Abstract
Single deletions of mitochondrial DNA (mtDNA) are associated with three major clinical conditions: Kearns-Sayre syndrome, a multisystem disorder; Pearson syndrome (PS), a disorder of the hematopoietic system; and progressive external ophthalmoplegia (PEO), primarily affecting the ocular muscles. Typically, single mtDNA deletions are sporadic events, since the mothers, siblings, and offspring of affected individuals are unaffected. We studied a woman who presented with PEO, ptosis, and weakness of pharyngeal, facial, neck, and limb muscles. She had two unaffected children, but another of her children, an infant son, had sideroblastic anemia, was diagnosed with PS, and died at age 1 year. Morphological analysis of a muscle biopsy sample from the mother showed cytochrome c oxidase–negative ragged-red fibers—a typical pattern in patients with mtDNA deletions. Southern blot analysis using multiple restriction endonucleases and probed with multiple mtDNA fragments showed that both the mother and her infant son harbored an identical 5,355-bp single deletion in mtDNA, without flanking direct repeats. The deletion was the only abnormal species of mtDNA identified in both patients, and there was no evidence for duplications. We conclude that, although the vast majority of single large-scale deletions in mtDNA are sporadic, in rare cases, single deletions can be transmitted through the germline.
Mitochondria, the main sources of cellular energy, are under the dual control of mtDNA and nuclear DNA. mtDNA, a small, 16.5-kb molecule, encodes 13 subunits of the respiratory-chain complexes, as well as 22 tRNAs and 2 ribosomal RNAs (Anderson et al. 1981). Large-scale single deletions of mtDNA are associated with three major clinical conditions: Kearns-Sayre syndrome (KSS [MIM 530000]), progressive external ophthalmoplegia (PEO [MIM 258450]), and Pearson syndrome (PS [MIM 557000]). Virtually all patients with KSS harbor single mtDNA deletions that are detectable in muscle and other tissues by Southern blot analysis (Zeviani et al. 1988; Moraes et al. 1989). Approximately 50% of patients with PEO and ragged-red fibers have single mtDNA deletions in muscle but not in other tissues (Moraes et al. 1989; Zeviani et al. 1990). In PS, a disorder mainly affecting the hematopoietic system, mtDNA deletions are abundant in white blood cells (Cormier et al. 1990; Rötig et al. 1990).
KSS, PEO, and PS are sporadic disorders; clinically unaffected mothers and siblings of patients do not have detectable mtDNA deletions. By contrast, large-scale mtDNA duplications are frequently transmitted maternally (Ballinger et al. 1992, 1994; Manfredi et al. 1997). There is only one previous report of a woman with PEO and a single mtDNA deletion who had an infant son with PS and the same deletion; however, the presence of mtDNA duplications, which could have been transmitted maternally, was not excluded in that family (Bernes et al. 1993).
Here, we describe a woman with sporadic PEO who harbored a single large-scale mtDNA deletion, which she transmitted to her infant son with PS. The deletion was the only abnormal species of mtDNA identified in both patients.
Patient 1, the mother, was always small. At age 18 years, she was 147 cm tall and weighed 50 kg. At age 23 years, after the birth of an unaffected son, she noted diplopia and began to lose weight. The following year, she had a second son (patient 2), who had severe sideroblastic anemia during infancy and died at age 1 year. At age 27 years, she had an unaffected daughter. Over the next 3–4 years, her voice became increasingly nasal, and she developed limb weakness. On examination at age 32 years, she was short and thin (weighing 33.5 kg), with a normal mental status. Funduscopy revealed no pigmentary retinopathy. She had overt ptosis, with severely impaired extraocular movements. Oropharyngeal and facial muscles were weak. Neck flexor and extensor muscles were weak against minimal resistance, and proximal and distal limb muscles were weak against moderate resistance. She was able to rise from a squatting position with mild difficulty. Sensory and cerebellar functions and tendon reflexes were normal. Resting venous lactate was 12 mg/dl (normal range 3–12 mg/dl). An electrocardiogram was normal. Cerebrospinal fluid (CSF) studies were normal, including protein 28 mg/dl (normal range 14–45 mg/dl), lactate 1.3 mM (normal range 0.6–2.2 mM), and pyruvate 0.10 mM (normal range 0.04–0.13 mM). Electromyography was consistent with a myogenic process. A muscle biopsy showed abundant ragged-red fibers.
