Abstract
High frequency oscillations (HFOs) called ripples (80–250 Hz) and fast ripples (FR, 250–500 Hz) can be recorded from intracerebral EEG macroelectrodes in patients with intractable epilepsy. HFOs occur predominantly in the seizure onset zone (SOZ) but their relationship to the underlying pathology is unknown. It was the aim of this study to investigate whether HFOs are specific to the SOZ or result from pathologically changed tissue, whether or not it is epileptogenic. Patients with different lesion types, namely mesial temporal atrophy (MTA), focal cortical dysplasia (FCD) and nodular heterotopias (NH) were investigated. Intracranial EEG was recorded from depth macroelectrodes with a sampling rate of 2000 Hz. Ripples (80–250 Hz) and Fast Ripples (250–500 Hz) were visually marked in 12 patients: five with MTA, four with FCD and three with NH. Rates of events were statistically compared in channels in four areas: lesional SOZ, non-lesional SOZ, lesional non-SOZ and non-lesional non-SOZ. HFO rates were clearly more linked to the SOZ than to the lesion. They were highest in areas in which lesion and SOZ overlap, but in patients with a SOZ outside the lesion, such as in NHs, HFO rates were clearly higher in the non-lesional SOZ than in the inactive lesions. No specific HFO pattern could be identified for the different lesion types. The findings suggest that HFOs represent a marker for SOZ areas independent of the underlying pathology and that pathologic tissue changes alone do not lead to high rates of HFOs.
Keywords: high frequency oscillations, focal cortical dysplasia, nodular heterotopia, temporal atrophy, seizure onset zone, intracranial EEG
Introduction
Improved MRI techniques, such as high resolution MRI, hippocampal volumetry, automatic detection of lesions and postprocessing image analysis have led to more patients with identified lesional epilepsy (Bernasconi et al., 2001; Huppertz et al., 2005). It also became clear that identifiable lesions on MRI are sometimes large or multifocal, or that MRI-detectable lesions may only represent the ‘tip of the iceberg’. They may not, therefore, necessarily contain the seizure onset zone (SOZ) and areas around or at distance from the visible lesion might actually be more epileptogenic than lesional areas (Boonyapisit et al., 2003). Results from EEG and functional imaging might also be contradictory and rarely match perfectly the lesion (Raymond et al., 1995; Li et al., 1997; Genow et al., 2004). Hence, in patients with symptomatic epilepsy, lesional and epileptogenic areas do not overlap necessarily and it remains unclear which part of the lesion and surrounding tissue is actually or potentially epileptogenic (Rosenow and Lüders, 2001).
Additionally, different lesion types seem to have various levels of intrinsic epileptogenicity and do not always contribute similarly to the epileptogenic network. Mesial temporal sclerosis is found in 40–60% of the surgical patients with mesial temporal lobe epilepsy (Radhakrishnan et al., 1998; Clusmann et al., 2004). The lesion is associated with neuronal loss in the hippocampus, most prominent in the fascia dentata, hippocampal areas CA1 and CA2 and the prosubiculum (Babb and Brown, 1987) and its intrinsic epileptogenicity is believed to derive from an imbalance between excitatory and inhibitory neurons following axonal sprouting and formation of new synapses caused by cell loss (Mathern et al., 1995). In patients with mesial temporal lesions, the SOZ is usually limited to the lesional area and removal of the affected mesial temporal structures results in a seizure free outcome in 60–80% of patients (Hennessy et al., 2001; Mohamed et al., 2001). However, some patients with unilateral mesial temporal sclerosis or atrophy show bi-temporal SOZ areas and in these patients the surgical outcome after removal of the lesion is poorer and it is unclear which role the lesional areas play in the whole epileptogenic network of these patients (Abosch et al. 2002; Holmes et al., 2003).
Malformations of cortical development (MCD) represent another group of very epileptogenic lesions, ranging from extensive lesions such as polymicrogyria to small localized structural abnormalities like nodular heterotopia (NH) and focal cortical dysplasia (FCD) (Barkovich and Raybaud, 2004). In focal cortical dysplasias surgical removal results in a seizure-free outcome in many patients even if the outcome is less promising than in other pathologies (Palmini et al., 1991). Intracranial EEG and functional imaging showed that FCD lesions are intrinsically epileptogenic (Palmini et al., 1995; O’Brien et al., 2004; Otsubo et al., 2005). As the removal of the complete dysplastic lesion is strongly correlated with a good outcome, it has been postulated that in FCD the epileptogenic area is congruent with the lesion (Chassoux et al., 2000). It has been hypothesized that the intrinsic epileptogenicity observed in FCD derives from abnormal synaptic interconnectivity as well as neurotransmitter changes within these lesions (Ferrer et al., 1992; Mattia et al., 1995; Chassoux et al., 2000).
Nodular heterotopia results from a migration disorder. Islands of grey matter consisting of normal neurons and glial cells no longer organized in layers are located within the white matter, most often next to the ventricles (Barth, 1987; Barkovich and Kjos, 1992). Most patients with NH suffer from drug resistant epilepsy but some do not show seizures (Raymond et al., 1994; Dubeau et al., 1995; Battaglia et al., 1997). It is still uncertain which role heterotopic lesions play for seizure onset and propagation and whether they are epileptogenic. Intracranial EEG investigations showed apparently contradictory results implying that seizures are generated sometimes by the heterotopias, sometimes by distant cortex and sometimes by both (Dubeau et al., 1995; Kothare et al., 1998; Aghakhani et al., 2005; Scherer et al., 2005; Tassi et al., 2005). However, neither the removal of the lesion alone nor mesial temporal resections lead to a favourable outcome, suggesting a more widespread epileptogenic network in these patients (Li et al., 1997; Tassi et al., 2005).
Traditionally, EEG frequencies are believed to be relevant up to the gamma band (40–100 Hz), but recent findings suggest that high frequency oscillations (HFOs) ranging between 100 and 500 Hz may be closely linked to epileptogenesis (Bragin et al., 2002). HFOs were first recorded in intracranial recordings of rats and humans using micro-wires and two event types were identified: ripples between 80 and 250 Hz and fast ripples above 250 Hz. Recently, our group demonstrated that ripples and fast ripples can also be recorded from clinical macro-electrodes (Jirsch et al. 2006; Urrestarazu et al., 2007). It is believed that ripples are physiological events generated in mesial temporal structures and closely linked to memory consolidation (Draguhn et al., 2000), but they could also represent pathological events in the SOZ of epilepsy patients (Bragin et al., 2004). On the other hand, fast ripples appear pathological and closely linked to epileptogenesis (Staba et al., 2002). While a clear correlation between high HFO rates and areas of seizure onset was observed (Bragin et al., 2004; Jacobs et al., 2008), the relationship between lesions and HFOs remains unclear. During a previous study of our group, we gained the impression that some pathologies, such as FCD, may be more likely to generate HFOs than others (Urrestarazu et al., 2007). Staba and coworkers (2007) showed that the degree of neuronal loss and tissue damage in patients with hippocampal sclerosis was closely linked to the generation of fast ripples. The majority of studies on HFOs have been performed in patients with temporal lobe epilepsy and mesial temporal sclerosis, where lesional and SOZ areas often overlap. It therefore remains open whether HFOs are mainly produced by pathologically changed tissue, thus reflecting damaged neuronal networks in general or whether they occur as a sign of intrinsic epileptogenic activity in SOZ areas independently of lesional pathology. This study evaluates this question in patients with temporal and extra-temporal lobe epilepsies and with different lesional pathologies, by analysing HFO activity in lesional and non-lesional SOZ areas.
Methods
Patient selection
Between September 2004 and October 2007, 41 patients underwent intracranial electrode implantation in the epilepsy unit of the Montreal Neurological Hospital. The decision for invasive EEG studies was made when no clear area of seizure onset could be determined with extensive non-invasive evaluation. Electrode placement was tailored to clinical history, seizure semiology and results of surface EEG investigation, neuroimaging and neuropsychological testing. The clinical intracranial recordings were interpreted independently of this study by an experienced neurophysiologist, who described in detail the different interictal findings in each channel and determined in which channels were the sites of seizure onset. We selected 12 patients with three types of lesional focal epilepsy as identified on MRI: five with unilateral mesial temporal atrophy (MTA), four with FCD and three with NH. The pathology, as defined on MRI, was the only selection criterion in this study. The clinical findings and electrode placements are described in Table 1. This study was approved by the Montreal Neurological Institute and Hospital Research Ethics Committee and all patients signed an informed consent.
Table 1.
