Abstract
Evaluation of candidate loci culminated in the identification of a heterozygous missense mutation (R67W) in KCNJ2, the gene encoding the inward-rectifying potassium current, Kir2.1, in 41 members of a kindred in which ventricular arrhythmias (13 of 16 female members [81%]) and periodic paralysis (10 of 25 male members [40%]) segregated as autosomal dominant traits with sex-specific variable expressivity. Some mutation carriers exhibited dysmorphic features, including hypertelorism, small mandible, syndactyly, clinodactyly, cleft palate, and scoliosis, which, together with cardiodysrhythmic periodic paralysis, have been termed “Andersen syndrome.” However, no individual exhibited all manifestations of Andersen syndrome, and this diagnosis was not considered in the proband until other family members were examined. Other features seen in this kindred included unilateral dysplastic kidney and cardiovascular malformation (i.e., bicuspid aortic valve, bicuspid aortic valve with coarctation of the aorta, or valvular pulmonary stenosis), which have not been previously associated. Nonspecific electrocardiographic abnormalities were identified in some individuals, but none had a prolonged QT interval. Biophysical characterization of R67W demonstrated loss of function and a dominant-negative effect on Kir2.1 current. These findings support the suggestion that, in addition to its recognized role in function of cardiac and skeletal muscle, KCNJ2 plays an important role in developmental signaling.
Polymorphic ventricular tachycardia is an arrhythmia characterized by a beat-to-beat alternating QRS axis and morphology at a rate slower than torsade de pointes; some individuals with polymorphic ventricular tachycardia manifest bidirectional ventricular tachycardia or ventricular ectopy. Recent reports of mutations in the cardiac ryanodine receptor gene (hRyR2), the calsequestrin 2 gene (CASQ2), and the inward-rectifying potassium channel Kir2.1 (KCNJ2) have established the genetic heterogeneity of polymorphic ventricular tachycardia (Lahat et al. 2001; Laitinen et al. 2001; Plaster et al. 2001; Priori et al. 2001). The periodic paralyses are a clinically and genetically heterogeneous group of disorders characterized by transient attacks of muscle weakness without impairment of nervous-system function (Jen and Ptacek 2001). Recently, mutations in KCNJ2 were reported to cause a cardiodysrhythmic type of periodic paralysis known as “Andersen syndrome” (MIM 170390) (Plaster et al. 2001).
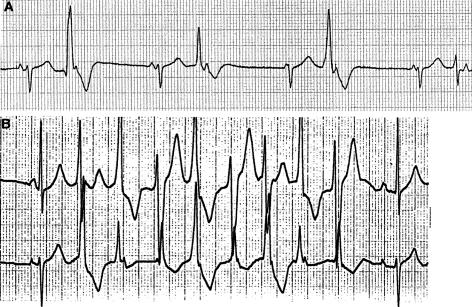
We evaluated a large kindred in which ventricular arrhythmia (in female members) and periodic paralysis (in male members) segregated as autosomal dominant traits. The proband, a white female 13-year-old, experienced a cardiac arrest during gym class. Valvular pulmonary stenosis (peak gradient of 35 mmHg) had been diagnosed at age 6 mo. At age 11 years, nonsustained, polymorphic ventricular tachycardia with a normal QT interval was diagnosed (fig. 1), and an exercise test showed suppression of ventricular ectopy when sinus heart rate exceeded 150 beats per minute (bpm) (not shown). At age 12 years, she was found unconscious, floating face down while swimming, and was revived with minimal effort. Ventricular fibrillation was induced during electrophysiological study at age 13 years (not shown), and a cardioverter defibrillator was implanted. Medical history, obtained from nearly 250 relatives, identified other female relatives with a history of ventricular arrhythmia. Male family members with a history of periodic paralysis brought on by physical exertion were also identified. Family members with ventricular arrhythmia or periodic paralysis, as well as their first-degree relatives, were invited to participate in a genetic study. Informed consent was obtained from all participants, in accordance with the Cincinnati Children's Hospital Medical Center Institutional Review Board. Participants were evaluated by history, review of medical records, physical examination, and 12-lead electrocardiogram. Two-dimensional transthoracic echocardiography with colorflow Doppler interrogation was performed in 23 individuals. Clinical studies were performed without knowledge of genotype.
Figure 1.
