Summary
Purpose
High-frequency oscillations (HFOs) known as ripples (80–250 Hz) and fast ripples (250–500 Hz) can be recorded from macroelectrodes inserted in patients with intractable focal epilepsy. They are most likely linked to epileptogenesis and have been found in the seizure onset zone (SOZ) of human ictal and interictal recordings. HFOs occur frequently at the time of interictal spikes, but were also found independently. This study analyses the relationship between spikes and HFOs and the occurrence of HFOs in nonspiking channels.
Methods
Intracerebral EEGs of 10 patients with intractable focal epilepsy were studied using macroelectrodes. Rates of HFOs within and outside spikes, the overlap between events, event durations, and the percentage of spikes carrying HFOs were calculated and compared according to anatomical localization, spiking activity, and relationship to the SOZ.
Results
HFOs were found in all patients, significantly more within mesial temporal lobe structures than in neocortex. HFOs could be seen in spiking as well as nonspiking channels in all structures. Rates and durations of HFOs were significantly higher in the SOZ than outside. It was possible to establish a rate of HFOs to identify the SOZ with better sensitivity and specificity than with the rate of spikes.
Discussion
HFOs occurred to a large extent independently of spikes. They are most frequent in mesial temporal structures. They are prominent in the SOZ and provide additional information on epileptogenicity independently of spikes. It was possible to identify the SOZ with a high specificity by looking at only 10 min of HFO activity.
Keywords: Epilepsy, High-frequency oscillations, Spikes, Seizure onset zone, Intracranial electrodes
In many patients with epilepsy, the area responsible for seizure generation, or the seizure onset zone (SOZ), may be difficult to define. Even in patients with identifiable lesions on brain MR imaging, noncongruent clinical and laboratory studies often indicate poor localization of the SOZ, which precludes a surgical approach in those patients with refractory epilepsy. Invasive intracranial EEG evaluation may help in those cases to finally obtain a good definition of the SOZ (Diehl & Lueders, 2000). These methods, however, do not always result in the finding of one clear SOZ, as seizures might be originating from more than one brain area and also because intracranial techniques are always spatially limited. For instance, seizures originating from areas not covered by electrodes but propagating to the actual electrode positions might lead to misinterpretation.
Additionally, interictal epileptiform discharges (IED) or spikes, which define the irritative zone (IZ), might be seen in several electrode positions outside the SOZ; it remains unclear how these discharges are related to epileptogenesis (Hufnagel et al., 2000; Rosenow & Lueders, 2000) and how much importance one should put on them to define the epileptogenic area. It is known that patients with spikes generated in multiple brain areas are less likely to become seizure free after surgery than patients with well-localized spikes (Bautista et al., 1999). This apparent multiplicity of epileptic generators may represent widespread disease or might be related to secondary epileptogenesis (Rosenow & Lueders, 2000). Therefore, new measurements for epileptogenicity are required additionally to spikes and the SOZ in stereo EEG (SEEG).
Traditionally, EEG oscillations are believed to be relevant up to frequencies in the gamma band (40–100 Hz) but recent findings suggest that high-frequency oscillations (HFOs) ranging between 100 and 500 Hz might be closely linked to epileptogenesis. They were studied in rodents as well as in humans using microwires (with a surface contact of 70 μm2) (Bragin et al., 1999; Staba et al., 2002) and more recently detected also in humans by using macroelecrode contacts, with a surface contact of 0.8 mm2 (Jirsch et al., 2006; Urrestarazu et al., 2006, 2007).
HFOs ranging from 100 to 250 Hz and described as ripples were seen in the hippocampus (Hc) and entorhinal cortex of normal rats (Chrobak & Buzsaki, 1996; Chrobak et al., 2000). In humans, similar oscillations generally showed a lower frequency range, between 80 and 160 Hz, and were found within epileptic tissue and outside in the less affected Hc of epileptic patients studied with bilateral temporal lobe intracerebral depth electrodes (Bragin et al., 1999; Staba et al., 2004). It was hypothesized that they are physiological rhythms closely linked to memory consolidation (Draguhn et al., 2000), but additional “pathological” ripples could be observed as well (Bragin et al., 2004). The differentiation between pathological and physiological events remains unclear (Le Van Quyen et al., 2006). HFOs between 250 and 500 Hz, called fast ripples, have been recorded from normal rodent and human brains (Curio et al., 1994, 1997; Jones et al., 2000; Ikeda et al., 2005). It was hypothesized that they are related to somatosensory stimulation and sensory information processing (Curio et al., 1997; Gobbele et al., 2004). Nevertheless, fast ripples in mesial temporal structures were more clearly linked to epileptogenesis than ripples (Staba et al., 2002).
Ripples and fast ripples occur frequently during IEDs and may reflect pathological hypersynchronous events. In general, these HFOs are seen more frequently during non-rapid-eye movement sleep (NREM) sleep compared to rapid-eye movement sleep (REM) sleep or wakefulness (Staba et al., 2004). Recently, it has been shown that HFOs may not exclusively be recorded from microwires but also from intracranial macroelectrodes (Jirsch et al., 2006; Urrestarazu et al., 2006). During ictal recordings, HFOs could be identified and occurred mostly in the region of primary epileptogenesis and less frequently in areas of secondary spread (Jirsch et al., 2006).
Analysis of interictal recordings showed a clear relation between spikes and fast ripples and again a close relation between HFOs and the SOZ (Urrestarazu et al., 2007). While most fast ripples were noted during spikes, others could be found independently. Fast ripples were more restricted to the presumed SOZ than ripples. In the studies with microwires, the relationship between HFOs and spikes was not specifically addressed but most examples of fast ripples show themoccurring during spikes.
The exact relationship between interictal discharges and HFOs, so far, remains unclear. All previous studies focused on channels that showed spiking activity in the first place. It is therefore unknown whether HFOs in general are produced by the same generators as IEDs or derive from independent structures. HFOs have never been measured in interictally inactive channels of epileptic patients, and their presence in these channels might provide additional information on epileptogenesis. Furthermore, HFOs occurring within spiking channels but independently of spikes need to be investigated more closely to gain information about the value of HFOs for the identification of the SOZ independently of spikes.
In this study we analyzed HFOs in spiking and nonspiking channels, looking at interictal intracerebral SEEG recordings of 10 patients with lesional focal epilepsy. Differences between HFOs occurring at the same time or independent of spikes, as well as the exact relationship between spikes and HFOs were investigated. We also compared the capacity of the limbic and neocortical (Nc) structures to generate fast oscillations, and, finally, we determined if HFOs could help identify the SOZ. We hypothesize that HFOs occur to a large extent independently of spikes and can add additional localizing information to the SEEG investigation.
Methods
Patient selection
Between September 2004 and June 2007, 37 patients underwent intracranial electrode implantation in the epilepsy unit of the Montreal Neurological Hospital. The decision for invasive EEG studies was undertaken individually when no clear area of seizure onset could be determined with extensive noninvasive evaluation. After informed consent, electrode placements were tailored according to the clinical history, seizure semiology and results of surface EEG investigation, neuroimaging, and neuropsychological testing. The clinical intracranial recordings were interpreted independently of this study by an experienced neurophysiologist, who also determined which channels were the sites of seizure onset. We selected patients with lesional focal epilepsies, spiking and nonspiking channels, one or at most two clear SOZs and electrode contacts placed clearly within and outside SOZs. Additionally, at least one night of good sleep recording was required. Ten patients fulfilled these criteria: in five patients, the seizure onset was in the mesial temporal lobe structures; in two, in the temporal neocortex; and in three, in other Nc areas. In two patients, two different SOZs could be identified; in all others all seizures originated from the same well-localized area.
This study was approved by the Montreal Neurological Institute and Hospital Research Ethics Committee and all patients signed an informed consent form.
Recording methods
Electrodes were implanted stereotactically using an image-guidance system (SSN Neuronavigation System, Mississauga, Ontario, Canada). A combination of depth and cortical surface electrodes was placed according to the methods of Olivier et al. (1994). All electrodes were manufactured onsite. A 10/1,000-inch wire of stainless steel was used as a central core and wrapped with a 3/1,000 inch steel wire. Each electrode had nine contacts, with the deepest contact (contact 1) consisting of the tip of the steel core stripped of insulation. This contact had a length of 1 mm, while all other contacts (2–9) were formed from stripped sections of the marginal wire that was tightly wound to create 0.5 mm long coils. The effective surface area was 0.80 mm2 for contact 1 and 0.85 mm2 for contacts 2 to 9. The localization of the electrodes is listed in Table 1.