Clinical data and implantation sites of all patients
| Patient # | Age/gender | Scalp EEG | MRI | No. of implanted electrodes | Implantation sites | Surgery | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 44/M | Ictal: LT Interictal: bi T |
L MTL atrophy | 6 | L- HC, L-PHC, L-A R-HC, R-PHC, R-A |
L sAHC | a |
| 2 | 49/Fe | Ictal: bi T Interictal: L T |
R MTL atrophy | 6 | L- HC, L-PHC, L-A R-HC, R-PHC, R-A Epidural E. L. First T gyrus |
None | |
| 3 | 45/M | Ictal: L T Interictal: L T |
L MTL atrophy L T pole atrophy |
5 | L- HC, L-PHC, L-A, L-OF, L T pole, Epidural E. L. First T gyrus |
None | |
| 4 | 54/Fe | Ictal: R T Interictal: R T |
R MTL sclerosis R temporal-occipital postraumatic contusion |
5 | R- HC, R-PHC, R-A, R- posterior-temporal junction in Le (R-Le1), R anterior occipital in Le (R-Le2) Epidural E: first T, angular and supramarginal gyrus |
R sAHC | 3A |
| 5 | 46/M | Ictal: bi T Interictal: bi T |
R MTL atrophy and sclerosis Malrotation R HC |
4 | L- HC, L-A, R-HC, R-A Epidural E: L and R first T gyrus |
R sAHC | 2A |
| 6 | 24/M | Ictal: R T Interictal: R T |
Two nodular Het in the right trigonal area | 5 | R-HC, R-PHC, R-A, R-AN (Anterior Le R trigonal area), R-PN (posterior Le R trigonal area) Epidural E over first T and supramarginal gyrus |
R sAHC | 2A |
| 7 | 25/M | Ictal: bi T Interictal: bi T |
Bilateral periventricular Het. | 9 | L-HC, L-A, L-O (occipial lobe within Het), L-S (L trigone and supra-marginal gyrus within Het) R-HC, R-PHC, R-A, R-O (Roccipital within Het), R-S (R trigonal area and supra-marginal gyrus within het) |
R sAHC | a |
| 8 | 20/Fe | Ictal: R P&T&O Interictal: R P&O |
Bilateral periventricular Het and malformed overlying cortex | 6 | R-HC, R-PHC, R-A, R-P (posterior to R-PHC towards the heterotopia) R-S and R-O inserted from occipital aiming obliquely at the heterotopia | Lesionectomy | a |
| 9 | 33/M | Ictal: bi T Interictal: bi T |
FCD R and L OF | 8 | L-A, L-HC, L-OF, L-AC R-A, R-HC, R-OF, R-AC |
Lesionectomy (limited) | 3A |
| 10 | 28/Fe | Ictal: R F & T Interictal: bi F & T |
FCD R F basal region and anterior insular cortex | 7 | L-AC, L-PC, L-OF R-AC, R-PC, R-OF, R-L (lesional trough superior F gyrus aiming at the anterior insular) |
None | |
| 11 | 36/M | Ictal: no changes Interictal: no changes |
FCD L second F gyrus | 7 | L-AC, L- anterior SMA, L-posterior SMA, L-Le second frontal gyrus; R-anterior SMA R-posterior SMA, R-second frontal gyrus |
Lesionectomy | 2A |
| 12 | 51/M | Ictal: no changes Interictal: CP L |
FCD L central region | 4 | L-SM superior motor, L-IM inferior motor, L-SPC superior post-central, L-IPC inferior post-central. |
Lesionectomy (limited) | 3A |
In patients with temporal lobe epilepsy a standard implantation consisting of three electrodes implanted orthogonally and pointing at the amygdala (A), Hippocampus (HC) and the posterior Hippocampus (PHC) was used.
Patients with <12 month postsurgical period.
A = amygdala; AC = anterior cingulate; bi = bilateral; F = frontal; Fe = female; HC = Hippocampus; HET = heterotopia; L = left; M = male; MTL = mesial temporal lobe; O = occipital; OF = orbito-frontal; P = parietal; PC = Posterior cingulate; PHC = posterior Hippocampus; R = right; sAHC = selective amygdalo-hippocampectomy; SMA = supplementary motor area; T = temporal.
Recording methods
Electrodes were implanted stereotactically using an image-guidance system (SSN Neuronavigation System, Mississauga, Ontario, Canada). A combination of depth and cortical surface electrodes was placed according to the methods of Olivier and coworkers (1994). All electrodes were manufactured on site. A 10/1000-in. wire of stainless steel was used as a central core and wrapped with a 3/1000 in. steel wire. Each electrode had nine contacts (5 mm apart), with the deepest contact (Contact 1) consisting of the tip of the steel core stripped of insulation. This contact had a length of 1 mm, while all other contacts (2–9) were formed from stripped sections of the marginal wire that was tightly wound to create 0.5 mm long coils. The effective surface area for contact 1 was 0.80 and 0.85 mm2 for Contacts 2–9.
SEEGs were recorded using the Harmonie monitoring system (Stellate, Montreal, Canada). The SEEG was low-pass filtered at 500 Hz and sampled at 2000 Hz. We also recorded the electro-oculogram (EOG) and electromyogram (EMG) to facilitate sleep staging. The analyses reported below were performed using a referential montage with an epidural reference electrode placed in the parietal lobe of the hemisphere least likely to include the main focus.
Channel selection and sampling
We analysed interictal samples of 10 min of slow-wave sleep, as HFOs and spikes occur more frequently during slow-wave sleep (Staba et al., 2004; Bagshaw et al., 2008). Sleep stages were selected using EEG, EOG and EMG. The Harmonie software was used to compute spectral trends in the delta, alpha and beta bands for SEEG channels selected for having no or minimal epileptic activity, and power of the chin EMG, with a 30-s time resolution. EEG sections with high delta and low EMG power were visually reviewed to con-firm that they were stage 3 or 4. Slow wave sleep was defined by at least 25% delta activity by visual inspection of 30 s epochs. Additionally, segments were only selected if they were recorded at least 6 h before and after a seizure, to reduce the influence of seizures on our analysis.
Lesional channels were identified by combining the information from the reconstructed positions of the electrodes from the Neuronavigation system, a CT obtained directly after the implantation and an MRI after explantation. Lesional borders were defined by the neurosurgeon and neuroradiologist after examination of all available clinical MRI images (global T1 with gadolinium, T1 sagittal, T2 axial and coronal, FLAIR coronal). All channels within lesions were selected for this study. If the SOZ was outside the lesion, channels in the SOZ were also analysed. The SOZ was defined as the contacts which showed the first ictal activity during the intracranial investigation, as identified by the clinical neurophysiologist reviewing the EEG for clinical purposes. Additionally, at least three channels distant from the lesion and from the SOZ were selected in each patient. Depending on the number of lesional and SOZ channels, this resulted in a selection of 8–23 channels per patient. A list of the channel positions and selection is provided in (Supplementary Table S1).
Identification of interictal abnormalities
Two examiners independently reviewed the selected 10 min EEGs to identify pathological changes and epileptiform patterns in the unfiltered EEG with normal time scale (10 s/page). The findings were discussed and a consensus was built between the two reviewers on the description of the identified changes.
Marking of spikes and HFOs
Spikes and HFOs were visually marked in two separate copies of the same EEG. It is necessary to mark spikes separately from HFOs because spikes become invisible in the filtered and extended time scale EEG necessary to mark HFOs, while HFOs are rarely visible in the unfiltered EEG. All spikes were identified within the selected sample using a common time scale (10 s/page).
For the identification of HFOs, channels were displayed with the maximum time resolution, which corresponded to ~0.6 s across the computer monitor (1200 samples of a signal sampled at 2000 Hz). The EEG was high-pass filtered at 80 and 250 Hz using a finite impulse response (FIR) filter to eliminate ringing. The computer display was split vertically with an 80 Hz high-pass filter on one side and a 250 Hz high-pass filter on the other side. A ripple was marked if an event was clearly visible on the side of the 80 Hz filter and did not occur or show the same shape on the side of the 250 Hz filter, as it is defined as a distinct event between 80 and 250 Hz. An event was regarded as a fast ripple if it was visible in the 250 Hz filter. After all events were marked, the EEG was re-reviewed a second time by the same reviewer for verification. Only events containing at least four consecutive oscillations were regarded, as HFOs and two events were considered distinct when they were separated by at least two non-HFO oscillations.
Statistical analyses
After marking all events, a MATLAB (The Mathworks Inc., Natick, Massachusetts, USA) program calculated for each channel rates of ripples and fast ripples per minute (computed for every 1-min interval in the data), the co-occurrence of spikes and HFOs (defined as the intersection between a spike marking and an HFO marking) and the average duration of HFOs.
All analysed channels in the 12 patients were grouped according to their localization in regard to the lesion (lesional versus non-lesional), the SOZ (SOZ versus NSOZ) as well as both variables together (lesional/SOZ versus lesional/NSOZ versus non-lesional/SOZ versus non-lesional/Non-SOZ). Rates and durations of HFOs were compared between the different groups of channels using ANOVA. Statistical results were corrected, if necessary, for multiple comparisons and were analysed with the post hoc Tukey’s HSD (Honestly Significant Differences) Test. The level of significance was set at 0.05. Additionally, the percentage of spikes co-occurring with HFOs was calculated.
In a second step, these statistics were repeated subdividing the lesional channels according to the type of lesion into the three subgroups: MTA, FCD and NH. We analysed the clinical EEG features, occurrence of spikes and HFOs as well as their distribution for each lesion type.
Results
The results from the 12 patients were grouped and channels were classified according to whether they were lesional or non-lesional and within or outside the SOZ. In total, 163 channels were analysed: 106 were within a lesion and 57 outside and 61 were in a SOZ area and 102 outside. Forty-six of the 61 channels in the SOZ were within a lesion while the remaining 15 SOZ channels were in areas that showed no abnormalities on the MRI.
Overall, the rate of spikes was 10.5 ± 18.5/min. Spikes co-occurred in 37.1% with ripples and in 21.9% with fast ripples. The rate of ripples was 11.9 ± 16.9/min and of fast ripples, 6.9 ± 17.9/min. A temporal overlap with spikes was seen in 63.1% of ripples and 36.3% of fast ripples. Ripples occurred in 53.6% independently of fast ripples. The mean duration of ripples was 88.6 ± 41.9 ms, higher than that of fast ripples, which was 36.2 ± 24.3 ms.
Comparison of lesional and non-lesional SOZ and non-SOZ channels
This first analysis investigated primarily whether event rates were more closely linked to lesional tissue or to seizure origin. For this purpose, all channels of all patients were grouped into four categories: lesional SOZ/non-lesional SOZ/lesional NSOZ and non-lesional NSOZ. The concept of these four groups is illustrated in Fig. 1. Event rates and durations were statistically compared. Figure 2 shows a graph with the event rates in the different areas. Figure 4 gives the significant differences of the post hoc analysis between the four compared groups in the form of arrows: vertical arrows comparing SOZ to non-SOZ are clearly predominant compared with horizontal arrows, comparing lesional to non-lesional areas. In the text below we will not report the numerical details of the interactions between lesional SOZ and non-lesional non-SOZ areas as well as between non-lesional SOZ and lesional non-SOZ, as it would be very tedious. These interactions are shown as the diagonal arrows in Fig. 4 and they are secondary results compared with the main comparisons (vertical and horizontal arrows in Fig. 4 and numerical results below).
