Abstract
Aspergillus fumigatus is the causative agent of invasive aspergillosis, leading to infection-related mortality in immunocompromised patients. We previously showed that the conserved and unique-to-fungi veA gene affects different cell processes such as morphological development, gliotoxin biosynthesis and protease activity, suggesting a global regulatory effect on the genome of this medically relevant fungus. In this study, RNA sequencing analysis revealed that veA controls the expression of hundreds of genes in A. fumigatus, including those comprising more than a dozen known secondary metabolite gene clusters. Chemical analysis confirmed that veA controls the synthesis of other secondary metabolites in this organism in addition to gliotoxin. Among the secondary metabolite gene clusters regulated by veA is the elusive but recently identified gene cluster responsible for the biosynthesis of fumagillin, a meroterpenoid known for its anti-angiogenic activity by binding to human methionine aminopeptidase 2. The fumagillin gene cluster contains a veA-dependent regulatory gene, fumR (Afu8g00420), encoding a putative C6 type transcription factor. Deletion of fumR results in silencing of the gene cluster and elimination of fumagillin biosynthesis. We found expression of fumR to also be dependent on laeA, a gene encoding another component of the fungal velvet complex. The results in this study argue that veA is a global regulator of secondary metabolism in A. fumigatus, and that veA may be a conduit via which chemical development is coupled to morphological development and other cellular processes.
Introduction
The filamentous fungus Aspergillus fumigatus is one of the most common human fungal pathogens found infecting a large population of immunodepressed patients. This group includes individuals with hematological malignancies, those with genetic immunodeficiencies, patients infected with HIV, and cancer patients treated with chemotherapy [1–6]. This immunodepressed population is currently increasing [7] due to the higher number of organ transplants performed, immunosuppressive and myeloablative therapies for autoimmune and neoplastic diseases, and the HIV pandemic [1,7–9]. The mortality rate resulting from A. fumigatus infections in immunodepressed patients ranges from 40% to 90% [7,9–12].
In a previous report we demonstrated that the global regulatory velvet gene veA controls A. fumigatus production of conidia [13], the main inoculum during infection [14,15], and production of gliotoxin [13], a compound with immunosuppressive properties [16–28] also found to inhibit phagocytosis in macrophage and to induce apoptosis [29,30].
veA orthologs have been identified and characterized in other fungi [31,32] including other Aspergillus species, such as A. flavus [33–35], A. parasiticus [36] and the model filamentous fungus A. nidulans [37]. These previous studies provided abundant evidence of the role of veA as a regulator of both fungal morphological development and secondary metabolism. In 2003 our group described for the first time the role of veA as a global regulator of secondary metabolism in A. nidulans, including production of the mycotoxin sterigmatocystin [37]. veA also regulates the biosynthesis of other mycotoxins, including aflatoxin, cyclopiazonic acid and aflatrem in Aspergillus flavus [33], the synthesis of trichothecenes in F. graminearum [38], and the production of fumonisins and fusarins in Fusarium spp, specifically F. verticillioides and F. fujikuroi [32,39,40]. However, veA also controls the synthesis of other secondary metabolites known for their beneficial medical applications, for example, the beta-lactam antibiotic penicillin in A. nidulans and P. chrysogenum [37,41] as well as cephalosporin C in Acremonium chrysogenum [42].
In-depth studies of A. nidulans veA and its gene product also revealed mechanistic details of its mode of action. For instance, it is known that the VeA protein is transported to the nucleus by the KapA α-importin, and that this transport is promoted in the absence of light [43,44]. In the nucleus, VeA interacts with light-sensing proteins that also affect secondary metabolism and fungal differentiation, such as the red phytochrome-like protein FphA, which interacts with the blue- responsive proteins LreA-LreB [31,45]. In the nucleus VeA also interacts with VelB and LaeA [46,47]. VelB is another protein in the velvet family [47], and LaeA is a chromatin modifying protein that, like VeA, is required for the synthesis of numerous secondary metabolites [48,49]. In addition, a LaeA-like putative methyltransferase was also described to interact with VeA [50].
A microarray-based transcriptome study showed that A. fumigatus laeA affects the expression of 13 secondary metabolite gene clusters [51]; however, at that time the extent of veA regulation of the activation of secondary metabolite gene clusters was mostly unknown. With the goal of elucidating the full extent of veA-regulation of the A. fumigatus genome, particularly with respect to genes involved in secondary metabolism, we performed RNA sequencing analyses [52] and chemical characterization of A. fumigatus cultures, obtaining results consistent with a global regulatory pattern. This study also contributes to uncovering the regulation of novel secondary metabolite gene clusters in A. fumigatus. For example, an important discovery in our study is that veA and laeA, both of which encode velvet complex components, regulate the recently discovered gene cluster responsible for the synthesis of fumagillin [53]. Fumagillin has been intensely studied due to its potential in the treatment of amebiasis [54], microsporidiosis [55] and most recently, for its anti-angiogenic activity as inhibitor of the human type 2 methionine aminopeptidase (MetAP2) [56,57].
Materials and Methods
Strains and culture conditions
Aspegillus fumigatus strains used in this study are listed in Table S1. Fungal strains were grown on Czapek Dox media (Difco), unless otherwise indicated, and supplements for the corresponding auxotrophies as needed [58]. Solid medium was prepared by adding 15 g/liter agar. Strains were stored as 30% glycerol stocks at -80°C.
RNA extraction
Total RNA was extracted as previously described [13]. Briefly, conidia from the wild type, deletion veA (∆veA), complementation and over-expression veA (OEveA) strains were inoculated in Czapek-Dox (approximately 107 spores/mL) and grown as liquid stationary cultures at 37°C in the dark. Mycelia were collected 48 h and 72 h after inoculation and RNA was extracted using TRIzol (Invitrogen) following the manufacturer’s instructions. RNA samples were further purified using QIAgen RNeasy mini kit as previously described [59].
Transcriptome analysis
Genome and transcriptome sequence versions
All A. fumigatus Af293 sequences used are from sequence version s03-m04-v01 from the Aspergillus Genome Database (AspGD) [60].
Library preparation and RNA sequencing
RNA-Seq libraries were constructed and sequenced at the Vanderbilt Genome Sciences Resource using the Illumina Tru-seq RNA sample prep kit as previously described [61,62]. In brief, total RNA quality was assessed via Bioanalyzer (Agilent). Upon passing quality control, poly-A RNA was purified from total RNA and the second strand cDNA was synthesized from mRNA. cDNA ends were then blunt repaired and given an adenylated 3’ end. Next, barcoded adapters were ligated to the adenylated ends and the libraries were PCR enriched, quantified, pooled and sequenced an on Illumina HiSeq 2000 sequencer.
Read alignment and quantification of gene expression
Illumina TruSeq adapters were trimmed from the 3’ end of reads using the scythe software package (available from Buffalo, V. at https://github.com/ucdavis-bioinformatics/scythe), and low-quality bases were trimmed using the sickle software package (available from Joshi, N. at https://github.com/ucdavis-bioinformatics/sickle). Reads were aligned to the transcriptome using the bowtie read alignment software for single-end reads, with the maximum mismatches per read set at 2 and a seed length of 28 [63]. Read count per gene was calculated using SAMtools idxstats software [64]. For each sample, gene expression was quantified using the reads per Kilobase of exon per million mapped reads (RPKM) metric [65]. Differential expression was calculated between ∆veA and wild type, between OEveA and wild type, and between the complementation and wild-type strains. Two cutoffs were used to determine differentially regulated genes [61,62]. The first cutoff compared the fold difference between genes by calculating the relative RPKM (rRPKM = RPKMsample1/RPKMsample2) for each gene. The second cutoff compared the proportion of reads mapping to a gene in different samples using Fisher’s exact test with Bonferroni’s correction for multiple comparisons. A gene was considered differentially regulated if the log2 rRPKM value was equal to or greater than 2 and the Bonferroni-corrected Fisher’s exact p-value was less than 0.05.
Gene ontology categorization
The gene ontology (GO) categorizations of differentially regulated genes were compared against the set of non-differentially regulated genes to identify GO categories that were specifically enriched in differentially upregulated or downregulated gene sets in the three strain comparisons. GO categorizations for each gene were obtained from the AspGD’s GOSlim mapper for A. fumigatus Af293 [60]. AspGD’s GOSlim mapper contains higher-order GO terms for the process, component, and function sections of GO. All comparisons were performed using Fisher’s exact test with Bonferroni’s correction for multiple comparisons.
Sliding window analysis
We used a sliding window analysis [61] to determine clusters of genes that were upregulated or downregulated in the A. fumigatus genome in ∆veA versus wild type and OEveA versus wild type comparisons. Briefly, each gene was encoded as upregulated, downregulated or not significantly differentially regulated according to our specified differential regulation cutoffs. Then, we calculated the cumulative binomial probability of observing every window of 24 genes along each chromosome. To account for multiple comparisons, we used an empirically derived false discovery rate (FDR) by randomly permuting the expression data 1,000 times and running the sliding window analysis on each permuted dataset. Our FDR cutoff was set conservatively at 0.01. After all clusters below this FDR cutoff were found, genomically overlapping windows were collapsed into a larger cluster. Due to the window size, regions at the beginning or end of these master clusters may contain stretches of non-differentially regulated genes. We have reported all clusters from the first to last differentially regulated gene found for each cluster and the start and end genes of all significant clusters.
Quantitative RT-PCR analysis
One microgram of total RNA was treated with RQI Dnase to remove possible DNA contamination. Then cDNAs were obtained by reverse transcription using Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega). Quantitative Real Time PCR was performed with an Agilent MX3000p thermocycler using SYBR green Jump Start Taq (Sigma). The primers used for gene expression analysis are listed on Table S2.
Metabolome analysis
Extractions of secondary metabolites
Secondary metabolites were extracted as previously described [13]. Briefly, liquid Czapek-dox stationary cultures of the wild type, ∆veA, complementation and OEveA strains were grown as described. Supernatants were collected by filtration through sterile Miracloth™ (Calbiochem, USA) from 72 h and 120 h cultures. Fifteen mL of the culture filtrate was extracted with same amount of chloroform. Extracts were allowed to dry and were resuspended in 500µL of methanol; 10 µL aliquots were used for LC-MS analysis.
