Abstract
Background
Human landing collections are currently the standard method for collecting onchocerciasis vectors in Africa and Latin America. As part of the efforts to develop a trap to replace human landing collections for the monitoring and surveillance of onchocerciasis transmission, comprehensive evaluations of several trap types were conducted to assess their ability to collect Simulium ochraceum sensu lato, one of the principal vectors of Onchocerca volvulus in Latin America.
Methodology/Principal Findings
Diverse trap designs with numerous modifications and bait variations were evaluated for their abilities to collect S. Ochraceum s.l. females. These traps targeted mostly host seeking flies. A novel trap dubbed the “Esperanza window trap” showed particular promise over other designs. When baited with CO2 and BG-lure (a synthetic blend of human odor components) a pair of Esperanza window traps collected numbers of S. Ochraceum s.l. females similar to those collected by a team of vector collectors.
Conclusions/Significance
The Esperanza window trap, when baited with chemical lures and CO2 can be used to collect epidemiologically significant numbers of Simulium ochraceum s.l., potentially serving as a replacement for human landing collections for evaluation of the transmission of O. volvulus.
Introduction
Onchocerciasis (river blindness) is caused by chronic infection with Onchocerca volvulus, a filarial nematode that is transmitted by Simulium spp. (Diptera: Simuliidae). The disease constitutes a serious public health concern and an enormous source of socio-economic loss in many developing countries, most severely in sub-Saharan Africa and, to a lesser extent, Latin America [1-5]. The current strategy for the elimination of onchocerciasis relies on mass treatment of endemic communities with ivermectin. A variety of treatment regimens, including quarterly, semi-annual and annual treatment have proven effective in focally interrupting transmission and eliminating the parasite in Latin America and in isolated foci in Africa over different time frames [6-12]. High coverage (≥ 85% of eligible persons) community-wide treatment of residents for a minimum of 15 years is believed to be sufficient to reduce the load of microfilariae in human hosts below the threshold that can sustain transmission by black fly vectors, thus locally eliminating the infection [7].
The elimination guidelines set forth by the Onchocerciasis Elimination Program for the Americas (OEPA) and the World Health Organization (WHO) use the prevalence of the infective stage of O. volvulus larvae in the black fly vectors as a major metric for determining whether or not transmission has been successfully interrupted in an endemic community [13,14]. The threshold used for declaring elimination is less than one O. volvulus per 2,000 female black flies per endemic community. At least 6,000 flies must be tested by Polymerase Chain Reaction (PCR-pool screening) in each endemic community to satisfy this standard [13-15]. To date, the collection of such large numbers of black flies has been problematic, as the primary method for collecting host-seeking black flies is the use of human landing collections [16-18]. This method involves stationing adult humans in areas of high Simulium densities and collecting black flies that attempt to land and blood-feed. Apart from the fact that this method has been a subject of criticism due to the potential risk of human collector’s exposure to infection during the early stages of a control program [19], it may be difficult for fly collectors to capture the large number flies needed to document that transmission has been interrupted where biting rates are low or highly seasonal. Thus, the development of trap(s) to replace human landing collections is becoming increasingly important as the focus in onchocerciasis shifts from control to elimination, and in some cases post-treatment surveillance [20].
Various attempts have been made to design black fly traps using both visual and chemical attraction techniques [21-25]. Most of these studies were conducted in West Africa where members of the Simulium damnosum species complex serve as the primary vectors for the causative agent of onchocerciasis, Onchocerca volvulus. In Latin America, Simulium ochraceum sensu lato is the most important vector of this parasite; until recently it was associated with ≥ 70% of transmission in the six countries where the disease occurred. The ecology and behavior of S. ochraceum s.l. is distinctly different from S. damnosum s.l., the primary vector in Africa [26-29], potentially complicating the prospects for development of a single trap that efficiently captures both species. As part of longitudinal studies in designing an improved black fly trap for monitoring and surveillance transmission of O. volvulus, field studies were carried out at endemic sites in Chiapas, Mexico, to evaluate different trap designs. Here we report the results of field trials and optimization of a promising design (Esperanza window trap) for the collection of host-seeking S. ochraceum s.l.
Materials and Methods
Ethics Statement
All procedures involving human landing collections were reviewed and approved by the appropriate institutional review boards of the countries involved. These included the Bioethics Committees of the Center for Research and Development in Health Sciences of the Autonomous University of Nuevo León (Monterrey, Nuevo León, Mexico), and an Institutional Review Boards of the University of South Florida. Written informed consent was obtained from all human landing collectors.
Strategic design
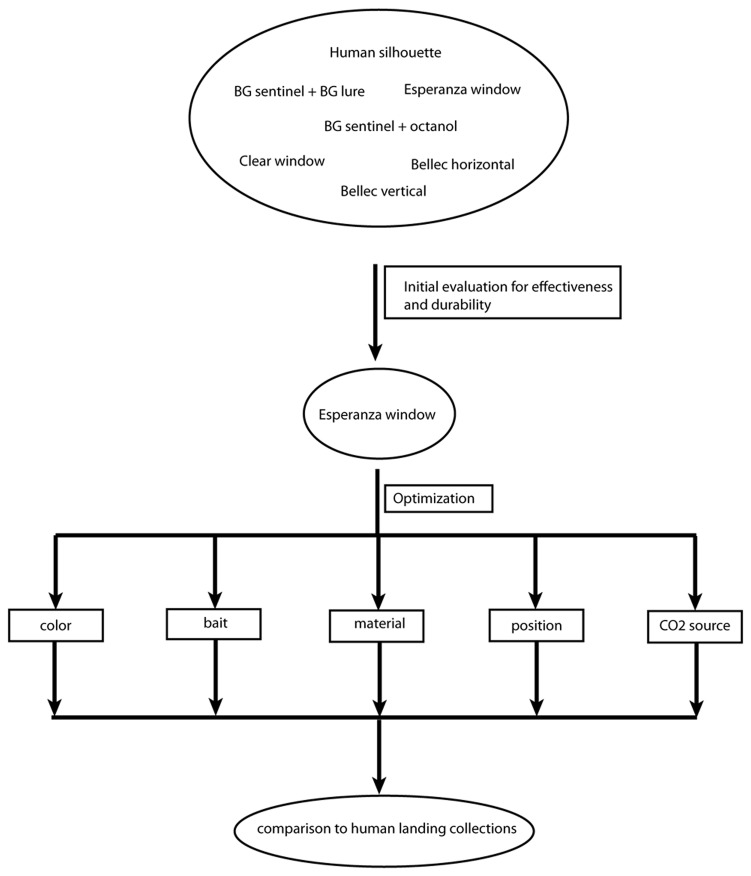
The strategy to design a trap to replace human landing collections is summarized in Figure 1. In the initial evaluations, diverse trap designs were compared, selecting those that showed initial promise and eliminating those that either collected few flies or were operationally impractical under field conditions. The platform showing the most promise in the initial evaluation was then optimized with different combinations of the factors that are known to contribute to trap effectiveness, especially with respect to visual and olfactory cues (trap color, olfactory lures). Collections from the optimized trap were compared to vector collectors employing a standard human landing collection protocol to estimate trap efficiency.