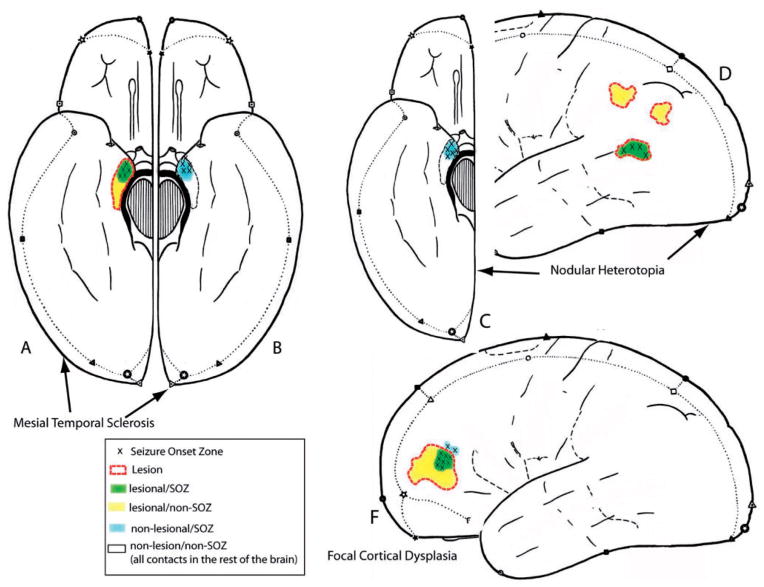
Figure 1.
This figure demonstrates the different relationships between SOZ and lesional areas in the different lesion types. In all patients contacts outside of lesion and SOZ were most frequent (non-Lesional/non-SOZ). (A and B) In MTA SOZ and lesional areas were most overlapping on the side of the lesional mesial temporal structure (lesional/SOZ) and some of the lesional areas did not show seizure onset (lesional/non-SOZ) (Part A). Contra-laterally to the lesion a second SOZ was seen in some patients (non-lesional/SOZ, Part B). (C and D) In Nodular Heterotopia most of the SOZ areas were not located in the lesion (nodule) but in non-lesional mesial temporal structures (non-lesional-SOZ) (Part C). Some SOZ areas were located within the nodule (lesional/SOZ) and many nodules did not show any seizure activity (lesional/non-SOZ) (Part D). (F) In patients with FCD the seizure onset mostly overlapped with parts of the lesion (lesional/SOZ) and in one patient extended outside the borders of the lesion on MRI (non-lesional/SOZ). Parts of the lesion could be inactive (lesional/non-SOZ).
Figure 2.
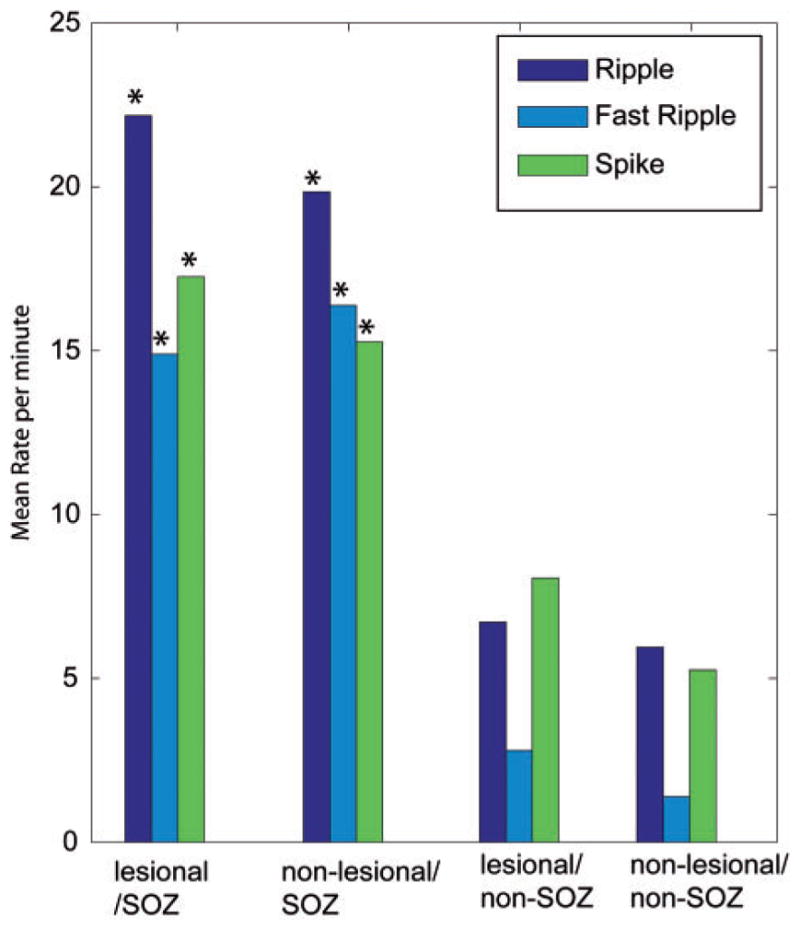
Rates per minute for the different events and the different regions as analysed, including all lesion types. Significant differences were found for the comparison SOZ versus non-SOZ but not for the comparison lesional versus non-lesional. In the ANOVA the rates of all event types were significantly higher in the lesional SOZ areas than in all areas outside the SOZ (*P<0.001). The same was observed for all rates of non-lesional SOZ areas compared with areas outside the SOZ (*P<0.001).
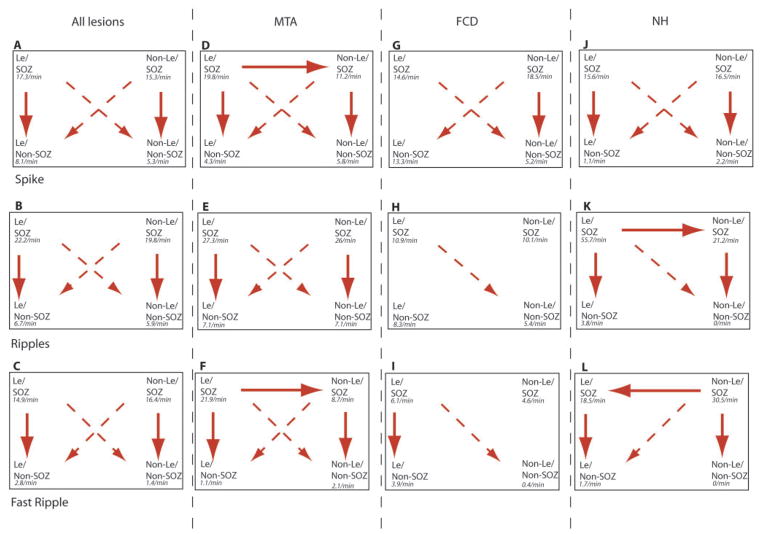
Figure 4.
Overview of the significant results in the post hoc analysis of all lesion together (A–C), and separately for MTA (D–F), FCD (G–I) and NH (J–K) patients. Arrows are given for all significant results (P<0.05), vertical arrows presenting the SOZ versus non-SOZ comparison and horizontal arrows the lesional versus non-lesional comparison. Diagonal arrows are shown with interrupted lines as their meaning can only be interpreted in combination with other arrows. The predominance of downward pointing vertical arrows underlines the conclusion of our results, that HFOs are predominant in SOZ areas and this statement is supported by the diagonal arrows.
Spikes
ANOVA results showed that there was a significant difference in rates of spikes for channels inside and outside the SOZ [F(1,1626) = 89.4, P<0.001] and for channels inside and outside the lesion [F(1,1626) = 5.6, P = 0.01].
In the following post hoc analysis, rates in the SOZ were significantly higher than rates in non-SOZ channels (vertical arrows Fig. 4A). Rates were as follows: SOZ-lesional (17.3 ± 12.8/min) and non-lesion (15.3 ± 15.6/min); non-SOZ-lesional (8.1 ± 24.1/min) and non-lesional (5.3 ± 9.5/min). However, there was no significant difference between the rates for lesional and non-lesional channels. Therefore the rate of spikes allowed to clearly differentiate SOZ areas from non-SOZ areas, but not lesional from non-lesional areas, when comparing the four groups.
HFOs
The results of the ANOVAs were very similar for HFOs to those for spikes. Significant differences were found in the comparison of SOZ channels versus non-SOZ channels independently of the relation to the lesion [ripples: F(1,1626) = 287.7, P<0.001; fast ripples: F(1,1626) = 202.6, P<0.001)], while no differences in rates of HFOs could be seen between lesional and non-lesional channels.
In the post hoc tests, the rate of ripples was significantly higher in SOZ channels than in channels outside the SOZ (vertical arrows Fig. 4B; lesional SOZ: 22.16 ± 22.4/min versus lesional—non-SOZ: 6.7 ± 10.4/min, P<0.001; non-lesional SOZ: 19.8 ± 17.4/min versus non-lesional non-SOZ: 5.9 ± 10.1/min, P<0.001). ‘Fast ripples’ showed a very similar distribution: they were more frequent in the SOZ (vertical arrows Fig. 4C; lesional 14.9 ± 23.9/min; non-lesional 16.4 ± 28.9/min) than in non-SOZ areas (lesional 2.8 ± 10.7/min; non-lesional 1.4 ± 4.2/min, P<0.001), but no difference could be found between lesional and non-lesional areas.
