Abstract
‘Jin Zhui’ is a spontaneous self-compatible mutant of ‘Ya Li’ (Pyrus bretschneideri Rehd. S21S34), the latter displaying a typical S-RNase-based gametophytic self-incompatibility (GSI). The pollen-part mutation (PPM) of ‘Jin Zhui’ might be due to a natural mutation in the pollen-S gene (S34 haplotype). However, the molecular mechanisms behind these phenotypic changes are still unclear. In this study, we identified five SLF (S-Locus F-box) genes in ‘Ya Li’, while no nucleotide differences were found in the SLF genes of ‘Jin Zhui’. Further genetic analysis by S-RNase PCR-typing of selfed progeny of ‘Jin Zhui’ and ‘Ya Li’ × ‘Jin Zhui’ progeny showed three progeny classes (S21S21, S21S34 and S34S34) as opposed to the two classes reported previously (S21S34 and S34S34), indicating that the pollen gametes of ‘Jin Zhui’, bearing either the S21- or S34-haplotype, were able to overcome self-incompatibility (SI) barriers. Moreover, no evidence of pollen-S duplication was found. These findings support the hypothesis that loss of function of S-locus unlinked PPM expressed in pollen leads to SI breakdown in ‘Jin Zhui’, rather than natural mutation in the pollen-S gene (S34 haplotype). Furthermore, abnormal meiosis was observed in a number of pollen mother cells (PMCs) in ‘Jin Zhui’, but not in ‘Ya Li’. These and other interesting findings are discussed.
Introduction
Gametophytic self-incompatibility (GSI) is a genetic mechanism in many flowering plants that prevents self-fertilization and promotes cross-fertilization. In Solanaceae, Plantaginaceae and Rosaceae, GSI is controlled by a single multi-allelic S locus, which is comprised of the pistil-S and pollen-S genes [1]. The pistil-S gene encodes a polymorphic ribonuclease (S-RNase) essential for rejection of self-pollen, and operates through inhibiting the growth of pollen tubes that possess the same S allele [2]–[5]. The pollen-S locus itself is a cluster of pollen-expressed S-locus F-box genes named SLF/SFBB [6]–[14]. Recent evidence in Petunia, Antirrhinum and Pyrus support a model in which the SLF functions as a component of the SCF E3 ubiquitin ligase complex that interacts with non-self S-RNases, leading to their degradation through the ubiquitin 26S proteasome proteolytic pathway [15]–[17]. However, an alternative model based on studies of Nicotiana suggests the resistance to non-self S-RNase by its sequestration from vacuolar compartments in compatible pollen tubes [18]. Synthesizing the above two models, a comprehensive hypothesis involving both S-RNase degradation and compartmentalization was recently proposed [19], though many details are yet to be clarified.
In recent studies, the use of induced or spontaneous self-compatible mutants have supported S-RNase and S-locus F-box genes as the S-determinants in Rosaceae, although functional approaches based on transgenic experiments have been proven problematic in this family. For instance, Okada et al. [20] described a self-compatible Japanese pear cultivar, Osa-Nijisseiki (S2S4). The cultivar had a 236 kb region deleted which included the S4-RNase of S4sm-haplotype. Whereas Li et al. [21] found that self-compatibility of ‘Yan Zhuang’ (Pyrus bretschneideri Rehd.) was caused by a single amino acid mutation in S21-RNase. The European pears (Pyrus communis) ‘Abugo’ and ‘Ceremeno’ were found to be self-compatible where S21°-RNase protein was absent in the style [22]. In sour cherry (Prunus cerasus), a Mu-like element insertion upstream of S6m-RNase reduced the level of expression of S-RNase, correlating with reduced accumulation of S-RNase in the pistil and reversal of the rejection mechanism [23]. These results all support S-RNase as pistil-S determinants in Rosaceae. Meanwhile, self-compatible pollen-part mutants with non-functional SFB proteins have been widely reported in Prunus. In self-compatible sweet cherry (Prunus avium), the SFB4 4 bp deletion caused a frame-shift resulting in defective SFB4 transcripts lacking two hypervariable regions. Similarly, a self-compatible Japanese apricot (Prunus mume) mutant was likely caused by insertion in the SFBf coding region, leading to a defective SFBf transcript that lacked the HVa and HVb containing C-terminus [9], [24]. These reports support the role of SFB as pollen-S determinants in prunus. Noteworthily, self-compatible pollen-part mutants with non-functional SLF/SFBB are little known in Solanaceae and the tribe Pyreae (apple, pear). Pollen-part self-compatibility (SC) in these species is due to the S-heteroallelic pollen effect [25]–[29]. The phenomenon is called competitive interaction (CI), since a pollen grain carrying two different pollen S alleles induces breakdown of pollen SI function [11], [27]. Recent functional analyses of SLFs/SFBBs in Solanaceae and Pyrus (Rosaceae) support a ‘non-self recognition by multiple factor SI system’ [14], [30]. Therefore, SC accessions reported in Pyrus are mostly related to S-allele duplications.
However, mutations in S-locus unlinked factors (also called modifier genes) that are required for GSI had also been associated with SC in Solanaceae and some genera of Rosaceae. In apricot cultivar ‘Canino’ (S2SC), a mutation was found in a modifier gene unlinked to the S-locus which independently caused the loss of pollen-S function [31], [32]. Results of Wu et al. [33] indicated that the SI breakdown in PPM ‘Katy’ apricot was associated with factors unlinked to the S-locus. Zuriaga et al. [34] reported another SC North-American apricot cv. Zaigers ‘Katy’, in which the mutated S-locus unlinked PPM was located at the distal end of chr.3. Pollen modifier factors have also been identified in Petunia, Pyrus and Prunus, such as the pollen-expressed Skp1-like1 proteins. As a modifier, Skp1-like1 proteins were proposed to be involved in a SCF complex [17], [35], [36]. Nevertheless, in Pyrus, the loss of function of S-locus unlinked PPM that leads to self-compatibility has never been reported. Therefore, the identification of S-locus unlinked PPM in Pyrus will be necessary to give a more complete picture of the self-incompatible mechanism.
‘Jin Zhui’ is a spontaneous self-compatible mutant of SI pear cultivar ‘Ya Li’. Li et al. [21] used genetic analysis of a small population to show that breakdown of SI in ‘Jin Zhui’ might be caused by pollen-S34 mutation [21]. However, it is not clear what form of mutation led to the breakdown of SI in ‘Jin Zhui’. In this work, genetic populations of selfed ‘Jin Zhui’ progeny and crossed progeny with ‘Jin Zhui’ were constructed. We analyzed the self-compatible ‘Jin Zhui’ using genetic and molecular approaches, with the compiled evidence suggesting that loss of function of the S-locus unlinked PPM was probably involved in pollen-S function breakdown. Further mapping will be necessary to identify the underlying mutant S-locus unlinked PPM.
Materials and Methods
Ethics Statement
No specific permits were required for the described observation or field studies. For locations and activities partaken, a. no specific permissions were required; b. locations were not privately-owned or protected in any way and c. no endangered or protected species were involved.