LC-MS
All solvents and other chemicals used were of analytical grade. All LC-MS analyses were performed on a Shimadzu 2010 EV LC-MS (Phenomenex® Luna, 5μ, 2.0 × 100 mm, C18 column) using positive and negative mode electrospray ionization with a linear gradient of 5–95% MeCN-H2O (0.1% formic acid) in 30 minutes followed by 95% MeCN for 15 minutes with a flow rate of 0.1 mL/min. The value of area under curve was observed by EIC (extracted ion chromatogram).
Generation of the fumR (Afu8g00420) deletion strain
Fusion Polymerase Chain Reaction (Fusion PCR) was used to create the deletion cassette of fumR as previously described [66]. First, Aspergillus parasiticus pyrG was PCR amplified from A. parasiticus genomic DNA using primers AparapyrGF-Linker and AparapyrGR-Linker (Table S2). The Aspergillus parasiticus pyrG fragment was ligated into pJET (Fermentas) yielding plasmid pSD38.1 Then, 1.5kb of 5’ UTR and 3’ UTR of fumR was amplified from A. fumigatus genomic DNA using primer pairs 420P1 & 420P2 and 420P3 & 420P4, respectively (Table S2). Aspergillus parasiticus pyrG was then amplified from pSD38.1 using primers 420P5 and 420P6. Three fragments were fused using primers 420P7 and 420P8 (Table S2). Protoplast mediated fungal transformation was done as previously described [66] using CEA17ku80 (gift from Robert Cramer) as the host strain. Aspergillus parasiticus pyrG was utilized as selectable marker, resulting in a complete gene replacement of fumR in CEA17ku80. Transformants were first screened using PCR (data not shown) and Southern blot analysis. Other DNA manipulations were done as previously described [67].
Generation of the laeA deletion strain
The laeA deletion DNA cassette was also generated by fusion PCR. Briefly, a 1.5 kb 5’ UTR fragment was first amplified from A. fumigatus genomic DNA with primers laeA_p1 and laeA_p2 (Table S2). A 1.3 kb 3’ UTR fragment was also amplified from genomic DNA with primers laeA_p3 and laeA_p4 (Table S2). Aspergillus parasiticus pyrG was amplified from pSD38.1 using primers laeA_p5 and lae_p6. The three fragments were fused using primers laeA_p7 and laeA_p8 (Table S2) as previously described [66]. The laeA deletion cassette was transformed into CEA17ku80. Transformants were first screened using PCR (data not shown) and Southern blot analysis.
Results
Hundreds of genes are differentially regulated by veA
Of the 9,784 genes in the A. fumigatus genome [60,68], 453 were upregulated and 1,137 were downregulated in the ∆veA strain when compared with the wild-type strain (Figure 1; Table S3). A similar pattern was observed in the OEveA versus wild type comparison, where 335 genes were upregulated and 908 genes were downregulated in the OEveA strain. In sharp contrast, comparison of the complementation strain and wild type showed that the two strains present very similar expression patterns.
Figure 1. Differentially regulated genes in comparisons of veA deletion, overexpression and complementation strains against the wild-type strain.
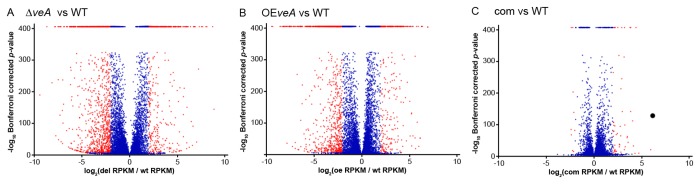
For each gene, the log2 rRPKM is reported along the x-axis and the negative log10 of the Bonferroni-corrected Fisher’s Exact p-value is reported along the y-axis. P-values with an infinite log10 value (p-values equal to zero) are reported as 400. Genes with a log2 rRPKM greater than 2 and a Bonferroni-corrected p-value less than 0.05 are considered differentially regulated and are represented by red dots, whereas genes below these thresholds are not considered to be differentially regulated and are represented by blue dots. (A) Comparison between the strain that carries a deletion of the veA gene (∆veA) and the wild-type (WT) strain; (B) Comparison between the veA over-expression strain (OEveA) and the WT strain; (C) Comparison between the veA complementation strain (com) and the WT strain.
Differentially regulated genes are dramatically enriched for secondary metabolism-related processes
Different GO process, component, and function categories are significantly enriched for both upregulated and downregulated genes in the two comparisons (Table 1). Importantly, enrichment analysis using GOSlim categories showed that differentially regulated genes in the ∆veA versus wild type and OEveA versus wild type comparisons have significant functional overlap (Table 1); specifically, 13 of the 19 GO categories that are enriched for either upregulated (3 categories) or downregulated (16 categories) genes in the ∆veA versus wild type comparison are also enriched and in the same direction in the OEveA versus wild type comparison. For example, both secondary metabolic process (GO:0019748) and toxin metabolic process (GO:0009404) GO function categories are enriched in downregulated genes from both comparisons.
Table 1. The List of GOSlim Categories That Are Significantly Enriched For Differentially Expressed Genes Between The veA Deletion (Del) And The Wild-Type (WT) Strain As Well As Between The veA Overexpression (OE) And The WT Strain.
| Comparison | GO Category ID | GO Category Description | p-value |
|---|---|---|---|
| GO Process Categories that are Significantly Enriched for Upregulated Genes | |||
| Del vs WT | GO:0042254 | ribosome biogenesis | 1.12E-06 |
| . | GO:0016070 | RNA metabolic process | 3.33E-02 |
| OE vs WT | GO:0005975 | carbohydrate metabolic process | 1.03E-02 |
| . | GO:0016070 | RNA metabolic process | 1.86E-02 |
| GO Process Categories that are Significantly Enriched for Downregulated Genes | |||
| Del vs WT | GO:0019748 | secondary metabolic process | 2.61E-34 |
| . | GO:0006996 | organelle organization | 1.22E-13 |
| . | GO:0016070 | RNA metabolic process | 1.24E-10 |
| . | GO:0009404 | toxin metabolic process | 1.38E-09 |
| . | GO:0006464 | cellular protein modification process | 3.31E-05 |
| . | GO:0042254 | ribosome biogenesis | 7.55E-05 |
| . | GO:0006259 | DNA metabolic process | 1.93E-04 |
| . | GO:0006810 | transport | 2.16E-04 |
| . | GO:0007049 | cell cycle | 5.40E-03 |
| . | GO:0005975 | carbohydrate metabolic process | 9.41E-03 |
| . | GO:0016192 | vesicle-mediated transport | 3.88E-02 |
| OE vs WT | GO:0019748 | secondary metabolic process | 5.21E-44 |
| . | GO:0006996 | organelle organization | 1.98E-13 |
| . | GO:0009404 | toxin metabolic process | 1.30E-09 |
| . | GO:0016070 | RNA metabolic process | 5.18E-06 |
| . | GO:0006810 | transport | 1.31E-05 |
| . | GO:0042254 | ribosome biogenesis | 1.20E-03 |
| . | GO:0006464 | cellular protein modification process | 1.38E-03 |
| . | GO:0007049 | cell cycle | 4.27E-03 |
| . | GO:0006259 | DNA metabolic process | 1.92E-02 |
| . | GO:0016192 | vesicle-mediated transport | 4.35E-02 |
| . | GO:0006950 | response to stress | 4.99E-02 |
| GO Component Categories that are Significantly Enriched for Upregulated Genes | |||
| Del vs WT | GO:0005730 | nucleolus | 4.57E-05 |
| OE vs WT | GO:0005886 | plasma membrane | 1.46E-04 |
| . | GO:0005576 | extracellular region | 1.67E-04 |
| . | GO:0005618 | cell wall | 1.72E-03 |
| GO Component Categories that are Significantly Enriched for Downregulated Genes | |||
| Del vs WT | GO:0005576 | extracellular region | 4.19E-18 |
| . | GO:0005634 | nucleus | 8.60E-06 |
| OE vs WT | GO:0005576 | extracellular region | 2.14E-07 |
| GO Function Categories that are Significantly Enriched for Upregulated Genes | |||
| OE vs WT | GO:0005215 | transporter activity | 4.01E-02 |
| GO Function Categories that are Significantly Enriched for Downregulated Genes | |||
| Del vs WT | GO:0016491 | oxidoreductase activity | 1.56E-06 |
| . | GO:0005198 | structural molecule activity | 1.32E-02 |
| . | GO:0005515 | protein binding | 3.63E-02 |
| OE vs WT | GO:0016491 | oxidoreductase activity | 5.25E-06 |
Differentially regulated genes are non-randomly distributed across the A. fumigatus genome
We used a sliding window analysis [2] to determine clusters of genes that were upregulated or downregulated for the ∆veA versus wild type and OEveA versus wild type comparisons. In total, 31 downregulated gene clusters and 6 upregulated gene clusters were identified (Figure 2). Ten downregulated clusters were found independently in both the ∆veA versus wild type and OEveA versus wild type comparisons, suggesting some similarity in phenotype between the αveA and OEveA strains. Twelve of the 31 downregulated clusters and 2 of the 6 upregulated clusters overlap with known or predicted secondary metabolic gene clusters [1,9]. For example, the gene clusters encoding for the secondary metabolites fumagillin, fumitremorgin G, and fumigaclavine C are all differentially regulated in at least one of the two strain comparisons (Figure 3). All 11 genes in the fumigaclavine C biosynthetic gene cluster are downregulated in both the ∆veA versus wild type and OEveA versus wild type comparisons. In both the ∆veA versus wild type and OEveA versus wild type comparisons, 13 of the 15 genes in the fumagillin gene cluster are downregulated. All genes involved in fumitremorgin G are upregulated on the OEveA versus wild type comparison, but none of these genes are differentially regulated in the αveA versus wild type comparison.