Therefore, as for spikes, the rates of ripples and fast ripples were clearly linked to SOZ areas. Lesional areas outside the SOZ had nearly as low rates of HFOs as areas outside lesion and SOZ (white regions in Fig. 1).
ANOVAs testing the durations of ripples and fast ripples showed significant differences in duration for both the SOZ and lesional effects. [ripples: F(1,20359) = 178.9, P<0.001, fast ripples: F(1,12103) = 141.6, P<0.001].
The following post hoc tests revealed that durations were longest in the lesional SOZ areas (ripples: 92.9 ± 43.8 ms, fast ripples: 40.6 ± 26.6 ms). Non-lesional SOZ and non-SOZ areas showed no significant difference in duration between them, but were both significantly shorter than the lesional SOZ channels (P<0.001). The shortest durations were observed in the lesional non-SOZ channels (ripples: 79.1 ± 40.2 ms, fast ripples: 26.6 ± 13.1 ms, P<0.001).
The durations of HFOs were therefore longest in the SOZ areas, and even significantly longer in those, which were lesional. However, in lesional areas that were not involved in the SOZ, the durations were the shortest. The durations of the events therefore seem to be closely linked to seizure generation and not to pathological tissue changes.
Effects of lesion type
The second part of our study aims to evaluate the effects of different types of lesion on the generation of HFOs.
Localization of the lesion and relation to the SOZ
Five patients (Patients #1–5) had unilateral hippocampal atrophy (the schematic representation of the relation between SOZ and lesional areas is given in Fig. 1A and B); in four, all seizures were generated in the mesial temporal structures on the atrophic side. The fifth patient had seizures from the atrophic mesial structures but also seizures starting in the contralateral hippocampus, which did not show any change on the MRI. One patient had marked atrophy and sclerosis but additionally a mal-rotation of the same hippocampus.
Four patients (Patients #6–9) with FCD were investigated; three had one localized lesion and their seizure onset was recorded in a part of this lesion, and in one of these patients, the seizure onset area also included a region outside the lesion. The fourth patient had several dysplastic lesions, all covered by depth electrodes and with seizures starting in only one of them (Fig. 1F).
Periventricular nodular heterotopia was found in three patients (Patients #10–12). In one, seizures started within the heterotopic nodule, in the second, seizures were recorded from both mesial temporal lobe structures and in the third, seizures started in a widespread area in dysplastic cortex above the nodule (Fig. 1C and D).
The clinical findings and the localization of the depth electrodes are summarized in Table 1.
Clinical SEEG-findings
We analysed the unfiltered EEGs in all patients (Supplementary Table S2). Patients with MTA showed no specific EEG changes compared with patients with other lesions. In all patients with MTA interictal epileptic discharges (IEDs) exceeded the extent of the lesion and in patients implanted in both MTLs, IEDs were bilateral and independent. Therefore no clear identification of the lesion was possible by looking at the patterns of interictal EEG.
Two of the four patients with FCD (Patients 11 and 12) did not show any abnormal EEG in the areas implanted outside the lesions. The interictal discharges however could be seen in all the lesional channels and were not specific to those belonging to the SOZ. In the two other patients, spikes were more widespread. One of these had multifocal dysplastic areas (Patient 9); interestingly, only the lesion involved in the SOZ showed interictal discharges. The other lesional areas did not show abnormal activity, but inter-ictal discharges were recorded from brain region without MRI changes, which may point towards subtler dysplastic areas invisible to MRI. In all patients, intervals of continuous spiking and frequent polyspikes, described as typical for this kind of lesion, could be observed.
IEDs were seen over widespread brain areas in patients with nodular heterotopias as well (Patients 6–8). In all of them, the implanted MTL structures showed interictal abnormalities even if they were not part of the lesion. IEDs were very abundant and poly-spikes occurred frequently. The latter were even found in lesional areas that were not part of the SOZ.
In conclusion, IEDs were limited to the lesional areas only in some patients with FCD. No specific EEG patterns were found for the different lesions except the pattern of continuous spiking or very frequent polyspikes, which was found in all patients with FCD and was then limited to the lesional areas.
Comparison of lesional and SOZ areas in the different types of lesion
The ANOVA compared the rates of all event types in all types of lesions across the four conditions: lesional SOZ, non-lesional SOZ, lesional non-SOZ and non-lesional non-SOZ. Significant differences were found for the rates of all event types [spikes: F(11,1618) = 18.8, P<0.001; ripples: F(11,1618) = 78.1, P<0.001; fast ripples: F(11,1618): 40.7, P<0.001]. The ANOVA also indicated significant differences in the durations of ripples [F(10,20 352) = 30.5, P<0.001] and fast ripples [F(10,12 096) = 161.7, P<0.001]. In the following, we report the post hoc results for the three lesion types separately comparing the four regions. Figures 3 and 4 summarize these comparisons.
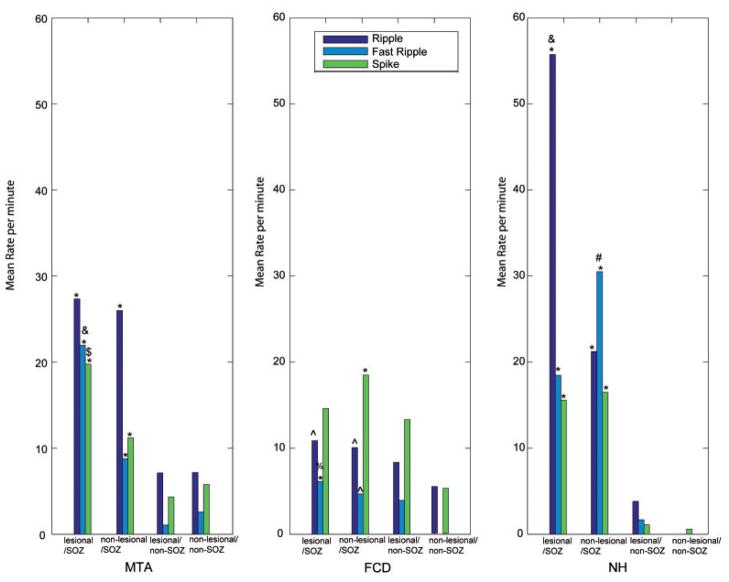
Figure 3.
Rates of the different events in the different regions for the three lesion types separately and statistical results of the post hoc test after ANOVA comparing the four different regions (lesional SOZ, non-lesional SOZ, lesional non-SOZ and non-lesional non-SOZ). In MTA all rates of the different events were significantly higher in the lesional and non-lesional SOZ areas compared with areas outside the SOZ (*P<0.001). The rate of fast ripples was significantly higher in lesional than in non-lesional SOZ areas (&P<0.001). The rate of spikes showed the same pattern but with less significance ($P = 0.04). In FCD rates of fast ripples in the lesional SOZ and rates of spikes in the non-lesional SOZ were significantly higher than in all areas of the SOZ (*P<0.001). Rates of ripples in the lesional SOZ and rates of ripples and fast ripples in the lesional and non-lesional SOZ were higher than in non-lesional areas outside the SOZ (^P<0.001), but not towards lesional areas outside the SOZ. Only fast ripple rates were higher in lesional SOZ areas than in lesional non-SOZ areas (%P = 0.04). In NH all rates of the different events were significantly higher in the lesional and non-lesional SOZ areas compared with areas outside the SOZ (*P<0.001). The rates of ripples and fast ripples were significantly higher in the non-lesional SOZ than in lesional SOZ areas (ripples &P<0.001; fast ripples #P<0.001).
MTA
Spikes were significantly higher in SOZ than in non-SOZ areas (vertical arrows Fig. 4D). There was also a difference between lesional and non-lesional SOZ areas (horizontal arrow, Fig. 4D). The rates were as follows: SOZ—inside the lesion (19.8 ± 13.5/min) and outside the lesion (11.2 ± 10/min); non-SOZ inside (4.3 ± 7.1/min) and outside the lesion (5.8 ± 7.7/min).
The rates of ripples were significantly higher in SOZ areas than in the non-SOZ areas (vertical arrows Fig. 4E) (SOZ lesional: 27.3 ± 19.9/min versus non-SOZ lesional: 7.1 ± 11.6/min, SOZ non-lesional 26.0 ± 22.9/min versus non-SOZ non-lesional 7.2 ± 10.2/min). No difference was seen between lesional and non-lesional areas (no horizontal arrow in Fig. 4E).
Fast ripples were also higher in SOZ areas than in the non-SOZ areas (vertical arrows Fig. 4F; SOZ lesional: 21.9 ± 29.4/min versus non-SOZ lesional: 1.1 ± 1.8/min, SOZ non-lesional 8.8 ± 8.8/min versus non-SOZ non-lesional 2.6 ± 5.9/min). Fast ripple rates were additionally significantly higher in lesional than in non-lesional SOZ areas (horizontal arrow Fig. 4F).
The durations of ripples and fast ripples were longest in lesional SOZ areas (P<0.001), but no significant differences could be found between the other areas.
For patients with MTA, all event types were therefore more closely linked to the SOZ than to the lesion. Spikes and fast ripples however were also linked to lesions as they were higher in lesional than non-lesional SOZ areas. This was not reflected in non-SOZ areas where lesional and non-lesional regions could not be separated by any of the event types. An example of a patient with bilateral SOZs and with very frequent HFOs in both mesial temporal structures is shown in Fig. 5, which demonstrates HFO activity in the lesional SOZ area.