Plant Material
Five cultivars of Chinese pear (Pyrus bretschneideri Rehd.), ‘Ya Li’ (SI, S21S34), ‘Jin Zhui’ (SC, bud mutant of ‘Ya Li’ ), ‘Jin Feng’ (SI, S19S34), ‘Han Hong’ (SI, S27S34), ‘Han Xiang’ (SI, S12S31), ‘Jin Zhui’ selfed progeny, and crossed progeny derived from ‘Ya Li’ × ‘Jin Zhui’, ‘Ya Li’ × ‘Jin Feng’, ‘Jin Zhui’ × ‘Jin Feng’, ‘Jin Zhui’ × ‘Han Hong’, ‘Ya Li’ × ‘Han Hong’, ‘Han Xiang’ × ‘Jin Zhui’ were used in this study. The pistils and pollen were collected and stored at −80°C for DNA and RNA extraction. Flower buds from ‘Jin Zhui’ and ‘Ya Li’ were collected in spring at the stage ideal for meiotic studies, and root-tips for the observation of chromosomal numbers of ‘Jin Zhui’ selfed progeny were obtained from the germinated seeds.
The trees were planted on the farm of Dasungezhuang Orchard (Shunyi, Beijing, China), and seeds from self-pollination and cross-pollination were used for segregation analysis of S-RNases.
Isolation of DNA and RNA
Genomic DNA from leaves and seedlings was isolated by the method described previously [37], [38], and then incubated with RNase I (Invitrogen, CA, USA) at 37°C for 2 hours to remove the RNA, after which it was quantified by spectrophotometer. RNA was extracted from leaves, styles, and pollen samples according to a modified SDS method [39] and digested with DNase I (TaKaRa, DaLian, China). RNA was detected by electrophoresis. Total RNA was used to synthesize first-strand cDNA using the SuperScript reverse transcriptase (Invitrogen, CA, USA) with Oligo-dT primer. The cDNA was then used as templates for PCR amplification.
Genetic Linkage Analysis between Pollen-expressed SLF Alleles and S-RNases
Primers were screened from the PpSFBB primers reported by KaKui et al. [14]. Four primer pairs (Table 1) were chosen for amplifying the pollen-expressed-SLF alleles in pollen cDNAs from ‘Ya Li’ and ‘Jin Zhui’. PCR conditions were as follows: 20 µL reaction system, 50 ng genomic DNA, 2 µL 10 × Ex Taq buffer (including 2 mM MgCl2), 0.2 mM dNTPs, 10 pM of each primer and 0.2 unit Ex Taq DNA polymerase (TaKaRa). PCR products were examined by 1% agarose gel, and purified using the Gel Extraction Kit (BioDev-Tech, Beijing, China) and then cloned into the pMD18-T vector (TaKaRa). Four independent clones of each PCR product were chosen for DNA sequencing. Sequence alignment was performed with MEGA version 5.0 [40]. Phylogenetic analyses of the amino acid sequences of SLF/SFBB were carried out using the neighbor-joining method implemented in MEGA, with 1000 bootstrap replicates. Homologs were identified by BLASTN searches of the National Center for Biotechnology Information database (NCBI; [41]). Specific primer pairs for SLF alleles were designed for genetic linkage analysis of SLF alleles (Table 1). A segregating population of 30 individuals from ‘Ya Li’ (S21S34) × ‘Jin Feng’ (S19S34) was used.
Table 1. Sequences of oligonucleotide primers used in this study.
| primer | Sequence(from5’ to 3′) | note |
| S12-F | AGTTGGTAATTATTTGGCCGAACG | This work |
| S12-R | TCAACCAATTCAGTCAATGATGTCC | This work |
| S19-F | GACCCAAAATATTGCAAGGCG | [17] |
| S19-R | TGGTTCTGTATTGGGGAAGACG | [17] |
| S21-F | ATATTGCAGGACAAGGAATCG | [17] |
| S21-R | ATATGGTGATCCGGGTAGAAAG | [17] |
| S31-F | AAGACCCAGAAGGTTGCAAGACACA | This work |
| S31-R | TTTCCAACTGGGGTTCGAGTATTTGC | This work |
| S34-F | ATGGGGATGACGGGGATGAT | [17] |
| S34-R | ATACTGAATACTATTGTTTGGGCAA | [17] |
| PActin-F | GGTGTCATGGTTGGTATGGGTC | [17] |
| PActin-R | TTCCGCAACCGCTTGAATAGA | [17] |
| PpSFBB1-F | GGACTTGTAGTTTGATTTAGTCTGG | [14] |
| PpSFBB1-R | AACGCCAGGCTATGAGTACTACTTC | [14] |
| PpSFBB2-F | TGGTGGTGTTTCCTATGTACAT | [14] |
| PpSFBB2-R | CATACAAATTAAATAGAAGAAAATG | [14] |
| PpSFBB3-F | AACCTTCATTATGATGTTAAGCC | [14] |
| PpSFBB3-R | TWAYGACAACAATAAGAAGTGATA | [14] |
| PpSFBB4-F | AAGAATTCTTGTGGAAAAAAAACT | [14] |
| PpSFBB4-R | CCTTATATCATGCATACAAATTAA | [14] |
| PbSLF3-S34-F | F: TTATCTTTCCATTTATAGTGACTAG | [17] |
| PbSLF3-S34-R | R: ACTATGATAAAATGTTCG | [17] |
| PbSLF6-S21-F | CATGAAAGTGAAACTCCTC | [17] |
| PbSLF6-S21-R | TACTTGAGATTTTCGGTACC | [17] |
| PbSLF1-S21-F | ATGTCCCTGGTGTATGAAAGT | This work |
| PbSLF1-S21-R | TATGTATTTCTCGCCATCGG | This work |
| PbSLF1-S34-F | ATGTCCCAGGTGCTTGAAAGG | This work |
| PbSLF1-S34-R | CAATCCCATTGCAATAGCCC | This work |
| PbSLF2-S34-F | AAATTTTGTCCAGGTTGCCAC | This work |
| PbSLF2-S34-R | GCAATCCAATAACAAAATCCCTTG | This work |
| PbSLF3-S34-F | TCTTCTATGCAATCCTTCG | This work |
| PbSLF3-S34-R | CAATAACAAAATCCCTTCG | This work |
Pollination Tests
Six cross-pollinations, ‘Ya Li’ × ‘Han Hong’, ‘Jin Zhui’ × ‘Han Hong’, ‘Han Xiang’ × ‘Jin Zhui’, ‘Ya Li’ × ‘Jin Zhui’, ‘Jin Zhui’ × ‘Jin Feng’, ‘Ya Li’ × ‘Jin Feng’ and two self-pollinations from ‘Ya Li’ and ‘Jin Zhui’, were performed in the field. Pollination was conducted as follows: all flowers were removed before anthesis except three per inflorescence, with the anthers in these three removed and insect-proof bags used to prevent accidental contamination by foreign pollen. Fruit set was recorded about 1 month later and fruits were collected up until harvest time, with the number of seeds and weight per fruit recorded.
Pollen Viability and Germination Tests
Pollen viability was estimated as the percentage of pollen grains stained with 0.5% fluorescein diacetate (FDA). Fresh pollen grains were germinated on a solid medium (0.01% boric acid, 1% agar, 8% sucrose) in petri dish for 40 min at 28°C, and then the solid medium with germinated pollen grains was cut into small patches and placed on a glass slide. The germinated pollen grains were observed directly under a microscope (Nikon Eclipse 80i, Japan) for germination tests. For tests of pollen viability, FDA (2.5 mg/ml) was added to solid medium for 10 min, and then the pollen grains were observed under a microscope (Nikon Eclipse 80i, Japan). A total of 500 pollen grains were observed.