Patient 2, the second son of patient 1, was diagnosed with PS. He was born after a full-term pregnancy without complications. At age 4 mo, he became increasingly irritable and pale, and a complete blood count revealed granulocytopenia and reticulocytopenic anemia. Bone marrow aspiration showed a hypocellular marrow with decreased erythroid precursors. At age 6 mo, he developed recurrent pneumonia, urinary-tract infections, gastroenteritis, and septic shock, from which he recovered. On examination at age 8 mo, he was small, was developmentally delayed, and had hepatomegaly. Investigations revealed aplastic anemia, persistent metabolic acidosis, organic acidemia, and hypophosphatemic rickets. At age 1 year, he developed sepsis and died.
Histochemical analysis of the muscle biopsy sample from patient 1 showed cytochrome c oxidase–negative fibers with abnormally increased succinate dehydrogenase (SDH) staining. Biochemical analysis showed decreased complex II + III and complex IV activities and increased activities of citrate synthase and SDH. When normalized to citrate synthase, a marker of mitochondrial volume, all complexes that contained mtDNA-encoded subunits had decreased activities.
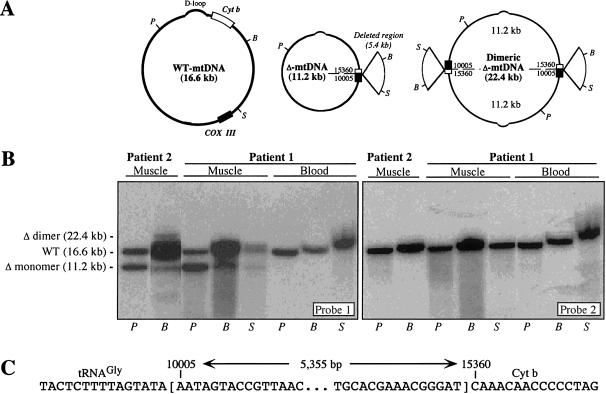
Southern blot analysis was performed using multiple restriction enzymes, and the blots were probed sequentially with two human mtDNA fragments that were generated by PCR: probe 1 (14.7 kb; nucleotides 12123–10256 [for mtDNA numbering, see Anderson et al. 1981]), which detects all mtDNA species; and probe 2 (1,331 bp; nucleotides 10762–12092), which hybridizes to normal mtDNA and, potentially, to duplicated mtDNA (dup-mtDNA), but not to the deleted mtDNA (Δ-mtDNA) in the patients whom we studied (fig. 1). This would have allowed us to detect unambiguously all three mtDNA species—wild-type mtDNA (WT-mtDNA), Δ-mtDNA (in monomeric and dimeric forms), and dup-mtDNA—even when they coexist in a heteroplasmic state (Tang et al. 2000a, 2000b). In patient 1, we found a single species of Δ-mtDNA (dimeric and monomeric forms) in muscle but not in blood. In patient 2, a single Δ-mtDNA species (monomeric and dimeric forms) was detected in DNA from muscle, the only tissue available for study (fig. 1). Both patients were heteroplasmic for the deletion: the proportion of Δ-mtDNAs in muscle was 60% in patient 1 and 40% in patient 2. There was no evidence for dup-mtDNA in either patient. Sequence analysis showed that the mother and her infant son had the same deletion (5,355 bp; nucleotides 10004–15359) without flanking direct sequence repeats (Mita et al. 1990). The deletion was also present in DNA that was isolated from the mother's urinary sediment, but it was not detectable in her buffy coat or skin fibroblasts.
Figure 1.
A, Maps of mtDNA species—namely, monomeric and dimeric Δ-mtDNA and WT-mtDNA. The protruding, pie-shaped section on the Δ-mtDNA denotes the deleted region. Only the genes involved in the rearrangement are shown—namely, cytochrome c oxidase subunit III (blackened box) and cytochrome b (unblackened box). A dashed line indicates the breakpoint. Also shown are the D-loop and the locations of the PvuII (P), BamHI (B), and SnaBI (S) restriction sites. Cyt b = cytochrome b. B, Southern blot analysis of mtDNA from muscle and blood from the patients whom we studied. DNA was digested with PvuII (P), BamHI (B), or SnaBI (S), and the blot was hybridized sequentially with probes 1 and 2 (see text). The identity of each hybridizing fragment and its size (in kb) are indicated at left. C, Sequence around the deletion breakpoints.
Patient 1 had PEO, and her infant son died of PS; both harbored an identical single large-scale mtDNA deletion. The mother had PEO, ptosis, and pharyngeal, facial, and limb weakness but had none of the extramuscular canonical features of KSS (Rowland et al. 1988). Specifically, she did not have pigmentary retinopathy, cardiac-conduction abnormalities, cerebellar signs, or increased CSF protein levels. In keeping with her clinical phenotype, a single Δ-mtDNA was identified in muscle but not in blood. The same deletion was found in muscle from her son with PS (patient 2). Although no blood was available from this infant son, who died 8 years before his mother sought medical attention, it can be assumed that he harbored the same deleted mitochondrial genome in blood.