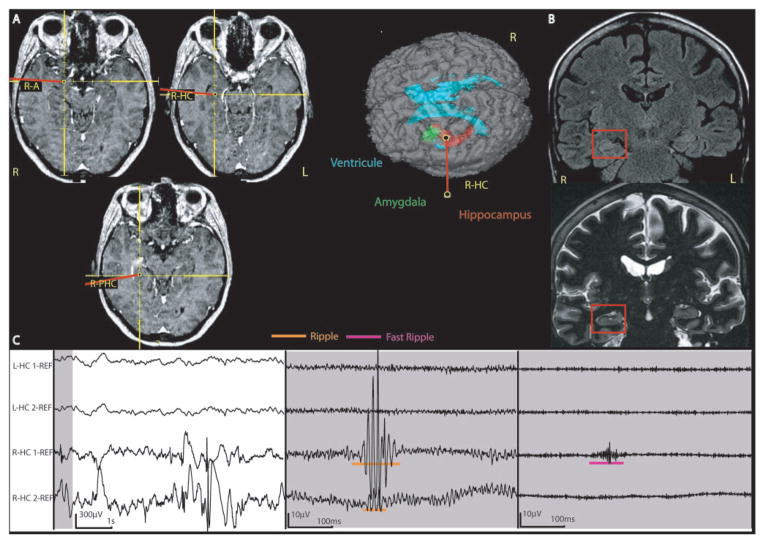
Figure 5.
Patient with right mesial temporal atrophy and bilateral SOZ areas (Patient #2). (A) implantation sites in relation to the lesional and SOZ contacts on the right side, showing the implantation of the mesial temporal structures as used in most patients. This patient had additionally the same implantation on the left side. (B) anatomical images (FLAIR and IRFSE) of the patient’s hippocampal atrophy, confirmed by volumetric measures. (C) intracranial EEG. The left panel shows the unfiltered EEG with a normal time scale; the middle shows the grey section from the left panel in extended time scale and high gain, and with a high pass filter of 80 Hz; and the right the same section with a filter of 250 Hz. A ripple in R-HC1 and 2 and a fast ripple in R-HC1 in the lesional/SOZ area are demonstrated. The left mesial temporal structures showed HFOs as well; this region was part of a SOZ and not of the lesional area. L = left; R = right; HC = Hippocampus; PHC = Parahippocampus; A = Amygdala.
Focal cortical dysplasia
This group differed from the two others as most of the investigated areas were outside the mesial temporal lobe structures, which may be a reason for the generally lower rates of events. Also, only one patient had channels in non-lesional SOZ areas and he therefore was the only one contributing to this category.
When comparing SOZ to non-SOZ, differences were limited to non-lesional areas (vertical arrow in Fig. 4G). Rates were as follows: SOZ- lesional (14.6 ± 13.5/min) and non-lesional (18.5 ± 25.5/min); non-SOZ lesional (13.3 ± 12.2/min) and non-lesional (5.2 ± 11.2/min). There was no difference beween lesional and non-lesional areas (no horizontal arrow on Fig. 4G).
For ripples no difference could be seen for the direct comparisons (SOZ versus non-SOZ and lesional versus non-lesional, Fig. 4H). Rates were as follows: SOZ—inside the lesion (10.9 ± 7.3/min) and outside the lesion (10.1 ± 6.7/min); non-SOZ inside (8.4 ± 10.3/min) and outside the lesion (5.4 ± 10.3/min).
For fast ripples, when comparing SOZ to non-SOZ, differences were limited to the lesional channels (vertical arrow Fig. 4I; lesional SOZ: 6.1 ± 10.7/min, non-lesional SOZ: 4.7 ± 5.4/min, lesional non-SOZ: 3.9 ± 14.0/min, non-lesional non-SOZ: 0.4 ± 0.9/min). There was no difference between lesional and non-lesional areas (no horizontal arrow on Fig. 4I). No significant differences in the durations of ripples and fast ripples were found for this group of patients.
Although patients with FCD had in general lower event rates, most of the SOZ was within a lesion and rates were clearly highest there, as shown in Fig. 6. In this group it remains unclear whether the events are linked to the lesion or the SOZ as there is a great overlap of both regions. This is especially the case for fast ripples, which seem to be linked to both lesion and SOZ, while they are not seen in unaffected areas.
Figure 6.
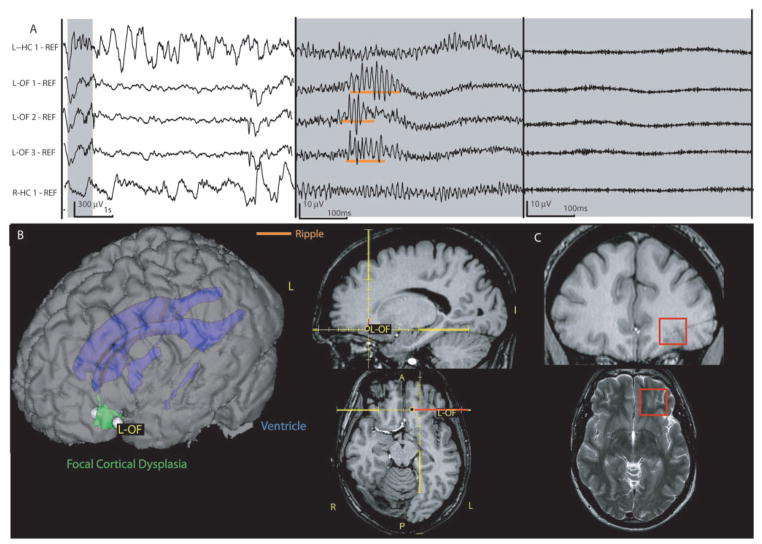
Patient with a focal cortical dysplasia in the left fronto-orbital region (Patient 9). The SOZ is limited to the lesion. (A) EEG displayed as in Fig. 5 showing highpass-filtered data extended time scale in the middle (80 Hz) and on the right (250 Hz). A ripple oscillation in the lesional/SOZ area LOF1-3 without a co-occuring fast ripple can be seen. No HFOs are observed over both hippocampal contacts during that time and rates were generally low in this patient, who did not show any seizures deriving from the mesial temporal structures. (B) implantation site of the electrode L-OF aiming at the lesion and SOZ. The patient had additional electrodes, not shown here, including bitemporal electrodes implanted as shown in Fig. 5. (C) FCD shown within the red frame on the axial T2 and the coronal T1 image. The T1 image also shows the electrode tract after explantation. L = left; R = right; OF = orbito-frontal.
Nodular heterotopia
This group differed from the two others as most of the SOZ areas were actually located outside the lesion and some lesions were completely ‘silent’, showing none of the event types.
Spikes were significantly higher in SOZ than in non-SOZ areas (vertical arrows Fig. 4J). They were highest in SOZ areas outside the lesion. No difference between lesional and non-lesional areas could be found (no horizontal arrow in Fig. 4J). The rates were as follows: SOZ-lesional (15.6 ± 14.5/min) and non-lesional (16.5 ± 9.4/min); non-SOZ lesional (1.1 ± 2.2/min) and non-SOZ non-lesional (0.6 ± 1.2/min).
Rates of ripples were higher in SOZ areas compared with non-SOZ areas (vertical arrows Fig. 4K; lesional SOZ: 55.7 ± 45.3/min, non-lesional SOZ: 21.2 ± 14.1, lesional non-SOZ: 3.8 ± 9.6/min and non-lesional non-SOZ: 1/min). A difference between lesional and non-lesional areas was limited to the SOZ areas (horizontal arrow Fig. 4K).
Fast ripples were highest in SOZ areas outside the lesion. They were significantly more frequent in SOZ channels than outside the SOZ (vertical arrows Fig. 4L; lesional SOZ: 18.5 ± 22.0/min, lesional non-SOZ: 30.5 ± 41.1/min, non-lesional SOZ 1.7 ± 5.1/min, non-lesional non-SOZ: 0/min). Rates of fast ripples were also significantly higher in SOZ areas outside the lesion that in those inside the lesion (left-pointing horizontal arrow Fig. 4L).
The durations of ripples were longest in non-lesional SOZ (89.1 ± 39.8 ms, P<0.001); it was significantly longer than in lesional SOZ areas (81.1 ± 31.8 ms, P<0.001) and all other areas (72.2 ± 29.4 ms, P<0.001). There were no significant differences in durations of fast ripples in the different areas.
Patients with NH often had SOZ areas outside the lesion. Fast ripples as well as spikes were clearly more linked to this SOZ than to the lesion. Ripples however showed a link to both lesional and SOZ areas and it remains unclear, which were more important for their occurrence. The rates of fast ripples and spikes were very low in nodules, which did not show any relation to the SOZ and HFOs did not occur at all in non-lesional/non-SOZ areas. An example of a patient with nearly no HFOs in his two nodules but frequent HFO activity in the mesial temporal SOZ areas is shown in Fig. 7.
Figure 7.
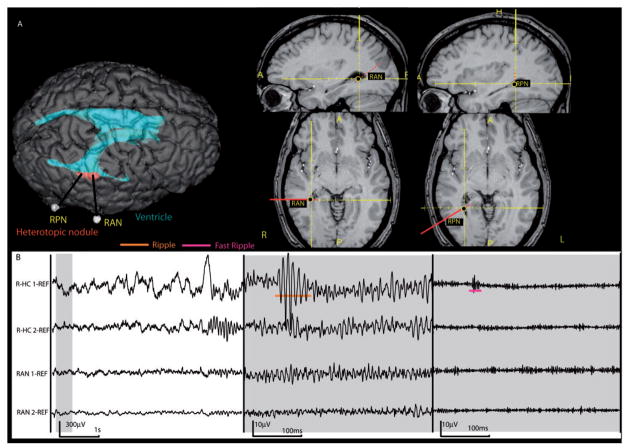
This patient (#6) had two nodular heterotopias close to the ventricles. His seizures all derived from the mesial temporal structures without involvement of the nodules. (A) implantation of the contacts RAN (anterior portion of the nodule) and RPN (posterior portion of the nodule). Additionally the patient had electrodes placed in the right mesial temporal structures similarly to those shown in Fig. 4. (B) EEG illustrated as in Fig. 5, showing a ripple oscillations as well as a co-occurring fast ripple oscillation in R-HC1. No HFOs were observed within the nodules. L = left, R = right, HC = hippocampus, AN = anterior nodule, PN = posterior nodule.