Chromosome Preparation and Observation
Upon reaching the optimal stage for meiotic studies, flower buds of ‘Jin Zhui’ and ‘Ya Li’ were fixed in carnoy's fluid (3 parts of ethanol plus 1 part glacial acetic acid) for 24 hours and then stored in 70% ethanol at 4°C until used. Pollen mother cells were stained with 4′, 6-diamino-2-phenylindole (DAPI) before observation. The chromosome numbers of ‘Jin Zhui’ selfed progeny were observed from root-tip cells with at least 30 cells observed per sample, fresh root-tips were harvested when 1∼2 cm long from germinated seeds and pretreated in 0.002 M 8-Hydroxyquinoline for 2 hours at room temperature, and then fixed with cannoy solution for 24 hours. After washing twice with distilled water, the root-tips were cut and kept in a solution of 2% cellulose and 1% pectinase at 37°C for 2 hours. Materials were washed twice with distilled water before being fixed again with cannoy solution and then stained on a pre-cooled slide with 4′, 6-diamino-2-phenylindole pyrrolindone (DAPI). All chromosomal images were captured under the Olympus BX53 fluorescence microscope used with a microCCD camera.
Results
Molecular Identification of SLFs in ‘Ya Li’ and ‘Jin Zhui’
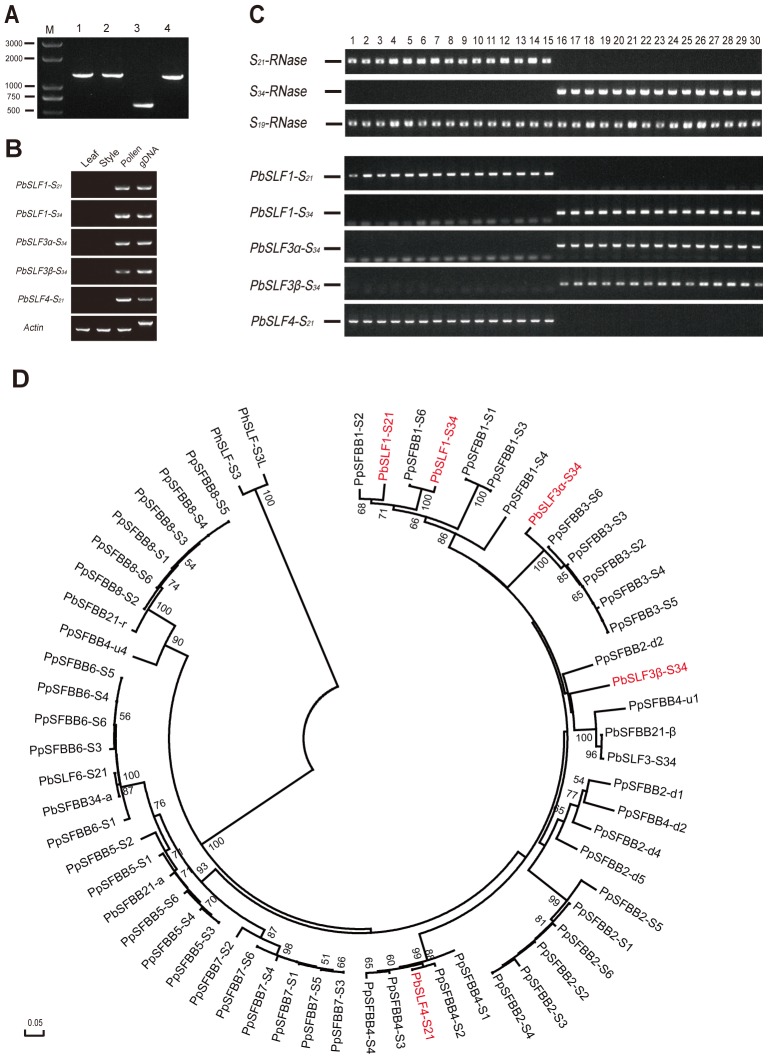
PCR amplification of the SLF alleles was performed using four different primer pairs as used for SFBB allele identification by Kakui et al. [14] (Table 1). Single bands were obtained when amplifying SLF alleles from the genomic DNA of ‘Ya Li’ (S21S34) using each primer pair (Fig. 1 A). Direct sequencing of these PCR products validated the five SLF candidates. Specific primer pairs for each SLF were generated on the basis of sequence alignments of the cloned SLFs (Table 1). The PpSFBB4 primers were specific for the S21-haplotype of the SLF gene. The expression analysis of these SLF genes in different tissues showed they were all expressed specifically in pollen (Fig. 1 B).
Figure 1. Molecular identification of SLFs in ‘Ya Li’.
A. PCR products from genomic DNA of ‘Ya Li’ for SLF allele identification. Lanes 1–5 represent PCR products using primers PpSFBB1, PpSFBB2, PpSFBB3, and PpSFBB4, respectively. M, DNA marker. B. Expression of PbSLFs in leaf, style and pollen in ‘Ya Li’. RT-PCR was performed with SLF-specific primers and synthesized cDNA was used as templates. Pear actin gene was used as internal control. C. Linkage analysis of PbSLFs and PbS-RNases. Genomic DNAs from 30 crossed progeny of ‘Ya Li’ (S21S34) and ‘Jin Feng’ (S19S34) were used as templates for linkage analysis. PbS21-RNase, PbS34-RNase and PbS19-RNase (top panel) and PbSLFs (lower panel) were detected by PCR using gene specific primers. Lanes 1–30 represent a progeny population of 30 individuals. D. Phylogenetic tree of deduced amino acid sequences of PbSLFs and other SLFs/SFBBs. A neighbor-joining tree was constructed from 49 SLF/SFBB proteins from Japanese pear and 11 PbSLF proteins from Pyrus bretschneideri. PhSLF-S3 and PhSLF-S3L from Petunia hybrida were used as an outgroup. Numbers on the branches showed bootstrap values above 50% from 1000 bootstrap replicates. Eight types of the SLF/SFBB proteins were classified by Kakui et al. [14]. PbSLFs obtained in this study were marked in red.
To confirm whether the five SLF genes were linked to the S locus, a population of 30 progeny from ‘Ya Li’ × ‘Jin Feng’ was used. When 15 progeny carrying S19S21 generated a single band which was absent in the other 15 progeny which carried S19S34, the SLF gene was assigned S21-haplotype-linkage. Similarly, if a single band only appeared in the progeny carrying S19S34, the SLF gene was assigned S34-haplotype-linked. Two of the SLF genes were detected in the S21-containing progeny only, and the other three were detected in the S34-containing progeny, suggesting linkage between the PbSLFs and PbS-RNases (Fig. 1 C). These SLFs were subject to phylogenetic analysis with SLF/SFBBs from Japanese pear and Chinese pear, based on which they were classified into three types: type-1, type-3 and type-4 according to Kakui et al. [14] (Fig. 1 D). Thus, they were named as PbSLF1-S21 (GenBank accession number KC569798), PbSLF1-S34 (GenBank accession number KC569799), PbSLF3α-S34 (GenBank accession number KC569800), PbSLF3β-S34 (GenBank accession number KC569801) and PbSLF4-S21 (GenBank accession number KC569802).