Figure 1. Strategic design of trap evaluation and optimization studies.
Study area
All field studies were conducted in the village of Las Golondrinas, Chiapas, (15°25´ 59˝ N; 92°39´ 06˝ W, elevation 890 m) Mexico. Las Golondrinas is located within the former Southern Chiapas focus of onchocerciasis, where transmission of O. volvulus was quite high, prior to recent elimination of the infection [30]. Field studies were conducted during the dry season to take advantage of the consistently high populations of Simulium ochraceum, the primary vector of O. volvulus in the region [32]. Populations of Simulium ochraceum reach maximum levels during this time, with daily human landing rates often exceeding 100 over a 4-5 month period in certain locations. Las Golondrinas is a rural community on the forested Pacific Ocean-facing slopes of the Sierra Madre Mountains in habitat well suited for cultivation of coffee and cacao. Community leaders were consulted and approved the use of the selected locations in the village and on nearby riverbanks as vector catching points. Endangered species were not involved in this work.
Trap evaluations against S. ochraceum s.l. in Chiapas, Mexico
Trap selection and first evaluation
Seven trap designs were initially selected for field studies, based on published reports and/or preliminary observations by the authors (Figure 2). These were (1), A human Silhouette: A 2-m high wooden human silhouette trap coated with Tangle-Trap Insect Trap Coating® (The Tanglefoot Company, Grand Rapids, MI) and baited with BG-Lure™ attractant (a mixture of compounds found in human skin secretions [Biogents AG, Regensburg, Germany]) (2), BG Sentinel with BG-Lure™: BG-Sentinel trap (Biogents AG, Regensburg, Germany) baited with BG-Lure™ attractant (3) BG Sentinel with octenol: BG-Sentinel trap baited with 1-octen-3-ol lure (AgriSense Ltd, UK); (4) Clear window trap: a flight intercept trap [31] consisting of a 0.5 x 0.25 m sheet of clear acrylic (3 mm thickness) suspended vertically above a collection tray partially filled with a nontoxic polysorbate surfactant (~1.0% Tween-20® non-ionic detergent in water); (5) Bellec vertical: An oviposition trap [21] consisting of a 1.0 m2 piece of fiberboard (3 mm thickness) covered in aluminum foil and coated with a film of Tangle-Trap adhesive, and suspended 0.6 m above ground, oriented vertically; (6) Bellec horizontal: A 1.0 x 0.5 m piece of plywood (3 mm thickness) covered in aluminum foil and coated with Tangle-Trap adhesive, and supported 0.5 m above ground, oriented horizontally; and (7) “Esperanza window trap”: a novel design consisting of a 1m2 piece of black satin fabric sandwiched between two sheets of 1m2 glass (3mm thickness) that were each coated on their outer faces with Tangle-Trap adhesive. The appearance of the Esperanza window trap differed between sides (shiny and dull) depending on the orientation of the satin fabric. The Esperanza window trap was baited with BG-lure and supported 0.6 m above ground with a wooden frame. The traps were evaluated concurrently at two forested plots within the village, each proximal to an S. ochraceum breeding site (Figure S1). With the exception of the BG-Sentinel (a trap designed for collecting mosquitoes) and the Esperanza window trap, the traps deployed have been previously used for collecting S. damnosum s.l. in Africa [21-23,25]. The Esperanza window trap was initially developed and evaluated by the authors during preliminary studies at La Esperanza (Latitude 17°37′40″ N and Longitude 96°22′10″ W, elevation 1,600 m), a community in the state of Oaxaca, an area with a history of endemic onchocerciasis.
Figure 2. Traps evaluated for potential in collecting Simulium ochraceum s.l. females in Mexico.
Panel A: vertical Bellec plaque. Panel B: Esperanza window trap (original). Panel C: BG Sentinel. Panel D: clear window trap. Panel E: Esperanza window trap (acrylic/hanging version). Panel F: horizontal Bellec plaque. Panel G: human silhouette trap (latex examination glove for scale).
The traps were evaluated simultaneously at two forested plots within the village for six consecutive days, with trap position rotated daily to avoid position bias. A small intermittent stream flowed through each plot, serving as a breeding site for S. ochraceum (data not shown). Seven trap positions were established at each plot, in a rough circle with the stream at its center. Trap positions were 10-20 m apart and 2-5 m from the stream. Collections were retrieved three times daily (1100 h, 1400 h, and 1700 h). Flies collected between 11:00 h and 14:00 h in each trap were dissected for parity and gravidity.
Optimization of Esperanza window trap
Following the initial trials that resulted in the selection of the Esperanza window trap as the most promising design, the Esperanza window trap was modified from the original prototype to increase its ease of use and its overall effectiveness (Figure 2, Panel E). Modifications included (1) replacing glass panes with acrylic sheeting (2 mm thickness), strengthening the trap and permitting easy movement of the trap in the field; (2) replacing the wooden stand with an aluminum frame, suspended from a tree branch (rather than standing on ground) (Figure 2); (3) evaluation of CO2 (two sources), octenol and worn shirts containing human secretions as potential attractants; (4) different colors of fabric; and (5) comparison with vector collectors (human bait).