Table 1.
Clinical data and implantation sites
| Patient #/age/gender | Seizure semiology | MRI | No. of electrodes (in brackets) and implantation sites |
|---|---|---|---|
| 1/44/m | CPS, automatisms, posturing of the right arm | L Hc atrophy | (6) Am, Hc and paraHc, L and R |
| 2/49/f | CPS, epigastric aura, cloni of the left face and arm | R Hc atrophy | (6) Am, Hc and paraHc, L and R |
| 3/45/m | CPS, oro-facial automatisms, bilateral hand movements | L T pole atrophy | (5) Am, Hc, paraHc, TL pole, and OF |
| 4/54/f | CPS, epigastric aura, left hand numbness, LOC. | R Hc atrophy and discrete R post. fusiform gyrus posttraumatic contusion |
(5) Am, Hc, paraHc, fusiform gyrus, and temporooccipital junction |
| 5/46/m | CPS, LOC, oral automatisms, agitation | R Hc malrotation and atrophy | (4) Am and Hc, L and R |
| 6/24/m | CPS, LOC, automatisms, urinary incontinence | Two nodular heterotopia, R trigone | (5) Am, Hc, paraHc, and two electrodes aiming at the nodules; AN and PN |
| 7/25/m | CPS, LOC, manual, and oral automatisms | Bil. Temporooccipital periventricular heterotopia. | (9) Am and Hc, L and R; paraHc, R; and four electrodes aiming at the nodules in the trigone and occipital lobe, L and R |
| 8/33/m | CPS, laughter, bilateral hand automatisms, shaking of the head | Tuberous sclerosis complex, 4 tuber in L OF, L ant T, R F and R P | (8) Am and Hc, L and R; AC and OF, L and R |
| 9/28/f | CPS, hypermotor seizures with urinary incontinence, mostly during sleep | R OF and ant. insular cortex FCD | (7) AC, midC and OF, L and R; ant. Insula, R |
| 10/36/m | CPS, rocking movement of the upper body and vocalization, mostly during sleep | L second F gyrus FCD | (7) Ant. and post. SMA, 2nd F gyrus, L and R; AC, L |
Am, amygdala; AC, anterior cingulate; bil., bilateral; CPS, complex partial seizure; F, frontal; f, female; FCD, Focal cortical dysplasia; Hc, Hippocampus, L, left, LOC, loss of contact; m, male; mesial TL, mesial temporal lobe; midC, mid cingulate gyrus; OF, orbitofrontal; R, right; SMA, supplementary motor area; T, temporal.
SEEGs were recorded using the Harmonie monitoring system (Stellate, Montreal, Quebec, Canada). The SEEG was low-pass filtered at 500 Hz and sampled at 2,000 Hz We also recorded the electrooculogram(EOG) and electromyogram (EMG) to allow sleep staging. The analyses reported below were performed using a referential montage with an epidural reference electrode placed in the parietal lobe of the hemisphere least likely to include the main focus.
Channel selection and sampling
HFOs and spikes occur more frequently during slow-wave sleep (Staba et al., 2004) and we therefore decided to analyze samples of 10 min of slow-wave sleep. Sleep stages were selected using EEG and the information of EOG and EMG. We did not select the stages according to the criteria of Rechtschaffen and Kales, as we had no scalp electrodes recording from the central areas. Such electrodes would have to be placed under the sterile bandage after implantation, increasing the risk of infection. The Harmonie software was used to compute spectral trends in the delta, alpha, and beta bands for the intracranial EEG and power of the chin EMG with a 30-s time resolution. EEG sections with high delta and low EMG power were visually reviewed to confirm the sleep stages. Slow wave sleep was defined by at least 25% delta activity by visual inspection of 30 s epochs. Additionally, segments were only selected if they were recorded at least 6 h before and after a seizure, to reduce the influence of seizures on our analysis.
A maximum of 14 spiking and nonspiking channels was analyzed per patient. During the visual review of the data, we found that a maximum of seven channels could be displayed simultaneously on the computer monitor in order to maintain a good screen resolution with the very high gains needed to see the HFOs. Fourteen channels could therefore be marked in two review sessions. Manual marking of the HFOs was time-consuming, taking up to 15 h for a 10-min segment of SEEG (Urrestarazu et al., 2007). In all patients, we tried to select equal numbers of spiking and nonspiking and SOZ and non-SOZ channels. The nonspiking channels did not show spikes in the selected 10-min segment or in the rest of the recording, while the spiking channels had spikes inside as well as outside of the 10-min segment. This resulted in 7 to 14 channels per patient, depending on the SOZs in each individual patient. Electrode and contact selection was guided by the images obtained from the neuronavigation system and only channels clearly located within gray matter were selected. Finally, if several channels were involved in the same type of spike, the channel in which the spike had the largest amplitude was chosen. A detailed list of all selected channels can be found in Tables 1 and 2.
Table 2.
Overview of the analyzed channels in each patient
| Patient # | Electrodes of SOZ | Selected SOZ channels | Total # of selected channels
|
# of channels with ripples
|
# of channels with fast ripples
|
|||
|---|---|---|---|---|---|---|---|---|
| Spiking | Nonspiking | Spiking | Nonspiking | Spiking | Nonspiking | |||
| 1 | L-HC 1–2 | L-HC 1 | 5/7 | 2/7 | 5/5 | 2/2 | 5/5 | 0/2 |
| L-A 1–2 | L-A 1 | |||||||
| 2 | R-PHC 1–2 | R-PHC 1 | 5/7 | 2/7 | 5/5 | 1/2 | 4/5 | 1/2 |
| R-HC 1–2 | R-HC 1 | |||||||
| L-HC 1–2 | LP-3 | |||||||
| L-PHC 1–2 | ||||||||
| 3 | L-A 1–2 | L-A 1 | 5/7 | 2/7 | 5/5 | 2/2 | 5/5 | 1/2 |
| L-HC 1–2 | L-HC 1 | |||||||
| 4 | R-HC 1–2 | R-PHC1 | 5/8 | 3/8 | 5/5 | 1/3 | 3/5 | 1/3 |
| R-A 1–2 | R-HC1 | |||||||
| R-PHC 1–2 | R-A1 | |||||||
| 5 | R-A 1–2 | R-A1 | 6/7 | 1/7 | 6/6 | 0/1 | 4/6 | 0/1 |
| R-HC 1–3 | R-HC1-3 | |||||||
| 6 | R-A 1–2 | R-A1 | 5/7 | 2/7 | 5/5 | 2/2 | 5/5 | 2/2 |
| R-HC 1–2 | RHC-1 | |||||||
| 7 | R-HC 1–2 | R-HC1 | 10/13 | 3/13 | 7/10 | 2/3 | 6/10 | 1/3 |
| R-A 1–2 | R-A 1 | |||||||
| L-HC 1–2 | L-HC 1 & 2 | |||||||
| L-A 1–2 | L-A1 | |||||||
| 8 | L-OF 1–4 | L-OF 1 | 5/8 | 3/8 | 5/5 | 2/3 | 5/5 | 3/3 |
| L-OF 4 | ||||||||
| 9 | R-OF 6–7 | R-OF 6 & 7 | 6/8 | 2/8 | 6/6 | 0/2 | 6/6 | 2/2 |
| 10 | L-Le 1–5 | L-Le 1 & 3 & 5 | 5/9 | 4/9 | 4/5 | 2/4 | 4/5 | 1/4 |
The number behind the electrode name indicates the contact (channel) that was used for the analysis. (1 being the innermost and 9 the most peripheral contact of the electrode.) Printed in bold letters is the predominant SOZ in patients with multiple SOZs.
A, Amygdala; Hc, Hippocampus; L, Left; Le, Lesion; OF, orbitofrontal; PHC, Parahippocampus; R, Right; SOZ, seizure onset zone.