To determine whether mutations or indels existed in the SLFs from ‘Jin Zhui’, seven SLFs including the five obtained in this study and two identified by Xu et al. [17] were cloned from pollen cDNA of ‘Jin Zhui’ and ‘Ya Li’. Comparative analysis of the seven SLFs showed no difference between ‘Ya Li’ and ‘Jin Zhui’ (data not shown).
Fruit Set and Genetic Analysis of Selfed and Crossed Progeny of ‘Jin Zhui’
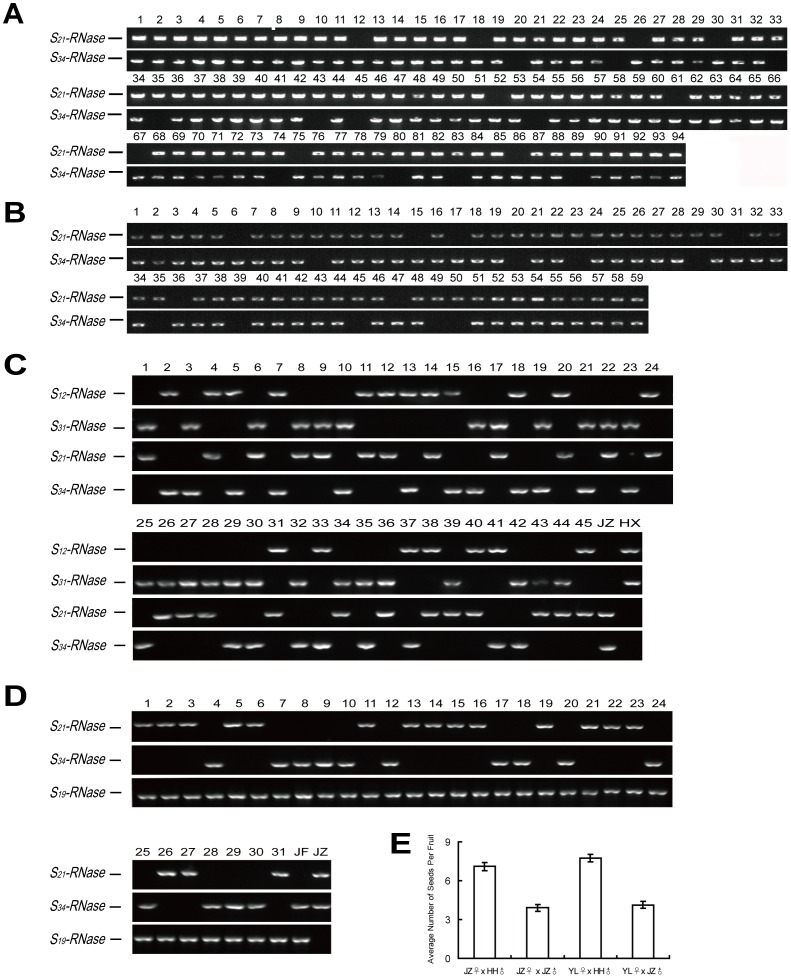
Li et al. [21] found that ‘Jin Zhui’ was a pollen-part mutant of ‘Ya Li’. Genetic analysis of 29 selfed progeny of ‘Jin Zhui’ indicated the SI breakdown in ‘Jin Zhui’ might be due to the pollen-S of the S34-haplotype. To further analyze the nature of the self-compatible mutant, pollination tests were performed. ‘Ya Li’ and ‘Jin Zhui’ were self-pollinated and reciprocally pollinated. The results from pollination tests showed that ‘Jin Zhui’, which had functional style and nonfunctional pollen, was self-compatible, while ‘Ya Li’, which had functional style and pollen, was self-incompatible (Table 2). Further genetic analysis of larger populations of selfed and crossed progeny of ‘Jin Zhui’ was performed. app:addword:respectiveSegregation of S-RNases in selfed progeny of ‘Jin Zhui’ and crossed progeny between ‘Ya Li’ and ‘Jin Zhui’ was investigated by PCR using the S-haplotype-specific S-RNase primers. The results showed that both the selfed progeny of ‘Jin Zhui’ and the crossed progeny between ‘Ya Li’ and ‘Jin Zhui’ consisted of three S-genotypes: S21S21, S21S34 and S34S34 (Fig. 2 A, B and Table 3). The results indicated that pollen carrying both pollen S-alleles could reach the ovule and achieve self-fertilization, which suggested that the pollen-part mutation in ‘Jin Zhui’ was unlinked to the S-locus.
Table 2. Fruit setting rates in self- or cross-pollination of three pear cultivars.
| Crosses | Pollinated flowers | Fruits set | Fruit-set per. (%) | Pollinated inflorescence No. |
| ‘Ya Li’ selfed | 117 | 3 | 2.6 | 39 |
| ‘Jin Zhui’ selfed | 150 | 119 | 79.3 | 50 |
| ‘Jin Zhui’♀ × ‘Ya Li’♂ | 106 | 5 | 4.7 | 36 |
| ‘Ya Li’♀ × ‘Jin Zhui’♂ | 150 | 124 | 82.7 | 53 |
| ‘Ya Li’♀ × ‘Han Hong’♂ | 133 | 103 | 77.4 | 50 |
| ‘Jin Zhui’♀ × ‘Han Hong’♂ | 120 | 104 | 86.7 | 42 |
Figure 2. Segregation of S-RNases in selfed and crossed progeny of ‘Jin Zhui’.
A. Segregation analysis of S21- and S34-RNases in selfed progeny of ‘Jin Zhui’. Lanes 1–94 represent a progeny population of 94 individuals. B. Segregation analysis of S21- and S34-RNases in crossed progeny between ‘Ya Li’ and ‘Jin Zhui’. Lanes 1–59 represents a progeny population of 59 individuals. C. Segregation analysis of S-alleles in crossed progeny between ‘Han Xiang’ and ‘Jin Zhui’. Lanes 1–45 represent a progeny population of 45 individuals. JZ, Jin Zhui; HX, Han Xiang. D. Segregation analysis of S-alleles in crossed progeny between ‘Jin Zhui’ and ‘Jin Feng’. Lanes 1–31 represent a progeny population of 31 individuals. JZ, Jin Zhui; JF, Jin Feng. E. Average numbers of seeds per fruit from selfed and crossed progeny of ‘Jin Zhui’. JZ, Jin Zhui; YL, Ya Li; HH, Han Hong.
Table 3. Segregation of S-genotypes in progeny of different self- or cross-pollinations.
| Crosses | Total | S-genotypes observed in progeny | Expectedsegregation ratioa | ?2 P-value | ||||||||
| S21S21 S21S34 S34S34 S19S21 S19S34 S12S21 S12S34 S21S31 S31S34 | ||||||||||||
| ‘Jin Zhui’ selfed | 94 | 11 | 74 | 9 | – | – | – | – | – | – | 1∶2∶1 | 31.11 (P<0.01) |
| ‘Ya Li’ × ‘Jin Zhui’ | 59 | 9 | 44 | 6 | – | – | – | – | – | – | 1∶2∶1 | 14.56 (P<0.01) |
| ‘Jin Zhui’ × ‘Jin Feng’ | 31 | – | – | – | 17 | 14 | – | – | – | – | 1∶1 | 0.29 (P>0.05) |
| ‘Han Xiang’ × ‘Jin Zhui’ | 45 | – | – | – | – | – | 10 | 9 | 14 | 12 | 1∶1;1∶1 | 1.31 (P>0.05) |
Expected ratios for a single mutation unlinked to the S-locus.