The original model of the Esperanza window trap was first tested against one with acrylic panes (instead of glass) and against a human landing collection. The traps were arranged in an array roughly 7 m from the breeding site, separated by a distance of 3 m from one another. Human landing collections were carried out approximately 5 m from the traps. These distances between traps and between traps and human landing collections minimized the interference among traps while still drawing from the same host-seeking black fly population. This arrangement permitted flies to choose amongst traps (including human bait), putting all the traps in competition. Human landing collections were carried out with two persons working as a team: one person with exposed upper body, while the other person collected the flies landing on the exposed skin of his colleague, as previously described [7]. The fly collector’s duties were rotated every counting period (180 minutes) to minimize the effect of individual attraction to the flies. Before the commencement of every trapping session, the traps were checked closely to remove any black flies that were trapped during non-experimental periods.
The acrylic-paned Esperanza window traps were then used in field trials to determine which chemical attractants could be employed to increase collections of S. ochraceum s.l. to levels rivaling that of vector collectors. Treatments included CO2 alone; CO2 plus octenol; CO2 plus BG-lure; CO2 plus a shirt worn by a farm worker for three consecutive days (without washing); ethanol (5mL dispensed from perforated plastic screw-cap vial); and completely unbaited. Traps were placed in a line roughly 7m from an S. ochraceum breeding site, and 3m from one another. The CO2 baited traps were connected to the source of CO2 (gas cylinders) with flexible tubing (Figure 2). The gas was released at each trap at a rate of 150-200 mL/min. Due to the limited supply and high dispense rate of CO2, it was not feasible to operate the traps throughout the entire day. Therefore, the traps were operated for 20 minutes per hour between 800 h and 1200 h, targeting the period of highest biting of S. ochraceum s.l. [32]. Trap positions were rotated daily, and trials were conducted four times daily for three consecutive days. Human landing collections were carried out as described above, at the same time and for the same duration (20-minute periods) as the trap evaluations.
The effectiveness of yeast-generated CO2 [33,34], as an alternative to commercial CO2, was investigated simultaneously with different colors of fabric [35]. Esperanza window traps, with black, blue, red or yellow fabric, and yeast-generated CO2, were evaluated over three days at the same plots as above. Fabric for the traps was obtained from Grupo Parisina S.A. de C.V. (Mexico City, MX). The product numbers for each fabric are as follows: Yellow 4917L72; Blue 4917L27; Black 4917L19; Red 4917L36. Descriptive colorimetric values for each fabric are provided in Table 1. Traps were operated for 20 minutes per hour between 800 h and 1200 h to conserve the limited supply of CO2.
Table 1. Colorimetric values of four nominal colors (red, yellow, blue, black) evaluated in Esperanza window traps in Chiapas, Mexico.
| System | Red | Yellow | Blue | Black |
|---|---|---|---|---|
| RGB | rgb(225, 48, 54) | rgb(249, 191, 84) | rgb(52, 85, 178) | rgb(23, 21, 22) |
| Hex | #e13036 | #f9bf54 | #3455b2 | #171516 |
| CIELab | L: 49.963, a: 66.294, b: 40.651 | L: 80.762, a: 10.162, b: 60.278 | L: 38.623, a: 20.177, b: -53.084 | L: 7.013, a: 1.162, b: -0.331 |
| Hunter-Lab | L: 42.881, a: 61.387, b: 22.672 | L: 76.185, a: 5.61, b: 40.516 | L: 32.313, a: 13.611, b: -57.134 | L: 8.811, a: 0.104, b: 0.324 |
| CMYK | cyan: 0, magenta: 0.787, yellow: 0.76, key: 0.118 | cyan: 0, magenta: 0.233, yellow: 0.663, key: 0.024 | cyan: 0.708, magenta: 0.522, yellow: 0, key: 0.302 | cyan: 0, magenta: 0.087, yellow: 0.043, key: 0.91 |
Statistical analysis
The statistical differences among effectiveness of different traps and their lures were evaluated using a t-test (for 2 treatments) or ANOVA (for 3 or more treatments). For the initial trap evaluations, ANOVA was first used to test for differences in the number of flies collected at different sampling periods for each trap type. If no significant differences were found, data from the three sampling periods were aggregated. Since trap-to-trap interference may violate the independence of samples assumption of ANOVA, we used the Kruskal-Wallis test to test for significant differences among traps for black fly captures and the Tukey test for post hoc multiple means comparison. All tests were performed using SAS 9.1.3 (SAS Institute, Cary, NC, USA). Since day-to-day variation in fly activity was substantial and relative performance of traps was more relevant than absolute performance, for the purposes of statistical analysis raw counts were converted to proportions (of total flies collected during a given period and plot). In the first evaluation of the Esperanza window trap in Mexico, counts from each face of the trap were analyzed separately, since each side of the fabric had a different visual quality (shiny vs. dull). Prior to statistical tests, all proportions were transformed using the angular transformation (arc sine of square root).