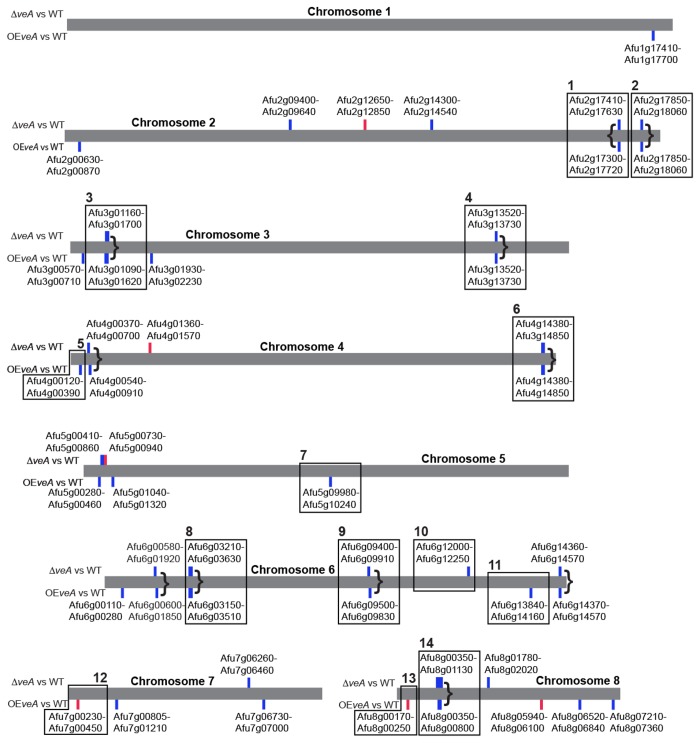
Figure 2. The genome-wide distribution of de novo identified gene clusters that appear to be regulated by veA.
De novo identification of gene clusters differentially regulated by the deletion versus wild-type (shown above each chromosome) and overexpression versus wild-type (shown below each chromosome) comparisons. Upregulated gene clusters are represented by red boxes and downregulated gene clusters are represented by blue boxes. Gene clusters identified in both comparisons whose boundaries overlap are denoted by bracket symbols. Previously reported secondary metabolism gene clusters [60,71] are boxed and numbered. 1: This cluster contains a conidial pigment biosynthesis cluster from Afu2g17530 – Afu2g17600 [82]; 2. This cluster contains the Fumigaclavine C cluster from Afu2g17960 – Afu2g18060 [83]; 3. Cluster of unknown function; 4. Cluster of unknown function; 5. This cluster contains an endocrocin secondary metabolism cluster from Afu4g00210 – Afu4g00230 [84]; 6. This cluster contains the pathway responsible for helvolic acid biosynthesis from Afu4g14770 – Afu4g14850 [85]; 7. Cluster of unknown function; 8. Cluster of unknown function; 9. This cluster contains the gliotoxin cluster from Afu6g09630 – Afu6g 09740 [86]; 10. This cluster contains the fumiquinazoline biosynthetic cluster from Afu6g12040 – Afu6g12110 [87]; 11. Cluster of unknown function; 12. Cluster of unknown function; 13. This cluster contains the fumitremorgin G cluster from Afu8g00260 – Afu8g00170 [76]; 14. This cluster contains the fumagillin gene cluster from Afu8g00370 – Afu8g00520 [53]; and the pseurotin A cluster from Afu8g00530 – Afu8g00570 [88].
Figure 3. Expression patterns of the fumagillin (A), fumitremorgin G (B), and fumigaclavine C (C) gene clusters in the ΔveA vs WT and OEveA vs WT comparisons.
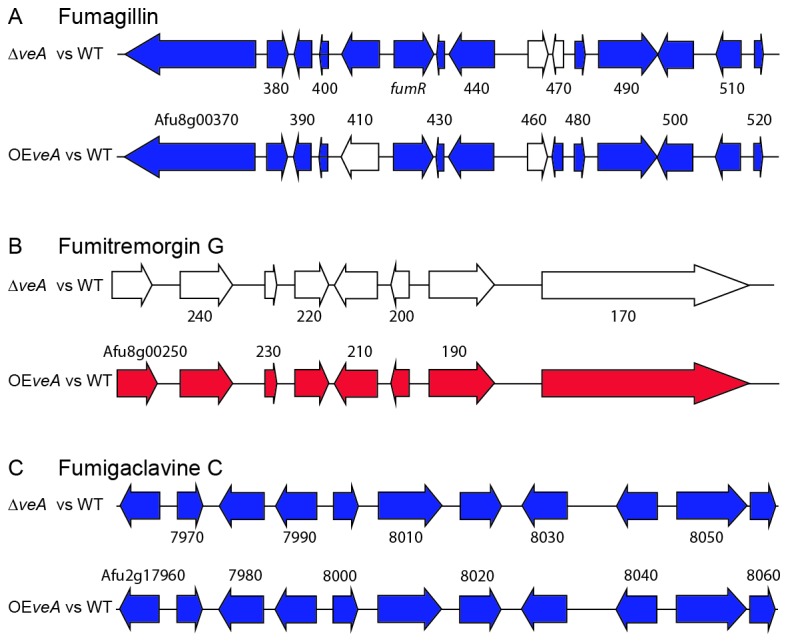
Genes labeled with numbers correspond to locus tags without the chromosomal prefix or trailing zeros (i.e., the gene labeled 380 in the fumagillin cluster corresponds to Afu8g00380). The sole exception is fumR (Afu8g00420), the name given to the regulatory gene for the fumagillin cluster characterized in this study. Genes are color-coded by differential expression; blue indicates downregulation, red indicates upregulation, and white indicates no differential regulation.
veA regulation profile of secondary metabolite gene clusters in A. fumigatus partially differs from the laeA profile
Previous studies of the model fungus Aspergillus nidulans demonstrated that the veA gene product, VeA, interacts with other proteins in cell nuclei [45,47], among them LaeA. Additionally, Park et al. [69] used tandem affinity techniques [47] to show that the A. fumigatus LaeA co-purifies with A. nidulans VeA, suggesting that A. fumigatus VeA might also interact with A. fumigatus LaeA. LaeA is a putative methyl transferase that affects chromatin conformation [48]. This VeA-interacting protein has also been described as affecting expression of secondary metabolite gene clusters in A. fumigatus [51]. Our results indicate that although the regulation patterns of the two proteins overlap, they are not identical (Table 2). Specifically, five out of the nine clusters that Perrin et al. report as being under full laeA regulation are found to be in windows of differentially regulated genes in both the ∆veA versus wild type and the OEveA versus wild type comparisons. Of the remaining four clusters, two are not found to be differentially regulated and two have different expression patterns in the two comparisons. The Afu6g12040–2080 laeA-regulated cluster was found to be part of a window of downregulated genes in the ∆veA versus wild type comparison but not in the OEveA versus wild type comparison. However, all five genes in this cluster are also downregulated in the OEveA versus wild-type comparison, and its lack of detection by the sliding window analysis is due to the gene cluster’s small size and the test’s stringency. Finally, of the four clusters Perrin et al. describe as being partially regulated by laeA, two are differentially regulated in both veA comparisons, one cluster is differentially expressed in the OEveA versus wild type but not in the ∆veA versus wild type comparison, and one cluster is not differentially expressed in either comparison.
Table 2. The correspondence between gene clusters defined in the studies by Perrin et al. and Inglis et al. with our sliding window analysis.