To further validate these observations, additional crosses were performed and analyzed (Fig. 2 C, D and Table 3). The crossed progeny of ‘Han Xiang’ × ‘Jin Zhui’ and ‘Jin Zhui’ × ‘Jin Feng’ fell into four classes (S12S21:S12S34:S21S31:S31S34) and two classes (S19S34: S19S21) by S-RNase genotyping, respectively. The observed ratios for S-genotype segregations fit with the expected ratios (χ2 values of 1.31, P>0.05 and 0.29, P>0.05). These results supported self-compatibility (SC) in ‘Jin Zhui’ was associated with S-locus unlinked PPM rather than S-allele duplications.
The homozygotes from selfed progeny of ‘Jin Zhui’ and crossed progeny of ‘Ya Li’ × ‘Jin Zhui’ were much fewer in number than heterozygotes (Table 3). The average number of seeds per fruit produced from selfed progeny of ‘Jin Zhui’ and crossed progeny of ‘Ya Li’ × ‘Jin Zhui’ was 3.91 and 4.13, respectively. This was much lower than the two cross-pollinations ‘Jin Zhui’ × ‘Han Hong’ (7.09) and ‘Ya Li’ × ‘Han Hong’ (7.75) (Fig. 2 E). The segregation ratios of the three classes (S21S21:S21S34:S34S34) from selfed progeny of ‘Jin Zhui’ and crossed progeny between ‘Ya Li’ and ‘Jin Zhui’ were 9∶74∶11 and 6∶45∶9, respectively. These segregation ratios did not fit the expected ratios of 1∶2∶1, with χ2 values of 31.11, P<0.01 and 14.56, P<0.01 (Table 3). The segregation distortion showed that there were significantly fewer homozygotes in the progeny than heterozygotes, and the reduction in homozygosity was probably due to postzygotic selection.
Significant Difference in Abortive Pollen between ‘Jin Zhui’ and ‘Ya Li’
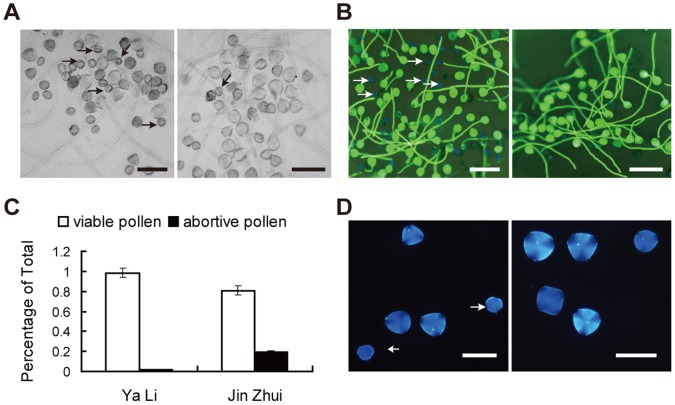
To investigate the pollen-part mutation of ‘Jin Zhui’, we observed the pollen germination ability of ‘Jin Zhui’ and ‘Ya Li’ (Fig. 3 A), where a significant difference was found. The number of abortive pollen grains was found to be much increased in ‘Jin Zhui’, with the abortive pollen grains incapable of both hydration and germination. The abortive pollen grains of ‘Jin Zhui’ and ‘Ya Li’ could not be stained by FDA, indicating they were inviable (Fig. 3 B). The percentage of abortive pollen grains in ‘Jin Zhui’ (about 20%) was much higher than in ‘Ya Li’ (about 1.6%) (Fig. 3 C), which was consistent with the results observed during pollen germinations. Further DAPI staining showed that the abortive pollen grains had no apparent nucleus (Fig. 3 D), and that they had already degenerated into shells. The increase in these types of abortive pollen grains in ‘Jin Zhui’ implied partial disruption of normal meiosis during the development of pollen.
Figure 3. Significant difference in abortive pollen grains between ‘Jin Zhui’ and ‘Ya Li’.
A. Pollen grains germination of ‘Jin Zhui’(left) and ‘Ya Li’ (right). Abortive pollen grains without hydration and germination are indicated with arrows. Scale bar: 100 µm. B. FDA staining of germinated pollen grains from ‘Jin Zhui’ (left) and ‘Ya Li’ (right). Abortive pollen grains not stained by FDA are indicated by arrows. Scale bar: 100 µm. C. Percentages of abortive pollen grains in FDA staining between ‘Jin Zhui’ and ‘Ya Li’. A total of 500 fresh pollen grains were examined in each case D. DAPI staining of pollen grains from ‘Jin Zhui’ (left) and ‘Ya Li’ (right). Abortive pollen grains without apparent nucleus are indicated by arrows. Scale bar: 50 µm.
Abnormal Meiosis of Pollen Mother Cells (PMCs) in ‘Jin Zhui’
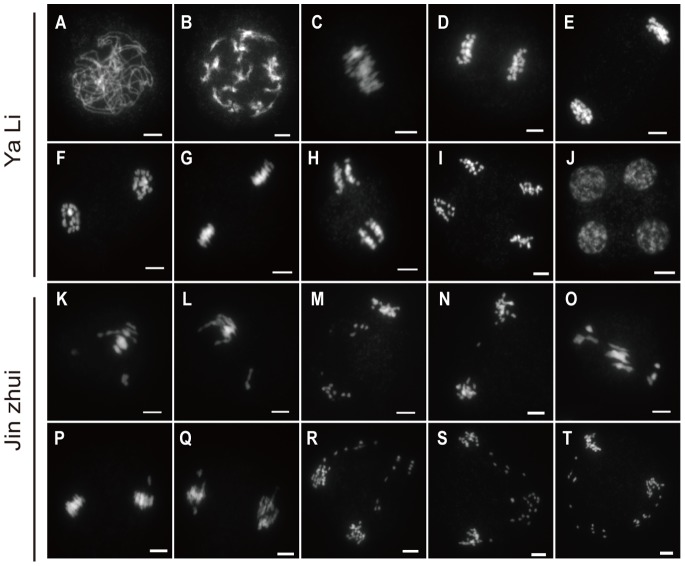
In order to investigate the abortive pollen grains in ‘Jin Zhui’, we analyzed the meiotic behavior of pollen mother cells (PMCs). As a self-incompatible cultivar, ‘Ya Li’ had normal meiotic behavior (Fig. 4 A–J). ‘Ya Li’ possesses 34 chromosomes, which during meiotic prophase I, undergo leptotene (not shown), zygotene (not shown), pachytene (Fig. 4 A), diplotene (not shown) and finally, the 17 bivalents condense through diakinesis (Fig. 4 B). The 17 bivalents then distribute on the equatorial plate at metaphase I (Fig. 4 C). During anaphase I, each group of homologous chromosomes separated from each other (Fig. 4 D), with each group reaching one pole of the cell (Fig. 4 E). Through meiotic prophase II (Fig. 4 F) and metaphase II (Fig. 4 G), the sister chromatids in each group were then separated in anaphase II (Fig. 4 H), generating four pools of 17 chromosomes (Fig. 4 I), which gave rise to four tetrads of microspores (Fig. 4 J). As a self-compatible pollen-part mutant of ‘Ya Li’, ‘Jin Zhui’ had abnormal meiosis in a number of PMCs (Fig. 4 K–T). Univalents appeared at metaphase I (Fig. 4 K and L), and laggards prevailed during anaphase I (Fig. 4 M, N, and O). During metaphase II, univalents also appeared (Fig. 4 P and Q), and then laggards and unbalanced separation were observed in anaphase II (Fig. 4 R, S and T). Unbalanced separation produced many abortive pollen grains which had no nucleus nor vitality. The number of meiotic abnormalities in ‘Jin Zhui’ PMCs was recorded (Table 4), and showed that abnormal meiosis existed in a number of PMCs of ‘Jin Zhui’. To test whether the abnormal meiosis affected the selfed progeny, we observed chromosome numbers of selfed progeny from ‘Jin Zhui’. Both the homozygotes and heterozygotes in the progeny had the usual (34) number of chromosomes (Fig. 5). Though abnormal meiosis was found in ‘Jin Zhui’ PMCs, the selfed progeny from ‘Jin Zhui’ had normal chromosome numbers.