Two other children of mothers with both PEO and single large-scale mtDNA deletions were clinically affected. One woman had a daughter with PEO, but this mother and her daughter harbored different single mtDNA deletions (Ozawa et al. 1988). A second woman had a son with a Pearson-like syndrome, and both harbored the 4,977-bp “common” mtDNA deletion (Bernes et al. 1993). Although the second case is similar to the family reported here, the deletion in the mother was documented only by PCR analysis; therefore, the presence of dup-mtDNA, which could have explained transmission from mother to child, was not excluded. It should be noted that what appears to be maternal inheritance may simply reflect low levels of age-related Δ-mtDNAs in the mother (Akaike et al. 1995). Several children of women with single mtDNA deletions were clinically normal and had no detectable deletions (Zeviani et al. 1990; Larsson et al. 1992; Graff et al. 2000), and we have followed a woman with KSS whose mother and son were clinically and genetically normal.
How was the Δ-mtDNA transmitted in the family that we studied? There is no direct answer, because we do not know the mtDNA genotype of the fertilized oocyte that developed into patient 2. However, several possible scenarios can be envisioned:
-
1.
Δ-mtDNA may have been transmitted directly from the mother’s oocyte to the child. This implies that the oocyte had relatively high levels of Δ-mtDNA, because the very narrow genetic bottleneck, possibly limited to a single mitochondrion, would make transmission of rare deletions unlikely (Chen et al. 1995; Marchington et al. 1998). Also, Δ-mtDNAs might make the oocyte nonviable or cause early embryonic developmental failure. Despite all this, a rare Δ-mtDNA might “slip through” the bottleneck without having significant deleterious effects on the embryo or the fetus, and the Δ-mtDNA might then segregate into different fetal tissues (Chen et al. 1995). In the case that we studied, if the Δ-mtDNA were truly transmitted from mother to child, then the mother must have harbored Δ-mtDNAs not only in her muscle but also in her oocytes (although Δ-mtDNA was not detected in blood, it was present in urinary sediment). The fact that only one of her three children inherited the Δ-mtDNA is probably due to pure chance of transmission through the genetic bottleneck. In a mouse model of mtDNA rearrangements, Δ-mtDNAs can indeed be transmitted (Inoue et al. 2000).
-
2.
dup-mtDNA, rather than Δ-mtDNA, may have been transmitted from mother to child. In the mouse model, an mtDNA duplication was identified in addition to the single mtDNA deletion, suggesting to Inoue et al. (2000) that the duplication, not the deletion, was transmitted maternally. It has been shown that, although dup-mtDNA per se does not impair mitochondrial oxidative phosphorylation (Tang et al. 2000b), it can recombine to generate Δ-mtDNA (Tang et al. 2000a). However, this is unlikely in the family that we studied, because dup-mtDNA was not detected in any tissue studied from either patient.
-
3.
A spontaneous de novo mtDNA deletion may have occurred during early embryonic development—perhaps secondary to a nuclear gene mutation. However, it is difficult to envision how a nuclear mutation could cause identical single mtDNA deletions in mother and son.
In addition to demonstrating that single mtDNA deletions can be transmitted maternally, our findings confirm that an mtDNA deletion may be associated with different clinical phenotypes, even in the same family. Differential tissue distribution probably accounts for this phenotypic variability.
Acknowledgments
The present study was supported by National Institutes of Health grants NS 11766, NS 28828, NS 39854, and HD 32062 and grants from the Muscular Dystrophy Association.