| Gene cluster borders defined by Perrin et al. | Gene cluster name defined by Inglis et al. | Gene cluster borders defined by SMURF analysis (Inglis et al.) | Gene cluster borders defined by antiSMASH analysis (Inglis et al.) | Gene cluster borders defined by Inglis et al. | Window of genes downregulated in αveA vs WT comparison | Window of genes downregulated in OEveA vs WT comparison | Additional notes |
|---|---|---|---|---|---|---|---|
| Full LaeA regulation | |||||||
| Afu1g10360–0390 | Afu1g10380 (nrps1) cluster | Afu1g10310–0380 | Afu1g10310–0420 | Afu1g10270–0380 | None | None | |
| Afu2g17510–7600 | Afu2g17600 cluster | Afu2g17511–7600 | Afu2g17490–7690 | Afu2g17480–7600 | Afu2g17410–7630 | Afu2g17300–7720 | conidial pigment biosynthesis cluster (Afu2g17530–7600) |
| Afu2g17960–8070 | Fumigaclavine C (fga) cluster | Afu2g17930–8070 | Afu2g17950–8070 | Afu2g17960–8060 | Afu2g17850–8060 | Afu2g17850–A8060 | |
| Afu3g12870–3010 | Afu3g12920 cluster | Afu3g12960–2750 | Afu3g13020–2820 | Afu3g12960–2890 | None | None | |
| Afu3g12930 cluster | Afu3g13000–2750 | Afu3g13020–2820 | Afu3g12960–2890 | ||||
| Afu6g03290–3490 | Afu6g03480 cluster | Afu6g03620–3430 | Afu6g03550–3400 | Afu6g03490–3430 | Afu6g03210–3630 | Afu6g03150–3510 | |
| Afu6g09580–9770 | Afu6g08560 cluster | Afu6g08540–8560 | Afu6g08520–8640 | Afu6g08550–8560 | Afu6g09400–9910 | Afu6g09500–9830 | |
| Afu6g12040–2080 | Afu6g12080 cluster | Afu6g12040–2160 | Afu6g11980–2145 | Afu6g12040–2080 | Afu6g12000–2250 | None | fumiquinazoline biosynthetic cluster (Afu6g12040–2110); all genes between Afu6g12030–2090 in the OEveA vs WT comparison are downregulated |
| Afu6g13920–4000 | Afu6g13930 cluster | Afu6g13830–4050 | Afu6g13820–4030 | Afu6g13920–4000 | None | Afu6g13840–4160 | |
| Afu8g00100–0720 | Afu8g00540 cluster | Afu8g00370–0370 | Afu8g00490–0310 | None | Afu8g00350–1130 | Afu8g00350–0800 | Afu8g00170–0250 is upregulated in OEveA vs WT comparison; fumitremorgin G (ftm) cluster (Afu8g00260–0170); fumagillin gene cluster (Afu8g00370–0520); pseurotin A cluster (Afu8g00530–0570) |
| Afu8g00620 cluster | Afu8g00640–0470 | Afu8g00720–0390 | None | ||||
| partial LaeA regulation | |||||||
| Afu3g01290–1600 | Afu3g01410 cluster | Afu3g01400–1560 | Afu3g01360–1560 | Afu3g01400–1480 | Afu3g01160–1700 | Afu3g00570–0710 | |
| Afu3g14560–4760 | Afu3g14700 cluster | Afu3g14880–4690 | Afu3g14820–4620 | Afu3g14730–4690 | None | None | |
| Afu4g00110–0280 | Afu4g00210 cluster | Afu4g00260–0210 | Afu4g00290–0150 | Afu4g00260–0200 | None | Afu4g00120–0390 | endocrocin secondary metabolism cluster (Afu4g00210–0230) |
| Afu4g14380–4850 | Afu4g14560 cluster | Afu4g14730–4420 | Afu4g14660–4440 | Afu4g14610–4450 | Afu4g14380–4850 | Afu4g14380–4850 | helvolic acid biosynthesis (Afu4g14770- 4850) |
veA regulates the synthesis of fumagillin, fumitremorgin G, fumigaclavine C and glionitrin A
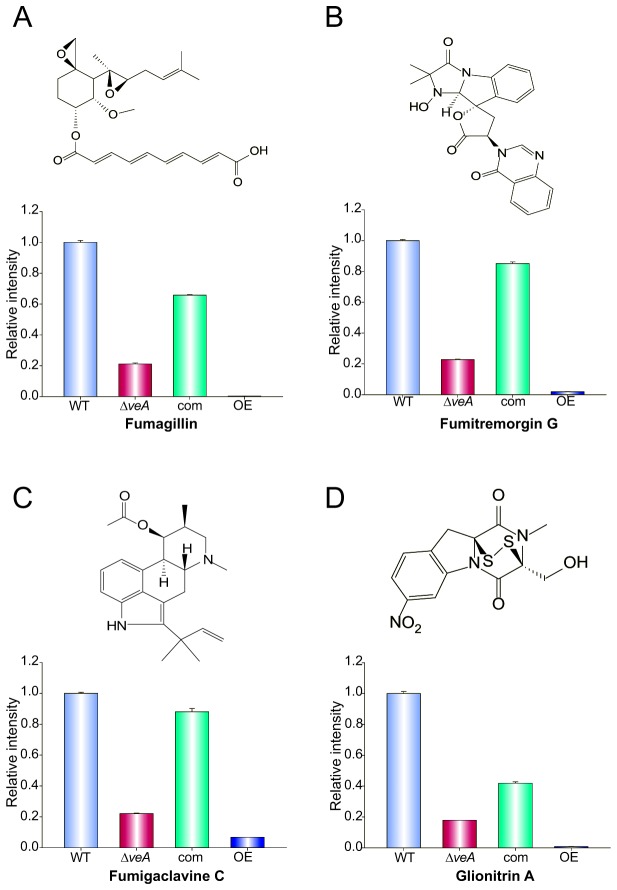
Aspergillus fumigatus has the potential to produce 226 bioactive secondary metabolites [70], and the genes responsible for their synthesis are commonly associated in the form of gene clusters. Recently, Inglis et al. described 39 secondary metabolite gene clusters in the A. fumigatus genome [71], some of which are experimentally characterized and some of which are computationally predicted. The production of a number of secondary metabolites has been shown to be under the control of veA orthologs in different fungal species [32,33,37–42]. In A. fumigatus we recently reported that the expression of gliotoxin genes and gliotoxin production are regulated by veA [13]. Additionally, in our current study, our RNA-seq data indicates that the expression of many secondary metabolite gene clusters is also veA-dependent, strongly suggesting that veA affects the synthesis of other natural products in A. fumigatus. For this reason, we also used LC-MS to analyze the production of other compounds in wild type, ∆veA, complementation strain and OEveA cultures. Our data revealed that production of four additional secondary metabolites was also dependent on veA under the experimental conditions assayed, specifically fumagillin, fumitromorgin G, fumigaclavine C and glionitrin A. The production of these four compounds was notably decreased in the ∆veA strain. Relative amounts of fumagillin, fumitremorgin G, fumigaclavine C and glionitrinA in the ∆veA strain were 18%, 23%, 22% and 18% compared to the wild type levels, respectively (Figure 4). The production of these compounds was also reduced in the OEveA with only 0.3%, 0.1%, 0.7% and 0.8% as compared to wild-type levels, respectively (Figure 4).
Figure 4. veA regulates the production of fumagillin, fumitremorgin G, fumigaclavine C and Glionitrin A.
Secondary metabolites were extracted from 120 h old Czapek-dox stationary liquid cultures of wild type (WT), ΔveA, complementation and OEveA strains. Extracts were analyzed with Shimadzu 2010 EV LC-MS as described in the materials and methods section. The predicted m/z [M+H]+ ratio was (A) fumagillin (m/z=459), (B) fumitremorgin G (m/z = 433) (C) fumigaclavine C (m/z = 299) and (D) Glionitrin-A (m/z = 354). The bars represent the mean of three samples and error bars represent standard error.
veA controls the fumagillin gene cluster and fumagillin production by regulating the expression of fumR (Afu8g00420)
Due to the medical applications of fumagillin and fumagillin-related compounds for their potential use in the treatment of amebiasis, microsporidiosis, and for their anti-angiogenic properties, we further characterized the genetic regulation of veA on the fumagillin gene cluster. RNA-seq analysis revealed that veA controls the fumagillin gene cluster, including fumR (Afu8g00420) (Figures 2 and 3), a gene encoding a putative C6 type transcription factor that our previous bioinformatics analysis identified within the fumagillin gene cluster, located in chromosome 8 [53]. We further validated these results by qRT-PCR analysis (Figure S1). The expression levels of fumR were 12% in ∆veA and 5% in OEveA with respect to the levels in the wild type strain. Expression of Afu8g00370, which encodes a polyketide synthase (PKS) in the fumagillin cluster [53], was also evaluated (Figure S1). We recently showed that expression of Afu8g00370 is necessary for the production of fumagillin, confirming the predicted role of Afu8g00370 as an indispensable PKS in fumagillin biosynthesis. Our data indicated that expression levels in ∆veA and OEveA strains were only 3% and 0.1%, respectively, compared to wild-type levels.
fumR is necessary for the expression of other genes in the fumagillin gene cluster
To gain insight into the function of the transcription factor encoded by fumR, this gene was deleted by gene replacement techniques using the A. parasiticus pyrG marker as described in the materials and methods section. The ∆fumR strain was confirmed by PCR (data not shown) and Southern blot analysis (Figure S2). Deletion of fumR did not change growth rate or development in A. fumigatus (data not shown).
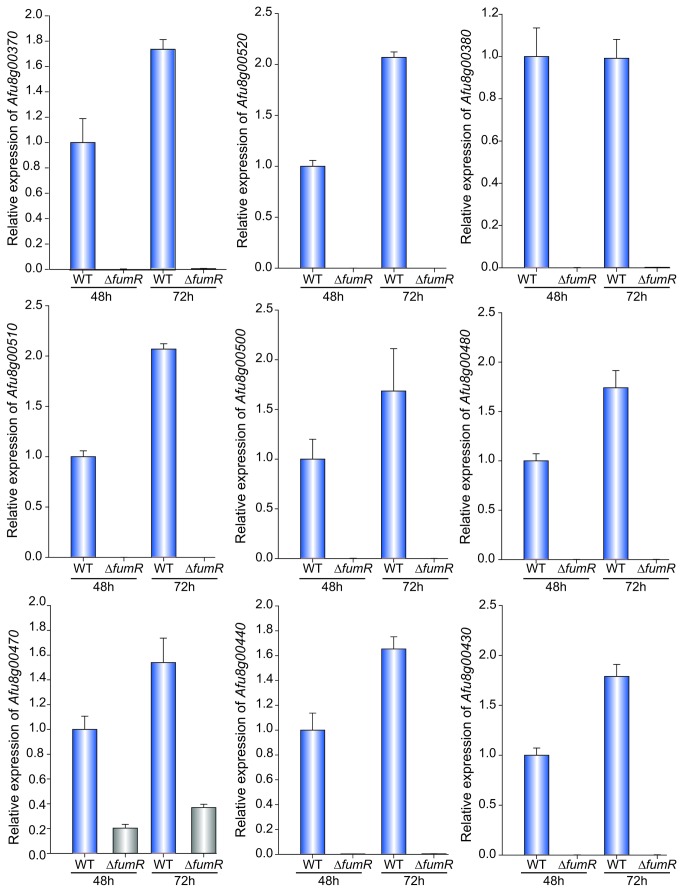
Our experiments showed that expression of the PKS gene, Afu8g00370, is regulated by fumR (Figure 5). The expression of Afu8g00370 in the ΔfumR strain and control strain was analyzed by qRT-PCR at 48 h and 72 h post inoculation. At both time points examined, there was only negligible expression of Afu8g00370 in the ∆fumR mutant as compared to the wild-type levels. Furthermore, our analysis revealed that the expression of other genes in the fumagillin cluster is also under the control of fumR (Figure 5). We examined the expression of Afu8g00520, the terpene cyclase involved in fumagillin biosynthesis [53] and Afu8g00380, an essential acyltransferase for fumagillin production [53]. In addition to the characterized genes in the fumagillin cluster, we analyzed the expression of other predicted genes in the cluster, including Afu8g00510, Afu8g00500, Afu8g00480, Afu8g470, Afu8g440 and Afu8g00430. Our results indicate that all genes were minimally expressed in the ∆fumR mutant compared to the wild type at both time points tested (Figure 5). Only Afu8g00470 showed slightly higher expression than the other genes in the cluster in ∆fumR (20% and 36% at 48 h and 72 h post inoculation, respectively) as compared to wild-type levels.
