrep.a138318. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. Journal of Neuroscience. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? Journal of Biological Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57:1245–1251. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Lee TM. Sex differences and effects of social cues on daily rhythms following phase advances in Octodon degus. Physiol Behav. 1995;58:205–213. doi: 10.1016/0031-9384(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness- eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Ito M, Kohsaka M, Fukuda N, Honma K, Honma S, Katsuno Y, Honma H, Kawai I, Morita N, Miyamoto T. Effects of menstrual cycle on plasma melatonin level and sleep characteristics. Japanese Journal of Psychiatry and Neurology. 1993;47(2):478–479. doi: 10.1111/j.1440-1819.1993.tb02157.x. [DOI] [PubMed] [Google Scholar]
- Kattapong KR, Fogg LF, Eastman CI. Effect of sex, menstrual cycle phase, and oral contraceptive use on circadian temperature rhythms. Chronobiology International. 1995;12:257–266. [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol (Lond) 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. Journal of Biological Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Lee Y, Czeisler CA, Kronauer RE. Linear demasking techniques are unreliable for estimating the circadian phase of ambulatory temperature data. Journal of Biological Rhythms. 1999;14:260–274. doi: 10.1177/074873099129000678. [DOI] [PubMed] [Google Scholar]
- Kostoglou-Athanassiou I, Treacher DF, Wheeler MJ, Forsling ML. Bright light exposure and pituitary hormone secretion. Clinical Endocrinology. 1998;48:73–79. doi: 10.1046/j.1365-2265.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- Lee KA. Circadian temperature rhythms in relation to menstrual cycle phase. Journal of Biological Rhythms. 1988;3:255–263. [Google Scholar]
- Lewy AJ, Sack RL, Singer CM. Immediate and delayed effects of bright light on human melatonin production: Shifting “dawn” and “dusk” shifts the dim light melatonin onset (DLMO) Annals of the New York Academy of Sciences. 1985;453:253–259. doi: 10.1111/j.1749-6632.1985.tb11815.x. [DOI] [PubMed] [Google Scholar]
- Mellette HC, Hutt BK, Askovitz SI, Horvath SM. Diurnal variations in body temperatures. Journal of Applied Physiology. 1951;3:665–675. doi: 10.1152/jappl.1951.3.11.665. [DOI] [PubMed] [Google Scholar]
- Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment: prevalence and correlates. Archives of General Psychiatry. 1985;42:225–232. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- Mills JN, Minors DS, Waterhouse JM. Adaptation to abrupt time shifts of the oscillator[s] controlling human circadian rhythms. Journal of Physiology (London) 1978;285:455–470. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe KE, Prinz PN, Vitiello MV, Marks AL, Larsen LH. Healthy elderly women and men have different entrained circadian temperature rhythms. Journal of the American Geriatrics Society. 1991;39:383–387. doi: 10.1111/j.1532-5415.1991.tb02904.x. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in Morningness-Eveningness. Journal of Biological Rhythms. 2004;19:248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Parry BL, Berga SL, Mostofi N, Klauber MR, Resnick A. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. Journal of Biological Rhythms. 1997;12:47–64. doi: 10.1177/074873049701200107. [DOI] [PubMed] [Google Scholar]
- Partinen M, Hublin C. Epidemiology of Sleep Disorders. In: Kryger MH, Roth TR, Dement WC, editors. Principles and Practice of Sleep Medicine. Elselvier Saunders; Philadelphia, PA: pp. 626–647. [Google Scholar]
- Pittendrigh CS. Some functional aspects of circadian pacemakers. In: Suda M, Hayaishi O, Nakagawa H, editors. Biological Rhythms and their Central Mechanism. Elsevier; pp. 3–12. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J Comp Physiol [A] 1976;106:291–331. [Google Scholar]
- Schull J, Walker J, Fitzgerald K, Hiilivirta L, Ruckdeschel J, Schumacher D, Stanger D, McEachron DL. Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol Behav. 1989;46:341–346. doi: 10.1016/0031-9384(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Shibui K, Uchiyama M, Okawa M, Kudo Y, Kim K, Liu X, Kamei Y, Hayakawa T, Akamatsu T, Ohta K, Ishibashi K. Diurnal fluctuation of sleep propensity and hormonal secretion across the menstrual cycle. Biol Psychiatry. 2000;48:1062–1068. doi: 10.1016/s0006-3223(00)00912-4. [DOI] [PubMed] [Google Scholar]
- Waterhouse J, Weinert D, Minors D, Atkinson G, Reilly T, Folkard S, Owens D, MacDonald I, Sytnik N, Tucker P. The effect of activity on the waking temperature rhythm in humans. Chronobiology International. 1999;16:343–357. doi: 10.3109/07420529909116863. [DOI] [PubMed] [Google Scholar]
- Webley GE, Leidenberger F. The circadian pattern of melatonin and its positive relationship with progesterone in women. Journal of Clinical Endocrinology and Metabolism. 1986;63(2):323–328. doi: 10.1210/jcem-63-2-323. [DOI] [PubMed] [Google Scholar]
- Wetterberg L, Arendt J, Paunier L, Sizonenko PC, van Donselaar W, Heyden T. Human serum melatonin changes during the menstrual cycle. Journal of Clinical Endocrinology and Metabolism. 1976;42:185–188. doi: 10.1210/jcem-42-1-185. [DOI] [PubMed] [Google Scholar]
- Wever RA. Sex differences in human circadian rhythms: Intrinsic periods and sleep fractions. Experientia. 1984;40:1226–1234. doi: 10.1007/BF01946652. [DOI] [PubMed] [Google Scholar]
- Wilson BW, Snedden W, Silman RE, Smith I, Mullen P. A gas chromatography-mass spectrometry method for the quantitative analysis of melatonin in plasma and cerebrospinal fluid. Anal Biochem. 1977;81:283–291. doi: 10.1016/0003-2697(77)90699-6. [DOI] [PubMed] [Google Scholar]
- Winget CM, DeRoshia CW, Vernikos-Danellis J, Rosenblatt WS, Hetherington NW. Comparison of circadian rhythms in male and female humans. Waking and Sleeping. 1977;1:359–363. [Google Scholar]
- Wright KP, Jr, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behavioural Brain Research. 1999;103:185–194. doi: 10.1016/s0166-4328(99)00042-x. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Daniels JE, Duffy JF, Klerman EB, Shanahan TL, Dijk DJ, Czeisler CA. Do plasma melatonin concentrations decline with age? American Journal of Medicine. 1999;107:432–436. doi: 10.1016/s0002-9343(99)00266-1. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol (Lond) 2000;526.3:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]