Discussion
The most important finding is that HFOs, ripples and fast ripples, are more closely linked to the areas involved in seizure generation than to lesional areas. These findings were especially clear in patients with MTA, in whom HFOs are frequently found in the non-lesional mesial temporal structures in which seizures occurred, and in patients with NH, in whom some of the heterotopia generated no identifiable HFOs and no seizure either.
Methodological considerations and HFOs in general
HFOs can be recorded with macro-electrodes with a surface of 0.8 mm2 (Urrestarazu et al., 2007; Bagshaw et al., 2008) and seem to have similar spatial patterns and frequencies as oscillations recorded with micro-electrodes (Bragin et al., 1999; Staba et al., 2002). However, recent studies using macro-electrodes with larger size (7.4 mm2) showed differences in the frequency and rate of HFOs below 250 Hz, when comparing macro- to micro-electrodes (Worrel et al., 2008). Studies comparing different electrode sizes are still pending to assess the differences between HFO findings in large macro-electrodes versus micro-electrodes. One advantage of the electrodes we used, however, is that they can record HFOs over their full length with standard EEG equipment (Jacobs et al., 2008); all contacts have the same size and can record HFOs from several areas including neocortex. This is especially important as we wanted to record from lesional and non-lesional locations in patients with neocortical lesions.
The relationship between HFOs and IEDs is not completely understood. There is evidence that both event types can occur together or separately (Urrestarazu et al., 2007). In an earlier study (Jacobs et al., 2008) we calculated rates of spikes, ripples and fast ripples and evaluated their overlap and co-occurrence. About 40% of ripples and 50% of fast ripples occur at different times than spikes regardless of whether HFOs are recorded in SOZ areas, lesional areas or in remote cortical structures. For this reason, only the rates of ripples, fast ripples and spikes were calculated and used for statistical comparison and not their co-occurrence. The rates for all three event types are similar to previous studies by our group (Bagshaw et al., 2008; Jacobs et al., 2008) but higher than the rates reported in micro-electrodes (Bragin et al., 1999). This may be related to the fact that macro-electrodes may record HFOs generated in a larger brain area than micro-electrodes. If HFOs are very focal events, it may be easier to record them with macro-electrodes, as they record events from a broader region than micro-electrodes.
HFOs can also be detected using automatic detection methods (Staba et al., 2002; Khalilov et al., 2005). In this study, we aimed to analyse not only the rate of HFOs but wanted to recognize differences in shape of HFOs of distinct lesion types, if they were present. Only visual analysis allows for this comparison. Additionally a distinction of HFOs within and outside spikes to our knowledge is not possible with existing HFO detection methods. Visual marking allowed us to perform this distinction (there was no significant differences in regard to lesion or SOZ for these two types of HFOs). The disadvantage of visual markings is the limitation of time periods that can be analysed, as the technique is very time consuming. In this study, one 10-min segment of slow wave sleep was evaluated. A previous study has shown that the localization and distribution of HFOs remains stable even when looking at EEG segments of different sleep stages (Bagshaw et al., 2008). There are indications that probably less than 10 min of EEG provide stable information on HFO rates and distribution (Zelmann et al., 2008). It is therefore likely that our results are representative for the patients’ HFO distribution.
There is no uniform definition of the frequency bands of ripples and fast ripples; we defined ripples as events between 80 and 250 Hz and fast ripples above this frequency (250–500 Hz), as suggested by Urrestarazu et al. (2007). While there seems to be agreement that fast ripples are an indicator of pathological tissue, having been recorded from lesional (Staba et al., 2007) and from epileptogenic areas (Bragin et al., 1999, 2002), ripples were variably defined and interpreted. In rats, ripples between 100 and 250 Hz were reported to be physiological oscillations of mesial temporal structures during memory consolidation (Chrobak and Buzsaki, 1996; Draguhn et al., 2000). At frequencies of 80–160 Hz, they were also found in the less epileptogenic hippocampus in humans and were interpreted as physiological rhythms (Bragin et al., 1999). On the other hand, we found a clear increase in the rates of ripples between 80 and 250 Hz inside SOZ areas compared with outside and concluded that at least some of these oscillations are linked to epileptogenicity (Urrestarazu et al., 2007; Jacobs et al., 2008). These findings were replicated in the present study in which ripples were clearly more frequent in SOZ areas, although to a lesser degree than fast ripples. It remains a challenge for future studies to develop criteria that allow a distinction between physiological and pathological HFOs within the mesial temporal structures in micro-electrodes as well as in macro-electrodes.
Studies in neocortical areas are limited. We found a high rate of ripples in SOZ areas outside the mesial temporal structures, as reported by Ochi et al. (2007), and ripples therefore seem pathological in some patients at least. Neocortical HFOs have only been reported as a result of somatosensory stimulation and not spontaneously (Hashimoto, 2000; Gobbele et al., 2004). It is therefore unlikely that our results in neocortical areas are significantly influenced by physiological HFOs, although a systematic exploration of normal cortex remains to be done. It has been hypothesized that pathological HFOs result from excitatory GABAergic interneurons or an imbalance between excitatory and inhibitory interneurons within epileptic tissue. While ripples in physiological conditions may result from synchronous GABAergic interneuron inhibition (Chrobak et al., 2000), ripples and fast ripples increased in rate and duration after applying GABA receptor antagonists (Bragin et al., 2002; Jones and Barth, 2002). Staba and co-workers analysed the degree of neuronal loss in patients with mesial temporal sclerosis and its relationship to HFO occurrence (Staba et al., 2007). They concluded that histopathologic changes as seen in this type of lesion can lead to the generation of fast ripples and, as a result, promote epileptogenicity in mesial temporal structures. Nevertheless, it has been unclear whether HFOs are a mirror for pathological changes such as neuronal loss, synaptic re-organization and abnormal neurons or whether they are specific products of epileptogenic tissue. This question could only be answered by looking at different types of lesion in which there are various degrees of overlap between lesional and SOZ areas. Our general analysis demonstrated that HFOs were more linked to the SOZ than the lesion with clear differences between SOZ (lesional or non-lesional) and non-SOZ areas, but no differences between lesional and non-lesional areas. It, however, has to be mentioned that patient groups for each pathology were relatively small. Repeated studies on larger groups are therefore desirable.
MTA and sclerosis
In patients with MTA, the lesion is believed to be intrinsically epileptogenic; in patients with bitemporal lobe seizures and unilateral lesions, the removal of the lesion is usually associated with good surgical outcome (Hennessy et al., 2001; Mintzer et al., 2004). The size of the electrodes in this study does not allow analysing the specific structures within the hippocampus, and HFOs can only be recorded with two contacts each from the amygdala, hippocampus and parahippocampus. We cannot therefore comment on the influence of cell loss on HFO generation in specific structures. In the analysed patients, the relationship between the lesion and the SOZ was not clear initially, which justified intracranial investigation. Even if the five patients of this group had a unilateral lesion, as defined by MRI, and one non-lesional hippocampus, two patients had bi-temporal seizure onsets, one had a very subtle lesion only detected after volumetric measurements and two had additional sclerotic lesions in the same temporal lobe. The potentially widespread epileptogenicity of tissue in those patients was also reflected by widespread IEDs, which were bilateral in the three patients explored bilaterally.
Ripples and spikes in this patient group were clearly linked to the seizure onset areas, as they were much less frequent in all non-SOZ areas. Lesional regions had comparably low rates if they did not include SOZ areas. Especially the sclerotic lesions outside the mesial temporal structures (Patients #3 and 4) had very low rates. One could argue that the difference is related to the fact that SOZ areas were all within mesial temporal structures, which generate more HFOs in general (Jacobs et al., 2008). There were however also several contacts in contralateral hippocampi outside the SOZ, which showed fewer HFOs than the ones generating seizures. Fast ripples were most frequent in the SOZ compared with all non-SOZ areas. They additionally showed a significant difference between lesional and non-lesional SOZ areas. No difference between the lesional and non-lesional non-SOZ areas was seen and the fast ripple rates were actually lowest in the lesional non-SOZ areas. For this reason it cannot be concluded that fast ripples are generated by pathologic tissue in general. A confounding factor in our study could be that mesial temporal structures, which show no MRI, abnormalities sometimes show tissue changes in pathology samples (Bote et al., 2008). Therefore we cannot exclude that some of our patients had subtle lesional changes on the contra-lateral side. Additionally two patients were only implanted unilaterally and conclusions about the contralateral side cannot be drawn. There was however no indication of bilateral SOZs during surface or depth EEG recordings in these patients. It is thus most likely that the lesional areas identified in this study are those with the most prominent lesional changes. The observation that surgery in patients with mesial temporal lesions may lead to a good seizure outcome even if some seizures are recorded from the contralateral hippocampus (Hirsch et al., 1991), suggests that lesional SOZ areas in mesial temporal lobe epilepsy are more epileptogenic than non-lesional SOZ areas.
Focal cortical dysplasia
In all patients with FCD the SOZ was located within the lesion and in only one patient it extended beyond the borders of the MRI lesion. However, IEDs extended beyond the lesion, as described in patients with FCD (Tassi et al., 2001). One patient even showed frequent interictal discharges in both mesial temporal regions. Continuous spiking and polyspikes are frequent in patients with FCD (Palmini et al., 1995; Chassoux et al., 2000). This pattern was seen in all our FCD patients and it was limited to the lesional areas.