Electrocardiogram of proband. A, Sinus bradycardia and multiform premature ventricular contractions obtained as a rhythm strip during recording of a standard electrocardiogram. B, Polymorphic ventricular tachycardia recorded during ambulatory electrocardiogram (Holter monitor).
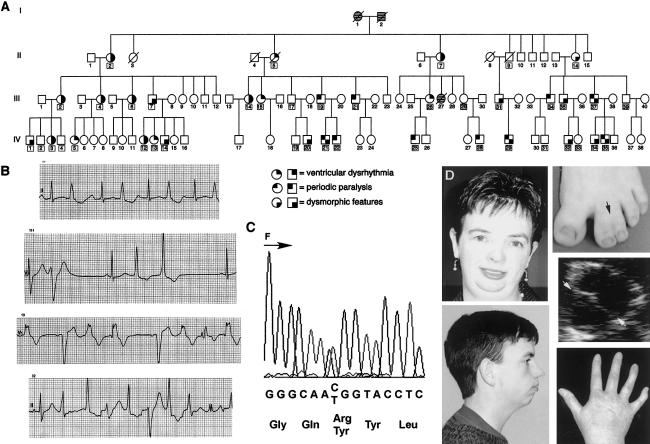
Pedigree analysis suggested that ventricular arrhythmia was an autosomal dominant trait with sex-specific variable expressivity (fig. 2A). Genomic DNA was isolated from lymphocytes of participants, and individuals with ventricular arrhythmia or whose offspring had ventricular arrhythmia were genotyped at candidate loci through use of polymorphic STRs (Benson et al. 1998). Using STRs located at chromosome 17q23 (D17S789, D17S949, D17S1786, and D17S840), we obtained a maximum two-point LOD score of 3.6 at recombination fraction 0.0, indicating linkage to chromosome 17q23, where the Kir2.1 channel, KCNJ2, is encoded. Two-point linkage analyses were performed using MLINK, with allele frequencies determined from family members and with a phenocopy rate of 0.001. Male subjects with periodic paralysis and female subjects with ventricular arrhythmia shared a common haplotype.
Figure 2.
Genotype-phenotype features. A, Pedigree. Square symbols depict male subjects, and circles depict female subjects. Cross-hatching indicates unknown status. The occurrence of ventricular arrhythmia, periodic paralysis and dysmorphic features are depicted by small darkened quadrants. A box around the identifying number denotes the presence of R67W. B, Electrocardiographic recordings from four female subjects with ventricular arrhythmia. C, C→T transition (C199T), which alters the coding sense from arginine to tryptophan (R67W), shown in the forward (F) direction. D, Dysmorphic features, including (clockwise, from bottom left) small mandible, hypertelorism, syndactyly of toes (black arrow), bicuspid aortic valve (the white arrows point to line of leaflet coaptation, indicating a bicuspid valve), and clinodactyly, observed in five different family members.
PCR was used to amplify the coding region of KCNJ2 from genomic DNA, as described elsewhere (Plaster et al. 2001), and products were prepared and sequenced in both the sense and antisense direction through use of an ABI PRISM 3700 DNA Analyzer (Applied Biosystems). During a survey of the KCNJ2 coding region, a C→T transition (C199T), which altered the coding sense from arginine to tryptophan (R67W), was identified (fig. 2C). Amplification-refractory mutation system analysis was utilized to identify the presence or absence of C199T in family members and in unrelated unaffected white control subjects (Little 1995). C199T is considered a mutation because it changes the coding sense of a conserved residue, it cosegregates with disease in this kindred, and it is absent in >100 chromosomes derived from unrelated unaffected subjects. R67W was identified in 41 of 77 genotyped family members (fig. 2A). Among 16 female carriers of R67W, 13 (81%) had ventricular arrhythmia, which was documented by electrocardiography at the time of the study (11 individuals) or from history and medical record review (2 individuals) (fig. 2B). Among individuals with ventricular arrhythmia, a history of syncope (3 individuals), cardiac arrest (1 individual), or sudden death (1 individual) was present. Periodic paralysis was diagnosed in 10 of 25 (40%) male subjects carrying R67W, on the basis of a characteristic history of weakness following exertion (8 individuals) and hypokalemia during symptoms of muscle weakness (2 individuals). A skeletal muscle biopsy had been obtained from one symptomatic individual (not shown). Facial dysmorphology, including hypertelorism (4 individuals) and small mandible (10 individuals), as well as hand anomalies, consisting of syndactyly of second and third digits of the hands and/or feet (9 individuals) or clinodactyly (12 individuals), affected both male and female subjects (fig. 2D). Surgically treated scoliosis (one female subject), cleft palate (one male subject), and unilateral hypoplastic kidney (one male subject) were also identified. Semilunar valve abnormalities, including pulmonary stenosis (one individual), bicuspid aortic valve (three individuals) (fig. 2D), and bicuspid aortic valve with coarctation of the aorta (one individual), were identified. In addition, a neonate who died in 1954 (individual III-27) was described as having a “missing heart valve”; medical records providing details were not available. First-degree heart block was noted in four male subjects and one female subject; the latter individual also demonstrated left-bundle-branch block. No genotype-positive individual exhibited electrocardiographic evidence of a long QT interval.