Figure 5. fumR is necessary for the expression of the genes in the fumagillin cluster.
The transcriptional pattern of the genes in the fumagillin cluster (Afu8g00370, Afu8g00520, Afu8g00370, Afu8g00510, Afu8g00500, Afu8g00480, Afu8g00470, Afu8g00440, Afu8g00430) was evaluated by qRT-PCR in the wild type (WT, TSD51.1) and αfumR strains. Total RNA was extracted from Czapek-Dox stationary liquid cultures incubated for 48 h and 72 h. The relative expression was calculated using 2-ΔΔCT as described by Schmittgen and Livak [89]. 18S gene expression was used as internal reference. Means of three replicates is shown. Values were normalized to WT expression at 48 h considered as 1. Error bar represents standard error.
fumR is required for fumagillin biosynthesis
Extracts from wild type and ∆fumR 72 h and 120 h cultures were subjected to LC-MS analysis as described in the material and methods section. The chemical analysis showed a peak corresponding to fumagillin in the wild type, with a retention time of 29.1 minutes (Figure 6). However, this peak was completely absent in the ∆fumR strain, indicating that A. fumigatus is unable to produce fumagillin in the absence of fumR. The amount of fumagillin production in the wild type increased over time (Figure 6).
Figure 6. fumR regulates fumagillin biosynthesis.
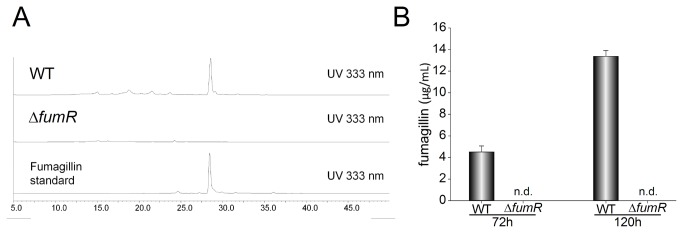
Aspergillus fumigatus wild type (WT TSD51.1) and ∆fumR strains were grown on Czapek-Dox as stationary liquid cultures for 72 h and 120 h. Culture supernatants were extracted with chloroform and analyzed by LC-MS (A) as described in materials and methods. (B) Quantitative data of the LC-MS analysis. The experiment was done with three replicates. Error bars represent standard error. No fumagillin was detected (n.d.) in ∆fumR mutants. A standard curve was obtained using commercial fumagillin (Sigma).
laeA regulates expression of fumR and the PKS gene Afu8g00370
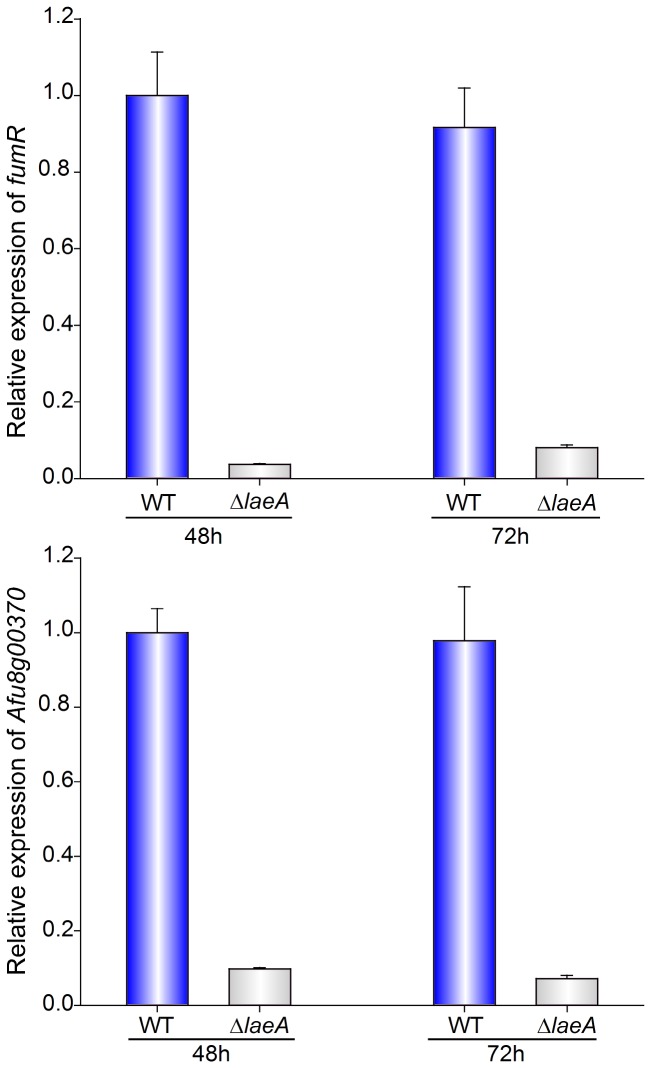
The identity of the cluster involved in fumagillin biosynthesis was not elucidated at the time of the microarray study previously carried out with a ∆laeA mutant [51]. In a recent study we described the A. fumigatus fumagillin gene cluster [53], and in the present study we have demonstrated that veA regulates fumR, and that expression of fumR is necessary for the activation of other genes in the cluster. We also examined whether the expression of fumR was also dependent on laeA, analyzing the expression of this gene in a ∆laeA mutant and corresponding control strain. The ∆laeA strain was constructed as described in material and methods, and the strain was verified by Southern blot analysis (Figure S3). qRT-PCR results indicated a near complete loss of fumR expression in ∆laeA under conditions that allowed its expression in the control strain (3% and 8% at 48 h and 72 h as compared to wild-type levels) (Figure 7). Expression of Afu8g00370 was also downregulated in ΔlaeA (9% and 7% at 48 h and 72 h as compared to wild-type levels).
Figure 7. Expression of fumR and PKS370 is regulated by laeA.
Total RNA was extracted from WT and ∆laeA strains, grown as stationary liquid Czapek-Dox cultures for 48 h and 72 h. Relative expression of fumR and PKS gene Afu8g00370 was calculated using 2-ΔΔCt method as described by Schmittgen and Livak [89]. Primers used for the expression analysis are listed in Table S2. The bar represents the mean of three replicates and error bars represent standard error. Expression of 18S was used as internal reference. Values were normalized to expression levels of wild-type levels considered as 1.
Discussion
Invasive aspergillosis is a disease caused by the ubiquitous opportunistic invasive mold Aspergillus fumigatus. In immunocompromised patients, the intraepithelial immune system of the lung is unable to properly eliminate the inhaled conidia, which then germinate [1–6]. Despite the prevalence of aspergillosis infection and the improvements in diagnosis, novel effective strategies to reduce Aspergillus infections are still needed. Deciphering the genetic mechanism controlling A. fumigatus cellular processes might provide the basis for the development of new strategies to prevent or treat aspergillosis.
Our study clearly established that veA is a global regulator of the A. fumigatus genome, affecting the expression of hundreds of genes, many of which are involved in secondary metabolism related processes, in non-random genomic locations. Secondary metabolites, also known as natural products, are part of the fungal chemical arsenal important for habitat adaptation. Some of them are considered virulent factors playing an important role in the pathogen-host interaction. The most studied of these secondary metabolites is gliotoxin, known for its immunosuppressive properties [16,18,72–74], for inhibiting phagocytosis in macrophage, and for induction of apoptosis [29,30]. We recently reported that expression of gliotoxin genes and concomitant gliotoxin production is dependent on veA in A. fumigatus [13]. In this study we demonstrate that veA controls the expression of 14 secondary metabolite gene clusters whose metabolite products are known and an additional 23 putative secondary metabolite gene clusters. Although veA exercised negative regulation on some gene clusters, overall veA acted as a positive regulator. The veA-dependent gene clusters regulatory pattern was not identical to that described for laeA [51], which encodes a VeA-interacting protein in the velvet complex [31,47,69], presenting some differences in their regulatory output. Among the gene clusters regulated by veA are those involved in the synthesis of fumitremorgin G, fumigaclavine C and fumagillin. Production of most of these compounds correlates with the veA regulatory pattern observed for the respective gene clusters, with the exception of fumitremorgin, suggesting that in this case other veA-dependent factors might be needed for production of this compound at wild-type levels. Fumitremorgin G, fumigaclavine C, and fumagillin, together with production of glionitrin A, currently an orphan compound without an associated gene cluster, have been described to be relevant in the A. fumigatus infection process and in other pathologies. Fumitremorgins are associated with dysfunction of the nervous system causing tremors, seizures and abnormal behavior in animals [75,76]. The alkaloid fumigaclavine also causes nervous system damage as well as alteration of the reproductive system [77]. Fumagillin has been associated with invasive aspergillosis due to its effect in slowing ciliary beat frequency [17] and inhibition of endothelial proliferation [17]. Some of these compounds, however, are bioactive molecules with potential or current applications, particularly anti-tumoral compounds such as glionitrin A [78], and the well-known fumagillin and related compounds, with applications against amebiasis [54], microsporidiosis [55], and with known anti-angiogenic activity as inhibitors of the human type 2 methionine aminopeptidase (MetAP2) [56,57]. Our study shows that the expression of most of the genes in the fumagillin gene cluster was negatively affected by either deletion or over-expression of veA. This corresponded with a decrease in fumagillin production in these two strains compared to the wild type.
Further insight into the mechanism regulating the secondary metabolite gene cluster in A. fumigatus may contribute to decreasing the detrimental effects of this fungus as well as increasing the production of valuable secondary metabolites, such as fumagillin. Our study indicated that the fumagillin gene cluster is regulated by a C6 transcription factor gene, now denominated fumR, located within the boundaries of this cluster. Other C6 type transcription factor genes have been found within other secondary metabolite genes clusters, such as the well-known gliZ in the A. fumigatus gliotoxin gene cluster [18] and aflR in the A. nidulans sterigmatocystin cluster [79,80], which have been demonstrated to regulate such clusters. fumR positively regulated the expression of the recently characterized PKS gene, Afu8g00370, the terpene cyclase gene, Afu8g00520, and the acyltransferase Afu8g00380 [53]. Furthermore, fumR also regulates all the other predicted genes in this cluster. Deletion of fumR resulted in complete absence of fumagillin production.