The originally described FCD of Taylor type was characterized by cortical disorganization, cytoskeletal anomalies and balloon cells (Taylor et al., 1971). Focal dysplasia can show varying degrees of pathological changes ranging from laminar cortical disruption only to lesions including also dysmorphic giant cells and undifferentiated balloon cells (Tassi et al., 2002). Abnormal synaptic connectivity and changes in neurotransmitter systems are believed to cause the high epileptogenicity of these lesions (Ferrer et al., 1992; Mattia et al., 1995; Spreafico et al., 1998) and may be mechanisms that lead to the generation of pathological HFOs.
The rates of HFOs in patients with FCD were in general lower than in the other patient groups, likely as a result of their location in the neocortex. Urrestarazu and co-workers (2007) described a very high rate of fast ripples in a patient with FCD, which occurred in the whole lesion and was not limited to the seizure onset area. In our study a clear indication of whether HFOs were more linked to the SOZ or the lesion was more difficult to obtain in this patient group than in the others. No fast ripples were seen in the control channels outside the lesion and outside the SOZ. Fast ripples were higher in lesional SOZ areas than in lesional non-SOZ areas; they therefore seem more clearly linked to the seizure origin than to the lesional areas. The high rate of all events in the remaining lesional tissue outside the SOZ, which was higher than in MTA and NH may be an indicator of the potential epileptogenicity of these parts of the lesion. Lesional non-SOZ areas might not be directly involved in the seizure origin at the time of the investigation but might turn into a seizure focus after the removal of primary epileptogenic tissue (Cohen-Gadol et al., 2004). Removal of the entire lesion and surrounding interictally active tissue may thus be necessary for long-term seizure relief (Hader et al., 2004; Alexandre et al., 2006). However, studies including the post-surgical outcome of our patients would be necessary to finally conclude on this issue.
Nodular heterotopia
Gray matter heterotopia are caused by a halt in neuronal radial migration and consist of clusters of neurons separated from the cortex by white matter and not organized in layers like normal cortex (Barkovich and Kjos, 1992). It has been discussed whether nodular heterotopia themselves are the areas of seizure onset or whether they are just part of a network with extended developmental damage and seizures occurring independently of these lesions. This question is raised as many patients with depth electrode investigations show a seizure onset outside their lesion (Dubeau et al., 1995; Kothare et al., 1998; Aghakhani et al., 2005; Tassi et al., 2005). We observed similar findings in our three patients; in only one, parts of the heterotopia were involved in the seizure onset. However, temporal lobe resections in patients with NH and temporal lobe seizures lead to worse seizure outcome compared with resections on patients with clear temporal lobe epilepsy (Li et al., 1997), suggesting that epileptogenicity in these patients is widespread. In a recent study, Tassi et al. (2005) showed that corticectomy including parts of the heterotopia that was active during the seizure, and the surrounding tissue that showed more epileptogenic activity than the nodule itself, resulted in a preferable outcome.
HFOs have not been described in patients with NH. It remains hypothetical that alterations in the GABAergic system within the nodules (Hannan et al., 1999; Kakita et al., 2002) may be expressed by the generation of pathological HFOs. Our findings, however, suggested that HFOs are linked primarily to seizure onset and are rarely generated within the NH. All event types were significantly more frequent in the SOZ areas. It is also remarkable that, in this group, fast ripples were most frequent in the non-lesional SOZ, while they were rare in lesional non-SOZ areas. In fact, fast ripples in the latter areas were only seen in one patient (Patient #8) and not in the NH but in the overlying malformed cortex. Ripples, however, did not show such a clear differentiation between lesional and SOZ areas. While they did not occur, as expected, in non-lesional non-SOZ areas, they were relatively frequent in all other areas. Whether this reflects a potential epileptogenicity of lesional non-SOZ areas remains open. Spikes occurred relatively widespread involving the mesial temporal structures and some lesional areas, their rate, however, seemed strongly linked to the SOZ.
This group of patients was small and relatively heterogeneous. We can, however, conclude that patients with NH seem to have different types of seizure onset, sometimes completely outside the lesion and sometimes with some overlap with the lesion, as described before. Fast ripples may be a good indicator for the SOZ in these patients.
In summary, HFOs, especially fast ripples, are closely linked to seizure onset areas, and seem to be relatively independent of lesional changes. However, in focal cortical dysplasia and sometimes in NH, HFOs occur in lesional areas that are not part of the SOZ, and they may indicate potential epileptogenicity of these lesions.
Supplementary Material
Acknowledgments
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The authors have no professional or financial affiliations that might be perceived as having biased the presentation.
Funding
Canadian Institutes of Health Research (MOP-10189); Preston Robb Fellowship, Montreal Neurological Institute (to J.J.).
Abbreviations
- EOG
electro-oculogram
- EMG
electromyogram
- FCD
focal cortical dysplasia
- FIR
finite impulse response
- FR
fast ripples
- HFOs
high frequency oscillations
- HSD
honestly significant differences
- IEDs
interictal epileptic discharges
- MCD
malformations of cortical development
- MTA
mesial temporal atrophy
- NH
nodular heterotopiaheterotopias
- SOZ
seizure onset zone
Footnotes
Supplementary material is available at Brain online.
For Permissions, please journals.permissions@oxfordjournals.org
References
- Abosch A, Bernasconi N, Boling W, Jones-Gotman M, Poulin N, Dubeau F, et al. Factors predictive of suboptimal seizure control following selective amygdalohippocampectomy. J Neurosurg. 2002;97:1142–51. doi: 10.3171/jns.2002.97.5.1142. [DOI] [PubMed] [Google Scholar]
- Aghakhani Y, Kinay D, Gotman J, Soualmi L, Andermann F, Olivier A, et al. The role of periventricular nodular heterotopia in epileptogenesis. Brain. 2005;128 (Pt 3):641–51. doi: 10.1093/brain/awh388. [DOI] [PubMed] [Google Scholar]
- Alexandre V, Jr, Walz R, Bianchin MM, Velasco TR, Terra-Bustamante VC, Wichert-Ana L, et al. Seizure outcome after surgery for epilepsy due to focal cortical dysplastic lesions. Seizure. 2006;15:420–7. doi: 10.1016/j.seizure.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Babb TL, Brown WJ. Pathological findings in epilepsy. In: Engel J Jr, editor. Surgical treatment of the epilepsies. New York: Raven Press; 1987. pp. 511–40. [Google Scholar]
- Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high frequency oscillations recorded from depth macro-electrodes in patients with focal epilepsy. Epilepsia. 2008 doi: 10.1111/j.1528-1167.2008.01784.x. Epub ahead of print 17 Sep 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Kjos BO. Gray matter heterotopias: MR characteristics and correlation with developmental and neurologic manifestations. Radiology. 1992;182:493–9. doi: 10.1148/radiology.182.2.1732969. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Raybaud CA. Neuroimaging in disorders of cortical development. Neuroimaging Clin N Am. 2004;14:231–54. doi: 10.1016/j.nic.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Barth PG. Disorders of neuronal migration. Can J Neurol Sci. 1987;14:1–16. doi: 10.1017/s031716710002610x. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Granata T, Farina L, D’Incerti L, Franceschetti S, Avanzini G. Periventricular nodular heterotopia: epileptogenic findings. Epilepsia. 1997;38:1173–82. doi: 10.1111/j.1528-1157.1997.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Bernasconi A, Antel SB, Collins DL, Bernasconi N, Olivier A, Dubeau F, et al. Texture analysis and morphological processing of magnetic resonance imaging assist detection of focal cortical dysplasia in extra-temporal partial epilepsy. Ann Neurol. 2001;49:770–5. [PubMed] [Google Scholar]
- Boonyapisit K, Najm I, Klem G, Ying Z, Burrier C, LaPresto E, et al. Epileptogenicity of focal malformations due to abnormal cortical development: direct electrocorticographic–histopathologic correlations. Epilepsia. 2003;44:69–76. doi: 10.1046/j.1528-1157.2003.08102.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–37. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–23. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52:407–15. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- Bote RP, Blázquez-Llorca L, Fernández-Gil MA, Alonso-Nanclares L, Muñoz A, De Felipe J. Hippocampal sclerosis: histopathology substrate and magnetic resonance imaging. Semin Ultrasound CT MR. 2008;29:2–14. doi: 10.1053/j.sult.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Chassoux F, Devaux B, Landré E, Turak B, Nataf F, Varlet P, et al. Stereo-electroencephalography in focal cortical dysplasia: a 3D approach to delineating the dysplastic cortex. Brain. 2000;123 (Pt 8):1733–51. doi: 10.1093/brain/123.8.1733. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–66. 127–37. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Lorincz A, Buzsaki G. Physiological patterns in the hippocampo-entorhinal cortex system. Hippocampus. 2000;10:457–65. doi: 10.1002/1098-1063(2000)10:4<457::AID-HIPO12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Ozduman K, Bronen RA, Kim JH, Spencer DD. Long-term outcome after epilepsy surgery for focal cortical dysplasia. J Neurosurg. 2004;101:55–65. doi: 10.3171/jns.2004.101.1.0055. [DOI] [PubMed] [Google Scholar]