To assess biophysical properties of wild-type (WT) and mutant KCNJ2 alleles, heterologous expression experiments were performed in both a human cell line (tsA201) and Xenopus oocytes. KCNJ2 expression plasmids were constructed in pCMV-Script (Stratagene) (for tsA201 expression) and a modified pSP64T vector (for Xenopus oocyte expression), from full-length coding-region cDNA (1,367 bp, including 43 bp of the 3′ UTR) obtained by PCR amplification of human genomic DNA (primers designed on the basis of GenBank accession number XM_008406). The R67W mutation was engineered by PCR-mediated site-directed mutagenesis. All constructs were sequenced in their entirety, to exclude polymerase errors and other cloning artifacts.
Expression of WT and R67W KCNJ2 was achieved by transient transfection of tsA201 cells through use of FUGENE-6 (Roche). Xenopus oocytes were manually defolliculated, incubated overnight in L-15 (Leibovitz’s media), injected with in vitro transcribed RNA (2–4 ng/oocyte in 50 nl water) or water (50 nl), and cultured for 3 d. Currents expressed in tsA201 cells or Xenopus oocytes were measured using the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981) or two-electrode voltage-clamp recording with a Warner OC-725B amplifier (Warner Instrument), respectively.
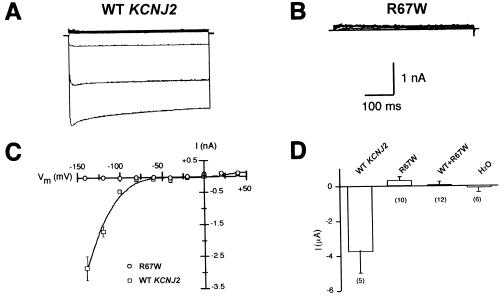
In tsA201 cells, expression of WT KCNJ2 gave rise to a large, inward-rectifying, Ba2+-sensitive current (fig. 3A and 3C) highly consistent with previous characterization of this potassium channel (Kubo et al. 1993), but transfection with R67W KCNJ2 exhibited no inward current (fig. 3B and 3C) consistent with a complete loss of channel function. To determine whether functional heterotetramers could be generated by mutant and wild-type subunits, we performed additional experiments in Xenopus oocytes injected with a mixture of WT KCNJ2 and R67W RNAs. Inward current measured in oocytes expressing WT KCNJ2 alone was robust, whereas no Ba2+-sensitive inward current was observed in oocytes injected either with R67W RNA alone or with an equal molar mixture of WT KCNJ2 and R67W RNAs (fig. 3D). These results are consistent with a strong dominant-negative effect of this mutant residue on KCNJ2 function.
Figure 3.
KCNJ2-induced currents in human tsA201 cells and Xenopus oocytes. Shown are representative Ba+2-sensitive potassium currents obtained from tsA201 cells expressing WT (A) and R67W (B) KCNJ2. The solid line next to the current traces indicates the zero current level. Currents were measured from a holding potential of −90 mV to potentials from −140 to +40 mV, in 20-mV steps. C, Current-voltage relationships for tsA201 cells expressing WT (squares) or R67W KCNJ2 (circles). Whole-cell currents were measured at 400 ms after the start of the voltage pulse. The expression of WT KCNJ2 generated current, whereas expression of the R67W mutant did not elicit detectable current. Data represent mean ± SEM (n⩾4 cells). D, Ba+2-sensitive K+ currents measured at −150 mV, 50 ms after the start of the voltage pulse in Xenopus oocytes injected with WT KCNJ2 (4 ng), R67W KCNJ2 (4 ng) or coinjected with WT (2 ng) and R67W (2 ng) RNAs. Injection of WT RNA generated current, whereas no currents were detected after injection of R67W RNA or coinjection of both WT and mutant RNAs. Current measured in water-injected oocytes is shown for comparison. Data represent mean ± SEM (n=5–12 oocytes).