Marking of interictal events
In a first step, all spikes and spike-and-slow-waves were visually identified within the selected sample. Spikes were defined as interictal epileptic discharges with a sharp component and a duration less than 70 ms. Slow waves were defined as the slow wave immediately following a spike and they were marked when present. The entire duration of the spikes and slow waves were marked. All EEG segments were reviewed twice to ensure that events were not missed. The EEG was displayed at a common time scale (10 s/page). The markers were then saved and made invisible to ensure that the marking of HFOs were not biased by the knowledge of spike localization. If we refer to HFOs in the subsequent manuscript we always refer to ripples or fast ripples or both, without differentiation. Channels were displayed with the maximum time resolution to visualize HFOs, which corresponded to approximately 0.6 s across the computer monitor (1200 samples of a signal sampled at 2,000 Hz). The EEG was high-pass filtered at 80 and 250 Hz using a finite impulse response (FIR) filter to eliminate ringing. The computer display was split vertically with an 80 Hz high-pass filter on one side and a 250 Hz high-pass filter on the other side. A ripple was marked if an event was clearly visible on the side of the 80 Hz filter and did not occur or show the same shape on the side of the 250 Hz filter, as it is defined as a distinct event between 80 and 250 Hz in frequency. An event was regarded as a fast ripple if it was only visible in the 250 Hz filter and therefore clearly had a frequency above 250 Hz. If ripples and fast ripples occurred at the same time they had to have different shapes to be regarded as separate events (Fig. 1). HFOs were visually identified and the duration of each event was marked. Only events containing at least four consecutive oscillations were regarded as HFOs. When the EEG is filtered with these characteristics, it is not possible to identify the presence of spikes (Figs. 2, 3, and4).
Figure 1.
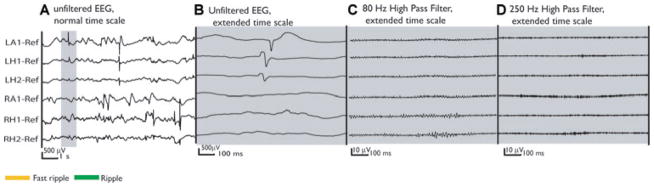
Patient 5 presented with interictal and ictal signs of bitemporal epilepsy on scalp EEG. He was implanted bitemporally aiming at the amygdala (LA, RA) and hippocampus (LH, RH). The MRI revealed a malrotation of the right hippocampus. All seizures originated from the right mesial temporal structures, where the highest rates of HFOs were found. (A) The unfiltered EEG shows frequent spikes in both mesial TL structures. The thin gray section in A is expanded in time and amplitude in B, C, and D demonstrating the cooccurrence of ripples and fast ripples outside spikes in channel RH1. Additionally, a ripple is seen in channel RH2. Note that C and D have a much higher gain than A and B.
Epilepsia © ILAE
Figure 2.
Same unfiltered EEG as in Fig. 1 (A). This time the selection includes two spikes over LA1 and LH1. Both spikes lied outside of the seizure onset zone and in the healthier mesial temporal lobe. The expanded sections of the unfiltered EEG (B) and filtered EEG (C, D) show no high-frequency oscillation during this time period.
Epilepsia © ILAE
Figure 3.
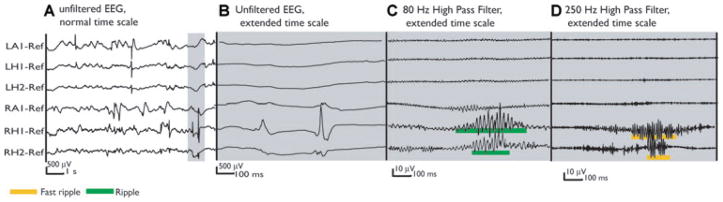
Same unfiltered EEG segment as in Figs. 1(A) and 2 (A). The gray selection shows two spikes recorded simultaneously at RH1 and RH2 in the SOZ. No oscillation is visible in the spike at RH1 in the extended unfiltered EEG (B), while the spike in RH2 shows a very short and small oscillation. The filtered EEG segments clearly reveal a ripple and fast ripple oscillation during these spikes (C, D). Compared with Fig. 2, this demonstrates that spikes which look similar in the unfiltered EEG might differ in whether they carry high-frequency oscillations or not. This difference is only visible after filtering.
Epilepsia © ILAE
Figure 4.
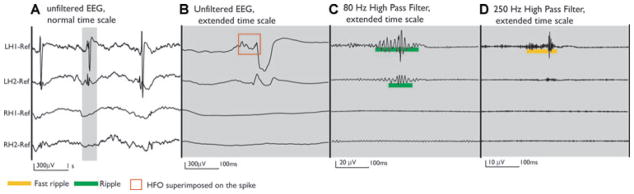
Patient 1 was implanted bilaterally in the mesial temporal structures. Bilateral interictal spikes were observed, but all recorded seizures started in the left mesial temporal structures. The unfiltered EEG shows three spikes over LH1 and LH2 (A). The selected gray segment shows the extended unfiltered EEG of one spike, which in LH1 showed a clear oscillation riding on the spike (B). When the EEG is filtered, ripples and fast ripples are seen at the time of the spike in LH1 and LH2 channels, but much more prominently at LH1 (C, D).
Epilepsia © ILAE
Statistical analyses
After marking all events, a MATLAB program calculated the cooccurrence of spikes and HFOs, rates per minute (computed for every 1-min interval in the data), the percentage of overlap between spikes and HFOs and the duration of HFOs. Spikes and HFOs were regarded as cooccurring if there was an overlap between these two events. This was independent of the duration or time sequence of both events. It is known that HFOs result to some extent from the high frequencies present in very sharp spikes (Urrestarazu et al., 2007). When this is the case, the filtered EEG typically shows a fast ripple cooccurring with a ripple and a spike. To prevent a bias introduced by this type of high-frequency activity, we specifically looked at the rates and durations of those fast ripples that cooccurred with a spike but not with a ripple. These fast ripples were regarded as true fast activity superimposed on a spike. Our focus in this study was the occurrence of HFOs independently of spikes, which was why we also calculated rates and durations for all HFOs that occurred outside spikes.
All analyzed channels in the 10 patients were grouped according to their anatomical localization, spiking activity, and relation to the SOZ. We then evaluated HFO activity by performing the following four comparisons: (1) spiking versus nonspiking (2) neocortex versus Hc versus amygdala (Am) (3) seizure onset versus non-SOZ and (4) SOZ/spiking versus SOZ/nonspiking versus non-SOZ/spiking versus non-SOZ/nonspiking channels. Rates and durations of HFOs, percentage of spike durations that overlap with HFOs, and the percentage of spikes and slow waves cooccurring with HFOs was compared between the different groups of channels using one way ANOVA. Statistical results were corrected, if necessary, for multiple comparisons and were analyzed with the post hoc Tukey’s honestly significant differences (HSD) Test. The level of significance was set at p < 0.05.
In order to assess if the rate of occurrence of HFOs could be used to distinguish between SOZ channels and non-SOZ channels, the distributions of the rates of HFOs in the SOZ channels and non-SOZ channels were plotted in histograms. To construct the histograms, all channels belonging to the SOZ were pooled and all those outside the SOZ were pooled, independently of patients. Histograms were obtained for each event type, spikes alone, spikes with ripples, spikes with fast ripples, ripples alone, and fast ripples alone. We evaluated the probability that a channel belonged to the SOZ given its event rate. First, thresholds were established to identify SOZ channels with 95% specificity for the entire group of patients. Then, sensitivities for the identification of SOZ channels were calculated on the group with those thresholds. We assumed the best predictor of the SOZ to be the rate with the highest sensitivity and specificity. The same thresholds were then used to calculate the sensitivities and specificities for each patient individually.
Results
In total, 81 channels were analyzed and 8,602 spikes, 3,119 slow waves, 10,530 ripples and 8,100 fast ripples were marked. All event types could be identified in all patients (Table 2). In channels with spikes, the mean rate of spikes was 10.6 ± 13.7/min and the mean rate of slow waves was 3.85 ± 5.6/min. In all but two patients, ripples were seen in more channels than fast ripples (R: 6.7 ± 0.9 vs. FR: 5.8 ± 1.5 channels). Their duration was also longer (R: 96.2 ± 45.5 vs. FR: 40.6 ± 26.7 ms). The mean rate of ripples per channel was 12.9 ± 11.2/min, and for fast ripples 9.9 ± 22.2/min. Fifty-five percent of the fast ripples occurred clearly outside of ripples. We will first present results on the cooccurrence of spikes and HFOs, then compare the occurrence of HFOs in spiking and nonspiking channels, and in different anatomical structures. Finally we will give results on relationships between HFOs and the SOZ.
Relationship between spikes and HFOs
Ripples and fast ripples cooccurred outside spikes as well as with spikes and slow waves.