Results
Initial trap evaluations
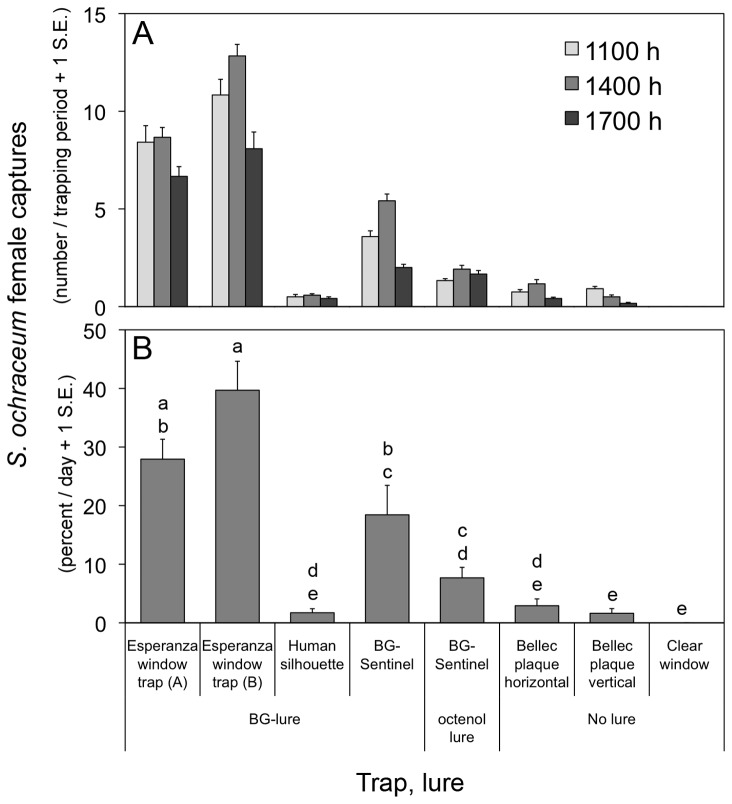
Significant differences in the number of S. ochraceum collected were observed among the different trap platforms that were initially evaluated at the field locations in Chiapas, Mexico (H=158.56; d.f.=7; P < 0.001, Kruskal–Wallis test). The Esperanza window trap and BG Sentinel traps consistently outperformed other traps, regardless of time of day (Figure 3). The number of females collected at 1100 h, 1400 h, and 1700 h was not significantly different for any trap type (Table 2). Collections on the two faces (shiny or dull) of the Esperanza window trap were also not significantly different (Figure 3). Other black fly species were also collected, namely S. metallicum s.l. and S. gonzalezi, but their numbers were low in comparison to those of S. ochraceum s.l. (data not shown).
Figure 3. Comparison of traps for collecting the onchocerciasis vector Simulium ochraceum s.l. in the community of Las Golondrinas, Chiapas in Mexico.
Traps were operated simultaneously, with positions rotated daily. Panel A: Mean females captured at each sampling time (1100 h, 1400 h, 1700h) over six days. Panel B: Percentage of total females captured each day by traps over six days. Letters denote significant differences among traps for the percentage of total flies captured each day.
Table 2. Variation in Simulium ochraceum females captured by traps at three sampling times (1100 h, 1400 h, 1700h) in Chiapas, Mexico.
| Flies per collection period |
|||||
|---|---|---|---|---|---|
| Trap | 1100 h | 1400 h | 1700 h | F | P |
| Esperanza window trap (both sides) | 19.25 | 21.50 | 14.75 | 0.59 | 0.5577 |
| Human silhouette (BG-lure) | 0.50 | 0.58 | 0.42 | 0.06 | 0.9379 |
| BG- Sentinel (BG-lure) | 3.58 | 5.42 | 2.00 | 3.1 | 0.0585 |
| BG- Sentinel (octenol lure) | 1.33 | 1.92 | 1.67 | 0.27 | 0.7661 |
| Bellec plaque horizontal | 0.75 | 1.17 | 0.42 | 0.54 | 0.5857 |
| Bellec plaque vertical | 0.92 | 0.50 | 0.17 | 1.41 | 0.2578 |
| Clear window trap | 0.00 | 0.00 | 0.00 | nd | nd |
Optimization of the Esperanza window trap
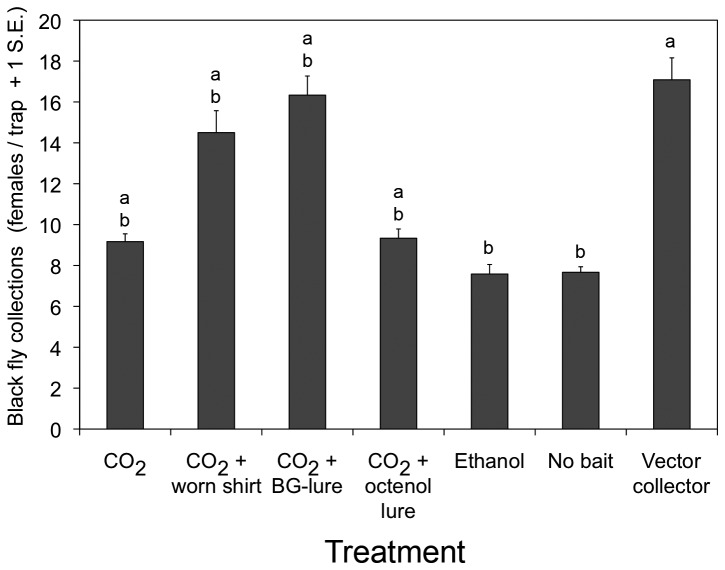
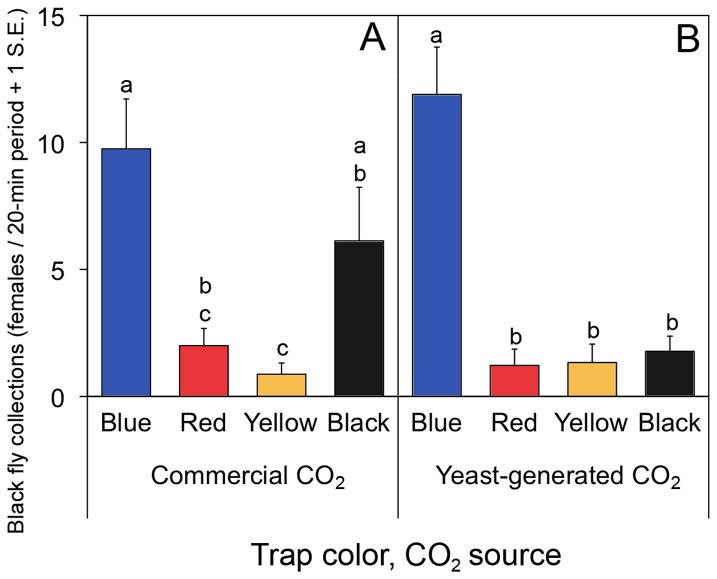
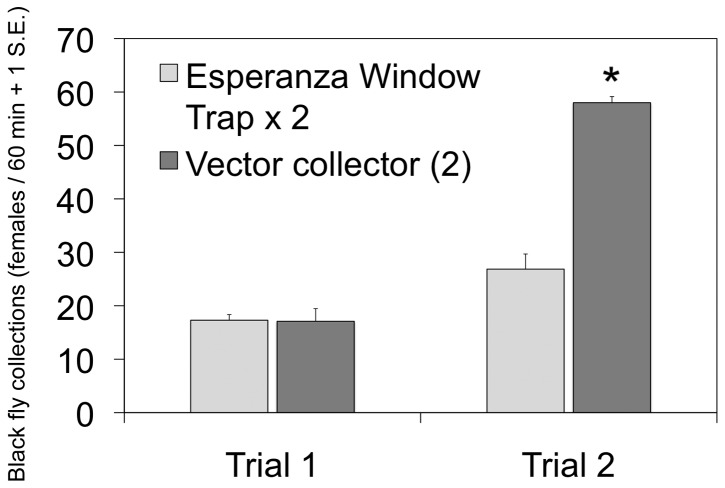
Optimization of the Esperanza window trap revealed that significant improvements and modifications of the original (prototype) design were feasible, facilitating deployment and increasing catches. Esperanza window traps with acrylic panes mounted in aluminum frames were far easier to handle and collected significantly more black flies (T=9.06, p<0.001) than those fitted with glass panes in wooden frames (acrylic: daily mean=52.7, s.d.=11.7; glass: daily mean=32.3, s.d.=4.45). Initial trials of various chemical lures showed that Esperanza window traps baited with CO2 and human scent (a worn shirt or BG-lure) could approach the attractiveness of a human landing collection (Figure 4). Esperanza window traps without baits and those baited with control substances (ethanol alone) caught the fewest number of flies (Figure 4). Significant differences in the number of flies captured were found when different colors of fabric used in Esperanza window traps baited with yeast-generated (F3,32=19.30, p<0.001) or bottled carbon dioxide (F3,32=10.12, p<0.001), with blue fabric traps generally outperforming traps with other colors (Figure 5). Black fly collections of Esperanza window traps baited with yeast-generated CO2 were not significantly different from those baited with commercial CO2 (T=2.14; p=0.054) (Figure 5). When blue Esperanza window traps baited with BG-lure and yeast-generated CO2 were compared with vector collectors (2 individuals working together) in two trials, the results showed that the number of flies captured on two traps was not significantly different than that captured by a vector collector team in the first trial (T=-0.24; p=0.82), but was about half that captured vector collector team in the second trial (T=-6.82; p<0.001) (Figure 6). Dissection of the flies captured by the traps or collection team revealed that the parity rates in the flies collected by both methods were not significantly different, exceeding 95% in both populations (data not shown).