The extended kindred presented here segregates a previously unreported heterozygous KCNJ2 mutation (R67W) with ventricular arrhythmia in female subjects and periodic paralysis in male subjects. In addition, some genotype-positive family members also exhibit dysmorphic features of hypertelorism, small mandible, syndactyly, clinodactyly, cleft palate, and scoliosis, which, together with cardiodysrhythmic periodic paralysis, have been termed “Andersen syndrome.” Additional dysmorphic features, including unilateral hypoplastic kidney and cardiovascular malformations, which have not previously been associated with the Andersen syndrome phenotype, were also observed. Nonspecific electrocardiographic abnormalities were identified in some individuals, but none had a prolonged QT interval. (For phenotypic features of genotype-positive family members, see online-only table.)online-only table.) Biophysical characterization of R67W demonstrated loss of function and a dominant-negative effect on Kir2.1 current, findings similar to those reported elsewhere (Plaster et al. 2001). Variation in expression of alleles, genetic background, or environmental factors that modify the clinical features could explain the pleiotropy manifested by the extreme intrafamilial variability in phenotype.
Table 1.
Phenotypic Features of Genotype-Positive Family Members[Note]
| ID | Sex | Ventricular Arrhythmia | Periodic Paralysis | Dysmorphic Featuresa | ECG Features |
| II-2 | F | Yes | 1, 3, 4 | ||
| III-2 | F | Yes | 1 | ||
| IV-1 | M | 2t | |||
| IV-2 | M | 1° heart block | |||
| IV-3 | F | Yesb | 8 | ||
| IV-4 | M | 1° heart block | |||
| III-4 | F | Yes | 2f, 2t, 4 | ||
| IV-5 | F | Yes | |||
| III-6 | F | Yes | 1, 4, 5, 9 | ||
| III-7 | M | 9 | |||
| IV-12 | F | Yes | 2f, 10 | ||
| IV-13 | F | Yes | |||
| IV-14 | M | 4 | |||
| II-5 | F | Yesc | |||
| III-14 | F | Yesb | 9 | ||
| III-15 | F | Yes | |||
| III-17 | M | ||||
| IV-19 | M | 1 | |||
| IV-20 | M | 1, 4 | |||
| III-19 | M | Yes | |||
| IV-21 | M | Yes | 4 | ||
| IV-22 | M | Yes | 1 | ||
| III-21 | M | Yes | |||
| II-7 | F | Yes | 1 | 1° heart block, left bundle branch block | |
| III-26 | F | Yes | 1 | ||
| IV-25 | M | Yes | |||
| III-29 | F | ||||
| IV-28 | M | Yes | |||
| II-9 | M | ||||
| III-31 | M | 1° heart block | |||
| IV-29 | M | Yes | 1, 3, 4, 6 | 1° heart block | |
| II-14 | F | 1 | |||
| III-34 | M | Yes | 4 | ||
| IV-31 | M | 2t, 4 | |||
| III-35 | M | 2t, 7 | |||
| IV-32 | M | 1, 2t | |||
| IV-33 | F | ||||
| III-37 | M | Yes | 1, 2f, 2t, 3 | ||
| IV-34 | M | 4 | |||
| IV-35 | M | Yes | |||
| III-39 | M |
Note.— Individual measures of corrected QT interval (QTc) are not shown, as all were normal. The mean ± SD QTc for genotype-positive (415 ± 15 ms) versus genotype negative (407 ± 16 ms) individuals is not significantly different.
1 = clinodactyly, 2f = finger syndactyly, 2t = toe syndactyly, 3 = hypertelorism, 4 = small mandible, 5 = scoliosis, 6 = cleft palate, 7 = hypoplastic left kidney, 8 = moderate pulmonary stenosis, 9 = bicuspid aortic valve, and 10 = bicuspid aortic valve with coarctation of aorta.