Ripples
A total of 62.3% of all ripples occurred within spikes. The mean% overlap between spikes and ripples, indicating how much of the total spike time was co-occurring with a ripple, was 69.9 ± 24.7%, calculated on the spikes that included ripples. In general, the duration of ripples was significantly longer when cooccurring with spikes than when not (100.8 ± 42.5 ms vs. 93.4 ± 46.1 ms, p < 0.001).
Fast ripples
A total of 48% of all fast ripples occurred within spikes. The mean percentage overlap between spikes and fast ripples (31.2 ± 19.1%) was lower than for ripples. Fast ripples cooccurring with spikes were longer (38.8 ± 27.2 ms) than those occurring independently (34.4 ± 21.9 ms). Fig. 1 shows a typical example for ripples and fast ripples occurring together but independently of spikes.
Spikes and slow waves
Spikes were more likely to cooccur with ripples than with fast ripples. A total of 44.3% of spikes carried ripples and the rate of spikes with ripples was 4.6 ± 6.9/min. The number of spikes with fast ripples was lower, as only 27.3% of all spikes cooccurred with fast ripples. The rate of spike with fast ripples was 2.9 ± 5.9/min. Figs. 2–4 give examples of spikes that may or may not cooccur with HFOs. Sometimes the HFOs are already visible within a spike on the extended time scale and unfiltered EEG as shown in Fig. 4, and at other times HFOs within a spike are only visible after filtering (Fig. 3). If filtering of a spike resulted in the occurrence of ripples and fast ripples, fast ripples would cooccur with ripples, most of them in their center. It is therefore unlikely that a fast ripple that results only from the filtering operation would be found cooccurring with a spike without cooccurring with a ripple. We found 405 fast ripples within spikes but not overlapping with any ripple (mean rate of 0.5 ± 1.6/min).
HFOs were less often generated during slow waves. Only 36.4% of slow waves showed ripples and 10.4% fast ripples. The rate of slow waves with ripples per channel was 1.4 ± 2.97/min and with fast ripples 0.4 ± 1.1/min. In 85.3% of the ripples within slow waves and 67% of the fast ripples within slow waves, the HFOs had already started in the spike preceding the slow wave and these HFOs therefore did not occur independently in the slow wave. This resulted in a rate of independent ripples in slow waves of 0.18 ± 0.62/min and of independent fast ripples of 0.15 ± 0.64/min. Due to the very low occurrence of independent HFOs within slow waves, we decided not to analyze these events separately in the following statistical comparisons.
Comparison of spiking versus nonspiking channels
Spikes could be observed in 57 of 81 channels and slow waves in 41 channels. Twenty-nine of the spiking channels were within the SOZ. Ripples and fast ripples were seen in 81% and 61% of the spiking channels, respectively, and both were seen independently in 63% of the nonspiking channels.
Ripples
The rate of ripples in spiking channels (18.4 ± 19.6/min) was significantly higher than in nonspiking channels (2.3 ± 5.3/min) (F[1; 808]: 175.5, p < 0.001). Additionally, the duration of ripples was significantly longer in spiking channels (96.6 ± 45.3 ms) compared to nonspiking channels (90.2 ± 48.2 ms) (F [1; 11053]: 12, p < 0.001). These differences were also observed when looking only at the ripples outside spikes.
Fast ripples
Fast ripples showed a very similar pattern in regard to spiking and nonspiking channels. They were significantly more common in spiking than nonspiking channels (mean rate: 14.0 ± 26.4/min vs. 1.8 ± 4.6/min) (F [1; 808]: 57.2, p < 0.001) and their duration was longer in spiking than nonspiking channels (42.1 ± 26.9 ms vs. 32.4 ± 19.9 ms) (F [1; 8451]: 50.1, p < 0.001). These highly significant differences between spiking and nonspiking channels might be linked to the seizure onset channels, all of which were spiking. However, when comparing rates of ripples and fast ripples in spiking and nonspiking channels outside the SOZ (R: 9.6 ± 13/min and FR: 2.1 ± 4.8 vs. R: 2.3 ± 5.3/min and FR 1.8 ± 4.5) these differences were reduced but still present (R: F [2; 809]: 185, p < 0.001; FR: F [2; 809]: 120, p < 0.001). This will be further discussed later.
Comparison of anatomical structures
We analyzed 39 channels in temporal and extratemporal Nc structures, 29 in the Hc and 13 in the Am.
Ripples
Ripples were significantly more common in Am and Hc compared to Nc structures (mean rate: Am = 19.25 ± 21/min; Hc = 21.9 ± 20.8/min; and Nc = 4.3 ± 7.25/min) (F [2; 809]: 114, p < 0.001). The overlap between spikes and ripples was significantly higher in the Am compared to Hc and neocortex (Am = 77.7 ± 19.5%; Hc = 67.5 ± 25.7%; and Nc = 65.3 ± 26.4%)(F [2; 3962]: 79.5, p < 0.001). This again correlated well with ripple durations (F [2; 11052]: 214.7, p < 0.001), which were significantly higher within the Am (105.29 ± 50.21 ms) than in the Hc (97.53 ± 44.22 ms, p < 0.001) or Nc structures (77.5 ± 36.7 ms, p < 0.001). These differences remained similar when looking separately at rates and durations of ripples coinciding and not coinciding with spikes.
Fast ripples
Fast ripple rates were significantly different between the anatomical structures (F [2; 809]: 58.3, p < 0.001). Fast ripples were more frequent in the Hc than in the Am and neocortex (mean rate: Hc = 19.8 ± 33.2/min; Am = 10.8 ± 15.4/min; and Nc= 2.2 ± 4.5/min). The percentage of overlap between spikes and fast ripples was in general smaller than with ripples, but followed the same pattern, being higher in the Am(38.1 ± 18.1%) and Hc (37 ± 19.4%), with no significant difference between these structures, than in Nc areas (26.9 ± 14.8%) (F [2, 2479]: 15.1; p < 0.001). The durations of fast ripples, however, differed significantly between all anatomical structures (F [2, 8450]: 135.3; p < 0.001), being longer in the Hc compared to Am and neocortex, and longer in Am than in neocortex (Hc = 43.1 ± 28.9 ms; Am=38.2 ± 19.4ms; and Nc= 28.3 ± 16.4ms). These same differences persisted for rates and durations of fast ripples occurring and not occurring with spikes (Table 3). Typical HFOs for Nc structures are shown in Figs. 5 and 6.
Table 3.
Rates of the different types of events, comparing seizure onset channels (SOZ) with channels outside the seizure onset zone (NSOZ)
| Event type | Rate SOZ | Rate NSOZ | Rate threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Spikes | 20.4 ± 15.1 | 5.2 ± 9.1 | 24.4 | 33.4% | 95% |
| Spikes with ripples | 9.6 ± 7.5 | 1.8 ± 4.7 | 13.3 | 30.2% | 95% |
| Spikes with fast ripples | 7.3 ± 7.7 | 0.5 ± 1.9 | 3.9 | 54.5% | 95% |
| Ripples | 25.8 ± 21.5 | 5.8 ± 10.5 | 27.6 | 37.5% | 95% |
| Fast ripples | 24.3 ± 32.4 | 1.9 ± 4.7 | 11.4 | 52.5% | 95% |
The threshold rate indicates above which rate channels could be placed in the SOZ with 95% specificity. The best sensitivity was found for the rate of spikes with fast ripples and fast ripples alone.
Figure 5.