Figure 4. Meiosis of ‘Ya Li’ and ‘Jin Zhui’. (A–J). DAPI staining of ‘Ya Li’ PMCs during meiosis.
(A)–(J) show pachytene, diakinesis, metaphase I, anaphase I, telophase I, prophase II, metaphase II, anaphase II, telophase II and tetrad, respectively. (K–T) DAPI staining of ‘Jin Zhui’ PMCs during meiosis. (K) and (L) show univalents in metaphase I, (M), (N) and (O) laggard chromosomes at anaphase I, (P) and (Q) univalents in metaphase II, (R), (S) and (T) laggard chromosome and unbalanced separation in anaphase II, respectively. Scale bar: 5 µm.
Table 4. Meiotic abnormalities in ‘Jinzhui’.
| Phases | Total number of cells | Number of abnormal cells | Abnormalities | Percentage of abnormal cells (%) |
| Metaphase I | 625 | 283 | univalents | 45.28 |
| Anaphase I | 731 | 172 | Laggards | 23.53 |
| Metaphase II | 563 | 169 | univalents | 30.01 |
| Anaphase II | 612 | 244 | laggards and unbalanced separation | 39.87 |
Figure 5. Normal chromosome numbers in selfed progeny of ‘Jin Zhui’.
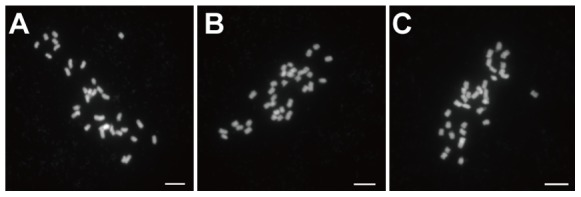
A, 34 chromosomes of an S21S21 homozygote representing a total of 12 progeny examined. B. 34 chromosomes of an S21S34 heterozygote representing a total of 21 progeny examined. C. 34 chromosomes of an S34S34 homozygote representing a total of 13 progeny examined. Scale bar: 5 µm.
Discussion
Identification and Comparative Analysis of SLF Genes between ‘Ya Li’ and ‘Jin Zhui’
In gametophytic self-incompatible (GSI) species of Solanaceae, Rosaceae and Plantaginaceae, multiple F-box genes within the S-locus region have been reported [6]–[8], [12], [42]. Through mutant analysis and transgenic approaches, the pollen S genes had been determined experimentally [9]–[11], [24], [43]. In Petunia and Pyrus, multiple SLFs/SFBBs as pollen S factors were proven to collaborate in recognizing non-self S-RNases and mediate their degradation [14], [30]. In previous studies, mutations or indels in pollen-S genes were always considered a reasonable explanation for self-compatible PPM, where Li et al. [21] reported that ‘Jin Zhui’ was a pollen-part mutant. The breakdown of self-incompatibility (SI) in ‘Jin Zhui’ might be caused by mutant pollen-S34 genes. Our study on pollen-part mutation of ‘Jin Zhui’ should help us to understand the function of the pollen-S gene. We cloned five F-box genes from pollen cDNA of ‘Ya Li’, with linkage analysis showing them to be S-Locus F-box (SLF) genes. However, comparative analysis of seven SLFs, including the two SLFs identified by Xu et al. [17], showed no differences between ‘Ya Li’ and ‘Jin Zhui’. Therefore, these seven SLFs were probably not directly responsible for the SI breakdown of ‘Jin Zhui’. Subsequent genetic analysis further supported this hypothesis.
Self-compatibility in ‘Jin Zhui’ is Associated with S-locus Unlinked PPM
‘Ya Li’ (S21S34) is a traditional Chinese pear (Pyrus bretschneideri) cultivar exhibiting self-incompatibility, and ‘Jin Zhui’ (S21S34) is a pollen-part mutant of ‘Ya Li’. Genetic analysis of 29 selfed progeny in ‘Jin Zhui’ showed that the pollen S34 allele might be mutated in ‘Jin Zhui’ [21].
To investigate the genetics of SC in ‘Jin Zhui’, genetic populations were constructed. ‘Jin Zhui’ (S21S34) was self-pollinated and reciprocally crossed with ‘Ya Li’ (S21S34). It is noteworthy that ‘Jin Zhui’ pollen tubes bearing either the S21- or the S34-haplotype were able to grow through ‘Jin Zhui’ and ‘Ya Li’ pistils and complete fertilization, producing three S-genotype classes (S21S21, S21S34 and S34S34) instead of the two classes observed previously (S21S21 and S21S34). However, no progeny were obtained in the reciprocal cross when using ‘Jin Zhui’ as the female parent. These results should support the PPM in ‘Jin Zhui’ was unlinked to the S-locus. The crosses between ‘Han Xiang’ (S12S31) and ‘Jin Zhui’ (S21S34), ‘Jin Zhui’ (S21S34) and ‘Jin Feng’ (S19S34) reinforced this conclusion.
Interestingly, in the ‘Jin Zhui’ × ‘Jin Zhui’ and ‘Jin Zhui’ × ‘Ya Li’ populations the number of seedlings homozygous for both the S21- and the S34-haplotype is significantly lower than that for the heterozygote S21S34 (see Table 3). Some explanations for this phenomenon may include linkage in coupling between the mutated allele of the S-locus unlinked PPM and the S-allele, or postzygotic selection against homozygous embryos. In the case of the former, the segregation ratios observed in different populations do not support linkage between the mutated factor and the S-locus. Whereas postzygotic selection would explain the significantly reduced numbers of S21S21 and S34S34 genotypes, and could also be a reasonable explanation that no S21S21 homozygotes were detected previously by Li et al. [21] in a small population from selfed progeny of ‘Jin Zhui’.
In Solanaceae, self-compatible pollen-part mutants may arise from S-allele duplications located in a centric fragment, in a non-S chromosome, or linked to the S-locus leading to the formation of S-heteroallelic pollen [27]. According to the segregations obtained in the crosses (including ‘Jin Zhui’ selfed, ‘Ya Li’ × ‘Jin Zhui’, ‘Jin Zhui’ × ‘Jin Feng’, ‘Han Xiang’ × ‘Jin Zhui’) performed here, S-allele duplications did not seem likely in ‘Jin Zhui’ (all descendants should have had the S21S34 genotype) (Fig. 2, Table 3). S-allele duplications may also result from polyploidy, but ‘Jin Zhui’ was confirmed as diploid by chromosome observation in selfed progeny (Fig. 5). These results rule out competitive interaction resulting from S-heteroallelic pollen as the cause of SC in ‘Jin Zhui’. Taken together, these results supported the hypothesis that an S-locus unlinked PPM was required in GSI. Loss of function in this S-locus unlinked PPM was responsible for the SI breakdown of ‘Jin Zhui’.