Electronic Database Information
Accession numbers and the URL for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for KSS [MIM 530000], PEO [MIM 258450], and PS [MIM 557000])
References
- Akaike M, Kawai H, Kashiwagi S (1995) A case of Kearns-Sayre syndrome whose asymptomatic mother had abnormal mitochondria in skeletal muscle. Rinsho Shinkeigaku 35:190–194 [PubMed] [Google Scholar]
- Anderson S, Bankier AT, Barrell BG, de Bruijn MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290:457–465 [DOI] [PubMed] [Google Scholar]
- Ballinger SW, Shoffner JM, Gebhart S, Koontz DA, Wallace DC (1994) Mitochondrial diabetes revisited. Nat Genet 7:458–459 [DOI] [PubMed] [Google Scholar]
- Ballinger SW, Shoffner JM, Hedaya EV, Trounce I, Polak MA, Koontz DA, Wallace DC (1992) Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet 1:11–15 [DOI] [PubMed] [Google Scholar]
- Bernes SM, Bacino C, Prezant TR, Pearson MA, Wood TS, Fournier P, Fischel-Ghodsian N (1993) Identical mitochondrial DNA deletion in mother with progressive external ophthalmoplegia and son with Pearson marrow-pancreas syndrome. J Pediatr 123:598–602 [DOI] [PubMed] [Google Scholar]
- Chen X, Prosser R, Simonetti S, Sadlock J, Jagiello G, Schon EA (1995) Rearranged mitochondrial genomes are present in human oocytes. Am J Hum Genet 57:239–247 [PMC free article] [PubMed] [Google Scholar]
- Cormier V, Rotig A, Quartino AR, Forni GL, Cerone R, Maier M, Saudubray JM, Munnich A (1990) Widespread multi-tissue deletions of the mitochondrial genome in the Pearson marrow-pancreas syndrome. J Pediatr 117:599–602 [DOI] [PubMed] [Google Scholar]
- Graff C, Wredenberg A, Silva JP, Bui TH, Borg K, Larsson NG (2000) Complex genetic counselling and prenatal analysis in a woman with external ophthalmoplegia and deleted mtDNA. Prenat Diagn 20:426–431 [PubMed] [Google Scholar]
- Inoue K, Nakada K, Ogura A, Isobe K, Goto Y, Nonaka I, Hayashi JI (2000) Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat Genet 26:176–181 [DOI] [PubMed] [Google Scholar]
- Larsson NG, Eiken HG, Boman H, Holme E, Oldfors A, Tulinius MH (1992) Lack of transmission of deleted mtDNA from a woman with Kearns-Sayre syndrome to her child. Am J Hum Genet 50:360–363 [PMC free article] [PubMed] [Google Scholar]
- Manfredi G, Vu T, Bonilla E, Schon EA, DiMauro S, Arnaudo E, Zhang L, Rowland LP, Hirano M (1997) Association of myopathy with large-scale mitochondrial DNA duplications and deletions: which is pathogenic? Ann Neurol 42:180–188 [DOI] [PubMed] [Google Scholar]
- Marchington DR, Macaulay V, Hartshorne GM, Barlow D, Poulton J (1998) Evidence from human oocytes for a genetic bottleneck in an mtDNA disease. Am J Hum Genet 63:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S, Rizzuto R, Moraes CT, Shanske S, Arnaudo E, Fabrizi GM, Koga Y, DiMauro S, Schon EA (1990) Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic Acids Res 18:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes CT, DiMauro S, Zeviani M, Lombes A, Shanske S, Miranda AF, Nakase H, Bonilla E, Werneck LC, Servidei S, Nonaka I, Koga Y, Spiro AJ, Brownell KW, Schmidt B, Schotland DL, Zupanc M, De Vivo DC, Schon EA, Rowland LP (1989) Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med 320:1293–1299 [DOI] [PubMed] [Google Scholar]
- Ozawa T, Yoneda M, Tanaka M, Ohno K, Sato W, Suzuki H, Nishikimi M, Yamamoto M, Nonaka I, Horai S (1988) Maternal inheritance of deleted mitochondrial DNA in a family with mitochondrial myopathy. Biochem Biophys Res Commun 154:1240–1247 [DOI] [PubMed] [Google Scholar]
- Rötig A, Cormier V, Blanche S, Bonnefont JP, Ledeist F, Romero N, Schmitz J, Rustin P, Fischer A, Saudubray (1990) Pearson's marrow-pancreas syndrome: a multisystem mitochondrial disorder in infancy. J Clin Invest 86:1601–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LP, Hausmanowa-Petrusewicz I, Bardurska B, Warburton D, Nibroj-Dobosz I, DiMauro S, Pallai M, Johnson WG (1988) Kearns-Sayre syndrome in twins: lethal dominant mutation or acquired disease? Neurology 38:1399–1402 [DOI] [PubMed] [Google Scholar]
- Tang Y, Manfredi G, Hirano M, Schon EA (2000a) Maintenance of human rearranged mitochondrial DNAs in long-term cultured transmitochondrial cell lines. Mol Biol Cell 11:2349–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Schon EA, Wilichowski E, Vazquez-Memije ME, Davidson E, King MP (2000b) Rearrangements of human mitochondrial DNA (mtDNA): new insights into the regulation of mtDNA copy number and gene expression. Mol Biol Cell 11:1471–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeviani M, Gellera C, Pannacci M, Uziel G, Prelle A, Servidei S, DiDonato S (1990) Tissue distribution and transmission of mitochondrial DNA deletions in mitochondrial myopathies. Ann Neurol 28:94–97 [DOI] [PubMed] [Google Scholar]
- Zeviani M, Moraes CT, DiMauro S, Nakase H, Bonilla E, Schon EA, Rowland LP (1988) Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology 38:1339–1346 [DOI] [PubMed] [Google Scholar]