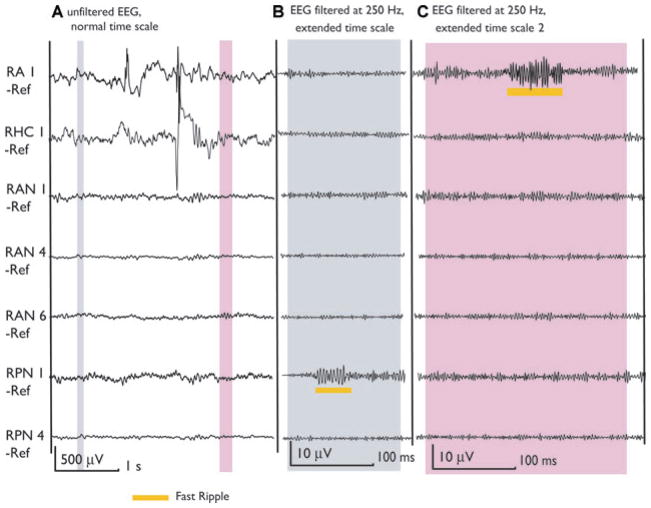
The MRI of patient 6 showed two heterotopic nodules located in the right trigonal area. Two contacts are inside these nodules (RAN1, RPN1). Ictal and interictal discharges were seen on the scalp over the right temporal lobe and the patient was implanted in the right hippocampus (RHC) and Amygdala (RA). Contacts RA1 and RHC1 were in the SOZ. This figure shows two selections (gray) of the unfiltered EEG. The first demonstrates a fast ripple outside spikes in a nonspiking channel (RPN1). This channel showed only fast ripples and no other epileptiform activity and none of the patient’s seizure was generated in this lesion. The second selection shows a fast ripple inside SOZ but outside a spike. It is important to notice that most of the HFOs in neocortical areas and outside spikes were shorter and of lower amplitude than those within spikes. This is visible when comparing with the HFOs in Fig. 1 inside the mesial temporal lobe or with the ones in Figs. 3 and 4 cooccurring with spikes.
Epilepsia © ILAE
Figure 6.
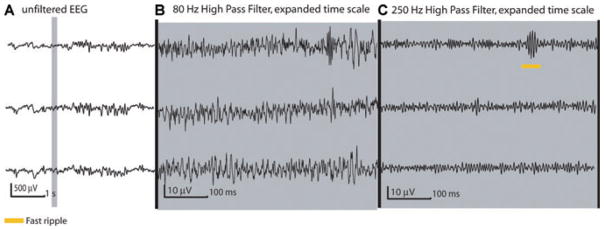
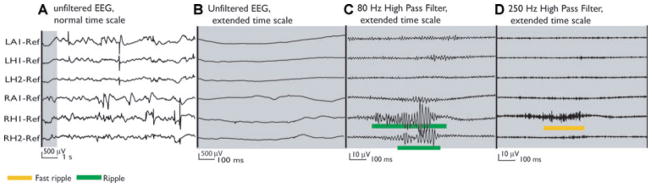
[Correction added after online publication July 11, 2008: The gain for the EEG traces in part B of Figure 6 was incorrect in the original publication. A corrected figure is provided above.] Patient 10 presented with frontal lobe seizures and a focal cortical dysplasia in the left second frontal gyrus. Electrode LS was implanted inside this area with contacts 1–5 in the lesion. All of these channels were spiking and we selected the three displayed in this figure for analysis. The selection shows a fast ripple (at LS1) occurring independently of spiking or ripple activity. Even though all of the patient’s seizures were generated within these channels, the rate of HFOs was generally low compared to that observed in the mesial temporal structures in other patients. As can be seen here the fast ripples were shorter and more discreet (compare with Fig. 1).
Epilepsia © ILAE
Spikes
The rate of spikes in general was significantly higher within the Am (14.7 ± 8.9/min) and the Hc (13.7 ± 8.1/min) than in Nc structures (3.1 ± 2.9/min) (F [1, 809]: 105.2; p < 0.001). Differences between Hc and Am were not significant. The percentage of spikes containing ripples and fast ripples was highest in the Am (R: 55.1 and FR: 48.4%) and Hc (54.3 and 42.9%) and lower in the Nc areas (45.1 and 22.9%).
Comparison of SOZ and non-SOZ
Twenty-nine channels within the SOZ were compared with 52 channels outside the SOZ. All channels within the SOZ were spiking channels whereas channels outside the SOZ included spiking and nonspiking channels. All differences remained similar when the data were analyzed separately for the different anatomical structures and therefore cannot be explained by the location of the channels.
Ripples
The rate of ripples was significantly higher in the SOZ (25.8 ± 21.1/min) than outside (5.8 ± 10.5/min) (F [1, 809]: 325, p < 0.001). No differences in the percentage of overlap between ripples and spikes were found, but ripples were significantly longer in the SOZ (98.6 ± 47.6 ms) than outside (90.1 ± 39.2 ms) (F [1, 11054]: 78.9, p < 0.001). The differences between channels inside and outside the SOZ could be observed independently of whether the ripples occurred within or outside spikes.
Fast ripples
Fast ripples occurred significantly more often in the SOZ (24.3 ± 32.4/min) than outside (1.9 ± 4.7/min) (F [1, 809]: 240; p < 0.001). This difference was highest when looking at the total rate of all fast ripples, but was also significant when looking only at fast ripples outside spikes or only those within spikes but outside ripples. No differences were seen for the percentage of overlap between spikes and fast ripples. The duration of fast ripples was significantly longer in the SOZ (41.7 ± 27.4 ms) than outside (33.1 ± 19.5 ms) (F [1,8451]: 96.3; p < 0.001).
Spikes
The rate of spikes was significantly higher in SOZ channels (20.4 ± 15/min) than non-SOZ channels (5.2 ± 9.1/min) (F [1, 809]: 230; p < 0.001). The same difference could be observed for the percentage of spikes carrying fast ripples, with 52.5% of the spikes cooccurring with fast ripples in the SOZ and only 34.7% of these spikes outside the SOZ. The percentage of spikes cooccurring with ripples was similar inside (53.7%) and outside the SOZ (50.3%).
Identification of the SOZ using HFOs
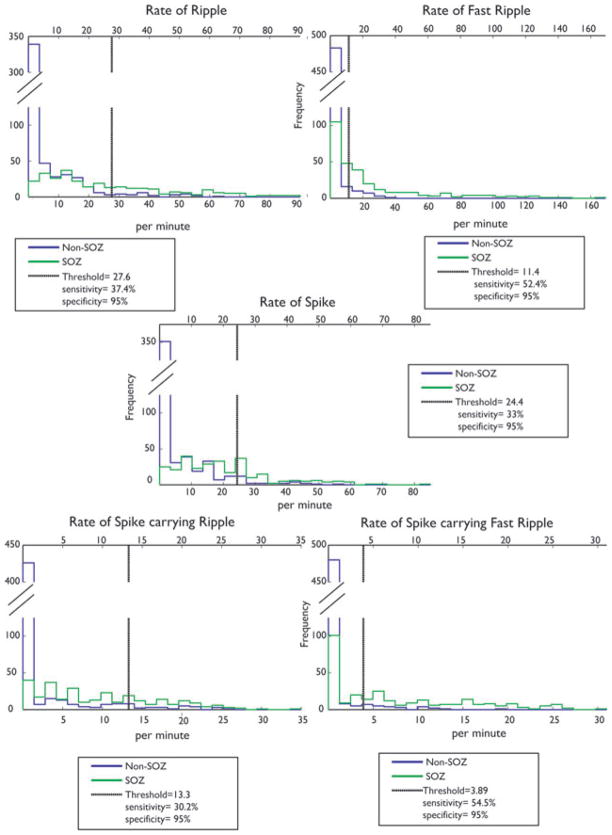
One goal of this study was to determine if using HFOs can help in the identification of the SOZ and to evaluate whether this provides additional information as opposed to looking only at spiking activity. The presence of HFOs independently of spikes suggests that they may provide distinct information concerning the SOZ. We established a threshold aimed at separating channels located inside the SOZ from channels located outside. We did not expect to find a threshold that would result in a perfect separation, but we aimed for a high specificity (identifying channels that are almost surely in the SOZ) and determined the sensitivity (proportion of SOZ channels that could be identified) for each event type. Therefore, we calculated a threshold for the different rates of events (ripples, fast ripples, and spikes), above which channels could be determined to belong to the SOZ with a specificity of 95% (Table 3), and we calculated the corresponding sensitivity. The best sensitivity was found for the rate of spikes with fast ripples (54.5%) and the rate of fast ripples alone (52.2%). This sensitivity was higher than when looking at ripples alone (37.5%), spikes alone (33.4%), or spikes with ripples (30.2%). Fig. 7 shows the distributions of the different rates of events for channels inside and outside the SOZ. The rates that would allow the identification of SOZ channels with a specificity of 95%, as calculated from the distributions, are also shown along with the associated sensitivities corresponding to the 95% specificity.
Figure 7.
Histograms comparing channels inside and outside the SOZ. The five histograms show the rates of spikes, ripples, fast ripples, spikes with ripples and spikes with fast ripples. Green indicates rates in SOZ and blue outside SOZ. The thresholds for 95% specificity are shown as black vertical lines. If the SOZ was not known and if a rate above this threshold was selected to separate SOZ from non-SOZ channels, the indicated sensitivity would result. The rate of fast ripples and of spikes with fast ripples showed the highest sensitivity in indicating the SOZ.