Our study showed that fumR is under the control of veA. Both deletion and over-expression of veA downregulated fumR transcription, suggesting that veA influences the activation of the fumagillin gene cluster through regulation of fumR. Over-expression of veA also had a marked negative effect on the expression of many A. fumigatus secondary metabolite gene clusters. We previously described a similar effect in the veA regulation of the gliotoxin gene cluster [13], showing a decrease of gliotoxin production in both deletion and over-expression strains with respect to wild-type levels. Our RNA sequencing data provide strong evidence supporting this pattern. The same pattern has also been observed in other Aspergillus species; for example, the production of penicillin has been demonstrated to be negatively affected by either deletion or over-expression of veA in A. nidulans [37,81]. Since VeA is part of a protein complex or complexes [31,45,47,50,69], we hypothesized that a balanced stoichiometry between VeA and other possible partners might be necessary for proper function, including the activation of secondary metabolite gene clusters. It is possible that other VeA-interacting proteins might also modulate the expression of the fumagillin gene cluster. In this current study we also examined whether laeA affects the expression of genes in this cluster. Our results revealed that this is indeed the case; the absence of laeA greatly decreases fumR and Afu8g00370 expression. This indicates that both VeA and LaeA, components of the fungal velvet protein complex, are indispensable for normal expression of the fumagillin gene cluster and fumagillin production in the opportunistic pathogen A. fumigatus.
Conclusion
In this study we have demonstrated that veA is a global genetic regulator in the opportunistic human pathogen A. fumigatus, controlling the expression of hundreds of genes. Among the genes governed by veA are numerous secondary metabolite gene clusters, some of them responsible for the synthesis of natural products considered to be virulent factors during A. fumigatus infection. Interestingly, we also showed that some of these veA-dependent gene clusters are associated with the production of important medical drugs, such as fumagillin, known for its anti-angiogenic properties among other relevant medical applications. All the genes in the fumagillin gene cluster are under the control of the endogenous regulator fumR, which is regulated by veA and laeA, both encoding interacting components in the velvet complex. The findings presented here provide further insight into the regulatory dynamics of the A. fumigatus genome, contributing to settinga basis for novel strategies to decrease the negative effects of A. fumigatus while increasing its potential to produce beneficial compounds.
Supporting Information
qRT-PCR validation of RNA sequencing analysis of the expression of fumR (A) and Afu8g00370 (B) in the wild type, ∆veA, complementation and OEveA. Total RNA was extracted using TRIzol from 72h old stationary cultures grown in Czapek-Dox medium. The relative expression was calculated using 2-ΔΔCt method as described by Schmittgen and Livak [89]. Primers used for expression analysis are listed in Table S2. The bar represents the mean of three replicates and error bars represent standard error. Expression of 18S was used as internal reference gene. Values were normalized to the expression levels of WT which was considered as 1.
(TIF)
Targeted fumR deletion. (A) Diagram showing SalI sites (S) in the wild-type fumR locus, and the same locus after gene replacement of fumR by the A. parasiticus pyrG gene used as selection marker for fungal transformation. The fragment used as probe templates for Southern blot analyses is also shown. (B) Southern blot analysis. The ∆fumR deletion construct was transformed in CEA17ku80 (Table S1). Additional transformants also presented the correct band pattern (data not shown).
(TIF)
Targeted laeA deletion. (A) Diagram showing XhoI sites (X) in the wild-type laeA locus, and the same locus after gene replacement of laeA by the A. parasiticus pyrG gene used as selection marker for fungal transformation. The fragment used as probe templates for Southern blot analyses is also shown. (B) Southern blot analysis. The ∆laeA deletion construct was transformed in CEA17ku80 (Table S1). Additional transformants also presented the correct band pattern (data not shown).
(TIF)
(DOC)
(DOC)
(XLSX)
Acknowledgments
We wish to thank Scott Grayburn and Xue-Huan Feng for technical support, as well as Travis Clark, Chelsea Baker, and the Vanderbilt Technologies for Advanced Genomics for Illumina library preparation and RNA sequencing.
Funding Statement
This work was funded by National Institutes of Health (NIH) R03AI079496 and Northern Illinois University to AMC, a NLM training grant 2T15LM007450 to ALL, and a Vanderbilt Discovery Grant to AR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Denning DW (1998) Invasive aspergillosis. Clin Infect Dis 26: 781-803. doi: 10.1086/513943. PubMed: 9564455. [DOI] [PubMed] [Google Scholar]
- 2. Pagano L, Girmenia C, Mele L, Ricci P, Tosti ME et al. (2001) Infections caused by filamentous fungi in patients with hematologic malignancies. A report of 391 cases by GIMEMA Infection Program. Haematologica 86: 862-870. PubMed: 11522544. [PubMed] [Google Scholar]
- 3. Kliasova GA, Petrova NA, Parovichnikova EN, Gotman LN, Isaev VG et al. (2005) [Invasive pulmonary aspergillesis]. Ter Arkh 77: 65-71. PubMed: 16116913. [PubMed] [Google Scholar]
- 4. Post MJ, Lass-Floerl C, Gastl G, Nachbaur D (2007) Invasive fungal infections in allogeneic and autologous stem cell transplant recipients: a single-center study of 166 transplanted patients. Transpl Infect Dis 9: 189-195. doi: 10.1111/j.1399-3062.2007.00219.x. PubMed: 17511828. [DOI] [PubMed] [Google Scholar]
- 5. Marr KA, Carter RA, Boeckh M, Martin P, Corey L (2002) Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100: 4358-4366. doi: 10.1182/blood-2002-05-1496. PubMed: 12393425. [DOI] [PubMed] [Google Scholar]
- 6. Wiederhold NP, Lewis RE, Kontoyiannis DP (2003) Invasive aspergillosis in patients with hematologic malignancies. Pharmacotherapy 23: 1592-1610. doi: 10.1592/phco.23.15.1592.31965. PubMed: 14695039. [DOI] [PubMed] [Google Scholar]
- 7. Latgé JP (1999) Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12: 310-350. PubMed: 10194462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hohl TM, Feldmesser M (2007) Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot Cell 6: 1953-1963. doi: 10.1128/EC.00274-07. PubMed: 17890370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sherif R, Segal BH (2010) Pulmonary aspergillosis: clinical presentation, diagnostic tests, management and complications. Curr Opin Pulm Med 16: 242-250. PubMed: 20375786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kontoyiannis DP, Bodey GP (2002) Invasive aspergillosis in 2002: an update. Eur J Clin Microbiol Infect Dis 21: 161-172. doi: 10.1007/s10096-002-0699-z. PubMed: 11957017. [DOI] [PubMed] [Google Scholar]
- 11. Oren I, Goldstein N (2002) Invasive pulmonary aspergillosis. Curr Opin Pulm Med 8: 195-200. doi: 10.1097/00063198-200205000-00008. PubMed: 11981308. [DOI] [PubMed] [Google Scholar]
- 12. Schmitt HJ, Blevins A, Sobeck K, Armstrong D (1990) Aspergillus species from hospital air and from patients. Mycoses 33: 539-541. PubMed: 2129435. [DOI] [PubMed] [Google Scholar]
- 13. Dhingra S, Andes D, Calvo AM (2012) VeA regulates conidiation, gliotoxin production, and protease activity in the opportunistic human pathogen Aspergillus fumigatus . Eukaryot Cell 11: 1531-1543. doi: 10.1128/EC.00222-12. PubMed: 23087369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rüchel R, Reichard U (1999) Pathogenesis and clinical presentation of aspergillosis. Contrib Microbiol 2: 21-43. doi: 10.1159/000060302. PubMed: 10523264. [DOI] [PubMed] [Google Scholar]
- 15. Samson RA (1999) The genus Aspergillus with special regard to the Aspergillus fumigatus group. Contrib Microbiol 2: 5-20. doi: 10.1159/000060298. PubMed: 10523263. [DOI] [PubMed] [Google Scholar]
- 16. Coméra C, André K, Laffitte J, Collet X, Galtier P et al. (2007) Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signalling pathways in human neutrophils. Microbes Infect 9: 47-54. doi: 10.1016/j.micinf.2006.10.009. PubMed: 17196420. [DOI] [PubMed] [Google Scholar]
- 17. Amitani R, Taylor G, Elezis EN, Llewellyn-Jones C, Mitchell J et al. (1995) Purification and Characterization of Factors Produced by Aspergillus-Fumigatus Which Affect Human Ciliated Respiratory Epithelium. Infect Immun 63: 3266-3271. PubMed: 7543879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bok JW, Chung D, Balajee SA, Marr KA, Andes D et al. (2006) GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect Immun 74: 6761-6768. doi: 10.1128/IAI.00780-06. PubMed: 17030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frame R, Carlton WW (1988) Acute Toxicity of Gliotoxin in Hamsters. Toxicol Lett 40: 269-273. doi: 10.1016/0378-4274(88)90050-1. PubMed: 2451322. [DOI] [PubMed] [Google Scholar]
- 20. Müllbacher A, Eichner RD (1984) Immunosuppression in vitro by a metabolite of a human pathogenic fungus. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences 81 pp. 3835-3837. PubMed: 6203127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan XQ, Harday J (2007) Electromicroscopic observations on gliotoxin-induced apoptosis of cancer cells in culture and human cancer xenografts in transplanted SCID mice. In Vivo 21: 259-265. PubMed: 17436574. [PubMed] [Google Scholar]