- Clusmann H, Kral T, Fackeldey E, Blümcke I, Helmstaedter C, von Oertzen J, et al. Lesional mesial temporal lobe epilepsy and limited resections: prognostic factors and outcome. J Neurol Neurosurg Psychiatry. 2004;75:1589–96. doi: 10.1136/jnnp.2003.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Bibbig A, Schmitz D. Ripple (approximately 200-Hz) oscillations in temporal structures. J Clin Neurophysiol. 2000;17:361–76. doi: 10.1097/00004691-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Dubeau F, Tampieri D, Lee N, Andermann E, Carpenter S, Leblanc R, et al. Periventricular and subcortical nodular heterotopia. A study of 33 patients. Brain. 1995;118 (Pt):1273–87. doi: 10.1093/brain/118.5.1273. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Pineda M, Tallada M, Oliver B, Russi A, Oller L, et al. Abnormal local-circuit neurons in epilepsia partialis continua associated with focal cortical dysplasia. Acta Neuropathol. 1992;83:647–52. doi: 10.1007/BF00299415. [DOI] [PubMed] [Google Scholar]
- Genow A, Hummel C, Scheler G, Hopfengärtner R, Kaltenhäuser M, Buchfelder M, et al. Epilepsy surgery, resection volume and MSI localization in lesional frontal lobe epilepsy. Neuroimage. 2004;21:444–9. doi: 10.1016/j.neuroimage.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Gobbelé R, Waberski TD, Simon H, Peters E, Klostermann F, Curio G, et al. Different origins of low- and high-frequency components (600 Hz) of human somatosensory evoked potentials. Clin Neurophysiol. 2004;115:927–37. doi: 10.1016/j.clinph.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hader WJ, Mackay M, Otsubo H, Chitoku S, Weiss S, Becker L, et al. Cortical dysplastic lesions in children with intractable epilepsy: role of complete resection. J Neurosurg. 2004;100 (2 Suppl):110–7. doi: 10.3171/ped.2004.100.2.0110. [DOI] [PubMed] [Google Scholar]
- Hannan AJ, Henke RC, Seeto GS, Capes-Davis A, Dunn J, Jeffrey PL. Expression of doublecortin correlates with neuronal migration and pattern formation in diverse regions of the developing chick brain. J Neurosci Res. 1999;55:650–7. doi: 10.1002/(SICI)1097-4547(19990301)55:5<650::AID-JNR12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hashimoto I. High-frequency oscillations of somatosensory evoked potentials and fields. J Clin Neurophysiol. 2000;17:309–20. doi: 10.1097/00004691-200005000-00008. [DOI] [PubMed] [Google Scholar]
- Hennessy MJ, Elwes RD, Rabe-Hesketh S, Binnie CD, Polkey CE. Prognostic factors in the surgical treatment of medically intractable epilepsy associated with mesial temporal sclerosis. Acta Neurol Scand. 2001;103:344–50. doi: 10.1034/j.1600-0404.2001.103006344.x. [DOI] [PubMed] [Google Scholar]
- Hirsch LJ, Spencer SS, Spencer DD, Williamson PD, Mattson RH. Temporal lobectomy in patients with bitemporal epilepsy defined by depth electroencephalography. Ann Neurol. 1991;30:347–56. doi: 10.1002/ana.410300306. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Miles AN, Dodrill CB, Ojemann GA, Wilensky AJ. Identifying potential surgical candidates in patients with evidence of bitemporal epilepsy. Epilepsia. 2003;44:1075–9. doi: 10.1046/j.1528-1157.2003.58302.x. [DOI] [PubMed] [Google Scholar]
- Huppertz HJ, Grimm C, Fauser S, Kassubek J, Mader I, Hochmuth A, et al. Enhanced visualization of blurred gray-white matter junctions in focal cortical dysplasia by voxel-based 3D MRI analysis. Epilepsy Res. 2005;67:35–50. doi: 10.1016/j.eplepsyres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008 May 9; doi: 10.1111/j.1528-1167.2008.01656.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Jones MS, Barth DS. Effects of bicuculline methiodide on fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2002;88:1016–25. doi: 10.1152/jn.2002.88.2.1016. [DOI] [PubMed] [Google Scholar]
- Kakita A, Hayashi S, Moro F, Guerrini R, Ozawa T, Ono K, et al. Bilateral periventricular nodular heterotopia due to filamin 1 gene mutation: widespread glomeruloid microvascular anomaly and dysplastic cyto-architecture in the cerebral cortex. Acta Neuropathol. 2002;104:649–57. doi: 10.1007/s00401-002-0594-9. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Le Van QM, Gozlan H, Ben-Ari Y. Epileptogenic actions of GABA and fast oscillations in the developing hippocampus. Neuron. 2005;48:787–96. doi: 10.1016/j.neuron.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Kothare SV, VanLandingham K, Armon C, Luther JS, Friedman A, Radtke RA. Seizure onset from periventricular nodular heterotopias: depth-electrode study. Neurology. 1998;51:1723–7. doi: 10.1212/wnl.51.6.1723. [DOI] [PubMed] [Google Scholar]
- Li LM, Cendes F, Watson C, Andermann F, Fish DR, Dubeau F, et al. Surgical treatment of patients with single and dual pathology: relevance of lesion and of hippocampal atrophy to seizure outcome. Neurology. 1997;48:437–44. doi: 10.1212/wnl.48.2.437. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15 (5 Pt 2):3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia D, Olivier A, Avoli M. Seizure-like discharges recorded in human dysplastic neocortex maintained in vitro. Neurology. 1995;45:1391–5. doi: 10.1212/wnl.45.7.1391. [DOI] [PubMed] [Google Scholar]
- Mintzer S, Cendes F, Soss J, Andermann F, Engel J, Jr, Dubeau F, et al. Unilateral hippocampal sclerosis with contralateral temporal scalp ictal onset. Epilepsia. 2004;45:792–802. doi: 10.1111/j.0013-9580.2004.35703.x. [DOI] [PubMed] [Google Scholar]
- Mohamed A, Wyllie E, Ruggieri P, Kotagal P, Babb T, Hilbig A, et al. Temporal lobe epilepsy due to hippocampal sclerosis in pediatric candidates for epilepsy surgery. Neurology. 2001;56:1643–9. doi: 10.1212/wnl.56.12.1643. [DOI] [PubMed] [Google Scholar]
- O’Brien TJ, So EL, Cascino GD, Hauser MF, Marsh WR, Meyer FB, et al. Subtraction SPECT coregistered to MRI in focal malformations of cortical development: localization of the epileptogenic zone in epilepsy surgery candidates. Epilepsia. 2004;45:367–76. doi: 10.1111/j.0013-9580.2004.54703.x. [DOI] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, et al. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–96. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- Olivier A, Germano IM, Cukiert A, Peters T. Frameless stereotaxy for surgery of the epilepsies: preliminary experience. Technical note. J Neurosurg. 1994;81:629–33. doi: 10.3171/jns.1994.81.4.0629. [DOI] [PubMed] [Google Scholar]
- Otsubo H, Iida K, Oishi M, Okuda C, Ochi A, Pang E, et al. Neurophysiologic findings of neuronal migration disorders: intrinsic epileptogenicity of focal cortical dysplasia on electroencephalography, electrocorticography, and magnetoencephalography. J Child Neurol. 2005;20:357–63. doi: 10.1177/08830738050200041501. [DOI] [PubMed] [Google Scholar]
- Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y. Focal neuronal migration disorders and intractable partial epilepsy: results of surgical treatment. Ann Neurol. 1991;30:750–7. doi: 10.1002/ana.410300603. [DOI] [PubMed] [Google Scholar]
- Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476–87. doi: 10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- Raymond AA, Fish DR, Sisodiya SM, Alsanjari N, Stevens JM, Shorvon SD. Abnormalities of gyration, heterotopias, tuberous sclerosis, focal cortical dysplasia, microdysgenesis, dysembryoplastic neuroepithelial tumour and dysgenesis of the archicortex in epilepsy. Clinical, EEG and neuroimaging features in 100 adult patients. Brain. 1995;118 (Pt 3):629–60. doi: 10.1093/brain/118.3.629. [DOI] [PubMed] [Google Scholar]
- Raymond AA, Fish DR, Stevens JM, Sisodiya SM, Alsanjari N, Shorvon SD. Subependymal heterotopia: a distinct neuronal migration disorder associated with epilepsy. J Neurol Neurosurg Psychiatry. 1994;57:1195–202. doi: 10.1136/jnnp.57.10.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124 (Pt 9):1683–700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan K, So EL, Silbert PL, Jack CR, Jr, Cascino GD, Sharbrough FW, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51:465–71. doi: 10.1212/wnl.51.2.465. [DOI] [PubMed] [Google Scholar]
- Scherer C, Schuele S, Minotti L, Chabardes S, Hoffmann D, Kahane P. Intrinsic epileptogenicity of an isolated periventricular nodular heterotopia. Neurology. 2005;65:495–6. doi: 10.1212/01.wnl.0000172350.25380.c7. [DOI] [PubMed] [Google Scholar]
- Spreafico R, Battaglia G, Arcelli P, Andermann F, Dubeau F, Palmini A, et al. Cortical dysplasia: an immunocytochemical study of three patients. Neurology. 1998;50:27–36. doi: 10.1212/wnl.50.1.27. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields T, Bragin A, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–8. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–52. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–15. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Tassi L, Colombo N, Garbelli R, Francione S, Lo Russo G, Mai R, et al. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain. 2002;125 (Pt 8):1719–32. doi: 10.1093/brain/awf175. [DOI] [PubMed] [Google Scholar]
- Tassi L, Colombo N, Cossu M, Mai R, Francione S, Lo Russo G, et al. Electroclinical, MRI and neuropathological study of 10 patients with nodular heterotopia, with surgical outcomes. Brain. 2005;128 (Pt 2):321–37. doi: 10.1093/brain/awh357. [DOI] [PubMed] [Google Scholar]
- Tassi L, Pasquier B, Minotti L, Garbelli R, Kahane P, Benabid AL, et al. Cortical dysplasia: electroclinical, imaging, and neuropathologic study of 13 patients. Epilepsia. 2001;42:1112–23. doi: 10.1046/j.1528-1157.2001.00501.x. [DOI] [PubMed] [Google Scholar]
- Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:369–87. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–66. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131 (Pt 4):928–37. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelmann R, Zijlmans M, Jacobs J, Chatillion C, Gotman J. Improving the visual identification of High frequency oscillations (Abstract) Epilepsia. 2008;49 (s7):29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.