Epilepsia © ILAE
The above calculations included all the channels from all patients, with each channel considered independently (all channels were pooled as belonging to the SOZ or not, independently of patients). In a second step, we retained the same thresholds, but calculated sensitivities and specificities for each patient (Table 4). In 7 of 10 patients, the SOZ was better predicted by looking at HFOs than at spikes alone (for 6, the fast ripples were the best predictor, for one it was ripples). The rates established by the average of all patients fail to predict the SOZ in three other patients (patients 8 to 10). They had Nc seizure onset and comparably lower rates of HFOs. To calculate valuable rates for these patients, it would probably be useful to establish separate thresholds of ripple and fast ripple rates in Nc and temporal onset patients in a larger group of patients.
Table 4.
General threshold applied on single patients (data expressed in %)
| Patient # | Rate of spikes Threshold: 24.4/min |
Rate of spikes with ripple Threshold: 13.3/min |
Rate of spikes with fast ripple Threshold 3.9/min |
Rate of ripples Threshold: 27.6/min |
Rate of fast ripples Threshold: 11.4/min |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |
| 1 | 30 | 98.3 | 67.5 | 89 | 85 | 78.9 | 75 | 97.3 | 69.4 | 96.9 |
| 2 | 6.3 | 100 | 11.3 | 100 | 41.1 | 100 | 30 | 100 | 44.1 | 100 |
| 3 | 31.7 | 95.6 | 27.5 | 95.8 | 98.6 | 91 | 55 | 93.3 | 53.8 | 99 |
| 4 | 90.6 | 100 | 70 | 100 | 100 | 100 | 34.4 | 98.3 | 100 | 99.2 |
| 5 | 12.9 | 98.2 | 47.5 | 72.5 | 66.4 | 75 | 73.3 | 75 | 64.7 | 76.7 |
| 6 | 13.3 | 100 | 35 | 100 | 95 | 100 | 53.3 | 100 | 81.3 | 75.6 |
| 7 | 30.3 | 100 | 33 | 100 | 49.4 | 100 | 39.3 | 100 | 58.6 | 100 |
| 8 | 0 | 83.3 | 0 | 88.3 | 0 | 100 | 0 | 83.3 | 0 | 96.7 |
| 9 | 50 | 86.4 | 2.5 | 100 | 0 | 100 | 1.7 | 100 | 30.3 | 100 |
| 10 | 69.4 | 100 | 0 | 100 | 15.2 | 100 | 0 | 100 | 7.5 | 100 |
| 1–10 | 33.5 | 96.2 | 29.4 | 94.6 | 55.6 | 94.5 | 36.2 | 94.7 | 50.9 | 94.5 |
This table shows the sensitivity and specificity of the threshold established in Table 3. The threshold for the different event types was applied on every patient separately. The results show clearly that for the last three patients the general threshold performs very badly in predicting the SOZ. This is probably related to the anatomical regions of seizure onset. Patients 8 to 10 are those with neocortical extra temporal lobe onset, which in general showed lower rates of ripples and fast ripples. The events which showed the highest sensitivity toward detecting the SOZ, are printed in bold.
Discussion
We have demonstrated that high-frequency oscillations between 80 and 500 Hz can be recorded with macroelectrodes and occur in large amounts independently of spikes: they were identified in nonspiking channels, and in spiking channels 38% of ripples and 52% of fast ripples were independent of spikes. Additionally, HFOs occurred more often inside than outside the SOZ and the identification of the SOZ was improved in 7 of 10 patients by using HFOs compared to spikes alone. Fast ripples distinguished between the two areas better than ripples. Moreover, we showed that ripples and fast ripples were more frequent and of longer durations in the Am and Hc compared to the neocortex. Finally, HFOs cooccurring with spikes were of longer duration than the ones outside spikes. The rate of cooccurrence of spikes and HFOs did not vary with respect to anatomical or seizure onset regions.
Methodological issues
In this study, we used macroelectrodes to detect HFOs (Urrestarazu et al., 2007), with a surface contact that is approximately 700 times larger than the microwires used in other human studies (Staba et al., 2004). These macroelectrodes most likely record HFOs generated synchronously over larger brain volumes. However, we could detect ripples and fast ripples in similar frequency ranges as described in microelectrodes and the rates of HFO occurrence were even higher than in the studies with microelectrodes. This higher rate might reflect the ability of larger electrodes to record highly synchronous events from a more extended brain area than microelectrodes, which are more limited in their spatial recording ability. Even if the durations of the HFOs were slightly longer in this study (R: 96 ms; FR: 40 ms) compared to microelectrodes (R ~ 57 ms; FR ~ 25 ms), all events were clearly identified as distinct oscillations and their shapes, as demonstrated in the figures, were similar to those seen with microelectrodes (Bragin et al., 1999; Draguhn et al., 2000).
HFOs are more often detected during slow wave sleep and it was hypothesized that this phenomenon is mainly the result of a higher synchronization of neuronal populations during slow wave sleep (Staba et al., 2004). For the same reason, HFOs are less frequent during REM sleep, where rhythmic discharge patterns are replaced by tonic firing patterns resulting in a reduced neuronal synchrony. We therefore selected segments of slow wave sleep for our study. This additionally ensured that there would be a large number of spikes as these are more likely to occur during sleep and usually are seen over more extended brain areas than during wakefulness (Sammaritano et al., 1991; Clemens et al., 2003).
HFOs in human epileptic brain often occur at the same time as IEDs (Bragin et al., 2002). In a human study with microelectrodes (Staba et al., 2004), the authors analyzed HFOs by filtering and thresholding the EEG and have not separated HFOs occurring during spikes from those occurring independently. Another problem is the issue of filtering. Fast transients such as spikes intrinsically include high frequencies; the sharper the transient, the more it includes high frequencies, which appear as HFOs when filtering out low frequencies (this is the case even if the digital filter is designed to avoid ringing). In a detailed visual analysis, our group showed that HFOs that represent an effect of filtering alone and do not reflect HFOs superimposed on spikes are rare (Urrestarazu et al., 2007). In the current study, spikes and HFOs were marked in two different copies of the same EEG sample, to avoid a bias in the selection of HFOs by the visualization of spikes. As fast ripples resulting from filtering spikes always cooccur with ripples, we also performed a subanalysis looking only at fast ripples occurring at the time of spikes but in the absence of ripples. The rate of such fast ripples was lower than the total rate but still all significant differences described in this study could be seen. It also has to be pointed out that sharp spikes resulting in HFOs in the filtered signal could still be interesting if they have a specific relevance in predicting the SOZ. For these reasons, all HFOs and spikes were used for analysis. The above mentioned phenomenon could also apply to the filtering of ripples which could include faster transients which then would occur as fast ripples when applying a 250 Hz filter. We cannot exclude that some of the reported fast ripples actually represent faster segments of a ripple event. However, 55% of the fast ripple occurred independent of ripples and subanalysis with only these events resulted in the same reported significant differences in the results. The differentiation between ripples and fast ripples derives from spectral analysis (Staba et al., 2002) and whether these events are always distinct from each other still remains to be clarified.
Relationship between spikes and fast oscillations
IEDs are believed to reflect summated membrane potentials from abnormally hypersynchronous neurons within epileptic tissue. Spikes probably represent paroxysmal depolarization shifts, while slow waves following spikes are caused by prolonged hyperpolarization (Matsumoto et al., 1964). HFOs have been mainly described within the spike itself, and the power at high frequencies was found to be reduced after the spike and within the slow waves (Urrestarazu et al., 2006). In this study, we looked at the overlap of spikes and slow waves with the HFOs and confirmed that HFOs are rarely generated within the slow wave. A high proportion of the HFOs occurring within slow waves had indeed started within the spike with only a small overlap with the slow wave, suggesting that the HFO is generated during the spike and stops with the occurrence of the slow wave.
In spite of a high degree of cooccurrence of spikes and HFOs, our study suggests that the two phenomena have also some degree of independence. A high percentage of HFOs occurred outside spikes and even in nonspiking channels. However the duration of HFOs within spikes was generally higher than outside. Differences in duration between HFOs at different times might be the result of different strengths of GABA-mediated inhibition after the HFO (Staba et al., 2002). This hypothesis was strengthened by the finding that HFOs were found in increased rates and durations after applying GABA receptor antagonists (Bragin et al., 2002; Jones & Barth, 2002). Hence, spikes generated in the same neuronal network as HFOs might increase the likelihood of occurrence and the duration of HFOs.