- 22. Piva TJ (1994) Gliotoxin Induces Apoptosis in Mouse L929 Fibroblast Cells. Biochem Mol Biol Int 33: 411-419. PubMed: 7524901. [PubMed] [Google Scholar]
- 23. Stanzani M, Orciuolo E, Lewis R, Kontoyiannis DP, Martins SLR et al. (2005) Aspergillus fumigatus suppresses the human ceflular immune response via ghotoxin-mediated apoptosis of monocytes. Blood 105: 2258-2265. doi: 10.1182/blood-2004-09-3421. PubMed: 15546954. [DOI] [PubMed] [Google Scholar]
- 24. Suen YK, Fung KP, Lee CY, Kong SK (2001) Gliotoxin induces apoptosis in cultured macrophages via production of reactive oxygen species and cytochrome c release without mitochondrial depolarization. Free Radic Res 35: 1-10. doi: 10.1080/10715760100300541. PubMed: 11697112. [DOI] [PubMed] [Google Scholar]
- 25. Tsunawaki S, Yoshida LS, Nishida S, Kobayashi T, Shimoyama T (2004) Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect Immun 72: 3373-3382. doi: 10.1128/IAI.72.6.3373-3382.2004. PubMed: 15155643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waring P (1990) DNA fragmentation induced in macrophages by gliotoxin does not require protein synthesis and is preceded by raised inositol triphosphate levels. J Biol Chem 265: 14476-14480. PubMed: 1696946. [PubMed] [Google Scholar]
- 27. Yamada A, Kataoka T, Nagai K (2000) The fungal metabolite gliotoxin: immunosuppressive activity on CTL-mediated cytotoxicity. Immunol Lett 71: 27-32. doi: 10.1016/S0165-2478(99)00155-8. PubMed: 10709782. [DOI] [PubMed] [Google Scholar]
- 28. Yoshida LS, Abe S, Tsunawaki S (2000) Fungal gliotoxin targets the onset of superoxide-generating NADPH oxidase of human neutrophils. Biochem Biophys Res Commun 268: 716-723. doi: 10.1006/bbrc.2000.2192. PubMed: 10679271. [DOI] [PubMed] [Google Scholar]
- 29. Eichner RD, Waring P, Geue AM, Braithwaite AW, Müllbacher A (1988) Gliotoxin causes oxidative damage to plasmid and cellular DNA. J Biol Chem 263: 3772-3777. PubMed: 2450088. [PubMed] [Google Scholar]
- 30. Richard JL, Dvorak TJ, Ross PF (1996) Natural occurrence of gliotoxin in turkeys infected with Aspergillus fumigatus, Fresenius. Mycopathologia 134: 167-170. doi: 10.1007/BF00436725. PubMed: 8981782. [DOI] [PubMed] [Google Scholar]
- 31. Calvo AM (2008) The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet Biol 45: 1053-1061. doi: 10.1016/j.fgb.2008.03.014. PubMed: 18457967. [DOI] [PubMed] [Google Scholar]
- 32. Myung K, Zitomer NC, Duvall M, Glenn AE, Riley RT et al. (2012) The conserved global regulator VeA is necessary for symptom production and mycotoxin synthesis in maize seedlings by Fusarium verticillioides . Plant Pathol 61: 152-160. doi: 10.1111/j.1365-3059.2011.02504.x. PubMed: 22247572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duran RM, Cary JW, Calvo AM (2007) Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl Microbiol Biotechnol 73: 1158-1168. PubMed: 16988822. [DOI] [PubMed] [Google Scholar]
- 34. Duran RM, Cary JW, Calvo AM (2009) The role of veA in Aspergillus flavus infection of peanut, corn and cotton. Open Mycology J 3: 27-36. doi: 10.2174/1874437000903010027. [DOI] [Google Scholar]
- 35. Cary JW, GR OB, Nielsen DM, Nierman W, Harris-Coward P, et al (2007) Elucidation of veA-dependent genes associated with aflatoxin and sclerotial production in Aspergillus flavus by functional genomics. Appl Microbiol Biotechnol 76: 1107-1118. doi: 10.1007/s00253-007-1081-y. PubMed: 17646985. [DOI] [PubMed] [Google Scholar]
- 36. Calvo AM, Bok J, Brooks W, Keller NP (2004) veA is required for toxin and sclerotial production in Aspergillus parasiticus . Appl Environ Microbiol 70: 4733-4739. doi: 10.1128/AEM.70.8.4733-4739.2004. PubMed: 15294809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kato N, Brooks W, Calvo AM (2003) The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot Cell 2: 1178-1186. doi: 10.1128/EC.2.6.1178-1186.2003. PubMed: 14665453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Merhej J, Urban M, Dufresne M, Hammond-Kosack KE, Richard-Forget F et al. (2012) The velvet gene, FgVe1, affects fungal development and positively regulates trichothecene biosynthesis and pathogenicity in Fusarium graminearum . Mol Plant Pathol 13: 363-374. doi: 10.1111/j.1364-3703.2011.00755.x. PubMed: 22013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Myung K, Li SJ, Butchko RAE, Busman M, Proctor RH et al. (2009) FvVE1 regulates biosynthesis of the mycotoxins fumonisins and fusarins in Fusarium verticillioides . J Agric Food Chem 57: 5089-5094. doi: 10.1021/jf900783u. PubMed: 19382792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wiemann P, Brown DW, Kleigrewe K, Bok JW, Keller NP et al. (2010) FfVel1 and FfLae1, components of a velvet-like complex in Fusarium fujikuroi, affect differentiation, secondary metabolism and virulence. Mol Microbiol 77: 972-994. PubMed: 20572938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoff B, Kamerewerd J, Sigl C, Mitterbauer R, Zadra I et al. (2010) Two components of a velvet-like complex control hyphal morphogenesis, conidiophore development, and penicillin Biosynthesis in Penicillium chrysogenum . Eukaryot Cell 9: 1236-1250. doi: 10.1128/EC.00077-10. PubMed: 20543063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dreyer J, Eichhorn H, Friedlin E, Kürnsteiner H, Kück U (2007) A homologue of the Aspergillus velvet gene regulates both cephalosporin C biosynthesis and hyphal fragmentation in Acremonium chrysogenum . Appl Environ Microbiol 73: 3412-3422. doi: 10.1128/AEM.00129-07. PubMed: 17400783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stinnett SM, Espeso EA, Cobeño L, Araújo-Bazán L, Calvo AM (2007) Aspergillus nidulans VeA subcellular localization is dependent on the importin alpha carrier and on light. Mol Microbiol 63: 242-255. doi: 10.1111/j.1365-2958.2006.05506.x. PubMed: 17163983. [DOI] [PubMed] [Google Scholar]
- 44. Araújo-Bazán L, Dhingra S, Chu J, Fernández-Martínez J, Calvo AM et al. (2009) Importin alpha is an essential nuclear import carrier adaptor required for proper sexual and asexual development and secondary metabolism in Aspergillus nidulans . Fungal Genet Biol 46: 506-515. doi: 10.1016/j.fgb.2009.03.006. PubMed: 19318129. [DOI] [PubMed] [Google Scholar]
- 45. Purschwitz J, Müller S, Kastner C, Schöser M, Haas H et al. (2008) Functional and physical interaction of blue- and red-light sensors in Aspergillus nidulans . Curr Biol 18: 255-259. doi: 10.1016/j.cub.2008.01.061. PubMed: 18291652. [DOI] [PubMed] [Google Scholar]
- 46. Bayram O, Braus GH (2012) Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol Rev 36: 1-24. doi: 10.1111/j.1574-6976.2011.00285.x. PubMed: 21658084. [DOI] [PubMed] [Google Scholar]
- 47. Bayram O, Krappmann S, Ni M, Bok JW, Helmstaedt K et al. (2008) VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320: 1504-1506. doi: 10.1126/science.1155888. PubMed: 18556559. [DOI] [PubMed] [Google Scholar]
- 48. Bok JW, Keller NP (2004) LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot Cell 3: 527-535. doi: 10.1128/EC.3.2.527-535.2004. PubMed: 15075281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reyes-Dominguez Y, Bok JW, Berger H, Shwab EK, Basheer A et al. (2010) Heterochromatic marks are associated with the repression of secondary metabolism clusters in Aspergillus nidulans . Mol Microbiol 76: 1376-1386. doi: 10.1111/j.1365-2958.2010.07051.x. PubMed: 20132440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Palmer JM, Theisen JM, Duran RM, Grayburn WS, Calvo AM et al. (2013) Secondary metabolism and development is mediated by LlmF control of VeA subcellular localization in Aspergillus nidulans . PLOS Genet 9: e1003193 PubMed: 23341778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perrin RM, Fedorova ND, Bok JW, Cramer RA, Wortman JR et al. (2007) Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLOS Pathog 3: e50. doi: 10.1371/journal.ppat.0030050. PubMed: 17432932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rokas A, Gibbons JG, Zhou X, Beauvais A, Latge JP (2012) The diverse applications of RNA-Seq for functional genomics studies in Aspergillus fumigatus . Ann N Y Acad Sci (in press). doi: 10.1111/j.1749-6632.2012.06755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin HC, Chooi YH, Dhingra S, Xu W, Calvo AM et al. (2013) The fumagillin biosynthetic gene cluster in Aspergillus fumigatus encodes a cryptic terpene cyclase involved in the formation of beta-trans-bergamotene. J Am Chem Soc 135: 4616-4619. doi: 10.1021/ja312503y. PubMed: 23488861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Killough JH, Magill GB, Smith RC (1952) The treatment of amebiasis with fumagillin. Science 115: 71-72. doi: 10.1126/science.115.2977.71. PubMed: 14913169. [DOI] [PubMed] [Google Scholar]
- 55. Molina JM, Tourneur M, Sarfati C, Chevret S, de Gouvello A et al. (2002) Fumagillin treatment of intestinal microsporidiosis. N Engl J Med 346: 1963-1969. doi: 10.1056/NEJMoa012924. PubMed: 12075057. [DOI] [PubMed] [Google Scholar]
- 56. Sin N, Meng L, Wang MQ, Wen JJ, Bornmann WG et al. (1997) The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase 94 MetAP- 2 Proceedings of the National Academy of Sciences of; the United States of America: . pp. 6099-6103 PubMed: 9177176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lefkove B, Govindarajan B, Arbiser JL (2007) Fumagillin: an anti-infective as a parent molecule for novel angiogenesis inhibitors. Expert Rev Anti Infect Ther 5: 573-579. doi: 10.1586/14787210.5.4.573. PubMed: 17678422. [DOI] [PubMed] [Google Scholar]