Implantible cardioverter defibrillator
Sudden death.
Andersen syndrome was previously mapped to chromosome 17q23, where the inward-rectifying potassium channel gene, KCNJ2, is encoded, and nine heterozygous KCNJ2 mutations were identified in 13 of 16 probands with the Andersen syndrome phenotype (Plaster et al. 2001). Heterologous expression of mutants revealed loss of function and dominant-negative effect. KCNJ2 mutations demonstrated reduced penetrance and variable expressivity, since 4 of 28 (14%) genotype-positive individuals had no obvious phenotype, and 13 of 28 (46%) demonstrated all manifestations (ventricular arrhythmia, periodic paralysis, and dysmorphic features) of Andersen syndrome. Among 41 genotype-positive individuals in the kindred we studied, the phenotype ranged from nonpenetrant gene carriers (8 individuals [20%]) to individuals exhibiting two of three characteristics (13 individuals [32%]). No individual exhibited all manifestations of Andersen syndrome, and this diagnosis was not considered in the proband until other family members were examined.
Inward-rectifying potassium channels have been identified in a wide range of tissues and are so named for their ability to allow the passage of inward current to a much greater extent than they allow the passage of outward current. To date, seven families of genes that share a similar proposed subunit structure and encode potassium inward rectifiers (Kir) have been identified. Kir2 family members are expressed in excitable tissue, and Kir2.1 function has been studied extensively in the heart (Zaritsky et al. 2001), where subunits form either homo- or heterotetramers (Tinker et al. 1996; Derst et al. 2001). However, as is evident from the Andersen syndrome phenotype, Kir2.1 function is not limited to excitable tissue and apparently has additional roles during development.
In the rat embryo, KCNJ2 mRNA is prominently expressed in cardiac and skeletal muscle, brain, metanephros, and developing bony structures of the craniofacial region, extremities, and vertebrae—a configuration similar to the distribution of abnormalities associated with Andersen syndrome (Karschin and Karschin 1997). The electrophysiological phenotypes of periodic paralysis and ventricular arrhythmia, which are characteristic features of Andersen syndrome, have been attributed to dominant-negative effects on Kir2.1 function that typically manifest in the 2nd decade of life. However, dysmorphic features associated with KCNJ2 mutation are present at birth, in both human and mouse. For example, Kir2.1 knockout mice exhibit narrowing of the maxilla and complete cleft of the secondary palate at birth (Zaritsky et al. 2000), and humans with Andersen syndrome also exhibit cleft palate and other craniofacial and skeletal anomalies (Anderson et al. 1971; Sansone et al. 1997; Canun et al. 1999; Plaster et al. 2001). We speculate that the dysmorphic features of unilateral hypoplastic kidney and cardiovascular malformations not previously associated with Andersen syndrome also result from R67W. How Kir2.1 mutation results in these developmental anomalies remains an open question. Two mechanisms appear possible: (1) reduced/absent inward-rectifier Kir2.1 current alters the function of precursor cells (e.g., osteoclasts), or (2) coassembly of mutant Kir2.1 in heteromultimeric combination may alter function of other Kir members and thereby result in malfunction of developmentally important cells. Additional support for the role of ion channels in development has come from studies of cerebellar hypoplasia in the weaver mouse, so named for its gait, which is due to a mutation in the pore region of the G protein–coupled inward-rectifier potassium channel GIRK2 (KCNJ6) and presumably leads to failure of granule cell migration (Patil et al. 1995).
Sex specificity of cardiac arrhythmias and periodic paralysis has not been previously described in Andersen syndrome. There has been an increasing recognition of specific electrocardiographic and electrophysiological differences—for example, resting heart rate and corrected QT intervals—between males and females, but the precise way in which sex and gonadal steroids contribute to these differences is not known (Pham and Rosen 2002; Wolbrette et al. 2002). Female subjects with R67W typically noted onset of ventricular arrhythmia after age 10 years. An increased incidence of arrhythmias has been reported during pregnancy, but female subjects with R67W reported reduced ventricular arrhythmias during pregnancy and after age 55 years, coinciding with menopause. Additional studies are required to determine whether the gender specificity is a specific effect of R67W or an uncommon manifestation of loss of the inward-rectifying K+ current, IK1.