The difference between pathologic and physiologic HFOs in humans remains unclear. In particular, ripples are known to occur physiologically and result from synchronous GABAergic interneuron inhibition (Chrobak & Buzsaki, 1996). However, the same interneurons are excitatory during brain development and in some circumstances can generate pathological HFOs in the ripple or fast ripple range (Le Van Quyen et al., 2006). For instance, in mesial temporal lobe epilepsy, spikes, and seizures may result from an imbalance between excitatory and inhibitory interneurons and dysfunctional GABA inhibition (Khazipov et al., 2004; Magloczky et al., 2005). It might be hypothesized that, in this disorder, pathological HFOs also result from excitatory GABAergic interneurons or an imbalance between excitatory and inhibitory interneurons within the epileptic tissue. HFOs are most frequent and largest in amplitude during epileptic seizures (Bragin et al., 2004; Jirsch et al., 2006); they therefore occur most frequently when inhibitory networks failed to a point that epileptic activity could no longer be restricted. IEDs and HFOs are possibly independent hypersynchronous excitatory events, generated within similar neuronal networks.
We observed ripples and fast ripples in nonspiking channels. Whether all of these marked events were actually pathological cannot be definitely demonstrated, as we are not able to identify healthy tissue with enough certainty. To our knowledge, fast ripples have not yet been reported to be physiologic oscillations during sleep. They were, however, described during wakefulness and during normal sensory information processing (Curio et al., 1997; Gobbele et al., 2004). We observed ripples and sometimes fast ripples in regions that were outside the presumed SOZ area and within apparently normal brain tissue as it appeared on the MRI. Whether these high-frequency oscillations represent potentially epileptogenic areas cannot be answered here.
HFOs in different anatomical areas
Ripples and fast ripples have first and most often been described in mesial temporal structures. Ripples are thought to be physiological and are usually seen in the Hc and entorhinal cortex, resulting from feedback networks and bursts of inhibitory GABAergic interneurons (Bragin et al., 1999; Buhl & Buzsaki, 2005). On the other hand, fast ripples appear to be the result of damaged “ripple networks” in epileptic mesial temporal areas (Staba et al., 2004), and studies evaluating the role of fast ripples in epileptogenic networks in humans were mainly focused on these anatomical structures. In recent studies, HFOs of lower frequencies (60–150 Hz) were identified in epileptic neocortex (Ochi et al., 2007) and previous studies by our group identified ripples (80–250 Hz) and fast ripples (250–500 Hz) in Nc SOZ areas (Jirsch et al., 2006; Urrestarazu et al., 2007). In this study, we included six patients with temporal lobe seizure onset and four with extratemporal Nc seizure onset and investigated Nc and mesial temporal channels (even if there were more seizure onset channels in the mesial temporal structures).
Temporal lobe epilepsy is the prototypic intractable focal epilepsy in humans and the Am and Hc are known to be epileptogenic more often than other brain areas. Whether this is caused by their anatomy or special vulnerability to tissue damage is not yet understood. It is first interesting to note that HFOs were as commonly found in the Am as in the Hc. Although the circuitry of the Hc seems to lend itself to the generation of HFOs, that of the Am, quite different, appears just as likely to generate HFOs. HFOs in the ripple range have been described physiologically in the Am (Ponomarenko et al., 2003), but never in relation to epileptic changes. However, the Am was characterized by high epileptogenic properties in the kindling model (McIntyre et al., 2002) and in vitro preparation (Benini et al., 2003).
Nearly all patients implanted in both temporal lobes show frequent bitemporal spiking, even if during the investigation and based on postsurgical results it is frequent that only one of their temporal lobes seems to be truly epileptogenic (Hamer et al., 1999). If HFOs and spikes need similar conditions and changes in neural excitability, it is not surprising that they occur most frequently in the same anatomical structures. The study of Staba and coworkers raised the question of whether pathological HFOs occurred from alteration of physiological HFOs (Staba et al., 2004). In this study, we showed that most of the recorded ripples were in the SOZ, and, like for the fast ripples, they increased in the area where the seizures were generated. These results suggest that ripples are also pathological and that fast ripples do not replace normal physiological ripples. Not only did we not see a decrease in ripples inside the SOZ, we actually saw an increase. It is therefore unlikely that fast ripples replaced physiological ripples. It is also unlikely that most of the observed ripples were physiological, since they were found more often in the SOZ.
HFOs occurred at a lower rate in neocortex compared to Am and Hc, but both ripples and fast ripples were more frequent in the Nc regions inside the SOZ than outside. The reason for this lower Nc propensity to generate HFOs is unclear. It is congruent with our earlier study showing that the high-frequency changes following a spike are different in mesial temporal structures and neocortex (Urrestarazu et al., 2006).
Identification of the SOZ
Many studies have shown that in general electrode contacts lying within the SOZ show spiking activity (Cendes et al., 1996; Bautista et al., 1999; Ray et al., 2007). This phenomenon was observed in our patients as well, and we could not identify seizure onset channels without concomitant spiking activity. However, spikes were also often observed in channels outside the SOZ. Therefore, spikes seem to be a highly sensitive but not very specific indicator of the SOZ. It has been discussed whether the localization, number, and multifocality of spikes seen in scalp and intracranial EEG have a predictive value for seizure outcome after surgery. This remains a matter of debate in both mesial temporal and Nc epilepsies (Bautista et al., 1999; Holmes et al., 2000; Hufnagel et al., 2000). We do not have the postsurgical seizure outcome of our patients, as they were all recorded recently. Additionally, we only could identify the seizure onset in the spatially limited area covered by the electrodes and this prevents the exact determination of the SOZ. However, all patients were selected to have localized SOZ and in eight patients, multifocal interictal activity was seen and, hence, spikes alone would not have allowed the identification of the SOZ. Our study showed that HFOs occurred to a high degree independently of spikes and, when they cooccurred with spikes, this seemed to be more often the case in SOZ areas. Therefore, it became interesting to investigate whether they predicted the SOZ better than spikes. Subsequently we demonstrated that incorporating information about HFOs increased our ability to detect the seizure onset channels: spikes with fast ripples and fast ripples themselves are more likely to localize the SOZ than spikes taken indiscriminately. We attempted to establish rate thresholds for HFOs, which would identify the SOZ, but these appear dependent on the potential area of seizure onset. As described above, temporal lobe structures generate manymore HFOs than other Nc brain areas. Therefore rate thresholds established in temporal regions cannot be used for other Nc regions. It might be possible to establish different rate thresholds of HFOs that enable to delineate the SOZ in patients with different types of epilepsies, but a larger number of subjects and postsurgical data are needed to test this hypothesis.
In this study, ripples and fast ripples behaved in parallel, increasing in the SOZ and spiking regions, but like in our previous study (Urrestarazu et al., 2007), we found that fast ripples were more specific to the SOZ region than ripples. We conclude that future analysis of HFOs should not focus only on fast ripples, as ripples seem to indicate SOZ areas as well. Our findings and that of others (Ochi et al., 2007) in some way contradict the findings of Staba and colleagues using microelectrodes in humans (Staba et al., 2004). This difference remains to be elucidated.
It appears that HFOs are an important electrophysiological manifestation of the epileptic tissue. They are correlated to the spiking region and to the SOZ but are partly independent of them. Whether they represent a region of possible epileptogenicity can only be assessed by postsurgical seizure outcome results. It also remains unclear whether HFOs actually contribute to the generation of seizures. In animal studies the occurrence of HFOs and their frequency could be clearly linked to the occurrence of spontaneous seizures and their latent period after injection of kainic acid (Bragin et al., 2004). There has also been evidence that HFOs might increase before low-Mg2+ seizure in the in vitro model (Khosravani et al., 2005). Therefore HFOs could contribute to seizure generation, but in an independent study of our group looking at HFOs during the preictal period, we so far could not find a clear change in the HFOs before the onset of seizures (Jirsch et al., 2007).