Figure 4. Comparison of baited and unbaited Esperanza window traps and vector collectors for collecting Simulium ochraceum s.l. females.
Treatments included CO2 (commercial grade), a shirt worn by a local villager, BG-lure, 1-octen-3-ol, ethanol (control), unbaited trap, and a vector collector team. Data are from 20-min collecting periods, at a site in the community of Las Golondrinas, Chiapas, Mexico, 2012. Letters denote significant differences among treatments for the percentage of total flies captured each day.
Figure 5. Evaluation of trap color and CO2 source of Esperanza window traps for collecting Simulium ochraceum s.l. females.
All traps were co-baited with the BG-lure. Data are from 20-min collecting periods in the community of Las Golondrinas, Chiapas. Letters denote significant differences among traps for the percentage of total flies captured each day. Panel A: Esperanza window traps baited with commercial CO2. Panel B: Esperanza window traps baited with yeast-generated CO2.
Figure 6. Comparison of Esperanza window traps and vector collectors for collecting Simulium ochraceum s.l. females.
Traps were baited with CO2 (yeast-generated) and BG-lure. Data are from 60-min collecting periods, during two trials at plots in the community of Las Golondrinas, Chiapas, Mexico. Asterisks denote significant differences among treatments for the percentage of total flies captured each day, as determined by t-test.
Discussion
The data presented here demonstrate the feasibility of using traps to replace human bait (vector collectors) in onchocerciasis monitoring and elimination programs. In initial field trials it was obvious that the several trap designs evaluated were not equally effective for collecting S. ochraceum s.l. females, but that some traps would be amenable to further development and experimentation (e.g. Figure 3). The Esperanza window trap showed the most initial promise, and also proved to be effective in subsequent tests (Figures 4-6). Since repeatability of results has been lacking in most black fly trapping studies [36], the consistent productivity of the Esperanza window trap was very encouraging.
After the first round trap comparisons field tests focused solely upon optimizing the Esperanza window trap for collecting S. ochraceum s.l. females. Modifications to the Esperanza window traps included replacing glass with acrylic panes and replacing the awkward wooden frame with aluminum, resulting in a lighter, and more manageable design that collected more black flies than the initial prototype. Additional experiments testing the effectiveness of available chemical lures (including CO2) indicated that olfactory attractants were key in trapping success.
It is interesting that Esperanza window traps with blue fabric collected more S. ochraceum s.l. females than did traps with other colors of fabric. Blue is reported to be attractive to tsetse flies (Glossina spp.) [37], and it is used as a dominant fabric color in tsetse trap fabrication [38]. Yeast-generated CO2 was as effective as commercial CO2. This is particularly important since onchocerciasis-endemic areas are often quite remote, and obtaining CO2 cylinders in these areas can pose a serious operational difficulty. Yeast-generated CO2 will likely conserve monetary resources, since the ingredients (yeast, sugar, water) and materials (plastic bottles, plastic tubing/hose) are easy to locate or improvise. Furthermore fermentation of sugar by yeast produces not only CO2, but also a variety of volatile organic compounds (alcohols, esters, aldehydes and fatty acids) that are known to attract the arthropods that are attracted to vertebrates [33].