Possible Role of the S-locus Unlinked PPM in Meiosis
Abnormal meiotic behaviors in PPM ‘Jin Zhui’ were observed in many PMCs (Fig. 4 and Table 4). Univalents, laggards and unbalanced separation were detected during the process of meiosis (Fig. 4). In anaphase II, unbalanced separation produced a number of abnormal microspores, which were degraded into shells (Fig. 3). Meanwhile, to test whether the abnormal meiosis affected the selfed progeny, we observed chromosome numbers of selfed progeny from ‘Jin Zhui’, and found that both the homozygotes and heterozygotes in the progeny had the usual 34 chromosomes (Fig. 5). Though abnormal meiosis was found in ‘Jin Zhui’ PMCs, the selfed progeny from ‘Jin Zhui’ had normal chromosome numbers and were not influenced by abnormal meiosis. Abnormal meiosis in PMCs was consistently caused by related mutant genes. In an Arabidopsis male sterile mutant, abnormal meiosis caused by a Ds insertion in the SKP1-LIKE1 gene was reported [44]. While the SKP1-LIKE1 homolog SSK (SLF-interacting Skp1-like1) was identified as a putative canonical SCFSLF complex, and proposed to degrade non-self S-RNase in the degradation model of Pyrus, prunus and Antirrhinum [17], [35], [36], [45]. In this work, we suspect the mutant S-locus unlinked PPM might influence the pollen development of ‘Jin Zhui’. Two SSKs, which had been identified in ‘Ya Li’ [17], were cloned from pollen cDNAs of ‘Jin Zhui’ and ‘Ya Li’, but no nucleotide difference was found (data not shown). Further studies focusing on the identification of S-locus unlinked PPM should help us to gain deeper insights into self-incompatibility in Pyrus.
Acknowledgments
We are very grateful to Dr Y. B. Xue (Laboratory of Molecular and Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences and National Center for Plant Gene Research, Beijing, China) for valuable advice and assistance. Also, we thank the two anonymous reviewers of this manuscript for valuable advice.
Funding Statement
This work was supported by Beijing Natural Science Foundation (6102017) and National Science Foundation for Young Scholars of China (31201606). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. De Nettancourt D (1997) Incompatibility in angiosperms. Sex Plant Reprod 10: 185–199 10.1007/s004970050087 [DOI] [Google Scholar]
- 2. Anderson MA, Cornish EC, Mau SL, Williams EG, Hoggart R, et al. (1986) Cloning of cDNA for a stylar glycoprotein associated with expression of self-incompatibility in Nicotiana-alata . Nature 321: 38–44 10.1038/321038a0 [DOI] [Google Scholar]
- 3. McClure BA, Haring V, Ebert PR, Anderson MA, Simpson RJ, et al. (1989) Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature 342: 955–957 10.1038/342955a0 [DOI] [PubMed] [Google Scholar]
- 4. Sassa H, Hirano H, Ikehashi H (1992) Self-incompatibility-related RNases in styles of Japanese pear (Pyrus serotina Rehd.). Plant Cell Physiol 33: 811–814. [Google Scholar]
- 5. Xue YB, Carpenter R, Dickinson HG, Coen ES (1996) Origin of allelic diversity in Antirrhinum S locus RNases. Plant Cell 8: 805–814 10.1105/tpc.8.5.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai Z, Ma WS, Han B, Liang LZ, Zhang YS, et al. (2002) An F-box gene linked to the self-incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol Biol 50: 29–42 10.1038/nature02523 [DOI] [PubMed] [Google Scholar]
- 7. Entani T, Iwano M, Shiba H, Che FS, Isogai A, et al. (2003) Comparative analysis of the self-incompatibility (S-) locus region of Prunus mume: identification of a pollen-expressed F-box gene with allelic diversity. Genes Cells 8: 203–213 10.1046/j.1365-2443.2003.00626.x [DOI] [PubMed] [Google Scholar]
- 8. Ushijima K, Sassa H, Dandekar AM, Gradziel TM, Tao R, et al. (2003) Structural and transcriptional analysis of the self-incompatibility locus of almond: Identification of a pollen- expr- essed F-box gene with haplotype-specific polymorphism. Plant Cell 15: 771–781 10.1105/tpc.009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ushijima K, Yamane H, Watari A, Kakehi E, Ikeda K, et al. (2004) The S haplotype-specific F-box protein gene, SFB, is defective in self-compatible haplotypes of Prunus avium and P. mume . Plant J 39: 573–586 10.1111/j.1365-313X.2004.02154.x [DOI] [PubMed] [Google Scholar]
- 10. Qiao H, Wang F, Zhao L, Zhou JL, Lai Z, et al. (2004) The F-Box protein AhSLF-S2 controls the pollen function of S-RNase-based self-incompatibility. Plant Cell 16: 2307–2322 doi http:dx.doi.org10.1105tpc.104.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sijacic P, Wang X, Skirpan AL, Wang Y, Dowd PE, et al. (2004) Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature 429: 302–305 10.1038/nature0-2523 [DOI] [PubMed] [Google Scholar]
- 12. Sassa H, Kakui H, Miyamoto M, Suzuki Y, Hanada T, et al. (2007) S locus F-Box brothers: Multiple and pollen-specific F-box genes with S haplotype-specific polymorphisms in apple and Japanese pear. Genetics 175: 1869–1881 10.1534/genetics.106.068858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Franceschi P, Pierantoni L, Dondini L, Grandi M, Sanzol J, et al. (2011) Cloning and mapping multiple S-locus F-box genes in European pear (Pyrus communis L.). Tree Genet Genomes 7: 231–240 10.1007/s11295-010-0327-5 [DOI] [Google Scholar]
- 14. Kakui H, Kato M, Ushijima K, Kitaguchi M, Kato S, et al. (2011) Sequence divergence and loss-of-function phenotypes of S locus F-box brothers genes are consistent with non-self recognition by multiple pollen determinants in self-incompatibility of Japanese pear (Pyrus pyrifolia). Plant J 68: 1028–1038 10.1111/j.1365-313X.2011.04752.x [DOI] [PubMed] [Google Scholar]
- 15. Hua Z, Kao T-h (2006) Identification and characterization of components of a putative Petunia S-locus F-box-containing E3 ligase complex involved in S-RNase-based self-incompatibility. Plant Cell 18: 2531–2553 10.1105/tpc.106.041061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang J, Zhao L, Yang Q, Xue Y (2006) AhSSK1, a novel SKP1-like protein that interacts with the S-locus F-box protein SLF. Plant J 46: 780–793 10.1111/j.1365-313X.2006.02735.x [DOI] [PubMed] [Google Scholar]
- 17. Xu C, Li M, Wu J, Guo H, Li Q, et al. (2012) Identification of a canonical SCF (SLF) complex involved in S-RNase-based self-incompatibility of Pyrus (Rosaceae). Plant Mol Biol 81(3): 245–57 10.1007/s11103-012-9995-x [DOI] [PubMed] [Google Scholar]