It has to be kept in mind that the additional information provided by HFOs is gained by looking at only 10 min of slow wave sleep. Moreover, the rates of ripples and fast ripples per minute remained quite stable, so this interval could probably be reduced. The identification of HFOs is time consuming but automatic methods are currently under development (Chander et al., 2006). The threshold we used to separate the channels inside from channels outside the SOZ was tailored to this particular group of patients; it would need to be validated on a new dataset and without any a priori knowledge of the SOZ. A future project will evaluate these findings by using postsurgical data and assess the best time interval for a robust HFO analysis.
Acknowledgments
This project was supported by grant MOP-10189 from the Canadian Institutes of Health Research.
Footnotes
Conflict of interest: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The authors have no professional or financial affiliations that might be perceived as having biased the presentation.
References
- Bautista RE, Cobbs MA, Spencer DD, Spencer SS. Prediction of surgical outcome by interictal epileptiform abnormalities during intracranial EEG monitoring in patients with extrahippocampal seizures. Epilepsia. 1999;40(7):880–890. doi: 10.1111/j.1528-1157.1999.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Benini R, D’Antuono M, Pralong E, Avoli M. Involvement of amygdala networks in epileptiform synchronization in vitro. Neuroscience. 2003;120:75–84. doi: 10.1016/s0306-4522(03)00262-8. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52:407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J. Spatial stability over time of brain areas generating fast ripples in the epileptic rat. Epilepsia. 2003;44:1233–1237. doi: 10.1046/j.1528-1157.2003.18503.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Buhl DL, Buzsaki G. Developmental emergence of hippocampal fast-field “ripple” oscillations in the behaving rat pups. Neuroscience. 2005;134:1423–1430. doi: 10.1016/j.neuroscience.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendes F, Dubeau F, Andermann F, Quesney LF, Gambardella A, Jones-Gotman M, Bizzi J, Olivier A, Gotman J, Arnold DL. Significance of mesial temporal atrophy in relation to intracranial ictal and interictal stereo EEG abnormalities. Brain. 1996;119(Pt 4):1317–1326. doi: 10.1093/brain/119.4.1317. [DOI] [PubMed] [Google Scholar]
- Chander R, Urrestarrazu E, Gotman J. Automatic detection of high frequency oscillations in human intracerebral EEG. Epilepsia. 2006;47(S4):37. [Google Scholar]
- Chrobak JJ, Buzsaki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Lorincz A, Buzsaki G. Physiological patterns in the hippocampo-entorhinal cortex system. Hippocampus. 2000;10:457–465. doi: 10.1002/1098-1063(2000)10:4<457::AID-HIPO12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Janszky J, Szucs A, Békésy M, Clemens B, Halász P. Interictal epileptic spiking during sleep and wakefulness in mesial temporal lobe epilepsy: a comparative study of scalp and foramen ovale electrodes. Epilepsia. 2003;44(2):186–192. doi: 10.1046/j.1528-1157.2003.27302.x. [DOI] [PubMed] [Google Scholar]
- Curio G, Mackert BM, Burghoff M, Koetitz R, Abraham-Fuchs K, Harer W. Localization of evoked neuromagnetic 600 Hz activity in the cerebral somatosensory system. Electroencephalogr Clin Neurophysiol. 1994;91:483–487. doi: 10.1016/0013-4694(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Curio G, Mackert BM, Burghoff M, Neumann J, Nolte G, Scherg M, Marx P. Somatotopic source arrangement of 600 Hz oscillatory magnetic fields at the human primary somatosensory hand cortex. Neurosci Lett. 1997;234:131–134. doi: 10.1016/s0304-3940(97)00690-3. [DOI] [PubMed] [Google Scholar]
- Diehl B, Lueders HO. Temporal lobe epilepsy: when are invasive recordings needed? Epilepsia. 2000;41(Suppl 3):S61–S74. doi: 10.1111/j.1528-1157.2000.tb01536.x. Review. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Bibbig A, Schmitz D. Ripple (approximately 200-Hz) oscillations in temporal structures. J Clin Neurophysiol. 2000;17:361–376. doi: 10.1097/00004691-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Gobbele R, Waberski TD, Simon H, Peters E, Klostermann F, Curio G, Buchner H. Different origins of low- and high-frequency components (600 Hz) of human somatosensory evoked potentials. Clin Neurophysiol. 2004;115:927–937. doi: 10.1016/j.clinph.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Najm I, Mohamed A, Wyllie E. Interictal epileptiform discharges in temporal lobe epilepsy due to hippocampal sclerosis versus medial temporal lobe tumors. Epilepsia. 1999;40(9):1261–1268. doi: 10.1111/j.1528-1157.1999.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Kutsy RL, Ojemann GA, Wilensky AJ, Ojemann LM. Interictal, unifocal spikes in refractory extratemporal epilepsy predict ictal origin and postsurgical outcome. Clin Neurophysiol. 2000;111(10):1802–1808. doi: 10.1016/s1388-2457(00)00389-8. [DOI] [PubMed] [Google Scholar]
- Hufnagel A, Dumpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia. 2000;41(4):467–478. doi: 10.1111/j.1528-1157.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Wang Y, Okada YC. Origins of the somatic N20 and high-frequency oscillations evoked by trigeminal stimulation in the piglets. Clin Neurophysiol. 2005;116:827–841. doi: 10.1016/j.clinph.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Jirsch JD, Chander R, Gotman J. Pre-Ictal High Frequency Oscillations (100–450Hz) in epileptogenic areas of patients with focal seizures. Epilepsia. 2007;48(s6):207. [Google Scholar]
- Jones MS, MacDonald KD, Choi B, Dudek FE, Barth DS. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2000;84:1505–1518. doi: 10.1152/jn.2000.84.3.1505. [DOI] [PubMed] [Google Scholar]
- Jones MS, Barth DS. Effects of bicuculline methiodide on fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2002;88(2):1016–1025. doi: 10.1152/jn.2002.88.2.1016. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19(3):590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Pinnegar CR, Mitchell JR, Bardakjian BL, Ferderico P, Carlen PL. Increased high-frequency oscillations precede in vitro Low-Mg2+ seizures. Epilepsia. 2005;46(8):1188–1197. doi: 10.1111/j.1528-1167.2005.65604.x. [DOI] [PubMed] [Google Scholar]
- LeVan Quyen M, Khalilov I, Ben-Ari Y. The dark side of high-frequency oscillations in the developing brain. Trends Neurosci. 2006;29(7):419–427. doi: 10.1016/j.tins.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Maglóczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28(6):334–340. doi: 10.1016/j.tins.2005.04.002. Review. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Marsan CA. Cortical cellular phenomena in experimental epilepsy: ictal manifestations. Exp Neurol. 1964;9:305–326. doi: 10.1016/0014-4886(64)90026-3. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Poulter MO, Gibly K. Kindling: Some old and some new. Epilepsy Res. 2002;50:79–92. doi: 10.1016/s0920-1211(02)00071-2. [DOI] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, Akizuki Y, Akiyama T, Imai K, Rutka JT, Snead OC., 3rd Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48(2):286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- Olivier A, Germano IM, Cukiert A, Peters T. Frameless stereotaxy for surgery of the epilepsies: preliminary experience. J Neurosurg. 1994;81(4):629–633. doi: 10.3171/jns.1994.81.4.0629. Technical note. [DOI] [PubMed] [Google Scholar]
- Ponomarenko AA, Korotkova TM, Haas HL. High frequency (200Hz) oscillation and firing patterns in the basolateral amygdala and dorsal endopiriform nucleus of the behaving rat. Behav Brain Res. 2003;141:123–129. doi: 10.1016/s0166-4328(02)00327-3. [DOI] [PubMed] [Google Scholar]
- Ray A, Tao JX, Hawes-Ebersole SM, Ebersole JS. Localizing value of scalp EEG spikes: a simultaneous scalp and intracranial study. Clin Neurophysiol. 2007;118(1):69–79. doi: 10.1016/j.clinph.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Lueders H. Presurgical evaluation of epilepsy. Brain. 2001;(Pt 9):1683–1700. doi: 10.1093/brain/124.9.1683. Review. [DOI] [PubMed] [Google Scholar]
- Sammaritano M, Gigli GL, Gotman J. Interictal spiking during wakefulness and sleep and the localization of foci in temporal lobe epilepsy. Neurology. 1991;41(2 Pt 1):290–297. doi: 10.1212/wnl.41.2_part_1.290. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Jirsch JD, LeVan P, Hall J, Avoli M, Dubeau F, Gotman J. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–1476. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]