Simultaneous testing of multiple trap types, as performed in our initial trap screening presents several operational and analytical challenges. Some of the traps tested present large visual cues (Bellec plaques and Esperanza window traps), while others presented primarily olfactory cues (e.g. BG-Sentinel). The Esperanza window trap presented a large visual target as well as olfactory lures. Testing this broad array of trap types, some with olfactory cues and others without makes it quite difficult to tease apart the subtle interplay of long and short-distance stimuli that ultimately result in a fly being captured by a particular trap. For example, a long distance olfactory cue emanating from one of the trap types might draw flies into the field plot. Once in the vicinity of the traps, a shorter distance signal (visual or olfactory) from a different trap type may cause a change in a fly’s trajectory so that the fly is actually captured by a trap that is different from the one that originally lured the fly to the experimental plot. In this scenario, the trap that ends up capturing the fly may perform better in the presence of a long-distance olfactory cue originating from a neighboring trap than if evaluated without other traps. However, given the temporal (day-to-day), spatial and environmental variation in fly activity, testing traps independently (on different days) and then trying to compare trap captures between traps across different days or locations would present its own set of analytical challenges. That said, our results indicate that the traps that utilized chemical lures caught the overwhelming majority of black flies (Figure 3). The four traps that utilized lures (Esperanza window trap, human silhouette, BG-Sentinel with human scent lure and BG-Sentinel with octenol) collected 95.4% of the captured flies, while the three unbaited traps (Bellec plaques horizontal and vertical and Clear window trap) captured only 4.6% of the total. This would suggest that flies are drawn into the trapping arena by olfactory cues emitted by baited traps, but traps with multimodal profiles, such as the Esperanza trap, outperformed unbaited traps in actually capturing flies.
Exactly what features of the Esperanza trap are responsible for its relative “success” in the field are not easy to ascertain. The optimized version of the trap utilized multiple visual and olfactory cues that likely worked in concert to maximize its attractiveness to S. ochraceum s.l. females. Trap color appeared to have a strong effect on black fly capture, when other variables were held constant. Experimental evidence suggests that spectral reflectance and polarization of reflected light are key determinants of how insects respond to visual stimuli [39]. For example, the black-and-white striped patterns of zebra coats result in alternating directions of reflected light polarization. Stripe width has a profound impact on attractiveness of zebras to tabanids, with attraction decreasing along with decreasing stripe width, due to disruptive patterns of reflected light polarization [40]. Spectral reflectance of visual stimuli (especially host silhouettes) is thought to be inversely related to attraction of host-seeking diurnal flies, including black flies [41] and tabanids [42]. The number of simuliids orienting and landing on silhouette traps of various reflectances was found to be greater when reflectance was lowest [41]. Tabanid traps treated with a product that reduced ultraviolet reflectance from cloth fabrics increased the catch of tabanids in canopy traps by 24% [42]. The optimized Esperanza window trap with clear acrylic panels (nearly eliminating UV reflectance [43]) collected significantly more females than the trap with glass panels, supporting the notion that spectral reflectance is inversely related to attractiveness of a visual stimulus to black flies. Though only a few hues (of the virtually limitless variety of colors) were evaluated in the current study, the results obtained here generally corroborate the findings of others – that blues and blacks are attractive to host-seeking simuliids [41].
Esperanza window traps, baited with olfactory attractants and CO2, were nearly as productive as a vector collector team (2 adults working together) in some trials. Considerable variation in trap effectiveness might be expected, based on site-specific conditions. Esperanza traps should therefore be evaluated at other locations with O. volvulus vectors to determine their effectiveness relative to human landing collections. However, it is probably not necessary that an effective trap be as productive as a vector collection team, since a single person can easily maintain five traps (data not shown). By deploying multiple traps, a person working alone can increase daily fly captures by at least threefold over those obtainable using standard collection methods.
While the data presented above suggest that the Esperanza window trap shows promise as a substitute for human landing collections, additional work will be necessary before they can be employed for monitoring and surveillance of onchocerciasis elimination. First, the bait used in the traps must be standardized and optimized. The BG-lure, which is a commercial product, successfully attracted S. ochraceum in fairly large numbers. However, the BG-lure, which employs ammonia, lactic acid, and caproic acid as active ingredients [44], was designed and optimized to attract Aedes spp. mosquitoes [45]. It is likely that this mixture of compounds was not an optimal attractant for black flies and that inclusion or substitution of other compounds found in human breath and sweat will result in an improved bait formulation.
Second, the models used to identify transmission thresholds for O. volvulus have all relied upon entomological indicators derived from human landing collections. Thus, it will be necessary develop algorithms to relate the trap collections to human biting rates and infective rates. In this regard, the fact that the traps appear to collect fly populations that exhibit parity rates which are not significantly different from those obtained by human landing collections carried out in parallel will simplify this process. Previous studies that have examined variability of infection rates associated with human landing collections in S. ochraceum s.l. [46] may prove to be a useful foundation in developing methods to relate trap collections to infective biting rates.
In conclusion, the Esperanza window trap, when baited with CO2 and olfactory lures collected substantial numbers of S. ochraceum s.l., one of the principal vectors of O. volvulus in Latin America. This trap has the potential for replacing humans as bait in the monitoring and surveillance of onchocerciasis vectors. Eventually, such a trap may prove to be an effective replacement for vector collectors in areas of Latin America and Africa where onchocerciasis remains endemic. These traps have the potential to be extremely useful tools in aiding in the certification of elimination of O. volvulus transmission and in the post-treatment surveillance era.
Supporting Information
Overhead view of a field plot: The photo includes a portion of one of the plots used to evaluate the trap types. A Bellec plaque and Esperanza window trap are visible.
(TIF)
Acknowledgments
The authors thank the residents of La Esperanza, Chiapas, Mexico for their support during these studies. We deeply appreciate the assistance of the individuals that assisted with the fieldwork: Rafael Vázquez-Sánchez (Centro Regional de Investigación en Salud Pública, Instituto Nacional de Salud Pública), Lizbeth and Emanuel Bustamante-Pérez (Laboratorio Estatal de Salud Pública de Oaxaca, Secretaría de Salud).