- 18. Goldraij A, Kondo K, Lee CB, Hancock CN, Sivaguru M, et al. (2006) Compartmentalization of S-RNase and HT-B degradation in self-incompatible Nicotiana . Nature 439: 805–810 10.1038/nature04491 [DOI] [PubMed] [Google Scholar]
- 19. Chen G, Zhang B, Zhao Z, Sui Z, Zhang H, et al. (2010) ‘A life or death decision’for pollen tubes in S-RNase-based self-incompatibility. J Exp Bot 61: 2027–2037 10.1093/jxb/erp381 [DOI] [PubMed] [Google Scholar]
- 20. Okada K, Tonaka N, Moriya Y, Norioka N, Sawamura Y, et al. (2008) Deletion of a 236 kb region around S4-RNase in a stylar-part mutant S4sm-haplotype of Japanese pear. Plant Mol Biol 66: 389–400 10.1007/s11103-007-9277-1 [DOI] [PubMed] [Google Scholar]
- 21. Li MF, Li XF, Han Zh H, Shu HR, Li T (2009) Molecular analysis of two Chinese pear (Pyrus bretschneideri Rehd.) spontaneous self-compatible mutants, Yan Zhuang and Jin Zhui. Plant Biol (Stuttg) 11: 774–783 10.1111/j.1438-8677.2008.00180.x [DOI] [PubMed] [Google Scholar]
- 22. Sanzol J (2009) Pistil-function breakdown in a new S-allele of European pear, S21, confers self-compatibility. Plant Cell Rep 28: 457–467 10.1007/s00299-008-0645-3 [DOI] [PubMed] [Google Scholar]
- 23. Yamane H, Ikeda K, Ushijima K, Sassa H, Tao R (2003) A pollen-expressed gene for a novel protein with an F-box motif that is very tightly linked to a gene for S-RNase in two species of cherry, Prunus cerasus and P. avium . Plant Cell Physiol 44: 764–769 10.5580/1f5e [DOI] [PubMed] [Google Scholar]
- 24. Sonneveld T, Tobutt KR, Vaughan SP, Robbins TP (2005) Loss of pollen-S function in two self-compatible selections of Prunus avium is associated with deletion/mutation of an S haplotype-specific F-box gene. Plant Cell 17: 37–51 10.1105/tpc.104.026963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crane M, Lewis D (1942) Genetical studies in pears. J Genet 43: 31–43 10.1007/BF02982745 [DOI] [Google Scholar]
- 26. Entani T, Iwano M, Shiba H, Takayama S, Fukui K, et al. (1999) Centromeric localization of an S-RNase gene in Petunia hybrida Vilm. Theor Appl Genet 99: 391–397 10.1007/s0012200512-49 [DOI] [PubMed] [Google Scholar]
- 27. Golz JF, Oh HY, Su V, Kusaba M, Newbigin E (2001) Genetic analysis of Nicotiana pollen-part mutants is consistent with the presence of an S-ribonuclease inhibitor at the S locus. Proc Natl Acad Sci 98: 15372–15376 10.1073/pnas.261571598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adachi Y, Komori S, Hoshikawa Y, Tanaka N, Abe K, et al. (2009) Characteristics of fruiting and pollen tube growth of apple autotetraploid cultivars showing self-compatibility. J Jpn Soc Hort Sci 78: 402–409. [Google Scholar]
- 29. Qi Y-J, Wu H-Q, Cao Y-F, Wu J, Tao S-T, et al. (2011) Heteroallelic diploid pollen led to self-compatibility in tetraploid cultivar ‘Sha 01′(Pyrus sinkiangensis Yü). Tree Genet Genomes 7: 685–695 10.1007/s11295-011-0366-6 [DOI] [Google Scholar]
- 30. Kubo K-i, Entani T, Takara A, Wang N, Fields AM, et al. (2010) Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science 330: 796–799 10.1126/science.119524-3 [DOI] [PubMed] [Google Scholar]
- 31. Vilanova S, Badenes ML, Burgos L, Martinez-Calvo J, Llacer G, et al. (2006) Self-compatibility of two apricot selections is associated with two pollen-part mutations of different nature. Plant Physiol 142: 629–641 10.1104/pp.106.083865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zuriaga E, Molina L, Badenes ML, Romero C (2012) Physical mapping of a pollen modifier locus controlling self-incompatibility in apricot and synteny analysis within the Rosaceae. Plant Mol Biol 79: 229–242 10.1007/s11103-012-9908-z [DOI] [PubMed] [Google Scholar]
- 33. Wu J, Gu C, Du YH, Wu HQ, Liu WS, et al. (2011) Self-compatibility of ‘Katy’ apricot (Prunus armeniaca L.) is associated with pollen-part mutations. Sex Plant Reprod 24: 23–35 10.1007/s00497-010-0148-6 [DOI] [PubMed] [Google Scholar]
- 34. Zuriaga E, Muñoz-Sanz JV, Molina L, Gisbert AD, Badenes ML, et al. (2013) An S-locus independent pollen factor confers self-compatibility in ‘Katy’Apricot. PloS ONE 8: e53947 10.1371/journal.pone.0053947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao L, Huang J, Zhao Z, Li Q, Sims TL, et al. (2010) The Skp1-like protein SSK1 is required for cross-pollen compatibility in S-RNase-based self-incompatibility. Plant J 62: 52–63 10.1111/j.1365313X.2010.04123.x [DOI] [PubMed] [Google Scholar]
- 36. Matsumoto D, Yamane H, Abe K, Tao R (2012) Identification of a Skp1-like protein interacting with SFB, the pollen S determinant of the gametophytic self-incompatibility in Prunus . Plant Physiol 159: 1252–1262 10.1104/pp.112.197343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doyle JJ, Dickson EE (1987) Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19: 11–15. [Google Scholar]
- 38. Doyle J, Doyle J (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13–15. [Google Scholar]
- 39. Cheng J, Zhang Y, Li T (2006) Quick SDS method for RNA isolation from apple and other plant tissues with room temperature centrifugation. Acta Horticulturae Sinica 33: 470–470. [Google Scholar]
- 40. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 doi 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 42. Wheeler D, Newbigin E (2007) Expression of 10 S-class SLF-like genes in Nicotiana alata pollen and its implications for understanding the pollen factor of the S locus. Genetics 177: 2171–2180 10.1534/genetics.107.076885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsukamoto T, Hauck NR, Tao R, Jiang N, Iezzoni AF (2006) Molecular characterization of three non-functional S-haplotypes in sour cherry (Prunus cerasus). Plant Mol Biol 62: 371–383 10.1007/s11103-006-9026-x [DOI] [PubMed] [Google Scholar]
- 44. Yang M, Hu Y, Lodhi M, McCombie WR, Ma H (1999) The Arabidopsis SKP1-LIKE1 gene is essential for male meiosis and may control homologue separation. Proc Natl Acad Sci 96: 11416–11421 10.1073/pnas.96.20.11416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen G, Zhang B, Liu L, Li Q, Zhang Y, et al. (2012) Identification of a ubiquitin-binding structure in the S-Locus F-Box protein Controlling S-RNase-based self-Incompatibility. J Genet and Genom 39: 93–102 doi 10.1016/j.jgg.2012.01.001. [DOI] [PubMed] [Google Scholar]