- 58. Käfer E (1977) Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv Genet 19: 33-131. doi: 10.1016/S0065-2660(08)60245-X. PubMed: 327767. [DOI] [PubMed] [Google Scholar]
- 59. Blatzer M, Barker BM, Willger SD, Beckmann N, Blosser SJ et al. (2011) SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus . PLOS Genet 7: e1002374 PubMed: 22144905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arnaud MB, Cerqueira GC, Inglis DO, Skrzypek MS, Binkley J et al. (2012) The Aspergillus Genome Database (AspGD): recent developments in comprehensive multispecies curation, comparative genomics and community resources. Nucleic Acids Res 40: D653-D659. doi: 10.1093/nar/gkr875. PubMed: 22080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gibbons JG, Beauvais A, Beau R, McGary KL, Latgé JP et al. (2012) Global transcriptome changes underlying colony growth in the opportunistic human pathogen Aspergillus fumigatus . Eukaryot Cell 11: 68-78. doi: 10.1128/EC.05102-11. PubMed: 21724936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gibbons JG, Salichos L, Slot JC, Rinker DC, McGary KL et al. (2012) The evolutionary imprint of domestication on genome variation and function of the filamentous fungus Aspergillus oryzae . Curr Biol 22: 1403-1409. doi: 10.1016/j.cub.2012.05.033. PubMed: 22795693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. doi: 10.1186/gb-2009-10-3-r25. PubMed: 19261174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078-2079. doi: 10.1093/bioinformatics/btp352. PubMed: 19505943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5: 621-628. doi: 10.1038/nmeth.1226. PubMed: 18516045. [DOI] [PubMed] [Google Scholar]
- 66. Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y et al. (2006) Fusion PCR and gene targeting in Aspergillus nidulans . Nat Protoc 1: 3111-3120. PubMed: 17406574. [DOI] [PubMed] [Google Scholar]
- 67. Sambrook J, Russell DW (2001) Molecular Cloning : A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 68. Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS et al. (2005) Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus . Nature 438: 1151-1156. doi: 10.1038/nature04332. PubMed: 16372009. [DOI] [PubMed] [Google Scholar]
- 69. Park HS, Bayram O, Braus GH, Kim SC, Yu JH (2012) Characterization of the velvet regulators in Aspergillus fumigatus. Mol Microbiol 86: 937-953. doi: 10.1111/mmi.12032. PubMed: 22970834. [DOI] [PubMed] [Google Scholar]
- 70. Frisvad JC, Rank C, Nielsen KF, Larsen TO (2009) Metabolomics of Aspergillus fumigatus . Med Mycol 47 Suppl 1: S53-S71. doi: 10.1080/13693780802307720. PubMed: 18763205. [DOI] [PubMed] [Google Scholar]
- 71. Inglis DO, Binkley J, Skrzypek MS, Arnaud MB, Cerqueira GC et al. (2013) Comprehensive annotation of secondary metabolite biosynthetic genes and gene clusters of Aspergillus nidulans, A. fumigatus, A. niger and A. oryzae . BMC Microbiol 13: 91. doi: 10.1186/1471-2180-13-91. PubMed: 23617571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cramer RA Jr., Gamcsik MP, Brooking RM, Najvar LK, Kirkpatrick WR et al. (2006) Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot Cell 5: 972-980. doi: 10.1128/EC.00049-06. PubMed: 16757745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Spikes S, Xu R, Nguyen CK, Chamilos G, Kontoyiannis DP et al. (2008) Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis 197: 479-486. doi: 10.1086/525044. PubMed: 18199036. [DOI] [PubMed] [Google Scholar]
- 74. Kwon-Chung KJ, Sugui JA (2009) What do we know about the role of gliotoxin in the pathobiology of Aspergillus fumigatus? Med Mycol 47 Suppl 1: S97-103. doi: 10.1080/13693780802056012. PubMed: 18608908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yamazaki M, Fujimoto H, Kawasaki T (1980) Chemistry of tremorogenic metabolites. I. Fumitremorgin A from Aspergillus fumigatus . Chem Pharm Bull 28: 245-254. doi: 10.1248/cpb.28.245. PubMed: 6988091. [DOI] [PubMed] [Google Scholar]
- 76. Maiya S, Grundmann A, Li SM, Turner G (2006) The fumitremorgin gene cluster of Aspergillus fumigatus: identification of a gene encoding brevianamide F synthetase. Chembiochem 7: 1062-1069. doi: 10.1002/cbic.200600003. PubMed: 16755625. [DOI] [PubMed] [Google Scholar]
- 77. Coyle CM, Panaccione DG (2005) An ergot alkaloid biosynthesis gene and clustered hypothetical genes from Aspergillus fumigatus . Appl Environ Microbiol 71: 3112-3118. doi: 10.1128/AEM.71.6.3112-3118.2005. PubMed: 15933009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park HB, Kwon HC, Lee CH, Yang HO (2009) Glionitrin A, an antibiotic-antitumor metabolite derived from competitive interaction between abandoned mine microbes. J Nat Prod 72: 248-252. doi: 10.1021/np800606e. PubMed: 19159274. [DOI] [PubMed] [Google Scholar]
- 79. Yu JH, Butchko RA, Fernandes M, Keller NP, Leonard TJ et al. (1996) Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus . Curr Genet 29: 549-555. doi: 10.1007/BF02426959. PubMed: 8662194. [DOI] [PubMed] [Google Scholar]
- 80. Fernandes M, Keller NP, Adams TH (1998) Sequence-specific binding by Aspergillus nidulans AflR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol Microbiol 28: 1355-1365. doi: 10.1046/j.1365-2958.1998.00907.x. PubMed: 9680223. [DOI] [PubMed] [Google Scholar]
- 81. Spröte P, Brakhage AA (2007) The light-dependent regulator velvet A of Aspergillus nidulans acts as a repressor of the penicillin biosynthesis. Arch Microbiol 188: 69-79. doi: 10.1007/s00203-007-0224-y. PubMed: 17375284. [DOI] [PubMed] [Google Scholar]
- 82. Tsai HF, Wheeler MH, Chang YC, Kwon-Chung KJ (1999) A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus . J Bacteriol 181: 6469-6477. PubMed: 10515939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wallwey C, Matuschek M, Xie XL, Li SM (2010) Ergot alkaloid biosynthesis in Aspergillus fumigatus: Conversion of chanoclavine-I aldehyde to festuclavine by the festuclavine synthase FgaFS in the presence of the old yellow enzyme FgaOx3. Org Biomol Chem 8: 3500-3508. doi: 10.1039/c003823g. PubMed: 20526482. [DOI] [PubMed] [Google Scholar]
- 84. Lim FY, Hou YP, Chen YM, Oh JH, Lee I et al. (2012) Genome-Based Cluster Deletion Reveals an Endocrocin Biosynthetic Pathway in Aspergillus fumigatus . Appl Environ Microbiol 78: 4117-4125. doi: 10.1128/AEM.07710-11. PubMed: 22492455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lodeiro S, Xiong Q, Wilson WK, Ivanova Y, Smith ML et al. (2009) Protostadienol biosynthesis and metabolism in the pathogenic fungus Aspergillus fumigatus . Org Lett 11: 1241-1244. doi: 10.1021/ol802696a. PubMed: 19216560. [DOI] [PubMed] [Google Scholar]
- 86. Gardiner DM, Howlett BJ (2005) Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus . FEMS Microbiol Lett 248: 241-248. doi: 10.1016/j.femsle.2005.05.046. PubMed: 15979823. [DOI] [PubMed] [Google Scholar]
- 87. Ames BD, Liu X, Walsh CT (2010) Enzymatic processing of fumiquinazoline F: a tandem oxidative-acylation strategy for the generation of multicyclic scaffolds in fungal indole alkaloid biosynthesis. Biochemistry 49: 8564-8576. doi: 10.1021/bi1012029. PubMed: 20804163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Maiya S, Grundmann A, Li X, Li SM, Turner G (2007) Identification of a hybrid PKS/NRPS required for pseurotin A biosynthesis in the human pathogen Aspergillus fumigatus . Chembiochem 8: 1736-1743. doi: 10.1002/cbic.200700202. PubMed: 17722120. [DOI] [PubMed] [Google Scholar]
- 89. Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101-1108. doi: 10.1038/nprot.2008.73. PubMed: 18546601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
qRT-PCR validation of RNA sequencing analysis of the expression of fumR (A) and Afu8g00370 (B) in the wild type, ∆veA, complementation and OEveA. Total RNA was extracted using TRIzol from 72h old stationary cultures grown in Czapek-Dox medium. The relative expression was calculated using 2-ΔΔCt method as described by Schmittgen and Livak [89]. Primers used for expression analysis are listed in Table S2. The bar represents the mean of three replicates and error bars represent standard error. Expression of 18S was used as internal reference gene. Values were normalized to the expression levels of WT which was considered as 1.
(TIF)
Targeted fumR deletion. (A) Diagram showing SalI sites (S) in the wild-type fumR locus, and the same locus after gene replacement of fumR by the A. parasiticus pyrG gene used as selection marker for fungal transformation. The fragment used as probe templates for Southern blot analyses is also shown. (B) Southern blot analysis. The ∆fumR deletion construct was transformed in CEA17ku80 (Table S1). Additional transformants also presented the correct band pattern (data not shown).
(TIF)
Targeted laeA deletion. (A) Diagram showing XhoI sites (X) in the wild-type laeA locus, and the same locus after gene replacement of laeA by the A. parasiticus pyrG gene used as selection marker for fungal transformation. The fragment used as probe templates for Southern blot analyses is also shown. (B) Southern blot analysis. The ∆laeA deletion construct was transformed in CEA17ku80 (Table S1). Additional transformants also presented the correct band pattern (data not shown).
(TIF)
(DOC)
(DOC)
(XLSX)