Funding Statement
Financial support for this work was provided by The Bill and Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Prost A (1986) The burden of blindness in adult males in the savanna villages of West Africa exposed to onchocerciasis. Trans R Soc Trop Med Hyg 80: 525-527. doi: 10.1016/0035-9203(86)90129-X. PubMed: 3810784. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (1995) Onchocerciasis and its control. WHO Expert Committee. Geneva: World Health Organization; Report #852 [Google Scholar]
- 3. Murdoch ME, Asuzu MC, Hagan M, Makunde WH, Ngoumou P et al. (2002) Onchocerciasis: the clinical and epidemiological burden of skin disease in Africa. Ann Trop Med Parasitol 96: 283-296. doi: 10.1179/000349802125000826. PubMed: 12061975. [DOI] [PubMed] [Google Scholar]
- 4. Mathers CD, Ezzati M, Lopez AD (2007) Measuring the burden of neglected tropical diseases: The global burden of disease framework. PLoS Negl Trop. Drosophila Inf Serv 1: e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sauerbrey M (2008) The Onchocerciasis Elimination Program for the Americas (OEPA). Ann Trop Med Parasitol 102 Suppl 1: 25-29. doi: 10.1179/136485908X337454. PubMed: 18718151. [DOI] [PubMed] [Google Scholar]
- 6. Lindblade KA, Arana B, Zea-Flores G, Rizzo N, Porter CH et al. (2007) Elimination of Onchocerca volvulus transmission in the Santa Rosa focus of Guatemala. Am J Trop Med Hyg 77: 334-341. PubMed: 17690408. [PubMed] [Google Scholar]
- 7. Rodríguez-Pérez MA, Lizarazo-Ortega C, Hassan HK, Domínguez-Vásquez A, Méndez-Galván J et al. (2008) Evidence for suppression of Onchocerca volvulus transmission in the Oaxaca focus in Mexico. Am J Trop Med Hyg 78: 147-152. PubMed: 18187798. [PubMed] [Google Scholar]
- 8. Rodríguez-Pérez MA, Lutzow-Steiner MA, Segura-Cabrera A, Lizarazo-Ortega C, Domínguez-Vázquez A et al. (2008) Rapid suppression of Onchocerca volvulus transmission in two communities of the Southern Chiapas focus, Mexico, achieved by quarterly treatments with Mectizan. Am J Trop Med Hyg 79: 239-244. PubMed: 18689630. [PMC free article] [PubMed] [Google Scholar]
- 9. Diawara L, Traore MO, Badji A, Bissan Y, Doumbia K et al. (2009) Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: First evidence from studies in Mali and Senegal. PLOS Neg Trop. Drosophila Inf Serv 3: e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katabarwa MN, Walsh F, Habomugisha P, Lakwo TL, Agunyo S et al. (2012) Transmission of onchocerciasis in Wadelai focus of Northwestern Uganda has been interrupted and the disease eliminated. J Parasitol Res, 2012: 2012: 748540. PubMed: 22970347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tekle AH, Elhassan E, Isiyaku S, Amazigo UV, Bush S et al. (2012) Impact of long-term treatment of onchocerciasis with ivermectin in Kaduna State, Nigeria: First evidence of the potential for elimination in the operational area of the African Programme for Onchocerciasis Control. Parasit Vect 5: 28. doi: 10.1186/1756-3305-5-28. PubMed: 22313631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Traore MO, Sarr MD, Badji A, Bissan Y, Diawara L et al. (2012) Proof-of-principle of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: Final results of a study in Mali and Senegal. PLOS Neg Trop. Drosophila Inf Serv 6: e1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization (2001) Criteria for Certification of Interruption of Transmission/Elimination of Human Onchocerciasis. Geneva: World Health Organization; p. 64 Report WHO/CDS/CPE/CEE/2001.18a [Google Scholar]
- 14. Program Coordination Committee of the Onchocerciasis Elimination. Program for the Americas (2012) Guide to detecting potential recrudescence of onchocerciasis during the posttreatment period: The American paradigm. Res Rep Trop Med 3: 21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basáñez MG, Razali K, Renz A, Kelly D (2007) Density-dependent host choice by disease vectors: epidemiological implications of the ideal free distribution. Trans R Soc Trop Med Hyg 101: 256-269. doi: 10.1016/j.trstmh.2006.08.009. PubMed: 17112556. [DOI] [PubMed] [Google Scholar]
- 16. Walsh JF, Davies JB, [!(surname)!], Grams R (1978) Standardization of criteria for assessing the effects of Simulium control in onchocerciasis control programs. Trans R Soc Trop Med Hyg 72: 675-676. doi: 10.1016/0035-9203(78)90039-1. PubMed: 734734. [DOI] [PubMed] [Google Scholar]
- 17. Davies JB, Seketeli A, Walsh JF, Barro T, Sawadogo R (1981) Studies on biting Simulium damnosum s.l. at a breeding site in the Onchocerciasis Control Programme area during and after an interruption of insecticidal treatments. Trop Med Parasitol 32: 17-24. [PubMed] [Google Scholar]
- 18. Porter CH, Collins RC (1988) Seasonality of adult black flies and Onchocerca volvulus transmission in Guatemala. Am J Trop Med Hyg 38: 153-167. PubMed: 3341518. [DOI] [PubMed] [Google Scholar]
- 19. Jacobi CA, Enyong P, Renz A (2010) Individual exposure to Simulium bites and intensity of Onchocerca volvulus infection. Parasit Vect 3: 53. doi: 10.1186/1756-3305-3-53. PubMed: 20565835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cupp EW, Sauerbrey M, Richards F (2011) Elimination of human onchocerciasis: History of progress and current feasibility using ivermectin Mectizan monotherapy. Acta Trop 120 Suppl 1: S100-S108. doi: 10.1016/j.actatropica.2010.08.009. PubMed: 20801094. [DOI] [PubMed] [Google Scholar]
- 21. Bellec C (1976) Captures d’adultes de Simulium damnosum Theobald, 1903 (Diptera,Simuliidae) á l’aide de plaques d’aluminum , en Afrique de l’Ouest. Cahiers ORSTOM sèrie Entomologie Mèdicale et Parasitologie 14: 209-217. [Google Scholar]
- 22. Bellec C (1974) Les methodes d’echantillonnage des populations adultes de Simulium damnosum Theobald, 1903 (Diptera: Simuliidae) en Afrique de L’Ouest: Universite de Paris-Sud. 237 p.