Acknowledgments
We are indebted to family members for their participation. The studies would not have been possible without the technical assistance of Laura Etter, Macaira Dyment, and Henry Lee, nor without the services, in the collection of patient materal, of Linda Boehm; Kerry Howell, M.S.; Carol Modica and staff at Joint Township District Memorial Hospital; Michael R. Epstein, M.D.; Wendi Long; Tamara Taggert; J. R. Bockhoven, M.D.; Kris Norris; and Holly Ippisch, M.D. This work was supported, in part, by National Institutes of Health grants HL61006 and HL/HD04300 (both to D.W.B.) and NS32387 and HL46681 (both to A.L.G.), as well as by National Research Service Award F32-GM20415 (to C.G.V.).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for primers designed on the basis of accession number XM_008406)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Andersen syndrome [MIM 170390])
References
- Andersen ED, Krasilnikoff PA, Overvad H (1971) Intermittent muscular weakness, extrasystoles, and multiple developmental anomalies: a new syndrome? Acta Paediat Scand 60: 559–564 [DOI] [PubMed] [Google Scholar]
- Benson DW, Sharkey A, Fatkin D, Lang P, Basson CT, McDonough B, Strauss AW, Seidman JG, Seidman CE (1998) Reduced penetrance, variable expressivity and genetic heterogeneity of familial atrial septal defect. Circulation 97:2043–2048 [DOI] [PubMed] [Google Scholar]
- Canun S, Perez N, Beirana LG (1999) Andersen syndrome autosomal dominant in three generations. Am J Med Genet 85:147–156 [DOI] [PubMed] [Google Scholar]
- Derst C, Karschin C, Wischmeyer E, Hirsch JR, Preisig-Muller R, Rajau S, Engel H, Grzeschik K-H, Daut J, Karschin A (2001) Genetic and functional linkage of Kir5.1 and Kir2.1 channel subunits. FEBS Letters 491:305–311 [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100 [DOI] [PubMed] [Google Scholar]
- Jen J, Ptacek L (2001) Channelopathies: episodic disorders of the nervous system. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th ed. McGraw-Hill Medical Publishing, New York, pp 5223–5240 [Google Scholar]
- Karschin C, Karschin A (1997) Ontogeny of gene expression of Kir channel subunits in the rat. Mol Cell Neurosci 10:131–148 [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Nung Y, Jan LY (1993) Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362:127–133 [DOI] [PubMed] [Google Scholar]
- Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M (2001) A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet 69:1378–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, Donarum EA, Marino M, Tiso N, Viitasalo M, Toivonen L, Stephan DA, Kontula K (2001) Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 103:485–490 [DOI] [PubMed] [Google Scholar]
- Little S (1995) Amplification-refractory mutation system (ARMS) analysis of point mutations. Current Protocols in Human Genetics. Unit 9.8.1-12. John Wiley & Sons, New York [DOI] [PubMed] [Google Scholar]
- Patil N, Cox DR, Bhat D, Faham M, Myers RM, Peterson AS (1995) A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet 11:126–129 [DOI] [PubMed] [Google Scholar]
- Pham TV, Rosen MR (2002) Sex, hormones, and repolarization. Cardiovasc Res 53:740–751 [DOI] [PubMed] [Google Scholar]
- Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu Y-H, Ptacek LJ (2001) Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell 105:511–519 [DOI] [PubMed] [Google Scholar]
- Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino VV, Danieli GA (2001) Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103:196–200 [DOI] [PubMed] [Google Scholar]
- Sansone V, Griggs RC, Meola G, Ptacek LJ, Barohn R, Iannaccone S, Bryan W, Baker N, Janas SJ, Scott W, Ririe D, Tawil R (1997) Andersen's syndrome: a distinct periodic paralysis. Ann Neurol 42:305–312 [DOI] [PubMed] [Google Scholar]
- Tinker A, Jan YN, Jan LY (1996) Regions responsible for the assembly of inwardly rectifying potassium channels. Cell 87:857–868 [DOI] [PubMed] [Google Scholar]
- Wolbrette D, Nacarelli G, Curtis A, Lehmann M, Kadish A (2002) Gender differences in arrhythmias. Clin Cardiol 25:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL (2000) Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ Res 87:160–166 [DOI] [PubMed] [Google Scholar]
- Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL (2001) The consequences of disrupting cardiac inwardly rectifying K+ current (IK1) as revealed by targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol 533:697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]