- 23. Thompson BH (1976) Studies on the attraction of Simulium damnosum s.l. (Diptera: Simuliidae) to its hosts. I. The relative importance of sight, exhaled breath, and smell. Tropenmed Parasitol 27: 455-473. PubMed: 1006802. [PubMed] [Google Scholar]
- 24. Thompson BH (1977) Studies on the attraction of Simulium damnosum s.l. (Diptera: Simuliidae) to its hosts. III. Experiments with animal-baited traps. Tropenmed Parasitol 28: 226-228. PubMed: 888186. [PubMed] [Google Scholar]
- 25. Thompson BH (1977) Studies on the attraction of Simulium damnosum s.l. (Diptera: Simuliidae) to its hosts. II. The nature of substances on the human skin responsible for atrractant olfactory stimuli. Tropenmed Parasitol 28: 83-90. PubMed: 871039. [PubMed] [Google Scholar]
- 26. Cupp EW, Collins RC (1979) The gonotrophic cycle in Simulium ochraceum . Am J Trop Med Hyg 28: 422-426. PubMed: 110158. [DOI] [PubMed] [Google Scholar]
- 27. Porter CH, Collins RC (1988) Biting activity of black flies in Guatemala: parity rates and differences between localities and habitats. Am J Trop Med Hyg 38: 142-152. PubMed: 3341517. [DOI] [PubMed] [Google Scholar]
- 28. Porter CH, Collins RC, Brandling-Bennett AD (1988) Vector density, parasite prevalence, and transmission of Onchocerca volvulus in Guatemala. Am J Trop Med Hyg 39: 567-574. PubMed: 3207177. [DOI] [PubMed] [Google Scholar]
- 29. Crosskey RW (1990) The Natural History of Blackflies. Chichester: John Wiley and Sons. [Google Scholar]
- 30. Rodríguez-Pérez M, Domínguez-Vázquez A, Unnasch TR, Hassan HK, Arredondo-Jiménez JI et al. (2013) Interruption of transmission of Onchocerca volvulus in the Southern Chiapas focus, México. PLOS Neg Trop. Drosophila Inf Serv 7: e2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jonsson E, Gardarsson A, Gislason G (1986) A new window trap used in the assessment of the flight periods of Chironomidae and Simulium (Diptera). Freshw Biol 16: 711-791. doi: 10.1111/j.1365-2427.1986.tb01012.x. [DOI] [Google Scholar]
- 32. Collins RC, Merino ME, Cupp EW (1981) Seasonal trends and diurnal patterns of man-biting activity of four species of Guatemalan black flies (Simuliidae). Am J Trop Med Hyg 30: 728-733. PubMed: 7196162. [DOI] [PubMed] [Google Scholar]
- 33. Smallegange RC, Schmied WH, van Roey KJ, Verhulst NO, Spitzen J et al. (2010) Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae . Malar J 9: 292. doi: 10.1186/1475-2875-9-292. PubMed: 20973963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guerenstein PG, Lorenzo MG, Núñez JA, Lazzari CR (1995) Baker’s yeast, an attractant for baiting traps for Chagas’ disease vectors. Experientia 51: 834-837. doi: 10.1007/BF01922439. PubMed: 7649243. [DOI] [PubMed] [Google Scholar]
- 35. Dar SC, Bhuyan M, Das NG (1984) Attraction of Simuliidae to different colors on human field trial. Mosq News 44: 79-80. [Google Scholar]
- 36. Walsh JF (1980) Sticky trap studies on Simulium damnosum s.l. in northern Ghana. Tropenmed Parasitol 31: 479-486. PubMed: 7233544. [PubMed] [Google Scholar]
- 37. Green CH (1980) The effect of colour in trap- and screen-oriented responses in Glossina palpalis palpalis (Robineau-Desvoidy)(Diptera: Glossinidae). Bull Entomol Res 78: 591-604. [Google Scholar]
- 38. Brightwell R, Dransfield RD, Kyorku C (1991) Development of a low-cost tsetse trap and odour baits for Glossina pallidipes and G. longipennis in Kenya. Med Vet Entomol 5: 153-164. doi: 10.1111/j.1365-2915.1991.tb00536.x. PubMed: 1768909. [DOI] [PubMed] [Google Scholar]
- 39. Kriska G, Bernáth B, Farkas R, Horváth G (2009) Degrees of polarization of reflected light eliciting polarotaxis in dragonflies (Odonata), mayflies (Ephemeroptera) and tabanid flies (Tabanidae). J Insect Physiol 55: 1167–1173. doi: 10.1016/j.jinsphys.2009.08.013. PubMed: 19699746. [DOI] [PubMed] [Google Scholar]
- 40. Egri A, Blahó M, Kriska G, Farkas R, Gyurkovszky M et al. (2012) Polarotactic tabanids find striped patterns with brightness and/or polarization modulation least attractive: an advantage of zebra stripes. J Exp Biol 215: 736-745. doi: 10.1242/jeb.065540. PubMed: 22323196. [DOI] [PubMed] [Google Scholar]
- 41. Bradbury WC, Bennett GF (1974) Behavior of adult Simuliidae (Diptera). I. Response to color and shape. Can J Zool 52: 251-259. doi: 10.1139/z74-030. [DOI] [PubMed] [Google Scholar]
- 42. Hribar LJ, [!(surname)!], Foil LD (1991) Increasing horse fly (Diptera: Tabanidae) catch in canopy traps by reducing ultraviolet light reflectance. J Med Entomol 28: 874-877. PubMed: 1770525. [DOI] [PubMed] [Google Scholar]
- 43. Dislich H (1979) Plastics as optical materials. Angew Chem Int Ed Engl 18: 49-59. doi: 10.1002/anie.197900491. [DOI] [Google Scholar]
- 44. Biogents (2008) Material Safety Data Sheet - BG Lure. Biogents. Regensburg. [Google Scholar]
- 45. Biogents (2013) BG-Lure: Mosquito lure for the professional monitoring of mosquitoes. Retrieved onpublished at whilst December year 1111 from http://www.mosquitotraps.eu/BG-Lure/en.
- 46. Basáñez MG, Rodríguez-Pérez MA, Reyes-Villanueva F, Collins RC, Rodríguez MH (1998) Determination of sample sizes for the estimation of Onchocerca volvulus (Filarioidea: Onchocercidae) infection rates in biting populations of Simulium ochraceum s.l. (Diptera: Simuliidae) and its application to ivermectin control programs. J Med Entomol 35: 745-757. PubMed: 9775604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overhead view of a field plot: The photo includes a portion of one of the plots used to evaluate the trap types. A Bellec plaque and Esperanza window trap are visible.
(TIF)